Movements of platypuses around and through instream structures and natural barriers in the Jenolan Karst Conservation Reserve, New South Wales
Anne Musser A B * , Tom Grant
A B * , Tom Grant  C and Eren Turak
C and Eren Turak  D
D
A
B
C
D
Abstract
Severe flooding in early 2020 and 2021 necessitated major desedimentation works at the iconic Blue Lake in the Jenolan Karst Conservation Reserve (JKCR). Movements and behaviour of platypuses were monitored before, during and after these works, using direct observations, remote cameras and environmental DNA (eDNA) sampling. Platypuses were observed along a 3 km reach of the Jenolan River, including the areas where works occurred, although in low numbers. In their use of the available waterways, platypuses negotiated artificial barriers, including a 10 m high dam, two smaller weirs and natural waterfalls and cascades. Overland movements were detected through vegetation tunnels, drainage pipes and culverts, and individuals were seen entering the cave system, where eDNA was also detected. Platypuses responded to the works activity by foraging outside the affected areas but also continued to traverse these areas from time to time. We describe movements around and through instream infrastructure and past natural barriers and report on other species detected by remote cameras. These observations could help planning and deployment of bypasses suitable for movement of platypuses around anthropogenic barriers and provide insights into impediments to dispersal and gene flow within stream systems.
Keywords: Blue Lake, caves, dispersal, genetic connectivity, habitat disturbance, instream barriers, instream infrastructure, Jenolan Karst Conservation Reserve, Monotremata, Ornithorhynchidae, platypus.
Introduction
The Jenolan Karst Conservation Reserve (JKCR) includes the Jenolan River and its headwater tributaries, the well-known Jenolan Caves and Caves House accommodation complex and the Blue Lake (https://en.wikipedia.org/wiki/Jenolan_Caves_House; Fig. 1). This iconic water body, coloured blue by dissolved calcite from the source water passing through caves in the Jenolan Limestone, is retained by a dam (area 3500 m2; average width 31 m2; length 153 m) representing a significant visitor attraction but also providing suitable habitat for a resident wild platypus population, frequently viewed over decades by many visitors to the area (https://bluemountainstoursydney.com.au/attractions/blue-lake/). Following destruction of much of the vegetation lining the slopes of the upper Jenolan River catchment by severe bushfires late in 2019, exceptional local rainfall in February 2020 resulted in a catastrophic flood that partially filled the Blue Lake with sand, gravel and rocks, as well as finer sediment. As a result, draining of the remaining water held behind the dam and major excavation was undertaken to re-establish the previous structure of the Lake during 2020. Unfortunately, in early 2021 a further flood event filled much of the Lake again with sand, gravel and rocks, resulting in the necessity for re-excavation (i.e. desedimentation works).
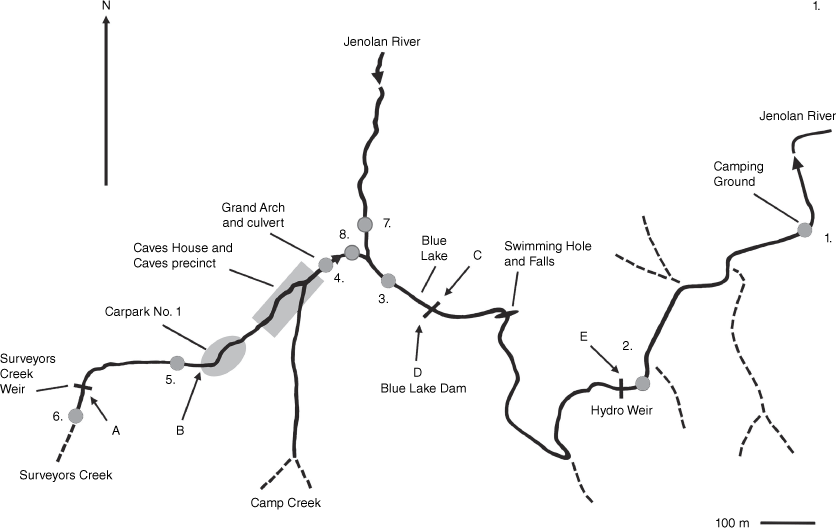
Map showing streams and locations of instream structures. Surveyors Creek flows in culverts under Car Park No. 1, beside parts of Jenolan Caves House and Caves Precinct and, in dry periods, descends into the limestone part way along the Grand Arch culvert. The Jenolan River descends into the Jenolan Caves themselves through the limestone several kilometres upstream of the Blue Lake. Locations of remote cameras (A–E) and eDNA sampling sites (1–8) are shown.
The possible impact of such works on the platypus population occupying the Blue Lake was unknown, and a program of monitoring before, during and after the first inundation, draining, excavation and refilling was undertaken. Fortunately, platypuses returned to the Blue Lake following this first flooding and the subsequent works. Monitoring was then continued before, during and initially following the second desedimentation works until it was abandoned due to weather and access issues in the middle of 2022.
We report on platypus movements within the natural steep, rocky and high energy reaches of the upper Jenolan River and its tributaries, including small rocky pools, boulder cascades and a high waterfall (~6 m), as well as a range of historical and current infrastructure associated with the establishment and operation of Caves House and Caves tourist and accommodation facilities from our monitoring through to June 2022. The infrastructure, including two water supply weirs, various culverts and pipes and a 10 m high dam, part of an early hydro-electricity scheme, was established in the late 1880s (Engineers Australia 1996). Movements around natural and anthropogenic barriers and structures are poorly understood and reported in the literature for the platypus. We also record the occurrence of other wildlife species detected by remote cameras around the instream infrastructure in our study area (Table 1, Supplementary Material).
| Site | Waterway | Location | Latitude/Longitude | Date sampled | PCRs | Result | Date sampled | PCRs | Result | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Jenolan River | Base of Old Campground Road | −33.8179909 | 09/10/2021 | 1 | Equivocal | 17/02/2023 | 1 | Equivocal | |
| 150.035779 | ||||||||||
| 2. | Jenolan River | River just past Hydro Dam | −33.8201649 | 09/10/2021 | 4 | Positive | 17/02/2023 | 1 | Equivocal | |
| 150.0320773 | ||||||||||
| 3. | Blue Lake | Blue Lake | −33.8196566 | 07/10/2021 | 1 | Equivocal | 17/02/2023 | 1 | Equivocal | |
| 150.0252616 | ||||||||||
| 4. | Surveyors Creek (flowing) + Camp Creek (dry) | Culvert, Grand Arch | −33.8194548 | 07/10/2021 | 1 | Equivocal | 17/02/2023 | 0 | Negative | |
| 150.0226665 | ||||||||||
| 5. | Surveyors Creek | Surveyors Creek @ Carpark One | −33.82144 | 09/10/2021 | 2 | Positive | 20/02/2023 | 0 | Negative | |
| 150.0190319 | ||||||||||
| 6. | Surveyors Creek | Surveyors Creek upstream of Surveyors Dam | −33.8217571 | 10/10/2021 | 1 | Equivocal | 20/02/2023 | 0 | Negative | |
| 150.0159457 | ||||||||||
| 7. | North side cave system | Imperial River | −33.818876 | 07/10/2021 | 0 | Negative | 23/02/2023 | 0 | Negative | |
| 150.023311 | ||||||||||
| 8. | South side cave system | Pool of Cerberus | −33.8194942 | 10/10/2021 | 2 | Positive | 23/04/2023 | 1 | Equivocal | |
| 150.02338926 |
Sampling locations Fig. 1. Equivocal category = only one assay (from a total of six assays from two water samples) returned a positive PCR result, indicating low levels of DNA; Positive = two or more assays positive; Negative = zero assays positive.
Materials and methods
The study site includes approximately 3 km of the upper Jenolan River, together with two of its headwater tributaries (Surveyors Creek and the ephemeral Camp Creek) within the Jenolan Karst Conservation Reserve (JKCR), about 170 km west of Sydney, New South Wales. These streams flow through steep gorge country and consist of a series of natural small pools, separated by boulder or cobble riffle sections with occasional gravel-bottomed or bedrock runs, and include three larger pools confined behind dams/weirs (Fig. 1). The riparian margins are mainly vegetated by indigenous and some introduced trees and shrubs, including River She-oaks (Casuarina cunninghamiana), a variety of Eucalyptus species and Soft Tree Ferns (Dicksonia antarctica). However, accumulated alluvial material consolidated by roots of riparian trees and shrubs, providing suitable banks for platypus burrows (Grant 2007, 2014), is restricted in occurrence. The Blue Lake represents the most suitable and extensive section of such bank habitat within the study area.
During desedimentation operations in the Blue Lake, works areas were under daily observation during working hours by one of us (AM) throughout the 2020 draining and excavation activities (16 July 2020 to 25 November 2020) and during the second excavation in 2022 (10 January 2022 to 28 May 2022). Additionally, morning and/or evening observations of the Blue Lake, the Jenolan River downstream of the Blue Lake and along Surveyors Creek were carried out on at least 3−4 days per week using the following equipment: camera and telephoto lens (Nikon D500/300 mm Nikon prime lens) and video camera (Sony AX700). Numerical and occurrence data arising from these surveys are to be collated, analysed and presented with reference to stages of the works in a later publication, but in this paper we present only details of our observations of platypuses negotiating infrastructure and natural barriers in our study area. Observations of platypuses negotiating artificial or natural barriers were recorded in field logs and/or on dated photographs or video records, including observations reported by other site workers. Images from remote cameras were collected and stored with corresponding dates and times.
Water samples (2 × 300 mL per site passed through attachable [leur lock] Sterivex© 0.22 µm filter units; lab analysis methods following Lugg et al. 2017) for detection of platypus environmental DNA (eDNA) were collected at locations shown in Fig. 1 (1–8) in October 2021 and February 2023. Sampling kits and protocols were provided by EnviroDNA, indicating no platypus DNA detected (Negative), low levels (Equivocal) or higher levels (Positive) detected (https://www.envirodna.com) (Table 1).
Remote cameras (Reconyx HC600 HyperFire Full Covert Camera) were placed at sites where platypuses had been seen negotiating barriers or where it was suspected they would do so (Fig. 1a–e). A single camera was placed at each site, which could detect movements in either direction past the barrier. However, on the north bank (left facing downstream; camera location C) at the Blue Lake, camera locations were changed to include the downstream end of a pipe connecting the upstream to the downstream side of the dam wall (termed the ‘Mousehole’). Water only flowed through this pipe at high lake levels and was used by other species as well as platypuses, including rodents, small marsupials and skinks, when it was dry (Supplementary Material). As well as some technical failures and loss through theft of one camera, cameras were actively recording images for varying periods across sites. As a result, data were calculated as numbers of platypus detections per full day of a camera deployment (and known to be recording) as the number of movements past the Blue Lake Dam or Hydro Weir walls per camera day and expressed as a percentage detection rate. Data on days when cameras failed, were not actively recording or had been moved or stolen were deleted from the data sets. Camera images where the direction of movement could not be determined were included only in calculation of the total number of platypus movements past the Blue Lake Dam wall in either direction. The frequency of camera days when one, two or three movements of platypuses past the Blue Lake Dam in either direction were detected for both banks was also recorded.
Results
Platypus occurrence within the study area
Platypuses were detected throughout the Jenolan study area, except for the usually dry Camp Creek, although the Blue Lake had by far the most platypus visits. Prior to the emptying and desedimentation works, 1–3 individual platypuses were frequently seen at this site. The Swimming Hole site below the waterfall (Fig. 1) was visited by platypuses next most frequently, with a single individual regularly seen and two observed on several occasions. Platypuses were seen, but less often, at other sites, including the Grand Arch culvert, Surveyors Creek/Caves House culvert, Jenolan River downstream of the Blue Lake and the remnant pool behind the Hydro Weir. Platypus visitation to these sites within the river system increased each time the Blue Lake had been emptied and when works activities were in progress there. The first eDNA determinations (October, 2021) confirmed the species’ distribution throughout the study site (three positive and four equivocal determinations across the eight sites sampled; Table 1). Surprisingly, platypus eDNA was detected only as Equivocal (one positive PCR from six assays) in the Blue Lake, where most observations of platypuses were made during the study, with the second sampling in February 2023 yielding equivocal results at four of the eight sites sampled, being negative at the other sites (Table 1).
Platypuses using the Caves
During the study, platypuses were observed on two occasions entering both the south (Pool of Cerberus) and north (Imperial) side (Supplementary Video S3) outflows from the cave systems without exiting during continuous observation from time of entry (0.5 and 1 h respectively). Water sampling in both October 2021 and February 2023 detected platypus eDNA in the Pool of Cerberus cave (2/6 positive PCR assays) but not in the Imperial Cave (Table 1).
Platypus movements around artificial barriers
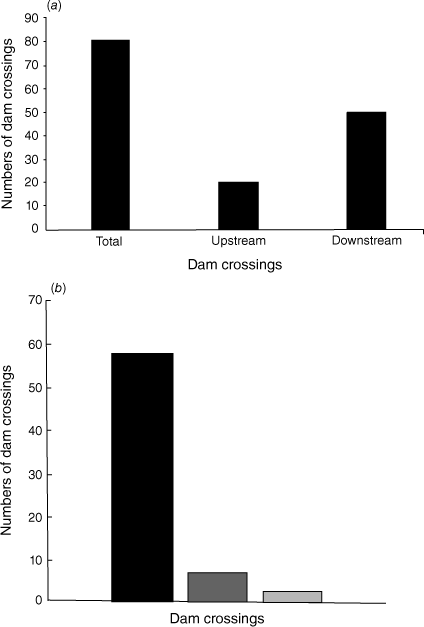
Table 2 and Fig. 2a, b show the frequency of platypus movements detected over, around or through a drainage hole (the ‘Mousehole’) in the vertical concrete wall of the Blue Lake Dam (Fig. 3a, b) and the frequency of dam wall crossings as a percentage of detections per day on which remote cameras were deployed and known to be working (camera days). A total of 81 crossings of the dam was recorded over 776 camera days. This total includes detected crossings where the direction of movement could not be determined (7), as well as days on which multiple crossings were detected. When adjusted for these multiple detections in a single camera day, at least a single crossing of the Blue Lake Dam wall occurred on 8.9% of days, including both upstream and downstream movements and for cameras on both sides of the dam. There were many fewer detections of platypuses crossing the dam wall moving upstream (21) compared with downstream (53) (Fig. 2a), and detections occurred more often on cameras on the left (northern; camera location C) bank than on the right (southern; camera location D) bank (Fig. 1 and Table 2). For the left (northern) bank, this suggests that there are alternative routes upstream to the Blue Lake (probably through vegetation), where crossings may not have been detected by the left bank remote cameras.
| Blue Lake Dam | Camera days | Moving upstream | Upstream detection % | Moving downstream | Downstream detection % | Total crossings | Total crossings detected % | Crossings at night % | |
|---|---|---|---|---|---|---|---|---|---|
| Left (north) bank cameras | 506 | 20 | 4.0 | 47 | 9.3 | 74 | 14.6 | 91 | |
| Right (south) bank cameras | 270 | 1 | 0.4 | 6 | 2.2 | 7 | 2.6 | 86 | |
| All cameras | 776 | 21 | 2.7 | 53 | 6.8 | 81 | 10.4 | – | |
| Hydro Dam | |||||||||
| Camera on right (south) bank. | 141 | 2 | 1.4 | 14 | 9.9 | 18 | 12.8 | 94 |
(a) Numbers of crossings of the Blue Lake Dam wall detected by the north and south bank remote cameras (776 camera days). Total movements in either direction (including those in which direction could not be established), and those in a definite upstream or downstream direction, are shown. (b) Total numbers of Blue Lake Dam wall crossings detected per camera day by the north and south bank remote cameras. The black column shows single crossings/day; medium grey, two crossings/day; light grey, three crossings in a 24 h period.
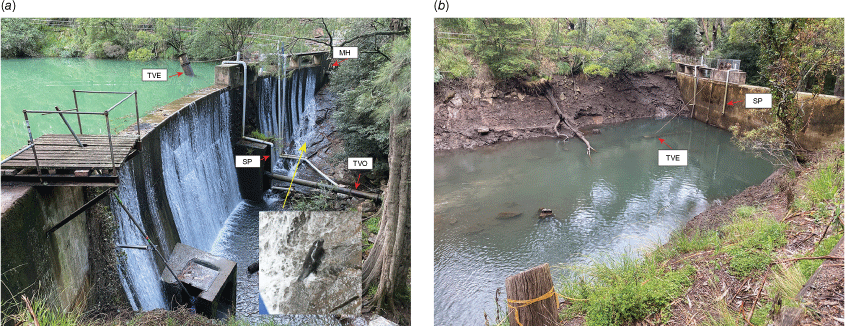
(a) Photo of the Blue Lake Dam wall showing the downstream face of the dam wall, its adjacent rock walls and the location of the ‘Mousehole’ (MH), siphon pipe (SP) and trunnion valve entrance (TVE) and outlet (TVO). The trunnion valve entrance is emergent, with its opening above the water level. Insert: platypus recorded climbing the wall (and subsequently going through the ‘Mousehole’ to the Blue Lake (Supplementary Video S2)). (b) Photo of the Blue Lake Dam wall from the upstream side, showing position and length of the siphon pipe (SP; vertical length approximately 6 m) used by a platypus to reach the Jenolan River downstream. The trunnion valve is lowered as it was when a platypus appears to have entered it to travel downstream of the dam.
Platypuses were detected, both visually and by remote cameras, negotiating past the main dam wall of the Blue Lake, ascending and descending the steep rocky banks by either keeping close to the base of the dam wall or through vegetated areas or ‘tunnels’. Movements through such vegetation ‘tunnels’ were detected on the south bank (right-hand side of the river looking downstream) (Fig. 1e, Supplementary Material) but ceased shortly after damage to vegetation on that bank during work at the base of the dam in February, 2021. Daily movements in either direction past the Blue Lake Dam wall varied in frequency, with most crossings of the dam wall occurring only once in a day, although as many as three crossings in a 24 h period were detected by the remote cameras (Fig. 2b). Throughout the study, platypuses were found to be frequently moving out of the stream onto land to move past the dam wall in both directions, although these bypasses were more frequent on the north bank, with many involving movements through the ‘Mousehole’ drain pipe. The majority (86–91%) of crossings of the Blue Lake dam wall occurred during the hours of darkness.
At least one individual platypus negotiated a siphon pipe (150 mm diameter) while it was siphoning water from the upper side of the dam, involving an ascent of over 6 m from the partially drained Blue Lake and a fall of nearly 10 m on the lower side through three right-angle bends, before exiting the horizontal downstream extension of the pipe into the river below. This was described by a worker, who witnessed it happening twice within a short period of time, possibly involving the same animal, which he described as being ‘small’. Observations of a larger platypus in the partially drained lake that suddenly disappeared from view suggest that it may have traversed through the trunnion valve (Fig. 3a, b), which was in the submerged position in the Blue Lake at the time, and its downstream pipe extension. The dam wall, its location and other structures discussed are shown in Fig. 3.
Platypuses were also found to be moving around the Hydro Weir wall (downstream height 4.15 m) (Figs 1 and 4a), where worn tracks or ‘slides’ were noted in the riparian vegetation, with a camera only deployed on the south bank and later in the study (camera location E; camera days 141; Table 2). Crossings of the Hydro Weir wall were detected on 12.8% of the days that the camera was deployed and known to be working, and 94% of these were during the hours of darkness.
Four photographs of other instream structures mentioned in the Results and Discussion sections: (a) Hydro Weir, including the historical fish ladder; (b) Surveyors Creek culvert, starting at Carpark No. 1; (c) Grand Arch culvert; and (d) a series of cascades in the Jenolan River with large boulders and/or bedrock.

Platypus movement through culverts
Opportunistic observations also detected platypuses moving through several culverts, including climbing over or under various structures associated with the culverts. One of us (AM) was able to observe a platypus first detected below the artificial waterfall downstream of the Carpark No. 1 section of Surveyors Creek culvert (Figs 1 and 4b), which subsequently travelled along the culvert system past Caves House and through the Grand Arch (Figs 1, 4c) to reach the Blue Lake. This individual, mentioned above, then entered the Imperial outflow exiting the south side cave system (Supplementary Video S3) and was not seen again despite an hour-long watch outside the outflow.
Movement of Platypuses past natural barriers (waterfalls and cascades)
The ~6 m high waterfall surrounded by rock cliffs at the Swimming Hole (Figs 1 and 5) represents the most significant natural barrier to platypus movement in our study area. Platypuses were regularly seen foraging in this pool. At least one individual platypus, identifiable by fur colouration around the ear/eye groove, was seen at the Blue Lake and downstream of the lake and the waterfall on a number of occasions. This observation, and the remote cameras detecting movements in both directions past the Blue Lake Dam wall, suggest that platypuses are likely to negotiate these falls during their normal movements within this relatively short reach of the river.
Swimming Hole site showing the observed route of one platypus bypassing the falls (Supplementary Video S1). FMR: Five Mile Road. Red stars indicate areas along the waterfall face where the platypus attempted to climb without success. Red dots indicate the observed route taken by the platypus up the rocky cliff.

Serendipitously, one of us (AM) was able to observe (and video) a platypus leaving the pool and climbing the sheer, vegetated rock cliffs of the valley adjacent to the falls. The platypus initially made several unsuccessful attempts to scale the waterfall and adjacent rock face, climbing repeatedly but falling back into the water on each attempt. It then swam over to an overhang and began to climb up the rock cliff, emerging on an open knoll well above the falls before continuing upward past a large pipe (part of the original hydro-electric infrastructure), after which it disappeared from sight. The platypus possibly continued around the top of the rock, which is almost level with the Five Mile Road (Fig. 5), before heading downhill to the Jenolan River and from there to the Blue Lake (the Swimming Hole was monitored for an hour after the overland traverse was videoed, and a platypus was seen in the Blue Lake upon return). The vertical distance from the pool to the crest of the rock wall is estimated to be 35–40 m. The platypus’s observed climb was mainly under vegetative cover; the platypus was out from under cover for just short periods of time relative to the total time of the climb (roughly 1.25 min out from under cover out of the total observed climb of just over 12 min) (Table 3). The videoed route taken is shown in Fig. 5, and a condensed video clip of the climb from the pool is available in the Supplementary Material section (Supplementary Video S1). Observations also showed platypus movements through the many smaller, but steep and fast-flowing, cascades within the streams in the study area (Fig. 5d).
| Clip number | Start of clip for climb only | End time on clip | Length of clip showing climb | Approximate time platypus exposed during climb | |
|---|---|---|---|---|---|
| C0443 | 1:18 | 11:04 | 9 min 14 s | 1 min 14 s | |
| C0444 | 0:00 | 1:11 | 1 min 11 s | – | |
| C0445 | 0:00 | 0:35 (lost sight of platypus at this point) | 0 min 35 s | – | |
| approx. 12 min climb observed (after which observer lost sight of platypus) |
Discussion
Historically, descriptions of favoured platypus habitat are of streams consisting of large pools, slow-flowing runs and cobbled riffle sections (suitable for foraging), with earth banks consolidated and overhung by riparian trees and shrubs (used for resting and nesting burrows) (Bennett 1860; Burrell 1927; Grant and Temple-Smith 1998). Such streams can support relatively large platypus populations (Grant and Carrick 1978; Grant 2007; Bino et al. 2015; Marchant and Grant 2015; Serena and Grant 2017). By contrast, the upper Jenolan River consists of a series of small and narrow pools, mainly separated by boulders, cascades and waterfalls, in a steep gorge landscape with rocky banks, mainly representing both poor foraging and burrowing habitat. It was therefore not surprising that the maximum number of individuals seen at any one time (in the Blue Lake prior to excavation) was usually only three, but observations, remote cameras and eDNA detected platypus occurrence throughout the approximately 3 km study area, with frequent movements around instream structures.
Use of caves
Two platypuses were observed swimming into the entrances to the cave system at the top end of the Blue Lake, remaining out of sight for some time (entrances observed for 30 min and 1 h respectively), suggesting they may have been foraging or resting inside the cave system. Although there have been no detailed observations, platypuses have been reported from caves in Tasmania, Victoria and NSW (Hamilton-Smith 1968; Lichon 1999), including Yarrangobilly Caves (Anne Musser, pers. obs.) and Wombeyan Caves (David Smith, pers. comm., with reporting in Tasmania of two apparent nesting sites (nesting material and platypus hair) 160 and 200 m inside a cave exit (Munks et al. 2004). There is at least one first-hand report of a platypus swimming inside a tourist cave (the Imperial Cave) upstream of the entrance to the Blue Lake during the late 1970s (Barry Richard, pers. comm.), and the occurrence of eDNA in the Pool of Cerberus cave in both sampling periods (Table 1) further suggests they may be using the caves for foraging, sheltering or possibly even nesting; however, this warrants further research.
Movements across barriers
In their modelling of the synergistic impacts of anthropogenic and natural influences on extinction risk to the platypus, Bino et al. (2020) ‘assumed that dams with a height of >5 m impeded movement along the river network between population units’ (p. 6). In our study, platypuses frequently moved in both directions past the 10 m high Blue Lake Dam wall and also past the waterfall (~6 m) at the Swimming Hole. Marked platypuses have also been recorded moving around the Belgrave Lake Weir on Monbulk Creek, near Melbourne in Victoria (Serena et al. 2023), and the Duckmaloi Weir in the central west of NSW (David Goldney, pers. comm.), with the walls of these weirs being just under 6 and 5 m high respectively. Armistead and Weeks (2009) and Griffiths and Weeks (2010) reported the movement of two separate marked individual male platypuses negotiating several high (up to ~35 m) waterfalls on the MacKenzie River (MacKenzie, Broken and Fish Falls), in the Grampians area of western Victoria, one moving upstream and the other downstream.
Mijangos et al. (2022) reported 4–20-fold greater genetic differentiation in platypuses captured above and below several high dams compared with platypuses captured along adjacent streams unregulated by large dams, also finding this differentiation increasing with the time since the dams were constructed. These authors suggest that such structures may be adversely affecting the population structure and possible long-term viability of platypus populations within catchments by restricting dispersal and gene flow. However, the size of a barrier may not be the only factor reducing population connectivity within a stream, especially with anthropogenic instream structures, where habitat changes around the structure may also be important. Furlan et al. (2013) reported that platypuses sampled in Olinda Creek, upstream of a relatively low weir forming Lillydale Lake (mean depth 3 m; https://en.wikipedia.org/wiki/Lillydale_Lake) exhibited genetic divergence from platypuses captured in other parts of the Yarra River catchment. Although our study of platypus movements in relation to instream barriers within a short section of a single stream, along with these genetic studies, sheds some light on the structuring of platypus populations, understanding of dispersal (juvenile and adult), gene flow and connectivity or fragmentation within and between platypus populations within streams awaits more extensive research in wild populations.
Movement through culverts, drains and pipes
Movement of platypuses through culverts, drains and pipes has been reported in a number of published studies and in anecdotal descriptions, particularly in Tasmania, where platypus road kills have been attributed to poorly designed culverts (Taylor et al. 1991; Otley and Le Mar 1998; Serena et al. 1999; Mooney and Spencer 2000; Magnus et al. 2004). Serena et al. (1999) reported radio-tracked platypuses readily moving in both directions through a concrete culvert (45 m in length, with a diameter of 1.35 m and a grade of 1 in 87.5), and Mooney and Spencer (2000) concluded that platypus avoidance of using culverts was unrelated to length, diameter, gradient or depth or permanency of water in the culvert, but rather to availability of access, with entry being restricted by undercutting at the downstream end of culverts under roads. Our study showed that platypuses are good climbers (Supplementary Material videoclips) but are unable to climb successfully if footholds are greater than about 150 mm apart (Tom Grant, pers. obs. on captive animals). However, Taylor et al. (1991) report six platypuses killed on roads in a number of places in Tasmania where apparently suitable culverts under the roads were present, suggesting reluctance of at least some individual platypuses to enter culverts.
The suggested movement through the relatively wide diameter (>400 mm) trunnion valve and associated pipes past the Blue Lake Dam wall was not surprising, but the reported movement of a platypus through a 150 mm siphon pipe was unexpected. Mainland platypuses were able to negotiate through by-catch reduction devices in fish traps of >55 mm diameter or square (Grant et al. 2004) but have been reported dead in PVC pipes of 100 mm diameter, where one end was blocked, indicating an inability to move backwards in a confined space or to turn around (Taylor et al. 1991). Platypuses are also unable to climb smooth surfaces, like PVC pipes (Tom Grant, pers. obs. of captive animals), so it must be assumed that the suction of water in the functioning siphon (150 mm diameter) permitted the platypus to ascend the pipe on the dam side of the siphon and that the water in the pipe cushioned its descent on the other side of the dam, where it dropped about m, then passing through three right-angle bends before reaching the bottom (Fig. 3) and out into the river.
It is unknown whether or not this platypus was drawn into the siphon or chose to enter the pipe. The workers reported a platypus exiting the bottom of the siphon pipe on two separate occasions on the same day, suggesting (based on the small size of both) that it could have been the same individual. At the time, a small juvenile male, recognised by its spur morphology (after slowing down and enlarging video footage of preening; see Grant 2007), was present in that section of the river and was seen investigating crevices and openings along the banks of the Blue Lake when it was empty. It was also videoed feeding in the pool below the dam in which the workers reporting the movement through the siphon pipe were standing, and climbing the rocky slope downstream of the dam up to the Blue Lake (Supplementary Video S2). Anecdotal reports of platypuses found in unusual locations (e.g. sewage treatment ponds, farm dams, ephemeral creeks and on land; Tom Grant, pers. obs.) are frequently of juvenile animals, and we wonder if movement through the siphon may represent investigative behaviour, more typical of younger than mature adult platypuses.
Movements across natural barriers
Movements overland are anecdotally reported, but platypuses normally forage in rivers, creeks, shallow lakes and wetlands, spending the rest of their time in burrows in the banks of these water bodies (Burrell 1927; Grant 2007). The single detection and videoing (Supplementary Video S1) of a platypus leaving the water and negotiating a circuitous overland route past the Swimming Hole waterfall (Figs 1 and 5), as well as remote camera detection of movements past both the Blue Lake and Hydro weir walls, suggest that movements past natural barriers in our study area probably occur quite frequently during foraging excursions. Whether or not platypuses also negotiate the long and hazardous route overland to travel downstream past the Swimming Hole waterfall is unknown, although the reporting of movement of animals through the trunnion valve and the siphon pipes makes us wonder if perhaps, except under flood conditions, platypuses may simply drop over the falls into the deep pool below.
Predators and possible predator avoidance behaviours
It has generally been assumed that platypuses mitigate predation pressure by either being in the water or in their burrows, but in situations like the upper Jenolan River, where foraging habitat is separated by cascades, falls and artificial structures, platypuses must frequently need to leave the water to get around these instream barriers despite the presence of a number of potential predators. Platypuses are occasionally reported being taken by raptors, including White-bellied Sea and Wedge-tailed eagles (Munday et al. 1998; Radick et al. 2001; Seale 2008), along with one report of platypus fur in three scats (<1% of samples) from a Spotted-tailed Quoll (Dasyurus maculatus) study (Dawson et al. 2007). Feral cats (Felis catus) and monitors (goannas) reportedly may also predate adult platypuses, and Water Rats (Rakali, Hydromys chrysogaster) may take young in burrows (Burrell 1927; Grant 2007; Serena et al. 2023). However, foxes (Vulpes vulpes) and dogs (feral and domestic; Canis lupus familiaris) are their most documented predators (Serena 1994; Connolly et al. 1998; Grant 2007; Serena and Williams 2010), with anecdotal reports of predation by these canids being more frequent during drought, when platypuses may move overland or through shallow riffles, seeking refuge pools in which to forage (Grant 2007).
In our study, remote cameras detected cats (F. catus), Rakali (H. chrysogaster) and a Lace Monitor (V. varius) at monitored bypass sites, along with a Spotted-tailed Quoll (D. maculatus) photographed investigating the drainage hole through the Blue Lake dam wall (‘Mousehole’), which was often used by platypuses and other indigenous and introduced small mammals and skinks (Table 1, Supplementary Material). The diurnal movement of a platypus overland past the Swimming Hole waterfall (Fig. 5) involved passage through vegetation, presumably to avoid detection (Supplementary Video S1). Platypuses were also detected moving past the Blue Lake Dam on both banks, with images showing the use of apparent ‘tunnels’ through the vegetation on the downstream side of the south bank (Fig. 1e, Supplementary Material), but no movements using this route were detected shortly after much of the ground vegetation was removed during work to replace the scour valve in the dam.
Facilitating passage across artificial barriers
Our study highlights the platypus’ ability to regularly leave its aquatic habitat to travel overland, to negotiate natural and artificial instream structures, and to move through structures such as culverts, drains and pipes, while avoiding predation by indigenous and introduced species often found across its current distribution. Their use of riparian vegetation as cover, moving predominantly during the hours of darkness and readily entering covered channels and pipes, appears to reduce predation and has allowed platypuses to access foraging and breeding resources along the Jenolan River, even during intrusive excavation works, facilitating distribution movement and presumably gene flow.
We suggest that by using a combination of vegetation plantings, tunnels, runnels, rock placements and/or pipes, it should be possible to design, test and implement simple and relatively low-cost structures to facilitate platypuses bypassing a range of pre-existing artificial instream structures. According to Mijangos et al. (2022), around 77% of the current high dams (>10–15 m) in Australia overlap the distribution of the platypus. Although the cost would be greater and require more complex planning, it should be possible to construct bypass routes for platypuses around at least some pre-existing high instream structures (Serena et al. 2023). We also encourage further research that would facilitate such an outcome and permit bypasses to be incorporated into the design of all new instream barrier structures.
Supplementary material
The Supplementary Material for this paper includes three figures: (1) platypus movements and behaviours; (2) platypus predators/potential predators/competitors; and (3) species previously unknown from JKCR captured on remote cameras. It also includes one table (a preliminary list of wildlife species other than platypuses detected by remote cameras) and three videos: (1) waterfall detour; (2) upward climb from the Jenolan River to the Blue Lake; and (3) platypus transit through culverts to south-side cave system. Supplementary material is available online.
Acknowledgements
We thank Jenolan Caves Reserve Trust and James Armstrong for partial funding for this project and for purchase of a Sony AX700 video camera. We thank Tom McAlister, Jason Madden, Cliff Kinchela and the Soil Conservation Service team for the care taken during excavation works. We acknowledge David Goldney for his personal communication relating to platypuses movements past the Duckmaloi Weir. We also thank Josh Griffiths of EnviroDNA, partly supported by funding awarded to EnviroDNA by the Australian Government through the Regional Bushfire Recovery for Multiregional Species, for providing environmental DNA sampling kits and analysis; Lochlan Dwyer (JCRT) for management of the remote cameras; Anne and Narawan Williams, who identified the small mammal species detected by remote cameras; and Barry Richards and David Smith, who provided information on platypuses in Jenolan and Wombeyan caves.
References
Bino, G., Grant, T. R., and Kingsford, R. T. (2015). Life history and dynamics of a platypus (Ornithorhynchus anatinus) population: four decades of mark-recapture surveys. Scientific Reports 5, 16073.
| Crossref | Google Scholar | PubMed |
Bino, G., Kingsford, R. T., and Wintle, B. A. (2020). A stitch in time - Synergistic impacts to platypus metapopulation extinction risk. Biological Conservation 242, 108399.
| Crossref | Google Scholar |
Connolly, J. H., Obendorf, D. L., Whittington, R. J., and Muir, D. B. (1998). Causes of morbidity and mortality in platypus (Ornithorhynchus anatinus) from Tasmania, with particular reference to Mucor amphibiorum infection. Australian Mammalogy 20, 177-187.
| Crossref | Google Scholar |
Dawson, J. P., Claridge, A. W., Triggs, B., and Paull, D. J. (2007). Diet of a native carnivore, the spotted-tailed quoll (Dasyurus maculatus), before and after an intense wildfire. Wildlife Research 34, 342-351.
| Crossref | Google Scholar |
Engineers Australia (1996). Jenolan Caves Engineering Works. Report on the Ceremony for the unveiling of the Historic Engineering Marker on Saturday 28 September 1996. Available at https://portal.engineersaustralia.org.au/system/files/engineering‐heritage‐australia/report‐title/Jenolan_Caves_Report.pdf
Furlan, E. M., Griffiths, J., Gust, N., Handasyde, K. A., Grant, T. R., Gruber, B., and Weeks, A. R. (2013). Dispersal patterns and population structuring among platypuses, Ornithorhynchus anatinus, throughout south-eastern Australia. Conservation Genetics 14(4), 837-853.
| Crossref | Google Scholar |
Grant, T. R. (2014). The platypus and the environmental impact assessment process: More cogitations of a consultant. Consulting Ecology (Newsletter of the Ecological Consultants Association of NSW) 33, 50-61.
| Google Scholar |
Grant, T. R., and Carrick, F. N. (1978). Some aspects of the ecology of the platypus, Ornithorhynchus anatinus in the upper Shoalhaven River, New South Wales. Australian Zoologist 20, 181-199.
| Google Scholar |
Grant, T. R., Lowry, M. B., Pease, B., Walford, T. R., and Graham, K. (2004). Reducing by-catch of platypuses (Ornithorhynchus anatinus) in commercial and recreational fishing gear in New South Wales. Proceedings of the Linnean Society of New South Wales 125, 259-272.
| Google Scholar |
Grant, T. R., and Temple-Smith, P. D. (1998). Field biology of the platypus (Ornithorhynchus anatinus) - historical and current perspectives. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 353, 1081-1091.
| Crossref | Google Scholar | PubMed |
Hamilton-Smith, E. (1968). Platypus in caves. Victorian Naturalist 85, 292.
| Google Scholar |
Lichon, M. (1999). Platypus in Tasmanian caves, and particularly in Croesus Cave. Journal of Mole Creek Caving Club 4, 21-27.
| Google Scholar |
Lugg, W. H., Griffiths, J., van Rooyen, A. R., Weeks, A. R., and Tingley, R. (2017). Optimal survey designs for environmental DNA sampling. Methods in Ecology and Evolution 9, 1049-1059.
| Crossref | Google Scholar |
Marchant, R., and Grant, T. R. (2015). The productivity of the macroinvertebrate prey of the platypus in the upper Shoalhaven River, New South Wales. Marine and Freshwater Research 66, 1128-1137.
| Crossref | Google Scholar |
Mijangos, J. L., Bino, G., Hawke, T., Kolomyjec, S. H., Kingsford, R. T., Sidhu, H., Grant, T., Day, J., Dias, K. N., Gongora, J., and Sherwin, W. B. (2022). Fragmentation by major dams and implications for the future viability of platypus populations. Communications Biology 5, 1127.
| Crossref | Google Scholar |
Mooney, N., and Spencer, C. (2000). Why did the platypus cross the road? Australian Mammalogy 21, 264.
| Google Scholar |
Munday, B. L., Whittington, R. J., and Stewart, N. J. (1998). Disease conditions and subclinical infections of the platypus (Ornithorhynchus anatinus). Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 353, 1093-1099.
| Crossref | Google Scholar | PubMed |
Munks, S., Eberhard, R., and Duhig, N. (2004). Nests of the platypus Ornithorhynchus anatinus in a Tasmanian cave. The Tasmanian Naturalist 126, 55-58.
| Google Scholar |
Otley, H. M., and Le Mar, K. (1998). Observation on avoidance of culverts by platypus. The Tasmanian Naturalist 120, 48-50.
| Google Scholar |
Rakick, R., Rakick, B., Cook, L., and Munks, S. (2001). Observations of a platypus foraging in the sea and hunting by a wedge-tailed eagle. The Tasmanian Naturalist 123, 2-4.
| Google Scholar |
Seale, J. (2008). Sea-eagle takes platypus. Field Notes. Boobook 26, 6.
| Google Scholar |
Serena, M. (1994). Use of time and space by platypus (Ornithorhynchus anatinus:Monotremata) along a Victorian stream. Journal of Zoology 232, 117-131.
| Crossref | Google Scholar |
Serena, M., and Grant, T. R. (2017). Effect of flow on platypus (Ornithorhynchus anatinus) reproduction and related population processes in the upper Shoalhaven River. Australian Journal of Zoology 65, 130-139.
| Crossref | Google Scholar |
Serena, M., and Williams, G. (2010). Factors contributing to platypus mortality in Victoria. Victorian Naturalist 127, 178-183.
| Google Scholar |
Serena, M., Williams, G. A., Thomas, J., and Worley, M. (1999). Effect of a flood retarding basin culvert on movements by platypus (Ornithorhynchus anatinus). Victorian Naturalist 116, 54-57.
| Google Scholar |
Taylor, R., Mooney, N., and Lange, K. (1991). Observation on platypus. The Tasmanian Naturalist 105, 1-3.
| Google Scholar |


