Saving rainforests in the South Pacific: challenges in ex situ conservation
Karen D. Sommerville A H , Bronwyn Clarke B , Gunnar Keppel C D , Craig McGill E , Zoe-Joy Newby A , Sarah V. Wyse F , Shelley A. James G and Catherine A. Offord AA The Australian PlantBank, The Royal Botanic Gardens and Domain Trust, Mount Annan, NSW 2567, Australia.
B The Australian Tree Seed Centre, CSIRO, Canberra, ACT 2601, Australia.
C School of Natural and Built Environments, University of South Australia, Adelaide, SA 5001, Australia.
D Biodiversity, Macroecology and Conservation Biogeography Group, Faculty of Forest Sciences, University of Göttingen, Büsgenweg 1, 37077 Göttingen, Germany.
E Institute of Agriculture and Environment, Massey University, Private Bag 11 222 Palmerston North 4442, New Zealand.
F Royal Botanic Gardens, Kew, Wakehurst Place, RH17 6TN, United Kingdom.
G National Herbarium of New South Wales, The Royal Botanic Gardens and Domain Trust, Sydney, NSW 2000, Australia.
H Corresponding author. Email: karen.sommerville@rbgsyd.nsw.gov.au
Australian Journal of Botany 65(8) 609-624 https://doi.org/10.1071/BT17096
Submitted: 24 May 2017 Accepted: 16 November 2017 Published: 11 January 2018
Journal compilation © CSIRO 2017 Open Access CC BY-NC-ND
Abstract
Rainforests in the South Pacific hold a considerable amount of plant diversity, with rates of species endemism >80% in some countries. This diversity is rapidly disappearing under pressure from logging, clearing for agriculture or mining, introduced pests and diseases and other anthropogenic sources. Ex situ conservation techniques offer a means to limit the loss of plant diversity. Seed banking is considered the most efficient and cost effective of these techniques but is applicable only to seed capable of tolerating desiccation and cold storage. Data on the degree of tolerance of these conditions was lacking for more than half of the 1503 South Pacific rainforest genera examined for this review. Of the 710 genera for which data were available, the storage behaviour of 324 was based on an assessment of only one or two species, although 76% of those genera contained at least 10 species. Many of the unstudied or poorly studied genera are shared across several South Pacific nations, providing an excellent opportunity for collaboration on future ex situ research and conservation. Of the 386 genera for which three or more species have been studied, 343 have a very high proportion of species (>95% of those tested) that are suitable for seed banking. Seed banking could therefore provide a suitable means for preserving a large proportion of the rainforest flora before it becomes extinct in the wild. Alternatives for preserving species that are not suitable for seed banking are also discussed.
Additional keywords: cryopreservation, living collections, orthodox, recalcitrant, restoration, seed banking, seed storage behaviour, tissue culture.
Introduction
The word ‘rainforest’ evokes images of deep green foliage, dripping leaves, tangled vines and the calls of a multitude of forest-dwelling fauna. Few vegetation types have captured the imagination and support of the public as well as rainforest, but while many people seem to be familiar with the high-profile, highly-threatened rainforests of the Amazon and South-East Asia, the rainforests of the South Pacific are less well known. Though mostly small in size compared with the Amazon, South Pacific rainforests support a wide variety of flora and fauna and are also, with less publicity, under great threat. To facilitate collaboration among members of the Pacific Community (http://www.spc.int/our-members/, accessed 27 November 2017) in conserving these forests, we present a review of the extent to which rainforest genera are shared among Pacific nations situated south of the equator. We discuss ongoing threats to rainforest habitat in the region, and look at options for ex situ conservation of seed-producing plants.
The scope of ‘rainforest’
Rainforests are usually defined as evergreen plant communities with a closed tree canopy (>70% projective foliage cover; Floyd 1990a), occurring in areas of relatively high annual rainfall (>1300 mm, Williams et al. 1984) and incorporating plant groups with special characteristics such as cauliflory, buttressed trunks, thick-stemmed vines, leaves with drip tips, and epiphytic growth (Harden et al. 2006). Climax rainforest species can be distinguished from non-rainforest plants by their dependence on humidity, especially in the early life-cycle stages, and their ability to regenerate in shade and in the absence of fire (Floyd 1990a; Baskin and Baskin 2014). The vegetation encompassed by the above definitions varies considerably with latitude, altitude, temperature, rainfall, soil type and the availability of shelter, with some rainforests able to survive in areas of much lower annual rainfall where shelter is sufficient, where there is access to groundwater, or where rainfall is concentrated in a wet season.
Mueller-Dombois and Fosberg (1998) described several rainforest types that are common across the tropical Pacific islands: ‘lowland tropical rainforest’, described as relatively tall, with multiple layers and many canopy-dwelling epiphytes; ‘seasonally dry evergreen forest’, which occurs on leeward, drier slopes and experiences a distinct dry season; ‘montane rainforest’, which occurs at higher elevations, is lower in stature but rich in shrubs and epiphytes; and ‘cloud forest’ (or mossy forest), which occurs at higher elevations in the cloud zone, has a very low canopy, is dominated by small-leaved species and rich in mosses and liverworts. The upper levels of a hierarchical scheme proposed by Webb (1968) suggested a similar classification: ‘evergreen vine forests’, occurring in wet tropical-subtropical habitats, ‘raingreen vine forests’, occurring in tropical–subtropical habitats and containing one or more species that are deciduous during the dry winter season; ‘fern forest’, occurring in submontane or warm temperate habitats; and ‘mossy forest’, occurring in montane or cool temperate habitats. The descriptions of these vegetation types correspond approximately to the commonly used Australian classifications of tropical to subtropical rainforest, dry rainforest and vine thickets (including monsoon vine forest), warm temperate rainforest, and cool temperate rainforest (as described by Beadle and Costin 1952; Harden et al. 2006; Metcalfe and Green 2017). A subcategory of tropical–subtropical rainforest is ‘littoral rainforest’ (Floyd 1990b), which grows in close proximity to the sea and often contains species distributed widely across the South Pacific (such as Guettarda speciosa, Hernandia nymphaeifolia, Hibiscus tiliaceus, Morinda citrifolia, Neisosperma oppositifolium and Heliotropium foertherianum; Mueller-Dombois and Fosberg 1998).
For simplicity, we here consider rainforests to be terrestrial forest communities, composed largely of evergreen species, with a tree canopy that is closed for either the entire year or during the wet season. Within this definition, we consider three broad rainforest biomes: tropical–subtropical rainforest, tropical–subtropical seasonal forest (also referred to as tropical dry forest, monsoon forest and dry rainforest), and warm to cool-temperate rainforest (Fig. 1). These rainforest types occur within the Köppen climate classifications of Af, Am and Aw (tropical), Cwa and Cfa (humid subtropical) and Cfb (temperate oceanic) (Köppen 1936; Peel et al. 2007). Although some might argue against the inclusion of tropical–subtropical seasonal forest as a type of rainforest, we include it here as many species occurring in that habitat often also occur in more humid rainforest (for examples, see Smith 1979; Harden et al. 2006; Munzinger et al. 2016) and seeds released during the wet season can have characteristics similar to those released in continuously moist rainforest (i.e. desiccation sensitivity; Daws et al. 2005).

|
Rainforest origins
The current distribution and composition of rainforest in the South Pacific has been strongly influenced by the area’s geological history. At the time Australia separated from Antarctica 30 million years ago (mya), much of it was covered in rainforest (Christophel 1989; Greenwood 1996). As the continent drifted north towards warmer latitudes, conditions became increasingly arid (Martin 2006; Byrne et al. 2008) and the moisture-dependent vegetation contracted in range. Today rainforests in Australia are mostly restricted to higher rainfall areas on the east and north coasts of the mainland and the west coast of Tasmania (Adam 1992). Species in the cool temperate rainforests of the south have their origins in the ancient Gondwanan forests (Dettmann 1989), whereas the tropical rainforests in the north contain many South-east Asian elements, which likely arrived through long-distance dispersal as Australia drifted closer to Asia (Burbidge and Whelan 1982; Byrne et al. 2011). Over the last 2 million years or so, both temperate and tropical rainforests repeatedly contracted to refugia during ice ages, which were accompanied by drier climates and lower sea levels (Schneider and Moritz 1999; Kooyman et al. 2013).
Island groups in the South Pacific have more complex geological histories, including various combinations of uplift of continental fragments (e.g. New Caledonia), formation of islands at plate boundaries due to subduction (e.g. Viti Levu in Fiji), formation of islands over volcanic hot spots (e.g. Samoa and Pitcairn Islands), and formation of coralline atolls built on subsided volcanoes (e.g. the Cook Islands; Mueller-Dombois and Fosberg 1998; Neall and Trewick 2008). New Caledonia and New Zealand, for example, were originally part of a continental fragment that began to separate from Gondwana 85 mya, became mostly (or possibly entirely) submerged, and began to re-emerge around 25 mya (Trewick et al. 2007). These two island groups contain several genera with Gondwanan ancestry (such as Araucaria in New Caledonia, and Nothofagus and Dacrydium in both countries) that have been considered relicts of the original Gondwanan flora but may be the result of long distance dispersal events post separation (Grandcolas et al. 2008).
The southern half of New Guinea resulted from uplift of the Australian continental plate while the northern part resulted from the convergence of several islands on the Pacific plate that were brought into contact with the southern half through continental drift (Hall 2001). The rainforests of New Guinea thus share some Gondwanan elements with Australia (Araucaria and Nothofagus) and many species originating from South-east Asia. The remaining islands in the South Pacific region steadily gained species through dispersal and speciation after their emergence – in some cases <5 mya – with South-east Asia and Australia as key source areas (Keppel et al. 2009; Franklin et al. 2013).
South Pacific rainforests today harbour floristic elements of both Gondwanan (e.g. Araucariaceae, Nothofagus, Proteaceae) and Asian origin (e.g. Lauraceae, Orchidaceae) in the west, with some elements from the Americas in the east (e.g. Fitchia and Oparanthus in the Asteraceae; van Balgooy et al. 1996; Keppel et al. 2009). The influence of these key sources varies among locations and ecosystems, with the Gondwanan influence being more pronounced in south-eastern Australia, New Caledonia and New Zealand, while the Asian influence is more pronounced in New Guinea and the Solomon Islands (Burbidge 1960; Keppel et al. 2009; Sniderman and Jordan 2011). The number and variety of species present on a given island has been influenced by the age of the island, its size, the complexity of its habitats, and its proximity to the key source points (Keppel et al. 2010; Gillespie et al. 2013; Keppel et al. 2016).
Diversity and importance
The biodiversity in the South Pacific is rich, with a high proportion of endemic species (Kier et al. 2009), and this is reflected in the six biodiversity hotspots (of 35 globally) that occur in the region: the Forests of East Australia, East Melanesian Islands, New Zealand, New Caledonia, Polynesia-Micronesia and the Galapagos Islands (Mittermeier et al. 2011). Though the common origins of the flora mean that many genera are shared among island groups (see Supplementary Material to this paper), isolation and speciation has led to a very high level of endemism. Among flowering plant species, for example, it has been estimated that 82% are endemic in New Zealand, 80% in New Caledonia, 70% in the Marquesas Islands and 61% in Fiji (Jaffré 1993). More than half of 552 orchid species known to occur on eight Pacific archipelagos are endemic to that region (Keppel et al. 2016).
Rainforest floras contribute a substantial amount to this biodiversity. Australian rainforests, for example, occupy less than 0.5% of the mainland (Stork et al. 2011) yet hold more than 3500 seed plant species representing at least 1219 genera in 198 families (AVH 2016). New Caledonia’s tropical rainforests and seasonal forests hold at least 2188 seed plants representing 451 genera in 116 families (Morat et al. 2012; Munzinger et al. 2016), and Fiji’s rainforests hold around 1024 seed plants representing 369 genera in 111 families (Smith 1979, 1981, 1985, 1988, 1991; Heads 2006). The diversity of tree species (with a diameter at breast height (dbh) ≥ 10 cm) in rainforests is particularly high, ranging from 81–85 ha–1 in the Wet Tropics of Australia (Tng et al. 2016), up to 96 ha–1 in the rainforests of New Caledonia (Ibanez et al. 2017) and up to 124, 131 and 167 ha–1 in Fiji, the Solomon Islands and Papua New Guinea, respectively (Table 1; Keppel et al. 2010).

|
The importance of this diversity cannot be overstated. Rainforests provide important ecosystem services (Haddad et al. 2015) and hold extensive carbon stocks (Wardell-Johnson et al. 2011; Vincent et al. 2015). They are also of great cultural importance and a vital source of food, fibres, timber, fuel, medicines and other materials important to Pacific Islanders (Thaman 1994; Whistler 2000). Many remote Pacific Island communities still greatly depend on these forests for daily living (Cox and Elmqvist 1993; Hviding 2003). Despite their importance, however, rainforests are disappearing at a rapid rate.
Threats and losses
In many regions of the South Pacific, the extent of primary (undisturbed) rainforest, particularly in lowland regions, has been greatly reduced by human activities. Initial disturbances were caused by the first human settlers (ancestors of the current indigenous inhabitants) through clearing for agriculture, increased fire frequency and the introduction of non-native plant and animal species. The rate and scale of disturbance has greatly increased since European settlement, particularly with the introduction of commercial logging and clearing for intensive agriculture or mining (Keppel et al. 2014). These disturbances have resulted in substantial losses of primary rainforest in many areas (Table 1), with some islands almost completely deforested (e.g. Tongatapu, Kingdom of Tonga; Wiser et al. 2002) or degraded by logging (e.g. New Georgia, Rendova and Isabel Province in the Solomon Islands; Pauku 2009; Katovai et al. 2015).
Logging and clearing for agriculture is still ongoing in areas such as the Solomon Islands (Pauku 2009) and Papua New Guinea (Corlett and Primack 2008; Bryan et al. 2015). The Solomon Islands were relatively untouched in the middle of the last century (Whitmore 1969), but the extent of primary rainforest has now been reduced by >40% (Table 1). In Papua New Guinea, 25% of commercially accessible (mainly lowland) rainforest on the mainland, and 62% on nearby islands, has now been degraded by logging (Bryan et al. 2015); the FAO (2015) estimated an overall reduction in primary rainforest of 56% over the period from 2009 to 2015. Given that the number of described species for these two nations is substantially less than the estimated number present (Pauku 2009; Gideon 2015), there is a great potential for species to become extinct before they have been identified.
The rainforest fragments remaining following logging and clearing are further threatened by secondary impacts. Fragmentation can reduce opportunities for breeding, particularly for species that were already sparsely distributed (Laurance et al. 2000, 2006) and the increase in exposed edges can increase the risk of fire and weed invasion (Ibanez et al. 2013). Invasive weeds, in turn, can limit the natural regeneration of native species (Meyer and Florence 1996; Minden et al. 2010; Wallace et al. 2017). On some of the smaller islands in the South Pacific, the number of non-native plant species now greatly exceeds the number of indigenous species. The Flora of Australia, for example, lists 125 indigenous but 273 naturalised angiosperms for Norfolk Island (Wilson 1994). Similarly, Sykes (2016) reported 187 indigenous but 424 naturalised angiosperms for the Cook Islands.
Loss of dispersal agents, or large distances between fragments, can limit opportunities for regeneration, particularly for larger-seeded species (Meehan et al. 2002; McConkey and Drake 2006; Rossetto et al. 2015; Goosem et al. 2016). Regeneration can also be limited by the activities of pest or agricultural animals. In New Zealand, for example, ground disturbance by wild pigs (Sus scrofa) can have a detrimental impact on plant establishment, plant growth and ecosystem structure, and can expedite invasion by weeds (Krull et al. 2016). Rodents such as rats and mice can limit plant establishment by consuming seed (Auld et al. 2010; McConkey et al. 2003), and browsing by herbivores such as red deer (Cervus elaphus scoticus) can alter plant growth and cause loss of seedlings (Forsyth et al. 2015). In Australia, six species of deer have established feral populations and the environmental degradation they cause through herbivory, trampling and weed dispersal has been listed as a ‘key threatening process’ potentially impacting rainforest plants and communities (http://www.environment.nsw.gov.au/determinations/FeralDeerKtp.htm, accessed 27 November 2017). In the Norfolk Island group, grazing by goats, pigs, rabbits and cattle has contributed to extensive losses of indigenous vegetation including rainforest communities (Mills 2009). Though the removal of rabbits in the 1980s, and pigs and goats in the 1990s, did allow for regeneration in some areas, the loss of native vegetation continues where feral animals cannot be excluded (Director of National Parks 2010).
Introduced diseases can also be problematic in disturbed rainforests. Myrtle rust (Austropuccinia psidii, Beenken 2017) has become a particular problem in rainforest habitats in Australia. This disease originated in South America and affects more than 300 species in the family Myrtaceae (Giblin and Carnegie 2014). Since it was first observed in New South Wales in 2010, Myrtle Rust has had such a devastating effect on two once common rainforest shrubs – Rhodamnia rubescens and Rhodomyrtus psidioides (Carnegie et al. 2016) – that they have now been nominated for listing as ‘Critically Endangered’ under the NSW Threatened Species Conservation Act. Myrtle rust has the potential to spread further through the South Pacific, affecting susceptible genera such as Metrosideros (Teulon et al. 2015) and Syzygium (Giblin and Carnegie 2014) that are present across several island groups (see Supplementary Material to this paper) and are often highly diverse (Ahmad et al. 2016). The disease has already progressed across water to Tasmania (http://dpipwe.tas.gov.au/biosecurity-tasmania/plant-biosecurity/pests-and-diseases/myrtle-rust, accessed 27 November 2017), to mainland New Zealand and to the remote Raoul Island (http://www.doc.govt.nz/news/media-releases/2017/myrtle-rust-found-in-new-zealand/, accessed 27 November 2017).
Diseases caused by various Phytophthora species are also having a serious impact in some areas. In New Zealand, Phytophthora agathidicida has caused extensive dieback in Kauri (Agathis australis, Scott and Williams 2014; Weir et al. 2015), with potential flow on effects for a suite of plant taxa dependent on the soil environment created by this species (Wyse et al. 2014). In Australia, Phytophthora cinnamomi has been linked to the decline of a variety of rainforest species in the Wet Tropics (e.g. Acronychia vestita (Rutaceae), Alphitonia petriei (Rhamnaceae), Carnarvonia araliifolia (Proteaceae), Cinnamomum oliveri (Lauraceae) and Polyosma alangiacea (Escalloniaceae); Brown 1999).
South Pacific rainforests face unprecedented threats and are declining in response to increasing population sizes and intensive exploitation (Woinarski 2010; Wardell-Johnson et al. 2011). Habitat loss and degradation, invasive species, overexploitation and pollution are the key threats to rainforest biodiversity in the region and island biotas are especially vulnerable (Kingsford and Watson 2011; Keppel et al. 2014). Current policies and management efforts appear inadequate in preventing vegetation and biodiversity loss, suggesting the need for new legislation and economic incentives (Wardell-Johnson et al. 2011). Implementing new policies is likely to be particularly challenging in developing Pacific Island nations, where past conservation efforts have often been unsuccessful, and administrative and land-tenure complexities add difficulty (Kingsford and Watson 2011; Keppel et al. 2012).
Conservation options
The rapid loss of rainforest diversity indicates that measures to conserve that diversity are needed urgently. In situ conservation such as the creation of national parks or reserves is often considered the most effective option; however, the declaration of a reserve does little to mitigate secondary pressures which can continue to act without immediately observable effects. The long lifespan of trees and shrubs, for example, can mean that the impacts of limited mates, absence of pollinators and barriers to regeneration can take a very long time to manifest (Cronk 2016).
On-ground conservation efforts have been successful in some areas where broad scale clearing has largely ceased, such as in the tropical and subtropical rainforests of Australia (Catterall et al. 2004), but have been difficult to implement in other areas of the South Pacific (Keppel et al. 2012). Secondary rainforests – rainforests that develop on previously cleared land or primary rainforest that has been partially degraded – can have great conservation value but their diversity is dependent on the availability of propagules and agents to disperse them (Putz and Romero 2014). Secondary rainforests developed on abandoned pastures in Queensland, for example, failed to match the diversity shown in nearby mature forests even after six decades of growth (Goosem et al. 2016). Elliott et al. (2013) noted that some tree planting is often required to restore full biodiversity in regenerating forests, particularly for species with large seeds that are poorly dispersed. Supplementary planting in turn requires a source of seed or other propagules and knowledge of appropriate methods of propagation. Ex situ conservation of plants is therefore a necessity – both as insurance against the loss of diversity and as a source of material that can be used to aid the restoration of diversity.
Challenges in ex situ conservation of rainforest species
Target 8 of the Global Strategy for Plant Conservation calls for 75% of threatened plant species to be held in ex situ collections by 2020, preferably in the country of origin, with at least 20% available for recovery and restoration programs (Convention on Biological Diversity 2012). The ex situ conservation options currently employed to meet this target include seed banking (the storage of dry seeds at subzero temperatures), the maintenance of whole plants in orchards and botanic gardens, the establishment of tissue cultures in vitro, and cryopreservation of tissue cultured material or seed embryos (Offord and Meagher 2009). The most appropriate option for a given situation is dependent on the biology of the species itself and the resources available to conserve it.
Seed banking is generally considered the most efficient and cost effective option in terms of capturing maximum diversity while minimising storage space and ongoing maintenance requirements (Offord and Meagher 2009). Long-term storage is accomplished by drying seeds to 3–7% moisture content and storing them in airtight containers at temperatures ≤ −18°C (Martyn et al. 2009). The method is therefore only suitable for seeds that are ‘orthodox’; that is, tolerant of drying and storage at cold temperatures (Roberts 1973). Tweddle et al. (2003) predicted that 50% of non-pioneer woody rainforest species were likely to be intolerant of drying (although recent modelling by Wyse and Dickie (2016) indicates this figure is likely to be much lower when herbaceous species are included). This prediction of desiccation intolerance has meant that, until recently, the seed banking of rainforest species has not been considered a priority, unless a species was particularly threatened or of economic importance. Initial testing of desiccation tolerance is the first logical step to overcome this barrier and, in light of the rapid destruction of rainforests globally (Corlett and Primack 2008), this research is imperative.
Although some predictions of desiccation tolerance can be made based on taxonomy, habitat, and certain seed characteristics, definitive results can best be achieved by experimentation. Over 98% of tested species (representing 34% of genera) in the Fabaceae, for example, are desiccation tolerant (Dickie and Pritchard 2002), but rainforest species from several previously untested genera in that family have recently been found to be desiccation sensitive (e.g. Archidendron, Sommerville et al. 2016; and Cynometra, Baskin and Baskin 2014). Work by Hamilton et al. (2013) showed that seed size can be a predictor of desiccation tolerance but the small seeds of whitewood (Endospermum medullosum) are just as sensitive to drying as the large seeds of hairy walnut (Endiandra pubens) (Fig. 2). The use of the seed coat ratio and dry seed weight to predict desiccation sensitivity (Daws et al. 2006) has some value; however, this method has not been found useful for predicting desiccation sensitivity in smaller rainforest seeds (KD Sommerville, G Errington, Z-J Newby, CA Offord, unpubl. data).
The expectation that a significant proportion of rainforest seeds are likely to be intolerant of drying has been borne out by recent assays of Australian mainland species. This proportion, however, has been less than expected with only a quarter of species tested showing full sensitivity to desiccation at 15% RH (Sommerville et al. 2016). Based on studies of the tropical Hawaiian flora, the proportion of desiccation sensitive seeds on remote oceanic islands is likely to be even lower. Of 207 taxa screened (including wet rainforest species), 78% were not sensitive to drying and a further 20% were categorised as ‘probably not sensitive’ (Yoshinaga and Walters 2003). The authors attributed this result (in part) to the inability of desiccation sensitive seeds to survive long-distance dispersal and successfully colonise remote islands.
Around one third of the Australian rainforest species found tolerant of drying to date have either not been tolerant of subsequent freezing or have been comparatively short-lived in storage. These species can still be conserved; however, a modification of standard seed banking protocols is necessary. Freezing-sensitive species, for example, can be held in a refrigerator until more effective long-term storage conditions can be identified. Differential scanning calorimetry coupled with an analysis of lipid content can aid in determining what those conditions should be (Crane et al. 2003). Short-lived species, in contrast, can be stored at much lower temperatures (e.g. –80 or −192°C) to extend their longevity (Sommerville and Offord 2015).
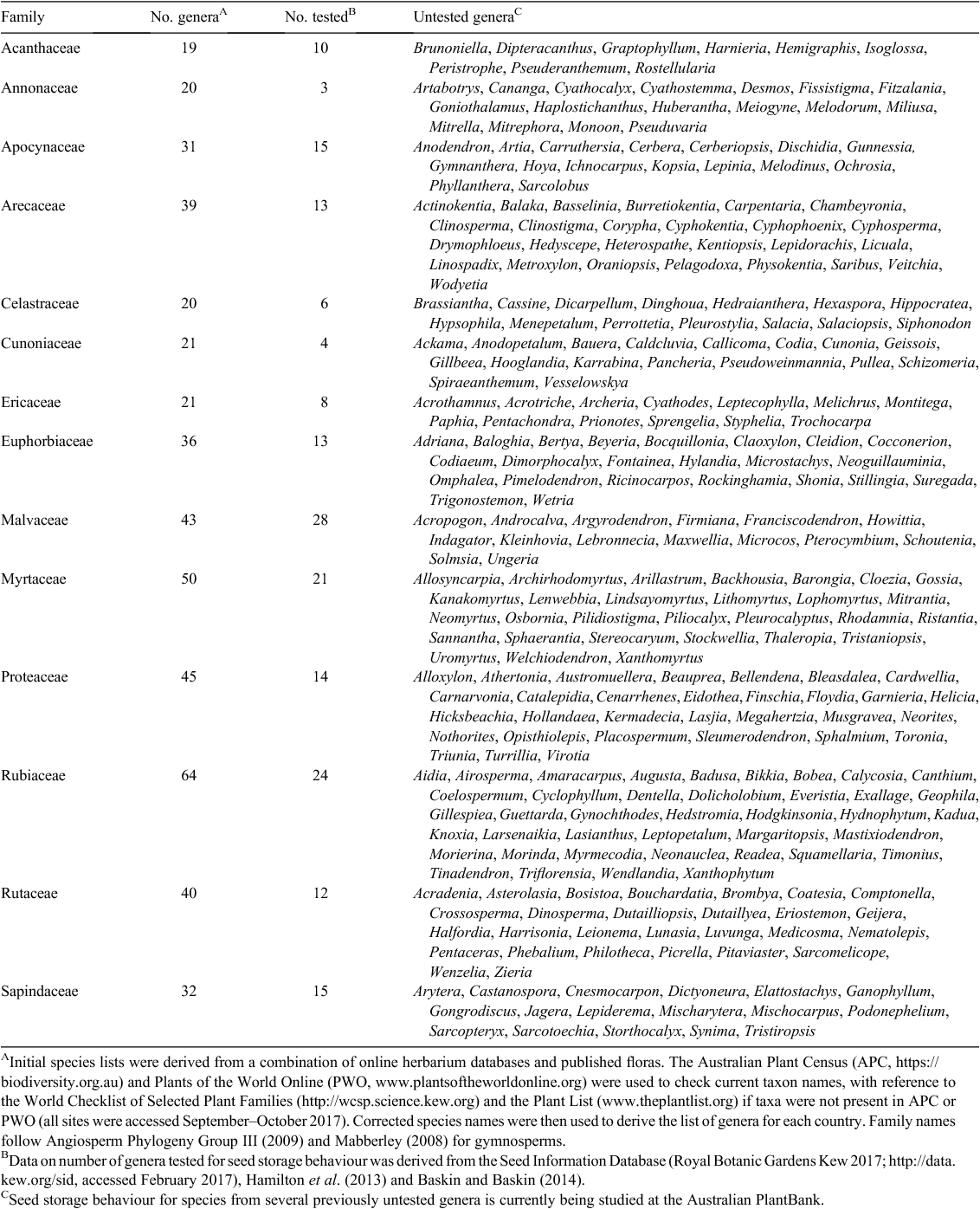
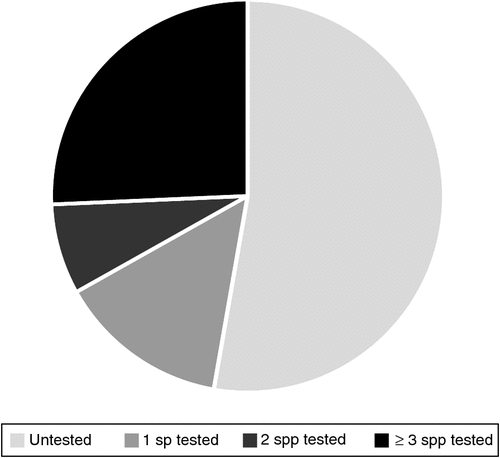
While the storage behaviour of some genera occurring in South Pacific rainforests is fairly well studied (e.g. Melaleuca (Myrtaceae), Plectranthus (Lamiaceae) and Hibiscus (Malvaceae)), little to no information is available for others (Table 2). Data on seed storage behaviour was lacking for more than half of the 1503 rainforest genera examined for this paper and nearly a quarter of the 209 families (Fig. 3, see Supplementary Material to this paper). Of the 710 genera for which storage behaviour data was found in the Seed Information Database (Royal Botanic Gardens Kew 2017), Hamilton et al. (2013) or Baskin and Baskin (2014), the behaviour of 324 genera was based on an assessment of only one or two species although 76% of those genera contained at least 10 species. Many of the unstudied or poorly studied genera are shared across several South Pacific nations, providing an excellent opportunity for collaboration on ex situ research and conservation. Of the 386 genera for which three or more species have been studied, 343 have a very high proportion of species (>95% of those tested) that are suitable for seed banking. However, the data available are biased towards species occurring in dryland areas (Wyse and Dickie 2016), so caution should be used in predicting the storage behaviour of rainforest species based on species already assayed in the same genus.
|
Determining whether rainforest seeds are suitable for storage can be frustrated by an inability to germinate the seed, either due to dormancy or lack of data on the optimum germination conditions. From germination studies performed using fresh seeds from non-pioneer rainforest trees, Baskin and Baskin (2014) inferred that half of over 1900 species tested had some form of dormancy. The seeds of some of these species took more than two years just to begin germinating. Germination experiments on rainforest plants can also be frustrated by the prolific growth of fungi, to which tropical fruit and seeds tend to be particularly susceptible (Berjak and Pammenter 2014).
The determination of storage behaviour can be further complicated by the difficulty in collecting rainforest seeds in sufficient quantity to enable useful testing. There are several factors contributing to this problem. For some species, individuals grow far apart (km) in the landscape so that finding and collecting from many individuals can take a considerable amount of time. In primary rainforests, fruiting of tree and vine species can occur high in the canopy which can be very difficult to access. Asynchronous ripening of fruit (i.e. ripening staggered over a long period) is a common feature of rainforest plants and can mean that multiple collecting trips may be required to make a useful collection. Given the enormous number of rainforest species yet to be studied, these difficulties point to the need for much greater collaboration among research institutions, government and conservation organisations if any headway is to be made in conserving species before they become extinct.
Options for conserving desiccation sensitive species
Species with seeds that do not tolerate any drying can be conserved by tissue culture (growing plants in a nutrient medium under sterile conditions, Box 1, Fig. 4) or cryopreservation of tissue-cultured material (involving pre-treatment with chemicals to reduce freezing injury and storage at temperatures ≤ −190°C). Tissue cultures can be initiated from plant material collected directly from the wild (eliminating the need to find fruiting specimens) and can even be initiated in the field if anti-microbial agents are used to control fungal and bacterial growth (Pence 2005). Alternatively, material for initiation can be obtained by germinating seeds in a sterile environment, or by growing seedlings in pots under controlled conditions; both methods reduce the amount of fungal and bacterial contamination encountered during initiation. Once established, tissue cultured material can be maintained by regular sub-culturing (transfer of shoot tips to fresh media). This is a labour-intensive process but the length of time between subcultures can be increased by storing the plantlets at low temperatures under low light (in vitro storage; Engelmann 2011).
| Box 1. Case study – Cryptocarya spp. |
| Cryptocarya (Lauraceae) is an important taxon in the rainforests of the South Pacific. The genus contains 47 Australian rainforest species, including some that are threatened (e.g. C. dorrigoensis, C. floydii, C. foetida, C. williwilliana). The seeds of two species (C. laevigata and C. rigida) were recently found to be desiccation sensitive during testing at the Australian PlantBank, and a third (C. microneura) was found to be desiccation tolerant. Freshly germinated seedlings resulting from germination trials of C. microneura, C. rigida and a fourth species, C. glaucescens, were transferred into potting mix and grown on, initially under glasshouse conditions and subsequently under 70% shade (V. Viler, pers. comm). Shoot tips were harvested from the species after ~1 years’ growth and used to initiate tissue cultures. All three species have grown well in culture and two have been successfully transferred from sterile culture back to potting mix and from pots to the field (A. Rollason, pers. comm). Material retained in tissue culture will now be used to develop cryopreservation protocols for desiccation sensitive species in the genus. |

|
Tissue cultures can also be used to produce buds for cryopreservation. This is a long-term storage option that requires far less maintenance than tissue culture but can require a considerable investment of time and resources at the outset, both to develop suitable protocols for culturing the species, and to develop protocols for cryopreservation (Ashmore et al. 2011). Though tissue culture and cryopreservation protocols have been developed for many economically important species (Reed 2008), relatively little work has been done on wild rainforest species in the South Pacific.
A promising option for conserving desiccation sensitive species that bypasses tissue culture is to excise the embryonic axis from a seed, dry the axis rapidly in a flow of gas with low humidity (e.g. air or nitrogen), and then preserve it in liquid nitrogen (Berjak and Pammenter 2014). This technique has been successfully applied to several desiccation sensitive horticultural species (Normah and Makeen 2008) but has so far proven difficult to optimise for individual rainforest species (see Box 2, case study on Dysoxylum spectabile).
| Box 2. Case study – Dysoxylum spectabile (kohekohe) |
| Seeds of the endemic New Zealand tree kohekohe (Dysoxylum spectabile, Meliaceae, Fig. 5) have been found to be relatively intolerant of drying, with germination declining from 93% at 50% seed moisture content (MC) to 58% after drying to 29% MC and 3% after drying to 21% MC (Park 2013). Though not wholly desiccation sensitive, kohekohe cannot be dried to the low seed moisture content required for standard seed banking. Rapid drying of excised embryos followed by cryopreservation may be an alternative option for conserving this species; while no excised embryo’s survived desiccation to 34% MC following slow drying, 10% remained viable following rapid drying to 10% MC. Another option for enhancing the survival of desiccation sensitive tissue is to encapsulate the tissue in a sodium-alginate bead. In kohekohe, encapsulation enabled around 17% of embryos to survive drying to a bead moisture content of around 17%. Pre-treatment with cryoprotectants can also protect tissue from freezing injury and enabled around 20% of excised kohekohe embryos to survive cryopreservation. Subsequent tissue culture of the embryos, however, failed to produce any normal seedlings (radicle elongation occurred but shoots failed to develop; Park 2013). This work suggests that desiccation sensitive species in the South Pacific may potentially be cryopreserved but that post-cryopreservation recovery protocols need to be developed. |
An alternative option for conserving desiccation sensitive species is to grow whole plants in pots, orchards or botanic gardens. Though establishing genetically diverse living collections requires a great deal more time and effort than seed banking, such collections can provide a valuable source of material for restoration (see Box 3, case study on Whitewood), and can also provide a readily accessible source of individuals for further research into issues related to conservation and management (Offord and North 2009). Land and resources for conservation are often limited, however, so this type of collection is only likely to be applied to species that are highly threatened or have some economic importance.
| Box 3. Case study – Endospermum medullosum (whitewood) |
| Endospermum medullosum (Euphorbiaceae) is economically important to Vanuatu because of its high-value timber (Settle et al. 2012); however, populations of the species are being degraded or lost through logging and land clearing for agriculture. The seed of this species is sensitive to drying, seed viability is characteristically low (13%) and refrigeration has not been found useful in extending the storage life of either the drupes or the cleaned seed (Page and Doran 2016). In the late 1990s, in an attempt to secure populations and provide easy access to high quality seed for replanting, seed was collected from 14 populations and used to establish stands suitable for ex situ conservation and seed production on the island of Santo (Fig. 6). In a follow-up project in 2010–2015, it was found that nine of the 14 populations originally sampled no longer existed as a result of changes in land use (Doran et al. 2012). These populations are now only represented in the ex situ stands. This highlights the importance of ex situ plantings for species where long-term seed storage has proven difficult. The conservation stands, however, are also at risk from land use changes and environmental disasters. Although research enabling the long-term storage of seed could provide another avenue for securing this species into the future, the establishment of a secure network of ex situ plantings across a range of sites, possibly extending to international locations, would provide the best security for the immediate future. |

|
Ex situ conservation facilities in the South Pacific
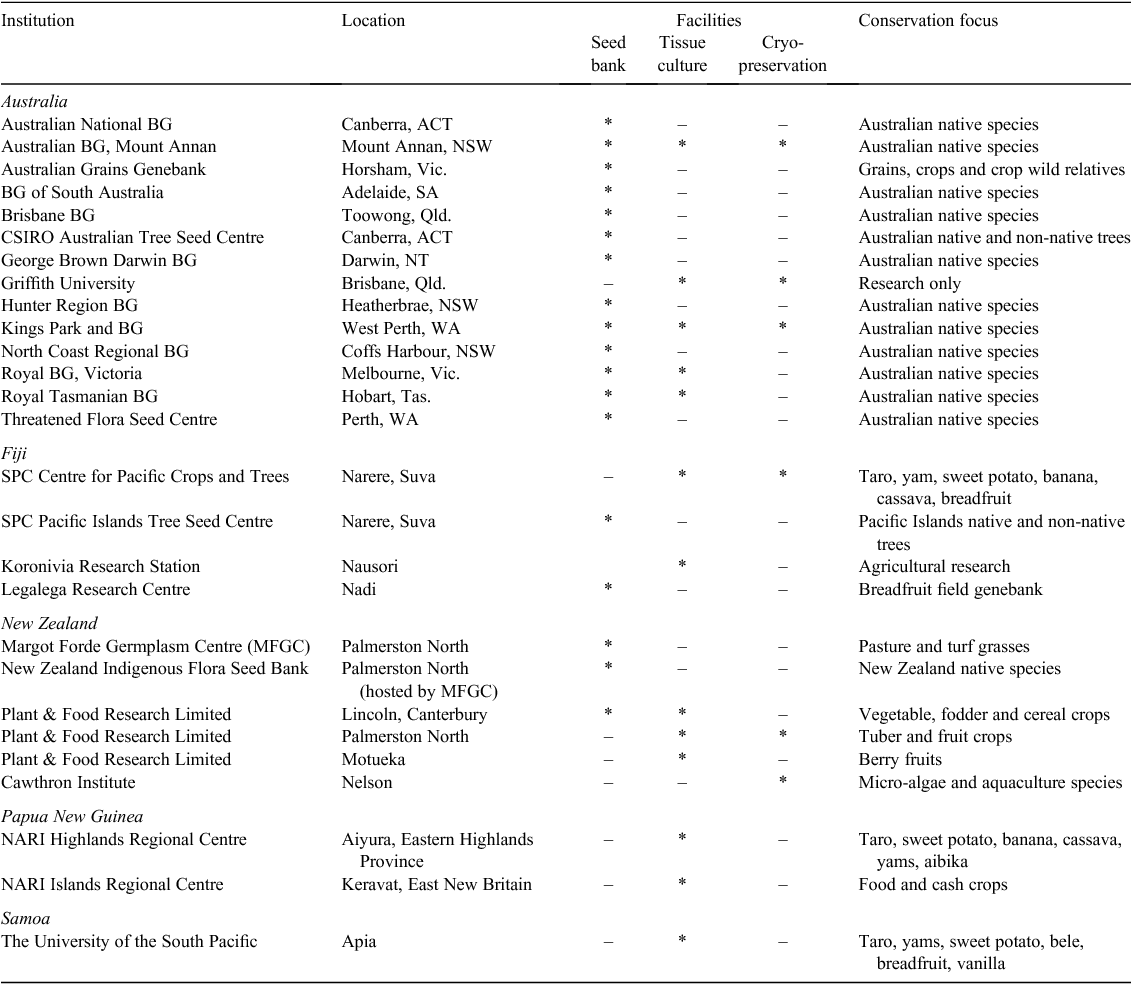
At present, the only long-term seed banking facilities available in the South Pacific are located in Australia, New Zealand and Fiji. The majority of these are situated within botanic gardens and are dedicated to the preservation of native plant diversity (Table 3). Three of these seed banks also house facilities for the tissue culture and cryopreservation of native species not suitable for seed banking. Tissue culture and cryopreservation facilities are also located in Fiji, Papua New Guinea and Samoa, but these are focussed on the conservation of food and cash crops such as taro, yams, sweet potato, cassava and breadfruit (Table 3).
Of the ex situ conservation facilities available, only the Australian PlantBank currently has a program dedicated to research into the conservation of rainforest species. The first stage of this program (The Rainforest Conservation Project, 2010–2012) involved screening over 100 rainforest species for desiccation tolerance (Hamilton et al. 2013). Stage two of the program (2013–2017) has focussed on screening a further 250 species, while collecting and storing species found to be desiccation tolerant, and developing alternative storage methods for species found to be sensitive to desiccation or freezing. The experience and knowledge accumulated under this program could now serve as the basis for expanding the work into other regions of the South Pacific. Given that desiccation sensitive species can be difficult to transport long distances without loss of viability, and permits to import species into other countries can be difficult to obtain (particularly for species with the potential to become weeds or to carry disease), such work would require collaboration with conservation entities within the country of origin to be effective.
The way forward
Considering the large number of rainforest species in the South Pacific, the high level of endemicity, and the rapidity with which primary forest is being lost or degraded, it would be beneficial to all South Pacific nations to invest greater resources in ex situ conservation of their native plant diversity. As conservation facilities for wild plants are limited at present (outside of Australia), one initial approach could be to utilise in-country forestry, agriculture and university facilities to conduct preliminary research and to house collections of orthodox seeds. Established seed bank facilities in Australia, New Zealand and Fiji could also be used to house orthodox seeds till in-country facilities could be established.
As pioneer rainforest species are most likely to have orthodox seeds (Tweddle et al. 2003), building up a diverse collection of such species could be a useful way to start a seed conservation program and would aid in efforts to begin restoring deforested areas. Working with genera that are common across several nations, that are important as crop wild relatives (e.g. genera in Rutaceae), or that are particularly threatened by disease (e.g. genera in Myrtaceae), could also be good ways to focus initial efforts and attract funding. Training in appropriate techniques could be provided at established facilities, or in-country by staff experienced in working with rainforest species.
Species that occur in multiple rainforest types (such as Crypotcarya triplinervis, Endiandra floydii, Litsea australis and Neolistea dealbata in Lauraceae), or multiple locations across the South Pacific (such as Alyxia stellata, Cerbera manghas, Neisosperma oppositifolium and Tabernaemontana pandacaquai in the Apocynaceae), raise the question of how many populations need to be sampled to adequately represent the genetic diversity of a species. Investigating the extent of genetic differentiation among populations of these species would provide useful information on the number of seed collections required to conserve the species effectively. The latter group of species also provide an opportunity to investigate survival following long distance dispersal in relation to seed storage behaviour.
Addressing the ongoing decline in rainforest diversity in the South Pacific demands knowledge, partnerships and facilities to support ex situ conservation. A potentially large number of rainforest plant species could be conserved using standard or modified seed banking techniques; however, our window of opportunity for utilising these techniques may be limited. The resources we invest in seed banking and conservation research over the coming years will determine whether we can conserve these species before their habitats are lost.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank the sponsors of the Rainforest Conservation Project – the Arcadia Fund, the Greatorex Foundation, HSBC Bank and several private benefactors – for funding the work of KD Sommerville and Z-J Newby. We thank Marion MacKay, Massey University, for assistance in identifying New Zealand rainforest genera. Gunnar Keppel acknowledges support from the Alexander von Humboldt Foundation in the form of a fellowship. We also thank two anonymous reviewers for their time and helpful suggestions.
References
Adam P (1992) ‘Australian rainforests.’ (Clarendon Press: Oxford)Ahmad B, Baider C, Bernardini B, Biffin E, Brambach F, Burslem D, Byng JW, Christenhusz M, Florens FV, Lucas E (2016) Syzygium (Myrtaceae): Monographing a taxonomic giant via 22 coordinated regional revisions. PeerJ Preprints 4, e1930v1
| Syzygium (Myrtaceae): Monographing a taxonomic giant via 22 coordinated regional revisions.Crossref | GoogleScholarGoogle Scholar |
Angiosperm Phylogeny Group III (2009) An update of the Angiosperm phylogeny group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161, 105–121.
| An update of the Angiosperm phylogeny group classification for the orders and families of flowering plants: APG III.Crossref | GoogleScholarGoogle Scholar |
Ashmore SE, Hamilton KN, Offord CA (2011) Conservation technologies for safeguarding and restoring threatened flora: case studies from eastern Australia. In Vitro Cellular & Developmental Biology. Plant 47, 99–109.
| Conservation technologies for safeguarding and restoring threatened flora: case studies from eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Auld TD, Hutton I, Ooi M, Denham AJ (2010) Disruption of recruitment in two endemic palms on Lord Howe Island by invasive rats. Biological Invasions 12, 3351–3361.
| Disruption of recruitment in two endemic palms on Lord Howe Island by invasive rats.Crossref | GoogleScholarGoogle Scholar |
AVH (2016) ‘Australia’s virtual herbarium.’ (Council of Heads of Australasian Herbaria) Available at http://avh.chah.org.au [Verified 21 November 2017].
Baskin CC, Baskin JM (2014) ‘Seeds: ecology, biogeography and evolution of dormancy and germination.’ (2nd edn) (Academic Press: San Diego, CA, USA)
Beadle NCW, Costin AB (1952) Ecological classification and nomenclature. Proceedings of the Linnean Society of New South Wales 77, 359–364.
Beenken L (2017) Austropuccinia: a new genus name for the myrtle rust Puccinia psidii placed within the redefined family Sphaerophragmiaceae (Pucciniales). Phytotaxa 297, 53–61.
| Austropuccinia: a new genus name for the myrtle rust Puccinia psidii placed within the redefined family Sphaerophragmiaceae (Pucciniales).Crossref | GoogleScholarGoogle Scholar |
Berjak P, Pammenter NW (2014) Cryostorage of germplasm of tropical recalcitrant-seeded species: approaches and problems. International Journal of Plant Sciences 175, 29–39.
| Cryostorage of germplasm of tropical recalcitrant-seeded species: approaches and problems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjtVCmtbc%3D&md5=71fd058526068f23f2e4b8cf37ceb4faCAS |
Brown BN (1999) Occurrence and impact of Phytophthora cinnamomi and other Phytophthora species in rainforests of the Wet Tropics World Heritage Area, and of the Mackay region, Queensland. In ‘Patch deaths in tropical Queensland rainforests: association and impact of Phytophthora cinnamomi and other soil borne organisms’. (Ed. PA Gadek) pp. 41–76. (Co-operative Research Centre for Tropical Rainforest Ecology and Management: Cairns, Qld)
Bryan JE, Shearman PL, Aoro G, Wavine F, Zerry J (2015) The current state of PNG’s forests and changes between 2002 and 2014. In ‘The state of the forests of Papua New Guinea 2014: Measuring change over the period 2002–2014’. (Eds JE Bryan, PL Shearman) pp. 7–42. (University of Papua New Guinea: Port Moresby, Papua New Guinea)
Burbidge NT (1960) The phytogeography of the Australian region. Australian Journal of Botany 8, 75–211.
| The phytogeography of the Australian region.Crossref | GoogleScholarGoogle Scholar |
Burbidge AH, Whelan RJ (1982) Seed dispersal in a cycad, Macrozamia riedlei. Australian Journal of Ecology 7, 63–67.
| Seed dispersal in a cycad, Macrozamia riedlei.Crossref | GoogleScholarGoogle Scholar |
Byrne M, Yeates DK, Joseph L, Kearney M, Bowler J, Williams MAJ, Cooper S, Donnellan SC, Keogh JS, Leys R, Melville J, Murphy DJ, Porch N, Wyrwoll K-H (2008) Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17, 4398–4417.
| Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cjhvFGruw%3D%3D&md5=a4db600222f090c769b66057aa78c855CAS |
Byrne M, Steane DA, Joseph L, Yeates DK, Jordan GJ, Crayn D, Aplin K, Cantrill DJ, Cook LG, Crisp MD, Keogh JS, Melville J, Moritz C, Porch N, Sniderman JMK, Sunnucks P, Weston PH (2011) Decline of a biome: evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota. Journal of Biogeography 38, 1635–1656.
| Decline of a biome: evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota.Crossref | GoogleScholarGoogle Scholar |
Carnegie AJ, Kathuria A, Pegg GS, Entwistle P, Nagel M, Giblin FR (2016) Impact of the invasive rust Puccinia psidii (myrtle rust) on native Myrtaceae in natural ecosystems in Australia. Biological Invasions 18, 127–144.
| Impact of the invasive rust Puccinia psidii (myrtle rust) on native Myrtaceae in natural ecosystems in Australia.Crossref | GoogleScholarGoogle Scholar |
Catterall CP, Kanowski J, Wardell-Johnson GW, Proctor H, Reis T, Harrison D, Tucker NIJ (2004) Quantifying the biodiversity values of reforestation: perspectives, design issues and outcomes in Australian rainforest landscapes. In ‘Conservation of Australia’s forest fauna’. (2nd edn) (Ed. D Lunney) pp. 359–393. (Royal Zoological Society of New South Wales: Mosman, NSW)
Christophel DC (1989) Evolution of the Australian flora through the Tertiary. Plant Systematics and Evolution 162, 63–78.
| Evolution of the Australian flora through the Tertiary.Crossref | GoogleScholarGoogle Scholar |
Commonwealth of Australia (2016) National vegetation information system v. 4.2. (Department of Environment and Energy: Canberra). Data licensed under the Creative Commons Attribution 4.0 International Licence available at http://www.ausgoal.gov.au/creative-commons. Available at https://www.environment.gov.au/land/native-vegetation/national-vegetation-information-system [Verified 21 November 2017].
Convention on Biological Diversity (2012) ‘Global strategy for plant conservation: 2011–2020.’ (Botanic Gardens Conservation International: Richmond, UK)
Corlett RT, Primack RB (2008) Tropical rainforest conservation: a global perspective. In ‘Tropical forest community ecology’. (Eds W Carson, S Schnitzer) pp. 442–456. (Wiley-Blackwell: Chichester, UK)
Corrigan H (2009) ‘Forest data and resource assessment of forestry in Vanuatu’ (Government of Vanuatu: Port Vila, Vanuatu)
Cox PA, Elmqvist T (1993) Ecocolonialism and indigenous knowledge systems: village controlled rainforest preserves in Samoa. Pacific Conservation Biology 1, 6–13.
Crane J, Miller AL, Van Roekel JW, Walters C (2003) Triacylglycerols determine the unusual storage physiology of Cuphea seed. Planta 217, 699–708.
| Triacylglycerols determine the unusual storage physiology of Cuphea seed.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmvFCisr8%3D&md5=fa45507f3043d29192404cf17ae8952cCAS |
Cronk Q (2016) Plant extinctions take time. Science 353, 446–447.
| Plant extinctions take time.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhtlSrs7nO&md5=61a7b6e33ee99e44f2292f77e2a6ec09CAS |
CSIRO (2010) ‘Australian tropical rainforest plants.’ Available at http://keys.trin.org.au/key-server/data/0e0f0504-0103-430d-8004-060d07080d04/media/Html/acknow.htm# [Verified 21 November 2017].
Daws MI, Garwood NC, Pritchard HW (2005) Traits of recalcitrant seeds in a semi-deciduous tropical forest in Panama: some ecological implications. Functional Ecology 19, 874–885.
| Traits of recalcitrant seeds in a semi-deciduous tropical forest in Panama: some ecological implications.Crossref | GoogleScholarGoogle Scholar |
Daws MI, Garwood NC, Pritchard HW (2006) Prediction of desiccation sensitivity in seeds of woody species: a probabilistic model based on two seed traits and 104 species. Annals of Botany 97, 667–674.
| Prediction of desiccation sensitivity in seeds of woody species: a probabilistic model based on two seed traits and 104 species.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD287ltVOntQ%3D%3D&md5=2f0128c234fd236dd648d7d7e8caa5dfCAS |
Dettmann ME (1989) Antarctica: Cretaceous cradle of austral temperate rainforests? Geological Society of London, Special Publications 47, 89–105.
| Antarctica: Cretaceous cradle of austral temperate rainforests?Crossref | GoogleScholarGoogle Scholar |
Dickie JB, Pritchard HW (2002) Systematic and evolutionary aspects of desiccation tolerance in seeds. In ‘Desiccation and survival in plants: drying without dying’. (Eds M Black, HW Pritchard) pp. 239–259. (CABI Publishing: Wallingford, UK)
Director of National Parks (2010) ‘Norfolk Island region threatened species recovery plan.’ (Department of the Environment, Water, Heritage and the Arts, Canberra, ACT)
Doran J, Bush D, Glencross K, Sethy M, Viji I (2012) Variation in growth traits and wood density in whitewood (Endospermum medullosum): a major timber species in Vanuatu. International Forestry Review 14, 476–485.
| Variation in growth traits and wood density in whitewood (Endospermum medullosum): a major timber species in Vanuatu.Crossref | GoogleScholarGoogle Scholar |
Elliott SD, Blakesley D, Hardwick K (2013) ‘Restoring tropical forests: a practical guide.’ (Royal Botanic Gardens: Kew, UK)
Engelmann F (2011) Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cellular & Developmental Biology. Plant 47, 5–16.
| Use of biotechnologies for the conservation of plant biodiversity.Crossref | GoogleScholarGoogle Scholar |
FAO (2015) ‘Global forest resources assessment 2015.’ (Food and Agriculture Organisation of the United Nations: Rome)
Floyd A (1990a) ‘Australian rainforests in New South Wales. Vol. 1.’ (Surrey Beatty & Sons: Chipping Norton, UK)
Floyd A (1990b) ‘Australian rainforests in New South Wales. Vol. 2.’ (Surrey Beatty & Sons: Chipping Norton, UK)
Forsyth DM, Wilson DJ, Easdale TA, Kunster G, Canham CD, Ruscoe WA, Wright EF, Murphy L, Gormley AM, Gaxiola A, Coomes DA (2015) Century-scale effects of invasive deer and rodents on the dynamics of forests growing on soils of contrasting fertility. Ecological Monographs 85, 157–180.
| Century-scale effects of invasive deer and rodents on the dynamics of forests growing on soils of contrasting fertility.Crossref | GoogleScholarGoogle Scholar |
Franklin J, Keppel G, Webb EL, Seamon JO, Rey SJ, Steadman DW, Wiser SK, Drake DR (2013) Dispersal limitation, speciation, environmental filtering and niche differentiation influence forest tree communities in West Polynesia. Journal of Biogeography 40, 988–999.
| Dispersal limitation, speciation, environmental filtering and niche differentiation influence forest tree communities in West Polynesia.Crossref | GoogleScholarGoogle Scholar |
Giblin F, Carnegie AJ (2014) ‘Puccinia psidii (myrtle rust) – Australian host list.’ Available at http://anpc.asn.au/myrtle-rust [verified 27 November 2017]
Gideon OG (2015) The flora of New Guinea: its origins, affinities and patterns of diversity and endemism. In ‘The state of the forests of Papua New Guinea 2014: Measuring change over the period 2002–2014’. (Eds JE Bryan, PL Shearman) pp. 115–135. (University of Papua New Guinea: Port Moresby, Papua New Guinea)
Gillespie TW, Keppel G, Pau S, Price JP, Jaffré T, O’Neill K (2013) Scaling species richness and endemism of tropical dry forests on oceanic islands. Diversity & Distributions 19, 896–906.
| Scaling species richness and endemism of tropical dry forests on oceanic islands.Crossref | GoogleScholarGoogle Scholar |
Gillespie TW, O’Neill K, Keppel G, Pau S, Meyer J-Y, Price JP, Jaffré T (2014) Prioritizing conservation of tropical dry forests in the Pacific. Oryx 48, 337–344.
| Prioritizing conservation of tropical dry forests in the Pacific.Crossref | GoogleScholarGoogle Scholar |
Goosem M, Paz C, Fensham R, Preece N, Goosem S, Laurance S (2016) Forest age and isolation affect the rate of recovery of plant species diversity and community composition in secondary rain forests in tropical Australia. Journal of Vegetation Science 27, 504–514.
| Forest age and isolation affect the rate of recovery of plant species diversity and community composition in secondary rain forests in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Grandcolas P, Murienne J, Robillard T, Desutter-Grandcolas L, Jourdan H, Guilbert E, Deharveng L (2008) New Caledonia: a very old Darwinian Island? Philosophical Transactions of the Royal Society B 363, 3309–3317.
| New Caledonia: a very old Darwinian Island?Crossref | GoogleScholarGoogle Scholar |
Greenwood D (1996) Eocene monsoon forests in central Australia? Australian Systematic Botany 9, 95–112.
| Eocene monsoon forests in central Australia?Crossref | GoogleScholarGoogle Scholar |
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD, Cook WM, Damschen EI, Ewers RM, Foster BL, Jenkins CN, King AJ, Laurance WF, Levey DJ, Margules CR, Melbourne BA, Nicholls AO, Orrock JL, Song D-X, Townshend JR (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Science Advances 1, e150052
| Habitat fragmentation and its lasting impact on Earth’s ecosystems.Crossref | GoogleScholarGoogle Scholar |
Hall R (2001) Cenozoic reconstructions of SE Asia and the SW Pacific: changing patterns of land and sea. In ‘Faunal and floral migrations and evolution in SE Asia-Australasia’. (Eds I Metcalfe, JMB Smith, M Morwood, ID Davidson) pp. 33–56. (Swets & Zeitlinger: Lisse, The Netherlands)
Hamilton KN, Offord CA, Cuneo P, Deseo M (2013) A comparative study of seed morphology in relation to desiccation tolerance and other physiological responses in 71 Eastern Australian rainforest species. Plant Species Biology 28, 51–62.
| A comparative study of seed morphology in relation to desiccation tolerance and other physiological responses in 71 Eastern Australian rainforest species.Crossref | GoogleScholarGoogle Scholar |
Harden GJ, McDonald WJF, Williams JB (2006) ‘Rainforest trees and shrubs: a field guide to their identification.’ (Gwen Harden Publishing: Nambucca Heads, NSW)
Heads M (2006) Seed plants of Fiji: an ecological analysis. Biological Journal of the Linnean Society 89, 407–431.
| Seed plants of Fiji: an ecological analysis.Crossref | GoogleScholarGoogle Scholar |
Hviding E (2003) Contested rainforests, NGOs, and projects of desire in Solomon Islands. International Social Science Journal 55, 539–553.
| Contested rainforests, NGOs, and projects of desire in Solomon Islands.Crossref | GoogleScholarGoogle Scholar |
Ibanez T, Hély C, Gaucherel C (2013) Sharp transitions in microclimatic conditions between savanna and forest in New Caledonia: insights into the vulnerability of forest edges to fire. Austral Ecology 38, 680–687.
| Sharp transitions in microclimatic conditions between savanna and forest in New Caledonia: insights into the vulnerability of forest edges to fire.Crossref | GoogleScholarGoogle Scholar |
Ibanez T, Chave J, Barrabé L, Elodie B, Boutreux T, Trueba S, Vandrot H, Birnbaum P (2017) Community variation in wood density along a bioclimatic gradient on a hyper-diverse tropical island. Journal of Vegetation Science 28, 19–33.
| Community variation in wood density along a bioclimatic gradient on a hyper-diverse tropical island.Crossref | GoogleScholarGoogle Scholar |
Jaffré T (1993) The relationship between ecological diversity and floristic diversity in New Caledonia. Biodiversity Letters 1, 82–87.
| The relationship between ecological diversity and floristic diversity in New Caledonia.Crossref | GoogleScholarGoogle Scholar |
Katovai E, Edwards W, Laurance WF (2015) Dynamics of logging in Solomon Islands: the need for restoration and conservation alternatives. Tropical Conservation Science 8, 718–731.
| Dynamics of logging in Solomon Islands: the need for restoration and conservation alternatives.Crossref | GoogleScholarGoogle Scholar |
Keppel G, Lowe AJ, Possingham HP (2009) Changing perspectives on the biogeography of the tropical South Pacific: influences of dispersal, vicariance and extinction. Journal of Biogeography 36, 1035–1054.
| Changing perspectives on the biogeography of the tropical South Pacific: influences of dispersal, vicariance and extinction.Crossref | GoogleScholarGoogle Scholar |
Keppel G, Buckley YM, Possingham HP (2010) Drivers of lowland rain forest community assembly, species diversity and forest structure on islands in the tropical South Pacific. Journal of Ecology 98, 87–95.
| Drivers of lowland rain forest community assembly, species diversity and forest structure on islands in the tropical South Pacific.Crossref | GoogleScholarGoogle Scholar |
Keppel G, Morrison C, Watling D, Tuiwawa M, Rounds IA (2012) Conservation in tropical Pacific Island countries: why most current approaches are failing. Conservation Letters 5, 256–265.
| Conservation in tropical Pacific Island countries: why most current approaches are failing.Crossref | GoogleScholarGoogle Scholar |
Keppel G, Morrison C, Meyer J-Y, Boehmer HJ (2014) Isolated and vulnerable: the history and future of Pacific Island terrestrial biodiversity. Pacific Conservation Biology 20, 136–145.
| Isolated and vulnerable: the history and future of Pacific Island terrestrial biodiversity.Crossref | GoogleScholarGoogle Scholar |
Keppel G, Gillespie TW, Ormerod P, Fricker GA (2016) Habitat diversity predicts orchid diversity in the tropical Southwest Pacific. Journal of Biogeography 43, 2332–2342.
| Habitat diversity predicts orchid diversity in the tropical Southwest Pacific.Crossref | GoogleScholarGoogle Scholar |
Kier G, Kreft H, Lee TM, Jetz W, Ibisch PL, Nowicki C, Mutke J, Barthlott W (2009) A global assessment of endemism and species richness across island and mainland regions. Proceedings of the National Academy of Natural Sciences, USA 106, 9322–9327.
| A global assessment of endemism and species richness across island and mainland regions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXot1Cgtb0%3D&md5=57afcf76b640fca971005c3055d7d303CAS |
Kingsford RT, Watson JEM (2011) Climate change in Oceania – a synthesis of biodiversity impacts and adaptations. Pacific Conservation Biology 17, 270–284.
| Climate change in Oceania – a synthesis of biodiversity impacts and adaptations.Crossref | GoogleScholarGoogle Scholar |
Kooyman RM, Rossetto M, Sauquet H, Laffan SW (2013) Landscape patterns in rainforest phylogenetic signal: isolated islands of refugia or structured continental distributions? PLoS One 8, e80685
| Landscape patterns in rainforest phylogenetic signal: isolated islands of refugia or structured continental distributions?Crossref | GoogleScholarGoogle Scholar |
Köppen W (1936) ‘Das geographische system der klimate.’ (Verlag von Gebrüder Borntraeger: Berlin)
Krull CR, Stanley MC, Burns BR, Choquenot D, Etherington TR (2016) Reducing wildlife damage with cost-effective management programmes. PLoS One 11, e0146765
| Reducing wildlife damage with cost-effective management programmes.Crossref | GoogleScholarGoogle Scholar |
Laurance WF, Delamônica P, Laurance SG, Vasconcelos HL, Lovejoy TE (2000) Rainforest fragmentation kills big trees. Nature 404, 836
| Rainforest fragmentation kills big trees.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjtVCktro%3D&md5=5e795c272ac5b2c631b511e5d1daf5d1CAS |
Laurance WF, Nascimento HEM, Laurance SG, Andrade AC, Fearnside PM, Ribeiro JEL, Carpetz RL (2006) Rainforest fragmentation and the proliferation of successional trees. Ecology 87, 469–482.
| Rainforest fragmentation and the proliferation of successional trees.Crossref | GoogleScholarGoogle Scholar |
Mabberley D (2008) ‘Mabberley’s plant-book: a portable dictionary of plants, their classifications and uses.’ 3rd edn. (Cambridge University Press: Cambridge, UK)
Martin HA (2006) Cenozoic climatic change and the development of the arid vegetation in Australia. Journal of Arid Environments 66, 533–563.
| Cenozoic climatic change and the development of the arid vegetation in Australia.Crossref | GoogleScholarGoogle Scholar |
Martyn AJ, Merritt DJ, Turner SR (2009) Seed banking. In ‘Plant germplasm conservation in Australia: strategies and guidelines for developing, managing and utilising ex situ collections’. (Eds CA Offord, PF Meagher) pp. 63–85. (Australian Network for Plant Conservation: Canberra, ACT)
McConkey KR, Drake DR (2006) Flying foxes cease to function as seed dispersers long before they become rare. Ecology 87, 271–276.
| Flying foxes cease to function as seed dispersers long before they become rare.Crossref | GoogleScholarGoogle Scholar |
McConkey KR, Drake DR, Meehan HJ, Parsons N (2003) Husking stations provide evidence of seed predation by introduced rodents in Tongan rainforest. Biological Conservation 109, 221–225.
| Husking stations provide evidence of seed predation by introduced rodents in Tongan rainforest.Crossref | GoogleScholarGoogle Scholar |
Meehan HJ, McConkey KR, Drake DR (2002) Potential disruptions to seed dispersal mutualism in Tonga, Western Polynesia. Journal of Biogeography 29, 695–712.
| Potential disruptions to seed dispersal mutualism in Tonga, Western Polynesia.Crossref | GoogleScholarGoogle Scholar |
Metcalfe DJ, Green PT (2017) Rainforests and vine thickets. In ‘Australian vegetation’. (Ed. DA Keith) pp. 257–280. (Cambridge University Press: Cambridge, UK)
Meyer J-Y, Florence J (1996) Tahiti’s native flora endangered by the invasion of Miconia calvescens DC. (Melastomataceae). Journal of Biogeography 23, 775–781.
| Tahiti’s native flora endangered by the invasion of Miconia calvescens DC. (Melastomataceae).Crossref | GoogleScholarGoogle Scholar |
Mills K (2009) ‘The vegetation of Phillip Island, Norfolk Island Group.’ Available at http://econorfolk.nf/pdf/Kevin%20Mills%20Report%20Body.pdf [Verified 21 November 2017].
Minden V, Jacobi JD, Porembski S, Boehmer HJ (2010) Effects of invasive alien kahili ginger (Hedychium garderianum) on native plant species regeneration in a Hawaiian rainforest. Applied Vegetation Science 13, 5–14.
| Effects of invasive alien kahili ginger (Hedychium garderianum) on native plant species regeneration in a Hawaiian rainforest.Crossref | GoogleScholarGoogle Scholar |
Mittermeier RA, Turner WR, Larsen FW, Brooks TM, Gascon C (2011) Global biodiversity conservation: the critical role of hotspots. In ‘Biodiversity hotspots: distribution and protection of conservation priority areas’. (Eds FE Zachos, JC Habel) pp. 3–22. (Springer-Verlag: Berlin)
Morat P, Jaffré T, Tronchet F, Munzinger J, Pillon Y, Veillon J-M, Chalopin M (2012) Le référentiel taxonomique FLORICAL et les caractéristiques de la flore vasculaire indigène de la Nouvelle-Calédonie. Adansonia 34, 179–221.
| Le référentiel taxonomique FLORICAL et les caractéristiques de la flore vasculaire indigène de la Nouvelle-Calédonie.Crossref | GoogleScholarGoogle Scholar |
Mueller-Dombois D, Fosberg FR (1998) ‘Vegetation of the tropical Pacific Islands.’ (Springer-Verlag: New York)
Munzinger J, Morat P, Jaffré T, Gâteblé G, Pillon Y, Tronchet F, Veillon J-M, Chalopin M (2016) FLORICAL: Checklist of the vascular indigenous flora of New Caledonia ver. 22.IV.2016. http://www.botanique.nc/herbier/florical [Verified 21 November 2017].
Neall VE, Trewick SA (2008) The age and origin of the Pacific islands: a geological overview. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 363, 3293–3308.
| The age and origin of the Pacific islands: a geological overview.Crossref | GoogleScholarGoogle Scholar |
Normah MN, Makeen AM (2008) Cryopreservation of excised embryos and embryonic axes. In ‘Plant cryopreservation: a practical guide’. (Ed. BM Reed) pp. 211–240. (Springer: New York)
Offord CA, Meagher PF (2009) ‘Plant germplasm conservation in Australia: strategies and guidelines for developing, managing and utilising ex situ collections.’ (Australian Network for Plant Conservation: Canberra, ACT)
Offord CA, North TG (2009) Living plant collections. In ‘Plant germplasm conservation in Australia: strategies and guidelines for developing, managing and utilising ex situ collections’. (Eds CA Offord, PF Meagher) pp. 149–161. (Australian Network for Plant Conservation: Canberra, ACT)
Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC, D’Amico JA, Itoua I, Strand HE, Morrison JC, Loucks CJ, Allnutt TF, Ricketts TH, Kura Y, Lamoreux JF, Wettengel WW, Hedao P, Kassem KR (2001) Terrestrial ecoregions of the world: a new map of life on Earth. Bioscience 51, 933–938.
| Terrestrial ecoregions of the world: a new map of life on Earth.Crossref | GoogleScholarGoogle Scholar |
Page T, Doran J (2016) ‘Development and delivery of germplasm for sandalwood and whitewood in Vanuatu and northern Australia.’ (ACIAR: Canberra)
Park MJ (2013) Seed storage behaviour of New Zealand’s threatened vascular plants. PhD thesis, Institute of Agriculture and Environment, Massey University) Available at https://mro.massey.ac.nz/handle/10179/4661 [Verified 27 November 2017]
Pauku RL (2009) ‘Solomon Islands forestry outlook study.’ (Food and Agriculture Organisation of the United Nations: Bangkok)
Peel MC, Finlayson BL, McMahon TA (2007) Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences 11, 1633–1644.
| Updated world map of the Köppen-Geiger climate classification.Crossref | GoogleScholarGoogle Scholar |
Pence VC (2005) In vitro collecting (IVC). I. The effect of collecting method and antimicrobial agents on contamination in temperate and tropical collections. In Vitro Cellular & Developmental Biology. Plant 41, 324–332.
| In vitro collecting (IVC). I. The effect of collecting method and antimicrobial agents on contamination in temperate and tropical collections.Crossref | GoogleScholarGoogle Scholar |
Putz FE, Romero C (2014) Futures of tropical forests (sensu lato). Biotropica 46, 495–505.
| Futures of tropical forests (sensu lato).Crossref | GoogleScholarGoogle Scholar |
Reed BM (2008) ‘Plant cryopreservation: a practical guide.’ (Springer: New York)
Roberts EH (1973) Predicting the storage life of seeds. Seed Science and Technology 1, 499–514.
Rossetto M, Kooyman R, Yap J-YS, Laffan SW (2015) From ratites to rats: the size of fleshy fruits shapes species’ distributions and continental rainforest assembly. Proceedings. Biological Sciences 282, 20151998
| From ratites to rats: the size of fleshy fruits shapes species’ distributions and continental rainforest assembly.Crossref | GoogleScholarGoogle Scholar |
Royal Botanic Gardens Kew (2017) Seed Information Database (SID) ver. 7.1. Available at http://data.kew.org/sid/ [Verified 17 November 2017].
Schneider CJ, Moritz C (1999) Rainforest refugia and evolution in Australia’s Wet Tropics. Proceedings. Biological Sciences 266, 191–196.
| Rainforest refugia and evolution in Australia’s Wet Tropics.Crossref | GoogleScholarGoogle Scholar |
Scott P, Williams N (2014) Phytophthora diseases in New Zealand forests. New Zealand Journal of Forestry 59, 14–21.
Settle DJ, Page T, Bush D, Doran J, Sethy M, Viji I (2012) Basic density, diameter and radial variation of Vanuatu whitewood (Endospermum medullosum): potential for breeding in a low density, tropical hardwood. International Forestry Review 14, 463–475.
| Basic density, diameter and radial variation of Vanuatu whitewood (Endospermum medullosum): potential for breeding in a low density, tropical hardwood.Crossref | GoogleScholarGoogle Scholar |
Smith AC (1979) ‘Flora vitiensis nova. Vol. 1.’ (National Tropical Botanical Garden: Lawaii, Hawaii)
Smith AC (1981) ‘Flora vitiensis nova. Vol. 2.’ (National Tropical Botanical Garden: Lawaii, Hawaii)
Smith AC (1985) ‘Flora vitiensis nova. Vol. 3.’ (National Tropical Botanical Garden: Lawaii, Hawaii)
Smith AC (1988) ‘Flora vitiensis nova. Vol. 4.’ (National Tropical Botanical Garden: Lawaii, Hawaii)
Smith AC (1991) ‘Flora vitiensis nova. Vol. 5.’ (National Tropical Botanical Garden: Lawaii, Hawaii)
Sniderman JMK, Jordan GJ (2011) Extent and timing of floristic exchange between Australian and Asian rain forests. Journal of Biogeography 38, 1445–1455.
| Extent and timing of floristic exchange between Australian and Asian rain forests.Crossref | GoogleScholarGoogle Scholar |
Sommerville KD, Offord CA (2015) Ex situ conservation techniques for Australian rainforest species. Acta Horticulturae 75–80.
| Ex situ conservation techniques for Australian rainforest species.Crossref | GoogleScholarGoogle Scholar |
Sommerville KD, Errington G, Newby Z-J, Offord CA (2016) The rainforest challenge – testing the ‘unstorable’ seed assumption. Australasian Plant Conservation 25, 10–11.
Stork NE, Goosem S, Turton SM (2011) Status and threats in the dynamic landscapes of Northern Australia’s tropical rainforest biodiversity hotspot: the Wet Tropics. In ‘Biodiversity hotspots’. (Eds NFE Zachos, JC Habel) pp. 311–332. (Springer-Verlag: Berlin)
Sykes WR (2016) ‘Flora of the Cook Islands.’ (National Tropical Botanical Garden: Kaua‘i, Hawai‘i)
Teulon DAJ, Alipia TT, Ropata HT, Green JM, Viljanen-Rollinson SLH, Cromey MG, Arthur K, MacDiarmid RM, Waipara MW, Marsh AT (2015) The threat of myrtle rust to Māori taonga plant species in New Zealand. New Zealand Plant Protection 68, 66–75.
Thaman RR (1994) Ethnobotany of Pacific Island coastal plants. In ‘Science of Pacific Island peoples: fauna, flora, food and medicine. Vol 3.’ (Eds J Morrison, P Geraghty and L Crowl) pp. 147–184. (Institute of Pacific Studies: Suva, Fiji)
Tng DYP, Apgaua DMG, Campbell MJ, Cox CJ, Crayn DM, Ishida FY, Laidlaw MJ, Liddell MJ, Seager M, Laurance SGW (2016) Vegetation and floristics of a lowland tropical rainforest in northeast Australia. Biodiversity Data Journal 4, e7599
| Vegetation and floristics of a lowland tropical rainforest in northeast Australia.Crossref | GoogleScholarGoogle Scholar |
Trewick SA, Paterson AM, Campbell HJ (2007) Hello New Zealand. Journal of Biogeography 34, 1–6.
| Hello New Zealand.Crossref | GoogleScholarGoogle Scholar |
Tweddle JC, Dickie JB, Baskin CC, Baskin JM (2003) Ecological aspects of seed desiccation sensitivity. Journal of Ecology 91, 294–304.
| Ecological aspects of seed desiccation sensitivity.Crossref | GoogleScholarGoogle Scholar |
van Balgooy MMJ, Hovenkamp PH, van Welzen PC (1996) Phytogeography of the Pacific – floristic and historical distribution patterns in plants. In ‘The origin and evolution of Pacific Island biotas, New Guinea to Eastern Polynesia: patterns and processes’. (Eds A Keast, SE Miller) pp. 191–213. (SPB Academic Publishing: Amsterdam)
Vincent JB, Henning B, Saulei S, Sosanika G, Weiblen GD (2015) Forest carbon in lowland Papua New Guinea: local variation and the importance of small trees. Austral Ecology 40, 151–159.
| Forest carbon in lowland Papua New Guinea: local variation and the importance of small trees.Crossref | GoogleScholarGoogle Scholar |
Wallace KJ, Laughlin DC, Clarkson BD (2017) Exotic weeds and fluctuating microclimate can constrain native plant regeneration in urban forest restoration. Ecological Applications
| Exotic weeds and fluctuating microclimate can constrain native plant regeneration in urban forest restoration.Crossref | GoogleScholarGoogle Scholar |
Wardell-Johnson GW, Keppel G, Sander J (2011) Climate change impacts on the terrestrial biodiversity and carbon stocks of Oceania. Pacific Conservation Biology 17, 220–240.
| Climate change impacts on the terrestrial biodiversity and carbon stocks of Oceania.Crossref | GoogleScholarGoogle Scholar |
Webb LJ (1968) Environmental relationships of the structural types of Australian rain forest vegetation. Ecology 49, 296–311.
| Environmental relationships of the structural types of Australian rain forest vegetation.Crossref | GoogleScholarGoogle Scholar |
Weir BS, Paderes EP, Anand N, Uchida JY, Pennycook SR, Bellgard SE, Beever RE (2015) A taxonomic revision of Phytophthora Clade 5 including two new species, P. agathidicida and P. cocois. Phytotaxa 205, 21–38.
| A taxonomic revision of Phytophthora Clade 5 including two new species, P. agathidicida and P. cocois.Crossref | GoogleScholarGoogle Scholar |
Whistler WA (2000) ‘Plants in Samoan culture: the ethnobotany of Samoa.’ (Isle Botanica: Honolulu, Hawaii)
Whitmore TC (1969) The vegetation of the Solomon Islands. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 255, 259–270.
| The vegetation of the Solomon Islands.Crossref | GoogleScholarGoogle Scholar |
Williams JG, Harden GJ, McDonald WJF (1984) ‘Trees and shrubs in rainforests of New South Wales and southern Queensland.’ (University of New England: Armidale, NSW)
Wilson AJG, (Ed.) (1994) ‘Flora of Australia. Vol. 49, Oceanic Islands 1.’ (Australian Government Printing Service: Canberra, ACT)
Wiser SK, Drake DR, Burrows LE, Sykes WR (2002) The potential for long-term persistence of forest fragments on Tongatapu, a large island in western Polynesia. Journal of Biogeography 29, 767–787.
| The potential for long-term persistence of forest fragments on Tongatapu, a large island in western Polynesia.Crossref | GoogleScholarGoogle Scholar |
Wiser SK, Hurst JM, Wright EF, Allen RB (2011) New Zealand’s forest and shrubland communities: a quantitative classification based on a nationally representative plot network. Applied Vegetation Science 14, 506–523.
| New Zealand’s forest and shrubland communities: a quantitative classification based on a nationally representative plot network.Crossref | GoogleScholarGoogle Scholar |
Woinarski JCZ (2010) Biodiversity conservation in tropical forest landscapes of Oceania. Biological Conservation 143, 2385–2394.
| Biodiversity conservation in tropical forest landscapes of Oceania.Crossref | GoogleScholarGoogle Scholar |
Wyse SV, Dickie JB (2016) Predicting the global incidence of seed desiccation sensitivity. Journal of Ecology
| Predicting the global incidence of seed desiccation sensitivity.Crossref | GoogleScholarGoogle Scholar |
Wyse SV, Burns BR, Wright SD (2014) Distinctive vegetation communities are associated with the long-lived conifer Agathis australis (New Zealand kauri, Araucariaceae) in New Zealand rainforests. Austral Ecology 39, 388–400.
| Distinctive vegetation communities are associated with the long-lived conifer Agathis australis (New Zealand kauri, Araucariaceae) in New Zealand rainforests.Crossref | GoogleScholarGoogle Scholar |
Yoshinaga AY, Walters C (2003) Conservation of tropical seeds: an example from Hawai’i. In ‘Seed conservation: turning science into practice’. (Eds RD Smith, JB Dickie, SH Linington, HW Pritchard, RJ Probert) pp. 956–963. (The Royal Botanic Gardens, Kew, UK)