Dispersal potential of Scaevola crassifolia (Goodeniaceae) is influenced by intraspecific variation in fruit morphology along a latitudinal environmental gradient
Lydia K. Guja A B D , David J. Merritt B C , Kingsley W. Dixon B C and Grant Wardell-Johnson AA Curtin Institute for Biodiversity and Climate, Curtin University, GPO Box U1987, Perth, WA 6845, Australia.
B Kings Park and Botanic Garden, West Perth, WA 6005, Australia.
C School of Plant Biology, Faculty of Science, The University of Western Australia, Crawley, WA 6009, Australia.
D Corresponding author. Present address: Centre for Australian National Biodiversity Research, CSIRO Plant Industry, Acton, ACT 2601, Australia. Email: Lydia.Guja@environment.gov.au
Australian Journal of Botany 62(1) 56-64 https://doi.org/10.1071/BT13290
Submitted: 3 December 2013 Accepted: 24 March 2014 Published: 28 April 2014
Abstract
Dispersal of plant propagules by ocean currents can result in long-distance dispersal and is important for the persistence of coastal species. However, the ability of such species to disperse via the ocean is often unknown because there is relatively little evidence that demonstrates that seeds or fruits can float and survive for extended periods in seawater. Furthermore, the seed or fruit traits, and intraspecific variation in these traits, that facilitate buoyancy remain largely unidentified. The genus Scaevola (L.) contains several widespread coastal species that may be capable of oceanic dispersal, such as S. crassifolia (Labill). We collected fruits of S. crassifolia along 700 km of a latitudinal environmental gradient. These fruits were used to determine the influence of fruit morphology and anatomy on fruit buoyancy. Morphological and anatomical variation in S. crassifolia was associated with dispersal potential. Our empirical data demonstrated that fruits with larger aeriferous mesocarp layers have greater buoyancy and, therefore, enhanced capacity for long range oceanic dispersal. Of three characters hypothesised to affect buoyancy (aeriferous mesocarp, air pockets in empty locules, and number of vascular cavities), only the properties of the mesocarp were significant. Intraspecific variation can significantly affect dispersal potential, and should not be overlooked in dispersal ecology.
Additional keywords: coast, hydrochory, oceanic dispersal, seed buoyancy.
Introduction
Seed dispersal is crucial to the formation and persistence of plant populations, and hydrochory (dispersal by water) is increasingly recognised as an important dispersal mechanism. Hydrochory, as with other dispersal mechanisms, significantly affects patterns and processes at genetic (e.g. gene flow and diversity), community (e.g. species richness and arrival of new species) and population (e.g. longevity and range size) levels (Nilsson et al. 2010). Capacity to undertake long-distance dispersal is likely to be increasingly important for the persistence of plant species as their ranges shift in response to continuing global climate change (Hughes 2000; Travis et al. 2013). Dispersal via ocean currents can potentially result in long-distance dispersal, with recent phylogenetic and biogeographic studies leading to a resurgence of support for oceanic hydrochory as a means of long-distance dispersal of plants (Howarth et al. 2003; de Queiroz 2005; Cousens et al. 2008; Dawson and Hamner 2008; Kokubugata et al. 2012). However, unlike Darwin’s seminal studies of oceanic hydrochory (Darwin 1856, 1859), recent hypotheses are generally not supported by evidence that fruits or seeds are capable of remaining buoyant and surviving in seawater for the extended periods required to achieve effective long-distance dispersal.
Buoyancy is a key factor that governs hydrochorous dispersal of seeds, which may be associated with intraspecific variation in morphological and anatomical traits of the dispersal unit (Lopez 2001; Leyer and Pross 2009; Vargas et al. 2014). However, differences in the morphology of seeds or fruits within a species may also influence dispersal capacity (Darling et al. 2008). Such variation may arise through genetic variation, the location of fruits in the infructescence, or the maternal environment during fruit development and maturation (Matilla et al. 2005; Donohue 2009). Such variation in seed or fruit morphology may greatly affect the dispersal potential of seeds (Darling et al. 2008). For example, infraspecific variation in subspecies of Bolboschoenus maritimus (L.) (Cyperaceae) affects the duration of achene buoyancy (Hroudova et al. 1997). B. maritimus subsp. compactus possesses well developed aeriferous tissue in the exocarp, resulting in greater buoyancy than the achenes of B. maritimus subsp. maritimus, which have thin or no aeriferous tissue in the exocarp (Hroudova et al. 1997). Further, the effect of intraspecific variation can be evident in seeds from the same parent plant. For example, at low wind speeds, the dispersal distance of winged seeds of Spergularia marina (L.) (Caryophyllaceae) was greater than that of seeds without wings, whereas in water, winged seeds were more frequently trapped in vegetation than seeds without wings (Telenius and Torstensson 1989). At a time when metadata analysis and multispecies models are increasingly common, intraspecific variation is often overlooked despite the potential to influence the frequency of rare events such as long-distance dispersal.
Intraspecific variation in seeds and fruits has been linked to differences in dispersal capacity and used to identify specific traits associated with large dispersal distances. Manipulation of seeds or fruits, or the construction of mimics (artificial seeds or fruits), is one method of investigating relationships between dispersal and intraspecific variation in seed or fruit traits such as mass, area and the presence of appendages (Augspurger and Franson 1987; Hughes and Westoby 1992; Yang et al. 2012). Although many seed and fruit traits are frequently proposed to be associated with particular dispersal vectors, the traits associated with hydrochorous dispersal have rarely been demonstrated experimentally. Seed characteristics associated with air chambers are commonly assumed to result in extended buoyancy (Higgins et al. 2003; Cousens et al. 2008) and some investigations have determined the particular traits that influence buoyancy in a small number of plant species. For example, aeriferous tissue in the exocarp of Bolboschoenus (Cyperaceae) (Hroudova et al. 1997), low specific weight (mass/volume) of 12 species from Panama (Lopez 2001), and the volume of an air pocket between embryonic cotyledons in Swartzia (Fabaceae) seeds (Williamson et al. 1999) are all associated with prolonged buoyancy. The effect of morphological traits on buoyancy requires further investigation, particularly for species that have diaspore morphologies different from those of the few species that have been studied.
The majority of Goodeniaceae genera are confined to the Australian continent, except for Scaevola, which is pantropical; 40 of 130 species are found outside Australia (Howarth et al. 2003). These species occur throughout the coastal regions of the Pacific and Indian Oceans, including the tropical Americas, Africa, Philippines, China, Marquesas and Hawaiian Islands (Howarth et al. 2003). Phylogenetic relationships within Scaevola suggest three independent colonisation events from the Australian continent to the isolated Hawaiian Islands (Howarth et al. 2003). This finding, combined with widespread distributions of several Scaevola species, implies a capacity for long-distance dispersal in Scaevola. This capacity may result from or be enhanced by fruit morphology. For example, it has been hypothesised that the widespread distribution of S. taccada may be due to its fleshy exocarp (facilitating bird dispersal), or its corky mesocarp (facilitating buoyancy in water) (Lesko and Walker 1969; Howarth et al. 2003). Certainly, the fruits of the widely distributed S. crassifolia and S. taccada are buoyant and survive in seawater for 42 and 120 days, respectively, without adverse effects on seed germination, demonstrating a capacity for oceanic dispersal (Lesko and Walker 1969; Guja et al. 2010).
The aeriferous, cork-like fruit coat of Scaevola species is often identified as a key trait that may determine oceanic dispersal ability (Lesko and Walker 1969; Howarth et al. 2003). However, there have been no experimental investigations of the capacity of the aeriferous fruit coat, or intraspecific variation in fruit morphology, to influence buoyancy. Fruits of all Scaevola species are indehiscent and drupe-like, with a hard endocarp towards the locules (Carolin 1966). Three layers are visible in most species and are referred to as epicarp, mesocarp and endocarp, although they do not correspond exactly to the layers as named in true drupes because the epicarp is likely to be derived from outer floral whorls rather than ovary tissue (drupe-like, cf. Carolin 1966). In fruits of S. crassifolia, the endocarp and mesocarp are not differentiated and are, henceforth, referred to as mesocarp. Unlike some Scaevola species that have succulent or fleshy epicarp and mesocarp layers, S. crassifolia fruits are dry with gradation to unthickened cells on the outermost part of the fruit (Carolin 1966). The outer mesocarp consists of air-filled parenchyma cells, which in some species is presumed to be the feature most likely to promote buoyancy (Lesko and Walker 1969; Howarth et al. 2003). Fruits of S. crassifolia have two (Carolin 1966), or occasionally three locules (L. K. Guja, pers. obs.). Often only one locule is filled (L. K. Guja, pers. obs.) and the resultant air pocket in the empty locule, surrounded by hard, dry mesocarp, may increase fruit buoyancy. False locules, and small cavities in the mesocarp, are formed by disintegration of vascular bundles (Carolin 1966) and these air pockets may also influence fruit buoyancy.
In the present study, we aimed to identify the anatomical features of Scaevola fruits that are most strongly associated with buoyancy, and to test for relationships between buoyancy and intraspecific morphological variation in fruits. We reasoned that the fruits of S. crassifolia possess the following three main anatomical traits that may be related to buoyancy: (1) aeriferous mesocarp, (2) air pockets in empty locules and (3) vascular cavities. We quantified anatomical and morphological variation of fruits along a latitudinal environmental gradient and investigated how this variation affected dispersal potential. Specifically, we hypothesised that (1) the anatomical structure of fruits would affect buoyancy, such that fruits with large aeriferous mesocarp and many vascular cavities would be most buoyant, and (2) fruits containing one seed and one empty locule would be more buoyant than fruits containing two filled locules.
Materials and methods
Study species and sites
Scaevola crassifolia is a shrub 0.1–1.5 m high, occurring on frontal coastal sand dunes and limestone cliffs in western and southern Australia (Western Australian Herbarium 1998; Rippey and Rowland 2004; Dixon 2011). The inflorescence is a terminal to subterminal spike of blue/mauve/white flowers present from July to February (Western Australian Herbarium 1998; Dixon 2011; Rippey and Rowland 2004). Fruits are more or less spherical, slightly compressed, hard, woody and minutely hairy (Marchant et al. 1987). The north–south distribution of S. crassifolia along the south-western Australian coastline extends for ~2000 km along a strong temperature and rainfall gradient – dry and hot in the north, to wet and cool in the south (Bureau of Meteorology 2013; CSIRO 2013). To examine morphological variation of fruits, collections of S. crassifolia fruits were made at eight distant localities along 700 km of the latitudinal temperature and rainfall gradient (Fig. 1). Average annual rainfall at the study sites is 400 mm in the north and 1200 mm in the south (Bureau of Meteorology 2013), and average annual daily mean temperature ranges from 21°C in the north to 15°C in the south (Bureau of Meteorology 2013). Climate data are based on 30-year climatology (1961–1990).
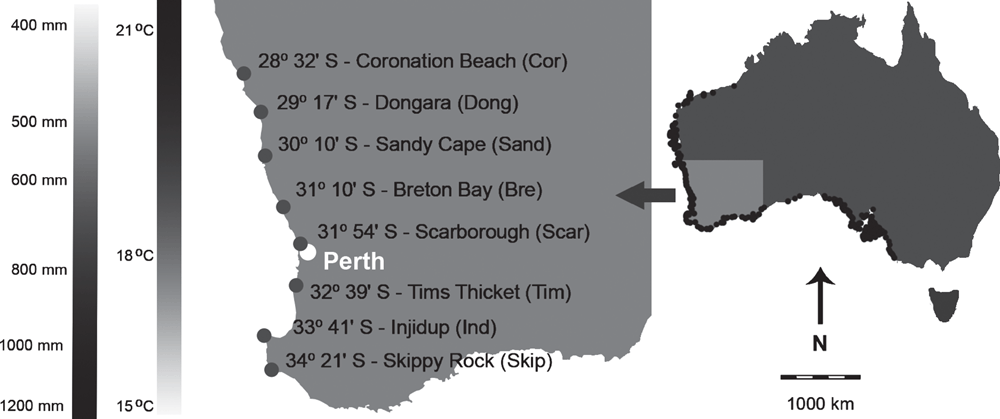
|
Fruit collection
Mature fruits (browned, woody, dry and easily detached with minimal force) were collected at the natural point of dispersal along the length of multiple infructescences from at least 10 plants at each site between January and April 2009. The average number of fruits collected per site was 23 000 (min 9750 at Breton Bay and max 39 000 at Tim’s Thicket). Herbarium vouchers for each collection were lodged at the Kings Park and Botanic Garden Herbarium (KPBG). Collector’s field numbers are Coronation Beach LKG003, Dongara LKG024, Scarborough LKG107, Sandy Cape LKG031, Breton Bay LKG060, Tim’s Thicket LKG066, Injidup LKG078, Skippy Rock LKG090. At the northern-most site, Coronation Beach, several fruits at the basal end of infructescences had already been released at the time of collection and only some fruits remained at the distal end. Fruits were stored within 2 days of collection in a controlled-environment room at 15°C and 15% relative humidity (RH). After 5 months of storage, when all fruits had equilibrated to 15% RH, fruits were then sealed in laminated aluminium-foil bags and stored at −18°C until used in experiments.
Sample selection
To identify fruits that comprised zero, one or two seeds, fruits were imaged using a digital X-ray (Faxitron MX-20, Faxitron X-ray, Lincolnshire, IL, USA). Fruits from each collection site were mounted upright on double-sided tape so that both locules were visible in X-ray images. For buoyancy experiments and subsequent morphological and anatomical measurements, 30 single-seeded fruits (three replicates of 10) were selected randomly from each collection. For the second experiment, a comparison of buoyancy of one- and two-seeded fruits, samples from all sites were pooled, X-rayed as above, and one- and two- seeded fruits (three replicates of 20 each) were randomly selected. Fruits containing more than two locules were rare and were not used in experiments.
Buoyancy of fruits
To determine buoyancy of fruits from each collection site, three replicates of 10 dry fruits were placed in 250 mL of seawater in plastic containers (11 cm in diameter, 4 cm high, Genfac Plastics, Melbourne, Victoria, Australia). To release surface tension, the water in each container was initially stirred for 30 s (Day 0). During the experiment, the container was topped up with deionised water to balance losses from evaporation and during this process all fruits were agitated (modified from Guja et al. 2010). The same procedure was followed to determine buoyancy of fruits containing one or two seeds for each replicate of 20 fruits (pooled across all sites). For both experiments, the number of buoyant fruits was recorded at 0, 3, 6 and 8 days, after stirring. After 8 days, ~50% of fruits remained buoyant and the experiment was terminated so that the buoyant and non-buoyant sample sizes were approximately equal for analysis.
Fruit morphology and anatomy
To study variation in the anatomical features of fruits from each site, sunken and buoyant fruits were removed from seawater, separated, and rinsed in deionised water to remove external salt. Fruits from each replicate were then dried by being placed in 10 g of silica gel for 2 days. Each fruit was weighed, height (Fig. 2) was measured with digital callipers, and each fruit was then cut transversely to expose both locules. One half of the section was mounted, with the cut surface facing upward, on carbon tape on an aluminium stub. Before examination in a scanning electron microscope (SEM), stubs were sputter-coated with gold at 25 mA for 2 min (Emitech K550X, Quorum Technologies, Ashford, Kent, UK). Fruits were imaged using a Jeol JCM6000 SEM under vacuum (Jeol, Sydney, NSW, Australia), with an accelerating voltage of 10 kV and magnification between ×28 and ×40, as required.
Measurements of the length (perpendicular to locules) and width (parallel to locules) (Fig. 2) of the fruit cross-section were made using the scaler function on the graphical user interface of the Jeol JCM6000 licenced software. In cross-section, measurements were taken at both the minimum and maximum to record the variation in the width of layers. The maximum and minimum width of the pericarp (from the inside edge of the locule to the outer edge of the epicarp), outer mesocarp (aeriferous cells) and inner mesocarp (hard layer) were measured (Fig. 2). Approximate total fruit volume (mm3) was calculated using the formula for an ellipsoid sphere (4/3π × length × width × height). Specific weight (mg µL–1) was calculated as mass/volume. Average layer width was calculated as (max + min)/2 for each layer. The ratio of outer to inner mesocarp was calculated by dividing the mean of the outer by the mean of the inner mesocarp. The number of distinct vascular cavities in each fruit was counted.
Statistical analysis
To investigate the extent of intraspecific variation in S. crassifolia fruits from different collection locations, each morphological and anatomical variable was analysed with ANOVA, with post hoc Tukey’s multiple comparisons of means (R version 2.15.1, R Foundation for Statistical Computing, Vienna). Differences in mean buoyancy (% after 8 days) of fruits from each collection site were also compared with one-way ANOVA, with post hoc Tukey’s multiple comparison of means. Where required, data were transformed and checked to ensure they met assumptions of normality and homogeneity of variance (Shapiro–Wilk test). Fruit width and the number of vascular cavities could not be transformed to normal and were therefore not included in the ANOVAs. Non-transformed data are shown in figures and tables.
To determine the effect of morphological and anatomical variables on buoyancy at 8 days, the two groups (buoyant versus sunken) were compared using binomial generalised linear models (GLM) with a logit link function. All variables were analysed because normality was not prerequisite. Binomial GLMs with a logit link function were also used to compare means for buoyancy of one- versus two-seeded fruits at each time.
Results
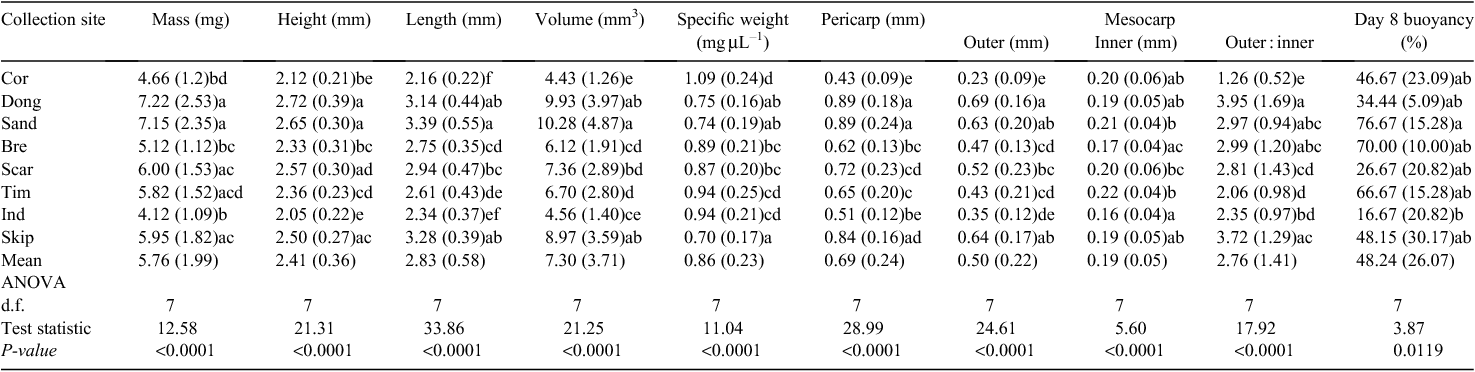
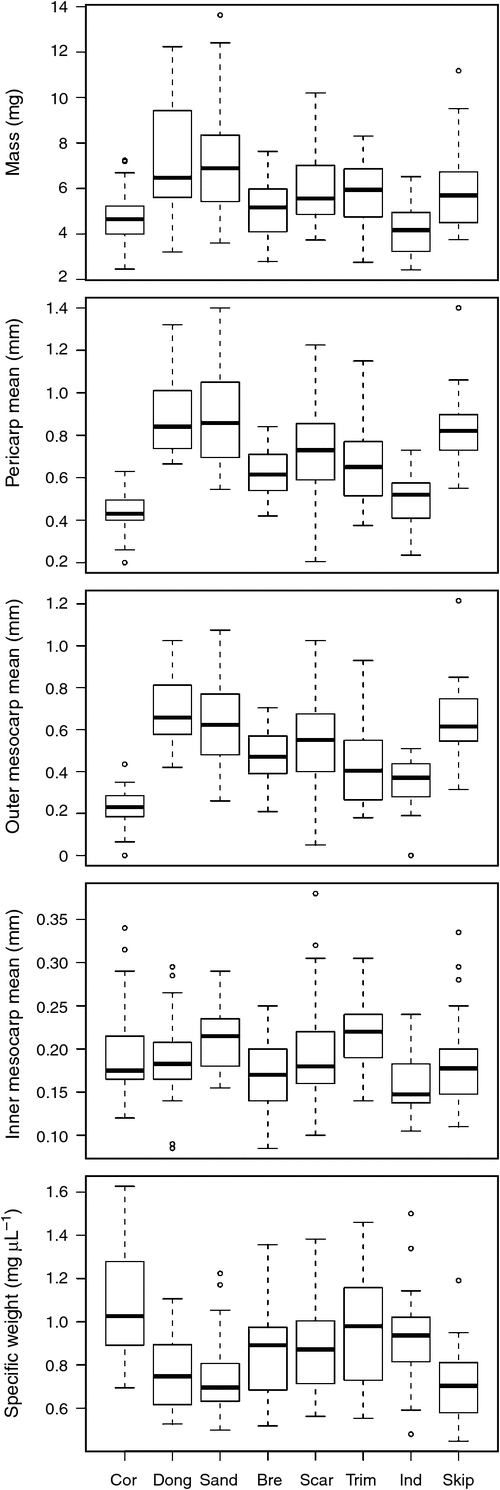
There were significant differences among all morphological and anatomical traits (that could be transformed to normal) of fruits from different collection locations (Fig. 2, Table 1). Post hoc tests revealed that fruits from Coronation Beach (northern-most study site) were generally the smallest and had the smallest length (2.16 ± 0.22 mm), volume (4.43 ± 1.26 mm3), pericarp (0.43 ± 0.09 mm), outer mesocarp (0.23 ± 0.09 mm) and mesocarp outer inner ratio (1.26 ± 0.52). These fruits were also the densest (highest specific weight 1.09 ± 0.24 mg µL–1) among all sites. Fruits from Dongara (~100 km south of Coronation Beach) were generally the largest and had the greatest mass (7.22 ± 2.53 mg), height (2.72 ± 0.39 mm), pericarp (0.89 ± 0.18 mm), outer mesocarp (0.69 ± 0.16 mm) and mesocarp outer inner ratio (3.95 ± 1.69) among all collections. Variation in fruit morphological and anatomical traits (i.e. mass, height, length, volume, specific weight, pericarp mean, outer mesocarp mean and mesocarp outer inner ratio, but not inner mesocarp mean) varied according to collection site, and south from Dongara, there was a general trend of smaller and denser fruits (to Injidup) (Table 1, Fig. 3, selected variables). However, this trend did not extend to the most northern (Coronation Beach) and southern (Skippy Rock) study sites.
|
|
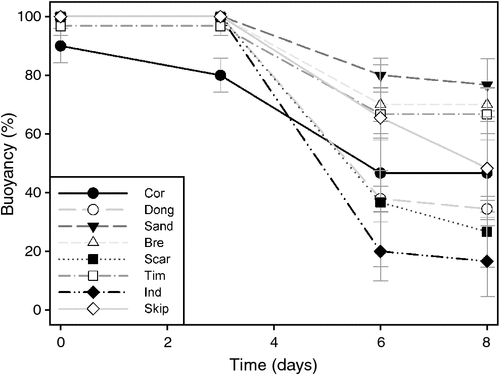
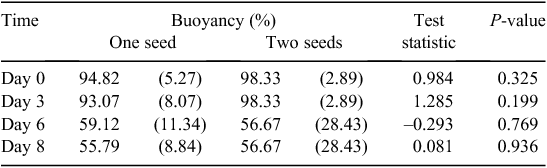
Initially, an average of 98% of all fruits were buoyant in seawater. After 8 days, an average of 48% of all fruits remained buoyant. The rate at which fruits sank varied with collection site (Fig. 4). After 8 days, 77% of fruits from Sandy Cape remained buoyant, being the highest percentage among all collection sites, whereas only 17% of fruits from Injidup remained buoyant, being the lowest percentage among all collection sites (Fig. 4). There was no significant difference between buoyancy of single-seeded versus double-seeded fruits at any of the time points investigated (Table 3).
|
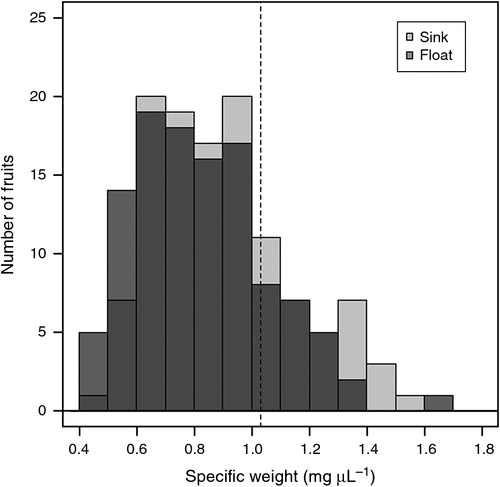
Across all collection locations, many morphological and anatomical variables were significantly different between buoyant and sunken fruits (as separated after 8 days in seawater). Length, volume, pericarp (mean, maximum and minimum), outer mesocarp (mean, maximum and minimum) and mesocarp outer inner ratio were significantly greater in buoyant fruits than in sunken fruits (Table 2). Mean mesocarp thickness was significantly greater for buoyant than for sunken fruits. The 25% and 75% quartiles for the outer mesocarp of buoyant fruits were 0.381 to 0.665 mm (n = 118), and 0.281 to 0.600 mm (n = 112) for sunken fruits. Buoyant fruits also had a lower specific weight than did sunken fruits (Table 2, Fig. 5). Mass, height, width, inner mesocarp and vascular cavities did not significantly affect fruit buoyancy at 8 days (Table 2).
|
|
Discussion
The present study has described large intraspecific variation in the morphology and anatomy of Scaevola crassifolia fruits along a latitudinal gradient, and resultant differences in dispersal potential of fruits from each collection site. The basic morphology and anatomical character of fruits was consistent. Yet, as hypothesised, intraspecific variation in the anatomical structure of fruits, particularly the size of the aeriferous outer mesocarp, was related to buoyancy. Here we demonstrated empirically, for the first time, that larger aeriferous mesocarp increases fruit buoyancy and can, therefore, increase the probability and distance of hydrochorous dispersal. These data empirically support propositions made in other studies that aeriferous or cork-like fruit and seed coats increase buoyancy potential (Lopez 2001; Cousens et al. 2008; Guja et al. 2010; Nilsson et al. 2010; Vargas et al. 2014). These data also demonstrated that the relationship between aeriferous tissue in the exocarp and buoyancy described for achenes (Hroudova et al. 1997) is similar for drupe-like woody fruits with multiple locules. Of the three fruit characteristics expected to affect buoyancy of S. crassifolia fruits (aeriferous mesocarp, empty locules and vascular cavities), only the mesocarp differed significantly between buoyant and non-buoyant fruits. A well developed aeriferous outer mesocarp extended the duration of fruit buoyancy, whereas lack of outer mesocarp resulted in denser fruits (high specific weight) that sank rapidly.
Specific weight is often reported to affect buoyancy (Lopez 2001; Cousens et al. 2008) and in a multi-species model, it was the only variable that consistently influenced buoyancy over time (L. K. Guja, M. J. Wallace, K. W. Dixon, G. Wardell-Johnson and D. J. Merritt, unpubl. data). In the present study, we have reported for the first time that a larger proportion of aeriferous tissue resulted in Scaevola fruits with lower specific weight and this was associated with prolonged buoyancy. The initial high buoyancy of fruits (98%) was likely to be due to their average specific weight being lower (0.9 ± 0.2 mg µL–1) than that of seawater (1.03 mg µL–1). Over extended periods, it is possible that fruits sink over time because of an increase in mass caused by water uptake. Water may be absorbed by the woody fruit coat, or by the seeds imbibing water that has entered through the woody fruit (Turner et al. 2009). Future investigations in dispersal ecology should quantify relationships between specific weights of imbibed and dry fruits, and their buoyancy relative to the specific weight of seawater.
Often investigations of dispersal ecology aim to predict dispersal ability, potential or distance by using simple plant, fruit or seed traits (Römermann et al. 2005; Will et al. 2007; Thomson et al. 2010). Hydrochory has received less attention than other dispersal modes, with knowledge of hydrochory in the Australian flora particularly lacking (Thomson et al. 2010), even though the continent has a large coastal fringe and numerous wetlands. Whereas we have previously shown that fruits of S. crassifolia can survive up to 42 days in seawater and are therefore capable of oceanic dispersal (Guja et al. 2010) here we have shown that several morphological variables differ significantly between buoyant and sunken fruits. However, the quantified variables are not all independent. This dependence limited the analysis and required each variable to be assessed individually, preventing the creation of models that account for the relative contributions of different variables to buoyancy potential. Further, the differences between buoyant and sunken fruits do not allow simple prediction of whether a fruit will sink or float. For example, the mean mesocarp thickness of buoyant fruits was significantly larger than that of sinking fruits, although there was also considerable overlap between mesocarp thickness of the two groups. Easily identifiable features of seeds and fruits that are indicative of hydrochorous dispersal remain to be discovered, as do diagnostic traits that would allow identification of buoyant and non-buoyant seeds.
Intraspecific variation can increase dispersal (Augspurger and Franson 1987; Greene and Johnson 1992; Hroudova et al. 1997; Higgins et al. 2003), affect patterns and processes at genetic, community and population levels (Darling et al. 2008; Nilsson et al. 2010) and may have ecological consequences, such as risk-spreading, enhancement of population stability and an increase in individual fitness in unpredictable habitats (Andersen 1992). A combination of variation in both seeds (intraspecific variation) and dispersal vectors (environmental variability) affects dispersal distance (Greene and Johnson 1992) and is likely to result in very important, but uncommon, long-distance dispersal events (Nathan 2006; Nathan et al. 2008). In the present study, even though intraspecific variation is described along an environmental gradient, it is uncertain whether variation in fruit morphology was driven by genetic or environmental effects or, more likely, by a combination of the two. Environmental variation influencing the maternal environment of the developing seed can affect seed germination, dormancy and response to environmental conditions or stress (Matilla et al. 2005; Donohue 2009). Such effects on germination are critical in a dispersal context because germination in new environmental conditions or under stress will be required for establishment of seedlings post-dispersal. Future research focused on the effects of intraspecific variation on dispersal potential, and germination or stress tolerance, will provide important insights for dispersal ecology. Intraspecific variation that imparts greater buoyancy and establishment potential may facilitate range shifts and assist coastal species to persist through disturbance or other significant changes to local climate.
Acknowledgements
Connor Weightman is thanked for measurements of fruit morphology and anatomy. Brendan Lepschi is thanked for advice on Goodeniaceae anatomy. Mark Wallace is thanked for comments on the manuscript and assistance with seed collection. Two anonymous reviewers and the editor are thanked for helpful comments on the manuscript. Seeds were collected under permit from the Western Australian Department of Environment and Conservation. LKG was funded by an Australian Postgraduate Award and Curtin University Postgraduate Scholarship. Fieldwork funds were provided by Curtin University, Centre for Ecosystem Diversity and Dynamics, 2008 Small Grant Scheme.
References
Andersen MC (1992) An analysis of variability in seed settling velocities of several wind-dispersed Asteraceae. American Journal of Botany 79, 1087–1091.| An analysis of variability in seed settling velocities of several wind-dispersed Asteraceae.Crossref | GoogleScholarGoogle Scholar |
Augspurger CK, Franson SE (1987) Wind dispersal of artificial fruits varying in mass, area, and morphology. Ecology 68, 27–42.
| Wind dispersal of artificial fruits varying in mass, area, and morphology.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2013) ‘Maps of average conditions.’ (Commonwealth of Australia: Canberra) Available at http://www.bom.gov.au/jsp/ncc/climate_averages. [Verified November 2013]
Carolin RC (1966) Seeds and fruit of the Goodeniaceae. Proceedings of the Linnean Society of New South Wales 91, 58–83.
Cousens R, Dytham C, Law R (2008) ‘Dispersal in plants: a population perspective.’ (Oxford University Press: New York)
CSIRO (2013) ‘The atlas of living Australia.’ Available at http://www.ala.org.au. [Verified November 2013]
Darling E, Samis KE, Eckert CG (2008) Increased seed dispersal potential towards geographic range limits in a Pacific coast dune plant. New Phytologist 178, 424–435.
| Increased seed dispersal potential towards geographic range limits in a Pacific coast dune plant.Crossref | GoogleScholarGoogle Scholar | 18194144PubMed |
Darwin C (1856) On the action of sea-water on the germination of seeds. Journal of the Proceedings of the Linnean Society of London Botany 1, 130–140.
Darwin C (1859) ‘The origin of species.’ (Oxford University Press: Oxford, UK)
Dawson MN, Hamner WM (2008) A biophysical perspective on dispersal and the geography of evolution in marine and terrestrial systems. Journal of the Royal Society, Interface 5, 135–150.
| A biophysical perspective on dispersal and the geography of evolution in marine and terrestrial systems.Crossref | GoogleScholarGoogle Scholar | 17626000PubMed |
de Queiroz A (2005) The resurrection of oceanic dispersal in historical biogeography. Trends in Ecology & Evolution 20, 68–73.
| The resurrection of oceanic dispersal in historical biogeography.Crossref | GoogleScholarGoogle Scholar |
Dixon KW (2011) ‘Coastal plants: a guide to the identification and restoration of plants of the Perth region.’ (CSIRO Publishing: Melbourne)
Donohue K (2009) Completing the cycle: maternal effects as the missing link in plant life histories. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 364, 1059–1074.
| Completing the cycle: maternal effects as the missing link in plant life histories.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXkslGgtrY%3D&md5=24b4f1465ae8634e1e42740818af4c52CAS | 19324611PubMed |
Greene DF, Johnson EA (1992) Can the variation in samara mass and terminal velocity on an individual plant affect the distribution of dispersal distances? American Naturalist 139, 825–838.
| Can the variation in samara mass and terminal velocity on an individual plant affect the distribution of dispersal distances?Crossref | GoogleScholarGoogle Scholar |
Guja LK, Merritt DJ, Dixon KW (2010) Buoyancy, salt tolerance and germination of coastal seeds: implications for oceanic hydrochorous dispersal. Functional Plant Biology 37, 1175–1186.
| Buoyancy, salt tolerance and germination of coastal seeds: implications for oceanic hydrochorous dispersal.Crossref | GoogleScholarGoogle Scholar |
Higgins SI, Nathan R, Cain ML (2003) Are long-distance dispersal events in plants usually caused by nonstandard means of dispersal? Ecology 84, 1945–1956.
| Are long-distance dispersal events in plants usually caused by nonstandard means of dispersal?Crossref | GoogleScholarGoogle Scholar |
Howarth DG, Gustafsson MHG, Baum DA, Motley TJ (2003) Phylogenetics of the genus Scaevola (Goodeniaceae): implication for dispersal patterns across the Pacific Basin and colonization of the Hawaiian Islands. American Journal of Botany 90, 915–923.
| Phylogenetics of the genus Scaevola (Goodeniaceae): implication for dispersal patterns across the Pacific Basin and colonization of the Hawaiian Islands.Crossref | GoogleScholarGoogle Scholar | 21659187PubMed |
Hroudova Z, Moravcova L, Zakravsky P (1997) Effect of anatomical structure on the buoyancy of achenes of two subspecies of Bolboschoenus maritimus. Folia Geobotanica et Phytotaxonomica 32, 377–390.
Hughes L (2000) Biological consequences of global warming: is the signal already apparent? Trends in Ecology & Evolution 15, 56–61.
| Biological consequences of global warming: is the signal already apparent?Crossref | GoogleScholarGoogle Scholar |
Hughes L, Westoby M (1992) Effect of diaspore characteristics on removal of seeds adapted for dispersal by ants. Ecology 73, 1300–1312.
| Effect of diaspore characteristics on removal of seeds adapted for dispersal by ants.Crossref | GoogleScholarGoogle Scholar |
Kokubugata G, Nakamura K, Forster PI, Hirayama Y, Yokota M (2012) Antitropical distribution of Lobelia species (Campanulaceae) between the Ryukyu Archipelago of Japan and Oceania as indicated by molecular data. Australian Journal of Botany 60, 417–428.
| Antitropical distribution of Lobelia species (Campanulaceae) between the Ryukyu Archipelago of Japan and Oceania as indicated by molecular data.Crossref | GoogleScholarGoogle Scholar |
Lesko GL, Walker RB (1969) Effect of sea water on seed germination in two Pacific atoll beach species. Ecology 50, 730–734.
| Effect of sea water on seed germination in two Pacific atoll beach species.Crossref | GoogleScholarGoogle Scholar |
Leyer I, Pross S (2009) Do seed and germination traits determine plant distribution patterns in riparian landscapes? Basic and Applied Ecology 10, 113–121.
| Do seed and germination traits determine plant distribution patterns in riparian landscapes?Crossref | GoogleScholarGoogle Scholar |
Lopez OR (2001) Seed flotation and postflooding germination in tropical terra firme and seasonally flooded forest species. Functional Ecology 15, 763–771.
| Seed flotation and postflooding germination in tropical terra firme and seasonally flooded forest species.Crossref | GoogleScholarGoogle Scholar | 24765572PubMed |
Marchant NG, Wheeler JR, Rye BL, Bennett EM, Lander NS, Macfarlane TD (1987) ‘Flora of the Perth region.’ (Western Australian Herbarium: Perth)
Matilla A, Gallardo M, Puga-Hermida MI (2005) Structural, physiological and molecular aspects of heterogeneity in seeds: a review. Seed Science Research 15, 63–76.
| Structural, physiological and molecular aspects of heterogeneity in seeds: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmtleltb8%3D&md5=b71c244883ef034961d49594984222b0CAS |
Nathan R (2006) Long-distance dispersal of plants. Science 313, 786–788.
| Long-distance dispersal of plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnvVyntr4%3D&md5=d288ca8c37c82270bcf1bfe0def7db06CAS | 16902126PubMed |
Nathan R, Schurr FM, Spiegel O, Steinitz O, Trakhtenbrot A, Tsoar A (2008) Mechanisms of long-distance seed dispersal. Trends in Ecology & Evolution 23, 638–647.
| Mechanisms of long-distance seed dispersal.Crossref | GoogleScholarGoogle Scholar |
Nilsson C, Brown RL, Jansson R, Merritt DM (2010) The role of hydrochory in structuring riparian and wetland vegetation. Biological Reviews of the Cambridge Philosophical Society 85, 837–858.
Rippey E, Rowland B (2004) ‘Coastal plants: Perth and the south-west region.’ 2nd edn. (University of Western Australia Press: Perth)
Römermann C, Tackenberg O, Poschlod P (2005) How to predict attachment potential of seeds to sheep and cattle coat from simple morphological seed traits. Oikos 110, 219–230.
| How to predict attachment potential of seeds to sheep and cattle coat from simple morphological seed traits.Crossref | GoogleScholarGoogle Scholar |
Telenius A, Torstensson P (1989) The seed dimorphism of Spergularia marina in relation to dispersal by wind and water. Oecologia 80, 206–210.
Thomson FJ, Moles AT, Auld TD, Ramp D, Ren S, Kingsford RT (2010) Chasing the unknown: predicting seed dispersal mechanisms from plant traits. Journal of Ecology 98, 1310–1318.
| Chasing the unknown: predicting seed dispersal mechanisms from plant traits.Crossref | GoogleScholarGoogle Scholar |
Travis JMJ, Delgado M, Bocedi G, Baguette M, Bartoń K, Bonte D, Boulangeat I, Hodgson JA, Kubisch A, Penteriani V, Saastamoinen M, Stevens VM, Bullock JM (2013) Dispersal and species’ responses to climate change. Oikos 122, 1532–1540.
| Dispersal and species’ responses to climate change.Crossref | GoogleScholarGoogle Scholar |
Turner SR, Commander LE, Baskin JM, Baskin CC, Dixon KW (2009) Germination behaviour of Astroloma xerophyllum (Ericaceae), a species with woody indehiscent endocarps. Botanical Journal of the Linnean Society 160, 299–311.
| Germination behaviour of Astroloma xerophyllum (Ericaceae), a species with woody indehiscent endocarps.Crossref | GoogleScholarGoogle Scholar |
Vargas P, Nogales M, Jaramillo P, Olesen JM, Traveset A, Heleno R (2014) Plant colonization across the Galápagos Islands: success of the sea dispersal syndrome. Botanical Journal of the Linnean Society 174, 349–358.
| Plant colonization across the Galápagos Islands: success of the sea dispersal syndrome.Crossref | GoogleScholarGoogle Scholar |
Western Australian Herbarium (1998) ‘FloraBase – the Western Australian flora.’ (Department of Parks and Wildlife). Available at http://florabase.dpaw.wa.gov.au/. [Verified April 2014]
Will H, Maussner S, Tackenberg O (2007) Experimental studies of diaspore attachment to animal coats: predicting epizoochorous dispersal potential. Oecologia 153, 331–339.
| Experimental studies of diaspore attachment to animal coats: predicting epizoochorous dispersal potential.Crossref | GoogleScholarGoogle Scholar | 17516091PubMed |
Williamson GB, Costa F, Vera CVM (1999) Dispersal of Amazonian trees: hydrochory in Swartzia polyphylla. Biotropica 31, 460–465.
| Dispersal of Amazonian trees: hydrochory in Swartzia polyphylla.Crossref | GoogleScholarGoogle Scholar |
Yang H, Lu Q, Wu B, Zhang J (2012) Seed dispersal of east Asian coastal dune plants via seawater – short and long distance dispersal. Flora 207, 701–706.
| Seed dispersal of east Asian coastal dune plants via seawater – short and long distance dispersal.Crossref | GoogleScholarGoogle Scholar |




