Platypus longevity: a new record in the wild and information on captive life span
Melody Serena A * , Gemma Snowball B , Jessica L. Thomas C , Geoff A. Williams A and Al Danger DA
B
C
D
Abstract
We report on a male platypus (Ornithorhynchus anatinus) who was tagged in November 2000 at the age of 1 year and recaptured in September 2023, when nearly 24 years old, in a small creek system in Melbourne’s southeastern suburbs. By comparison, a female platypus recently reached the age of 30 years in captivity, though with signs of ageing that may have precluded her survival if she were living in the wild. Ten other captive individuals have lived to an age of more than 20 years in recent decades.
Keywords: Dandenong Creek catchment, Healesville Sanctuary, male reproductive costs, Monbulk Creek, monotreme, Ornithorhynchidae, platypus life history, platypus survival in zoos, senescence.
Introduction
By definition, long-lived mammals are subject to relatively low mortality risk as adults. Positive associations between species-specific longevity and a low metabolic rate or adaptations to survive hypoxia have also been posited, possibly because these attributes select for biochemical features that also tend to protect animals against disease and ageing (Omotoso et al. 2021; Shilovsky et al. 2022). Both attributes plausibly apply to the platypus (Ornithorhynchus anatinus), which can remain submerged for at least 11 min (Evans et al. 1994) and expends metabolic energy at around half the rate of amphibious eutherian mammals of similar size (Grant and Dawson 1978; Bethge et al. 2001). To date, the maximum known life span for a wild platypus (a female who was lactating when last captured in the upper Shoalhaven River in New South Wales) is 21 years (Grant 2004).
We report here on a new longevity record for a platypus living in the wild and identify some possible contributing factors. We also provide a comprehensive list of platypus life spans recently exceeding 20 years in captivity.
Methods
Study area
Monbulk Creek is a small water course (typically 1–4 m wide) that arises in Dandenong Ranges National Park and flows for approximately 15 km to join Ferny Creek in Melbourne’s southeastern suburbs (Fig. 1). Median daily discharge from 1 January 2000 to 30 June 2023 at an automated gauging station located roughly midway along its length was 6.2 ML (range = 0–1085 ML) (station 228229 operated by Melbourne Water; records available for 81% of days in the period of interest). Mark–recapture studies indicate that Monbulk Creek supports a small platypus population, comprising 12–29 resident animals in the period from 1997 to 2007 (Serena et al. 2014). The population is believed to be effectively isolated: the only other platypus population found within the same drainage basin since 2000 occupied the Dandenong Creek headwaters until it apparently became extinct ~10 years ago (Melbourne Water, unpubl. data); it was segregated from the Monbulk Creek population by >20 km of badly degraded stream channel or underground concrete piping.
Capture, marking and measurements
Platypus survey nets were set by the Australian Platypus Conservancy (APC) at 18 sites distributed along the length of Monbulk Creek from February 1996 to February 2007 (mean sampling effort = 20.5 nights site−1), and by Ecology Australia (EA) at five sites distributed along 4.1 km of Monbulk Creek’s upper reaches in both April and September 2023. On all occasions, fyke nets were deployed in pairs (facing upstream and downstream) at several sites in the afternoon and attended overnight at intervals of ≤2 h (Serena and Williams 2012). Each platypus was marked when first captured with a uniquely coded transponder tag implanted subcutaneously between the scapulae (Grant and Whittington 1991). Age and sex were identified based on the appearance and size of calcaneal spurs (measured in a straight line along the outer aspect) and/or spur sheaths, with three male age classes (juvenile 1–12 month, subadult 13–24 month, adult ≥25 month) and two female age classes (juvenile 1–12 month, adult or subadult ≥13 month) identified; a standard hatching date of 1 November was assumed to apply (Williams et al. 2013). Animals were weighed to ±10 g (using a hand-held spring balance from 1996 to 2007) or ±1 g (using an electronic balance in 2023); dorsal body length from bill tip to tail tip was estimated to the nearest mm using dial or electronic callipers (bill and bill shield) and a small flexible tape measure (rest of the body). To estimate physical condition, tail thickness and turgidity were manually assessed and assigned to a standard five-point ordinal scale (Tail Volume Index, TVI) (Grant and Carrick 1978).
Captive life span assessment
The following pieces of information were extracted from the Zoo and Aquarium Association Australasia (ZAA) platypus studbook in November 2023 (n = 69 animals held in captivity since 1975) for individuals living >20 years: sex; source (wild- or captive-hatched); known or (for wild juveniles entering captivity when ≤6 month old) presumed hatching date; institution where first held; and life span/current age.
Results and discussion
Platypus longevity in the wild: a new record
In total, 62 O. anatinus were marked and released along Monbulk Creek from 1996 to 2007, including 43 adults or subadults (23 males, 20 females) and 19 juveniles (14 males, 5 females); 6 adults or subadults (4 males, 2 females) were marked and released in 2023.
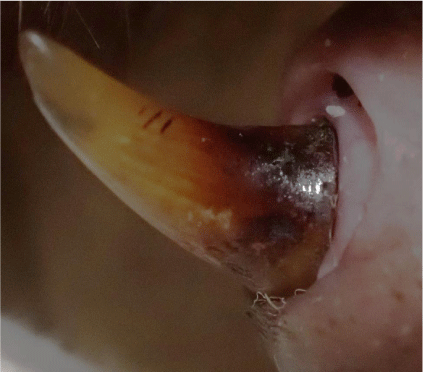
Male 01F6-03FF was first captured on 17 November 2000 at a site located ~3.5 km upstream of the Ferny Creek confluence. Based on spur morphology, he was classified as a newly subadult male (12.5 month old) with ‘very sharp’ spurs. He weighed 1750 g and his condition was rated as above average (TVI = 2) (Table 1). He was recaptured on 17 April 2023 at a site located ~9.2 km upstream of his initial capture site (similar to distances achieved by other males recaptured in this system: Serena et al. 1999) and again on 25 September 2023 (~0.7 km downstream of his April capture site), when he was 23.9 years old. His weight declined modestly (by ~8%) from April to September 2023, consistent with previous findings that adult male condition is lower in spring than in other months (Gust and Handasyde 1995; Connolly et al. 2016). His spurs were only about half as long in 2023 as compared with 2000, presumably due to gradual abrasion by the channel substrate (Williams et al. 2013), but still appeared to be sharp enough to be used to compete for mates; dark staining as seen around the spur base is a typical feature of sexually mature adult males (Fig. 2).
| Date | Weight (g) | TVI | Total length (mm) | Spur length (mm) | |
|---|---|---|---|---|---|
| 17.11.2000 | 1750 | 2 | 473 | 18.7, 18.6 | |
| 17.04.2023 | 2006 | 3 | 479 | ||
| 25.09.2023 | 1850 | 4 | 490 | 9.5, 9.6 |
TVI can vary from 1 (very fat) to 5 (very thin). See Methods for details of how measurements were obtained.
Mating effort has been inferred to drive male mortality in a wide range of polygynous mammals, due to aggressive interactions, reduced foraging effort and/or altered activity patterns that in turn contribute to adverse health outcomes and increased predation risk (e.g. Lukas and Clutton-Brock 2014; Thompson and Georgiev 2014). For example, in the case of the platypus, the upper Shoalhaven River in NSW supports a comparatively dense platypus population (~12 resident animals km−1), with females comprising a mean 84% of adults or subadults and males generally not encountered beyond the age of 7 years (Grant 2004; Serena and Grant 2017). Following on from findings for other mammals, these attributes are plausibly linked, insofar as high female density is predicted to contribute both to the value of a male defending an area containing multiple potential mates and to likely high costs of sexual competition (Serena and Grant 2017). By comparison, Monbulk Creek supports many fewer animals per unit length of channel (with a recorded maximum of 1.6 platypus km−1: Serena et al. 2014) and has a fairly even sex ratio (this study), suggesting that each male may typically seek to defend at most one or two females during the breeding season. Other factors potentially contributing to relatively low male reproductive costs in Monbulk Creek include its narrow channel (which in theory should make it easier for a male to defend a receptive female efficiently and exclude intruders: Gardner and Serena 1995; Gust and Handasyde 1995) and the fact that this population is spatially isolated (thereby presumably reducing the total number of intruders). However, further studies are needed to confirm the relevance of these factors to male longevity in the wild.
Platypus longevity in captivity
The ZAA studbook lists nine known-age individuals (either captive-bred or acquired from the wild as juveniles ≤6 month old) that have lived >20 years, including four males and five females (Table 2). Two other animals originally acquired from the wild as adults died in captivity at a minimum age of 21.4 years (male) and 23.7 years (female). The longest-lived male and female were respectively born in captivity (in 1998) and the wild (in 1993) and are still alive in November 2023, at 25 and 30 years of age. The 30-year-old female continues to feed normally and is healthy, apart from arthritis developing in one wrist and cataracts occurring in both eyes; her muted response to loud noises suggests that she is also becoming deaf (JLT, pers. obs.). These observations suggest that an age of 24 years, as documented along Monbulk Creek, may approach the maximum longevity expected in the wild due to the onset of age-related physical dysfunction.
Data availability
The data that support this study will be shared upon reasonable request to Jessica L. Thomas (captive records) or the corresponding author (records from the wild).
Declaration of funding
Platypus live-trapping studies were funded by Melbourne Water, City of Knox, Primelife Corporation and Yarra Ranges Council from 1996 to 2007, and by Melbourne Water in 2023. JLT’s involvement was facilitated by funding provided to Healesville Sanctuary by Dr Audrey Harvey, Dr Dennis Wilson and Helen Wilson.
Acknowledgements
We thank the many volunteers (too numerous to name individually) and Matthew Linn (EA) and Alice Ewing (EA) for assisting with platypus live-trapping activities, and Alice Ewing for providing permission to reproduce the spur image used in Fig. 2. We also thank the Zoo and Aquarium Association Australasia for authorising our use of platypus studbook records, and Tom Grant and Ross Goldingay for helpful feedback on an earlier draft of this manuscript. Research activities in Monbulk Creek were authorised by the Victorian Department of Energy, Environment and Climate Action (Wildlife Research Permits 95-208 through 10003546, APC; Wildlife Research Permit 10010423, EA) and the Victorian Fisheries Authority (Fisheries Permit RP 553, APC; Fisheries Permit RP 1142, EA), with annual oversight and approvals provided by the Australian Platypus Conservancy AEEC (Project 95/1) and Ecology Australia AEEC (Project 08.22).
References
Bethge, P., Munks, S., and Nicol, S. (2001). Energetics of foraging and locomotion in the platypus Ornithorhynchus anatinus. Journal of Comparative Physiology B 171, 497-506.
| Crossref | Google Scholar | PubMed |
Connolly, J. H., Claridge, T., Cordell, S. M., Nielsen, S., and Dutton, G. J. (2016). Distribution and characteristics of the platypus (Ornithorhynchus anatinus) in the Murrumbidgee catchment. Australian Mammalogy 38, 58-67.
| Crossref | Google Scholar |
Evans, B. K., Jones, D. R., Baldwin, J., and Gabbott, G. R. J. (1994). Diving ability of the platypus. Australian Journal of Zoology 42, 17-27.
| Crossref | Google Scholar |
Gardner, J., and Serena, M. (1995). Spatial organisation and movement patterns of adult male platypus, Ornithorhynchus anatinus (Monotremata: Ornithorhynchidae). Australian Journal of Zoology 43, 91-103.
| Crossref | Google Scholar |
Grant, T. R. (2004). Captures, capture mortality, age and sex ratios of platypuses, Ornithorhynchus anatinus, during studies over 30 years in the upper Shoalhaven River in New South Wales. Proceedings of the Linnean Society of New South Wales 125, 217-226.
| Crossref | Google Scholar |
Grant, T. R., and Carrick, F. N. (1978). Some aspects of the ecology of the platypus, Ornithorhynchus anatinus, in the upper Shoalhaven River, New South Wales. Australian Zoologist 20, 181-199.
| Google Scholar |
Grant, T. R., and Dawson, T. J. (1978). Temperature regulation in the platypus, Ornithorhynchus anatinus: production and loss of metabolic heat in air and water. Physiological Zoology 51, 315-332.
| Crossref | Google Scholar |
Grant, T. R., and Whittington, R. J. (1991). The use of freeze-branding and implanted transponder tags as a permanent marking method for platypuses, Ornithorhynchus anatinus (Monotremata: Ornithorhynchidae). Australian Mammalogy 14, 147-150.
| Crossref | Google Scholar |
Gust, N., and Handasyde, K. (1995). Seasonal variation in the ranging behaviour of the platypus (Ornithorhynchus anatinus) on the Goulburn River, Victoria. Australian Journal of Zoology 43, 193-208.
| Crossref | Google Scholar |
Lukas, D., and Clutton-Brock, T. (2014). Costs of mating competition limit male lifetime breeding success in polygynous mammals. Proceedings of the Royal Society B 281, 1786.
| Crossref | Google Scholar | PubMed |
Omotoso, O., Gladyshev, V. N., and Zhou, X. (2021). Lifespan extension in long-lived vertebrates rooted in ecological adaptation. Frontiers in Cell and Developmental Biology 9, 704966.
| Crossref | Google Scholar | PubMed |
Serena, M., and Grant, T. R. (2017). Effect of flow on platypus (Ornithorhynchus anatinus) reproduction and related population processes in the upper Shoalhaven River. Australian Journal of Zoology 65, 130-139.
| Crossref | Google Scholar |
Serena, M., and Williams, G. A. (2012). Effect of sex and age on temporal variation in the frequency and direction of platypus (Ornithorhynchus anatinus) captures in fyke nets. Australian Mammalogy 34, 75-82.
| Crossref | Google Scholar |
Serena, M., Williams, G., Thomas, J., and Worley, M. (1999). Effect of a flood retarding basin culvert on movements by platypus Ornithorhynchus anatinus. The Victorian Naturalist 116, 54-57.
| Google Scholar |
Serena, M., Williams, G. A., Weeks, A. R., and Griffiths, J. (2014). Variation in platypus (Ornithorhynchus anatinus) life-history attributes and population trajectories in urban streams. Australian Journal of Zoology 62, 223-234.
| Crossref | Google Scholar |
Shilovsky, G. A., Putyatina, T. S., and Markov, A. V. (2022). Evolution of longevity as a species-specific trait in mammals. Biochemistry (Moscow) 87, 1579-1599.
| Crossref | Google Scholar | PubMed |
Thompson, M. E., and Georgiev, A. V. (2014). The high price of success: costs of mating effort in male primates. International Journal of Primatology 35, 609-627.
| Crossref | Google Scholar |
Williams, G. A., Serena, M., and Grant, T. R. (2013). Age-related change in spurs and spur sheaths of the platypus (Ornithorhynchus anatinus). Australian Mammalogy 35, 107-114.
| Crossref | Google Scholar |