Burrowing seabirds drive decreased diversity and structural complexity, and increased productivity in insular-vegetation communities
Wesley J. Bancroft A D , J. Dale Roberts A and Mark J. Garkaklis B CA School of Animal Biology, M092, University of Western Australia, Crawley, WA 6009, Australia.
B Western Australian Department of Conservation and Land Management, PO Box 1167, Bentley Delivery Centre, Bentley, WA 6983, Australia.
C School of Biological Sciences and Biotechnology, Murdoch University, Murdoch, WA 6150, Australia.
D Corresponding author. Email: wes@graduate.uwa.edu.au
Australian Journal of Botany 53(3) 231-241 https://doi.org/10.1071/BT04079
Submitted: 2 June 2004 Accepted: 25 November 2004 Published: 26 May 2005
Abstract
Burrow-nesting seabirds, such as the wedge-tailed shearwater (Puffinus pacificus (Gmelin)) physically and chemically engineer the soil of their colonies in a manner that is likely to affect plant growth and ecology. We examined this functional interaction by measuring the diversity, vertical structure and productivity of vegetation in shearwater colonies on Rottnest Island, Western Australia, and by comparing these with those in the adjacent, non-colonised heath. The colony supported a distinct, less diverse vegetation community and was dominated by short-lived, succulent exotics. An ecotone was present between the two communities. Across all species, vegetation was shorter and denser in the colony and individual species that co-occurred in both locations were stunted in the colony. The percentage of bare soil in the colony was double that of the heath. The productivity of a phytometer (Rhagodia baccata) was significantly higher in colony soil than in heath soil. In a glasshouse experiment, cuttings grown in colony soil had 337% of the root mass and 537% of the foliage mass of plants grown in heath soil. Field measurements demonstrated increased leaf set and foliage extension in colony plants. Seed germination from the colony soil (2674 seedlings m–2) greatly exceeded that of the heath (59 seedlings m–2). Dense, productive and species-poor colony vegetation supports the assemblage-level thinning hypothesis as the mechanism for vegetation change, but we argue that prominent colony species are simply better adapted to high nutrient loads and frequent disturbance. A model of vegetation succession is also proposed.
Introduction
The association between burrowing seabirds and temperate-island vegetation has been relatively poorly researched. Most studies have concentrated on non-burrowers or non-temperate climates and have largely focussed on the chemical inputs through the deposition of guano (Gillham 1960a, 1961, 1962, 1963a, 1963b; Brown et al. 1993; Walsh et al. 1997; Abbott et al. 2000; Stern et al. 2000; Garcia et al. 2002; Rippey et al. 2002b; Low 2003). Recent work has recognised that the birds act as an important nutrient vector, affecting plant and animal communities by the transfer of allochthonous nutrients from ocean to land (Anderson and Kristensen 1991; Sanchez-Pinero and Polis 2000). Burrowing seabirds such as the Procellaridae (fulmars, prions, petrels and shearwaters), however, introduce another major factor to their colonies, physical biopedturbation. The birds physically alter their habitat by constructing burrows, which has led to their classification as allogenic ecosystem engineers (Jones et al. 1994; Bancroft et al. 2004c). Through their actions, ecosystem engineers affect plant and animal communities by modifying resource flows within the ecosystem (Jones et al. 1994). On Rottnest Island, wedge-tailed shearwaters (Puffinus pacificus) have displaced 210 t of soil ha–1 to form their current burrows (Bancroft et al. 2004c). Their biopedturbation has altered a range of physical soil characteristics such as temperature, bulk density, water content, water repellency, infiltration and soil strength, as well as chemical properties such as nutrient content, soil pH and conductivity (Bancroft et al. 2004a). These soil properties are important in determining vegetation composition and structure (Chapman 1986; Crawley 1986a) and we predict that the vegetation of the biopedturbated colonies should differ from that of adjacent, undisturbed areas.
In studies of the association between vegetation and insular seabird colonies, species richness has been commonly used to quantify vegetation (Walsh et al. 1997; Rippey et al. 1998, 2002b; Abbott et al. 2000), yet very few have extended to measure the vertical structure of the vegetation, as used in other disturbance studies (e.g. mining, Chaffey and Grant 2000). Few studies have compared measures of community composition, vertical structure and plant productivity between seabird colonies and undisturbed vegetation at the within-island scale (Mulder and Keall 2001).
We should observe strong differences between the vegetation community and adjacent, unbiopedturbated areas. Allogenic ecosystem engineers such as burrowers generally reduce habitat complexity by reducing plant density and species richness (Crooks 2002). Regular soil disturbance favours smaller, annual plants that can rapidly grow and reproduce in brief periods of reduced disturbance (Grime 1979; Rippey and Rowland 1995). Burrowing alters the soil-water dynamics and guano deposition increases nutrient levels (Anderson and Polis 1999; Garcia et al. 2002; Bancroft et al. 2004a). Together these drive greater plant productivity and, in areas where soil fertility is naturally poor, they favour exotic plant species over natives that are adapted to low nutrient levels (Lamont 1984; Anderson and Polis 1999; Garcia et al. 2002). Soil overlaying burrows is often drier and can cause plant dwarfism (Gillham 1956; Bancroft et al. 2004a). Physical damage, atmospheric or light exposure to roots, dry soil and burial of surface vegetation favour succulent shrubs over woody heath species (Gillham 1956, 1961).
Given these mechanisms and trends, we predict that the vegetation community of a burrowing-seabird colony should be less diverse, comprise more exotic, short-lived succulents, have fewer heath and woody species, have a lower maximum height, and colony plants should have increased productivity. Here we test these predictions by quantifying the community composition, vertical vegetation structure and plant productivity of the vegetation community of wedge-tailed shearwater colonies, and adjacent heath, on Rottnest Island.
Methods
Study site
The study was conducted on Rottnest Island, Western Australia (32°00′00″S, 115°31′00″E), a 1900-ha, limestone island, 18 km off the coast of Western Australia (Playford 1983; Rippey and Rowland 1995). The island experiences a cool, wet winter and a ‘distinctly dry and warm summer’ (Stern et al. 2000), with a mean annual rainfall of 717 mm, and mean daily maximum/minimum temperatures of 26.9/19.3°C and 17.2/11.6°C for summer and winter, respectively (Rippey and Rowland 1995). There are six separate wedge-tailed shearwater colonies on Rottnest Island, the largest of which, the Radar Reef colony (Bancroft et al. 2004b), was the site of this study. We worked only at a single site and acknowledge that pseudoreplication may be an issue. We ensured, however, that our sampling methods were such that we could confidently assume independence of each sampling point. The Radar Reef colony was visually representative of all other colonies on the island. We defined three locations within this site, following Bancroft et al. (2004a):
-
colony—areas within the shearwater colony, where burrow density was greater than 0.15 burrows m–2;
-
edge—the transition area between colony and adjacent heath, where burrow density was between 0 and 0.15 burrows m–2; and
-
heath—heath adjacent to the shearwater colony where burrow density was zero.
Community composition
Surveys were made in May, July, October and December 2002, and February 2003. At each time, 20 1-m2 quadrats were scored within each of the three locations defined above. Randomly generated compass bearings and distances were used to position quadrats. In each quadrat, plant species were recorded as present/absent. We distinguished between live and dead plants. Richness data were analysed by a 2-factor (location, time) ANOVA for total richness, and a 2-factor (location, time) MANOVA for live and dead species (the variables). Quadrat data were used to calculate Shannon–Wiener diversity indices (H), and subsequently the equitabilty (E, (see Krebs 1994) for each of the communities studied at each sampling occasion. Similarly, the quadrat data were also used to calculate two community-comparison indices; the coefficient of Jaccard (Sj) and percentage similarity (P, Krebs 1999). Mean H, E, Sj and P were compared separately by a single-factor (location) ANOVA. Student Newman–Keuls (SNK) post hoc tests were used where appropriate.
Vertical vegetation structure
Vertical vegetation height was measured in the same months as species richness, and followed a modification of the method described by Chaffey and Grant (2000). A 2-mm-diameter levy pole, marked at 15-cm intervals to 120 cm (in excess of the maximum vegetation height), was placed vertical to the ground and the number of touches of each plant species, at each interval, was recorded. Live and dead material was distinguished. Poles where no vegetation touches were recorded were considered ‘bare soil’. Two hundred poles were placed, using random bearings and distances, in the colony and heath locations for each time point. Overall and strata-by-strata densities (as measured by the number of vegetation touches) were analysed separately using two factor (location, time) ANOVA. Student–Newman–Keuls (SNK) post-hoc tests were used where appropriate. Paired t-tests were used to compare the mean number of touches per strata and mean percentage bare ground (calculated for each month sampled; the pairings) between sites.
Plant productivity and seedling germination
Plant productivity (assimilation of biomass per unit time, Chapman 1986) was assessed in the glasshouse and in the field. A single species, Rhagodia baccata, was chosen, on the basis of species richness data, as a phytometer (see Mulder and Keall 2001). A native, chenopod shrub, R. baccata, was the third-most common species across all sampling quadrats and the most evenly spread species among locations. The two more common species, Acanthocarpus preissii and Threlkeldia diffusa, were not selected for glasshouse trials because they were unevenly represented among sampling locations or thought to be poorly suited to the propagation process.
For glasshouse trials, R. baccata cuttings were propagated from a single mother plant. Sixty established cuttings were then randomly assigned to two soil treatments: heath or colony. Surface soils (up to 10-cm depth) were collected from colony and heath from 10 randomly assigned points at the Radar Reef site in June 2003 and were stored in sealed drums. Once in the glasshouse, soils from each separate treatment were thoroughly mixed by mat rolling. Plants were potted in 500 g of soil in plastic-lined, 10-cm-diameter pots and maintained at field water capacity by watering to weight for 7 weeks (49 days). Initially, each pot was randomly arranged within a block on glasshouse benches, and then rearranged weekly. Plants were exposed to the natural photoperiod (11.75 h increasing to 12.75 h) for the duration of the study. The number of leaves on each plant was counted at 2 and 5 weeks (Day 14 and Day 35). After 49 days, each plant was harvested, washed free of soil, separated into root and foliage material and then dried at 55°C for 4 days. Dry biomass for the root and foliage material was recorded. We also recorded the number of seedlings of all plant species that germinated in the glasshouse pots. These seedlings were removed throughout the study to avoid confounding the productivity experiment.
For field trials, 30 stems from plants in both the colony and heath locations were marked with coloured wool at the 10th node from the stem tip in September 2003. The length, to the nearest millimetre, from the 10th node to the stem tip was also recorded. The number of nodes and length from the mark to the stem tip was remeasured in January 2004.
Leaf set on plants was analysed with a single-factor (soil type) repeated measures ANOVA. Biomass and field data were separately analysed with a single-factor (soil type) MANOVA for root and foliage biomass, and node number and stem length, respectively (variables). Student–Newman–Keuls (SNK) post hoc tests were used where appropriate. A t-test was used to compare mean seed germination among soil types.
Taxonomy
Nomenclature and common names follow Rippey et al. (2003) and Rippey and Rowland (1995). Species are listed in the taxonomic order provided by the Bureau of Flora and Fauna (2002).
Results
Community composition
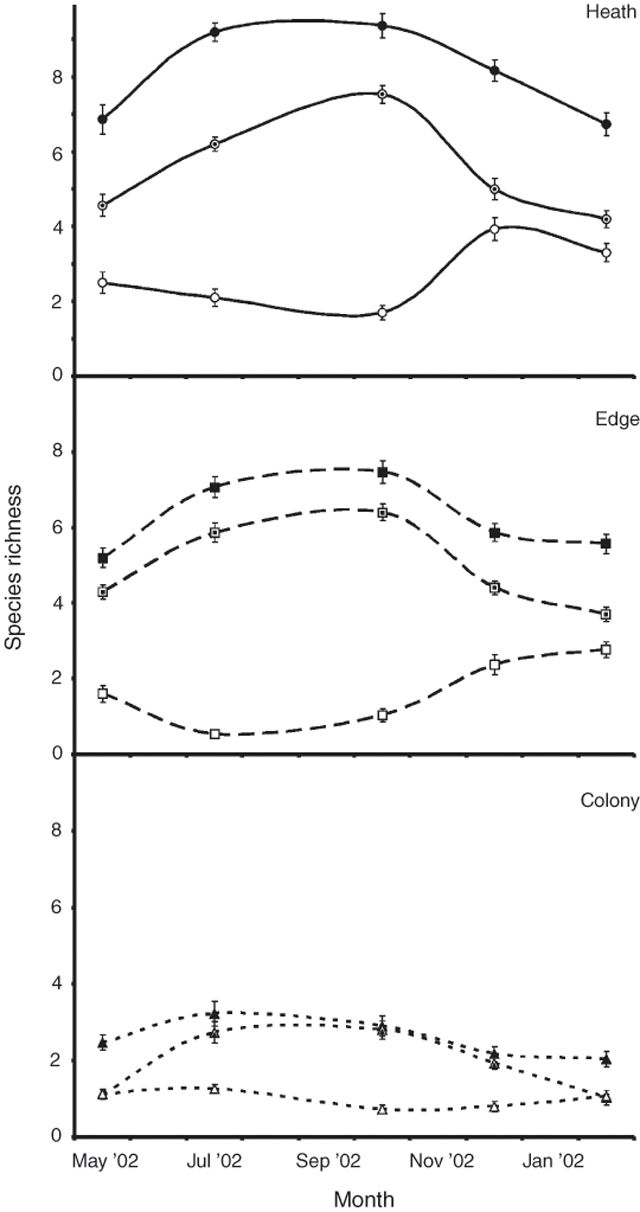
Twenty-eight live, vascular plant species were observed at the study locations. More species were observed in the heath (21) than the edge (18) and colony (15). Nineteen species were encountered during the quadrat and vertical vegetation surveys (Table 1). Mean species richness per quadrat (Fig. 1) differed significantly among locations (ANOVA, F2,435 = 511.6, P = 0.00) and among sampling months (ANOVA, F4,435 = 29.8, P < 0.001), with richness greater in the heath than the edge and colony, and the edge richness greater than the colony (SNK, P < 0.001 in all cases). Across all locations, species richness was significantly greater in July and October, than December, and greater in December than February and May (SNK, P > 0.016). There was an overall significant difference in species richness among locations (MANOVA, F4,868 = 173.3, P < 0.001) and months (MANOVA, F8,868 = 43.4, P < 0.001) for the mean number of live and dead species recorded per quadrat. The seasonal fluctuation in live and dead species appeared similar across locations (Fig. 1), so it is likely that the significant interaction term (MANOVA, F16,868 = 5.8, P < 0.001) reflects the concurrent increase in the number of live species and the decrease in dead species (Fig. 1). The mean number of live and dead species per quadrat followed the same significant trends among locations as total richness (SNK, P < 0.001 in all cases). Mean species diversity (Shannon–Wiener diversity index, H) was significantly greater in the heath (H = 1.04 ± 0.04 s.e.) and the edge (H = 0.95 ± 0.03 s.e.) than in the colony (H = 0.67 ± 0.03 s.e.; ANOVA, F2,12 = 44.4, P < 0.001; SNK, P < 0.001 in both cases). Evenness (equitability, E) followed the same, significant trends: heath (E = 0.90 ± 0.01 s.e.) and edge (E = 0.88 ± 0.01 s.e.) were more even than the colony (E = 0.77 ± 0.03 s.e.; ANOVA, F2,12 = 11.974, P = 0.001; SNK, P = 0.002, 0.003, respectively). There was no significant difference between the species diversity (SNK, P = 0.054) or evenness (SNK, P = 0.428) of the heath and the edge.
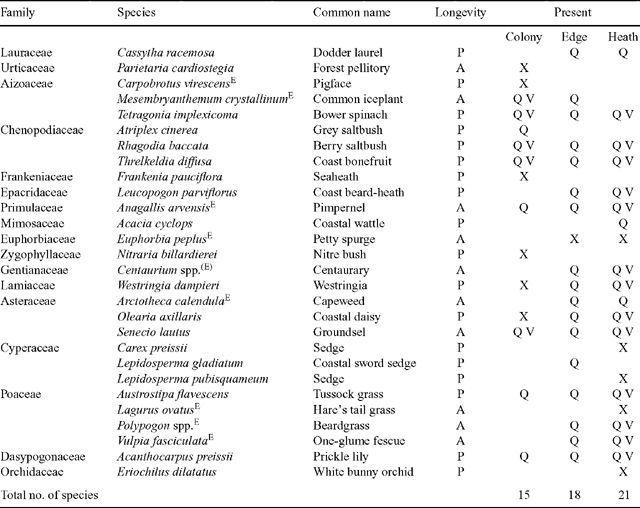
|
|
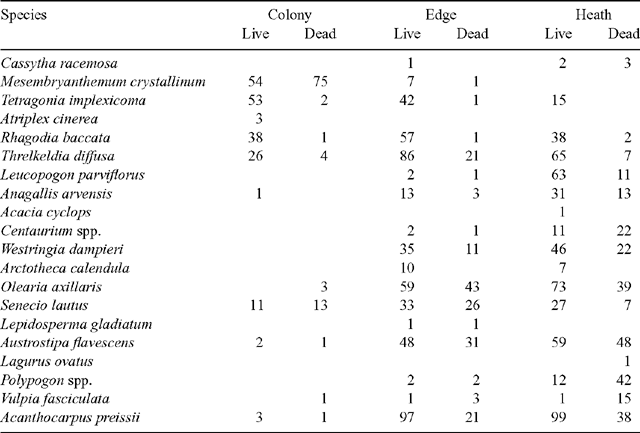
The community composition for each location, based on data from all months, is presented in Table 2. Heath vegetation was dominated by Acanthocarpus preissi, Olearia axillaris, Threlkeldia diffusa, Lecuopogon parviflorus and Austrostipa flavescens, the edge community by A. preissi, T. diffusa, O. axillaris, and Rhagodia baccata, and the colony community by Mesembryanthemum crystallinum and Tetragonia implexicoma. Levy-pole data confirmed the dominance of O. axillaris (30% of all touches) and A. preissi (24%) in the heath, and M. crystallinum (62%) and T. implexicoma (16%) in the colony. Measures of community similarity for presence/absence data (coefficient of Jaccard, Sj) and accounting for the proportion of quadrats in which species were present (percentage similarity, P) are shown in Table 3. Both measures were consistent, and there was a significant difference among the three community comparisons for Sj (ANOVA, F2,12 = 41.0, P < 0.001; SNK, P < 0.020 in each case) and P (ANOVA, F2,12 = 63.1, P < 0.001; SNK, P < 0.007 in each case). The greatest similarity was between the heath-vegetation community and the edge, followed by the edge and the colony, and then by the colony and the heath.
|

|
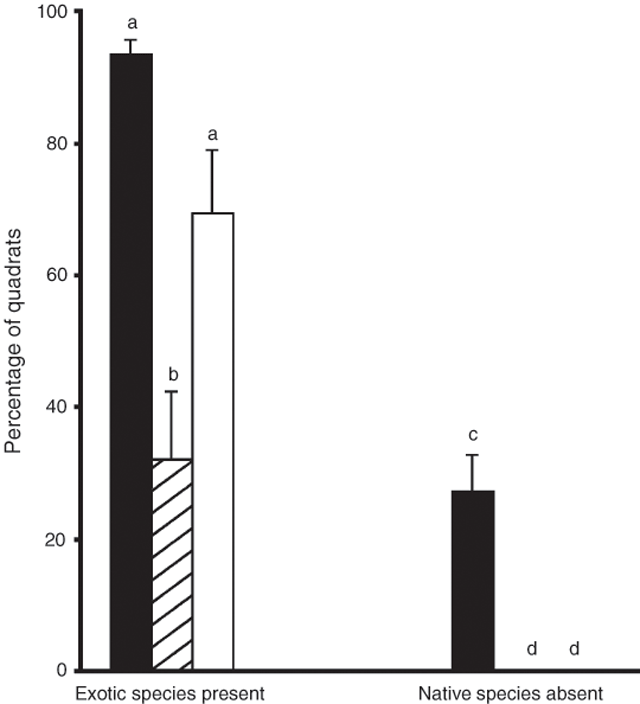
On average, exotic species (Table 1) were recorded in 93% ± 2.4 s.e. of colony and 69% ± 10.3 s.e. of heath quadrats, but in only 32% ± 9.7 s.e. of edge quadrats (Fig. 2). There were significantly more quadrats containing exotic species in the colony and heath than in the edge (Fig. 2, ANOVA, F2,12 = 13.9, P < 0.001; SNK, P = 0.001, 0.008, respectively). The colony and heath did not significantly differ (SNK, P = 0.063). All quadrats in the edge and heath contained at least three native species, but native species were absent from a significant number of colony quadrats (mean 27% ± 5.5 s.e.; Fig. 2; ANOVA, F2,12 = 24.5, P < 0.001; SNK, P < 0.001 in both cases).
|
Vertical vegetation structure
Heath vegetation was taller than colony vegetation (Fig. 3). For heath, the median vegetation touch fell in the 15–30-cm strata, and this was also the modal value. For the colony, both the median touch and modal value occurred in the 0–15-cm strata. Across all height strata, vegetation was significantly (ANOVA, F1,990 = 291.5, P < 0.001) more dense in the heath than in the colony, the total number of touches differed significantly (ANOVA, F4,990 = 7.5, P < 0.001) among months and there was a significant (ANOVA, F4,990 = 3.3, P = 0.010) interaction between the two (Fig. 3). The latter is most likely explained by the dramatic increase in the density of live vegetation (in particular Mesembryanthemum crystallinum) in the colony in October, following winter rain, and the subsequent die-back of this vegetation through summer and autumn (February, May; Fig. 3). At both locations, vegetation was denser near ground level. There were significantly (ANOVA, F7,64 = 38.8, P < 0.001; SNK, P < 0.001 in all cases) more touches at the 0–15-cm strata. The 15–30-cm and 30–45-cm strata had a similar number of touches (SNK, P = 0.217) but these both had significantly more touches than all strata above 45 cm (SNK, P < 0.005). To account for variation in vegetation height, and consequently the number of height strata at each location, the mean number of touches per stratum was calculated. This standardised calculation revealed that the colony vegetation was denser (1.7 ± 0.3 s.e. touches per stratum) than the heath vegetation (1.1 ± 0.2 s.e. touches per stratum), although there was no significant difference (paired t-test, t4 = 2.4, P = 0.073). Within a species, heath plants were taller than those occurring in the colony. Three of the four species recorded in both the colony and heath (Table 1) had a greater maximum height in the heath (Rhagodia baccata, Senecio lautus and Threlkeldia diffusa). Tetragonia implexicoma had an equal maximum height in both locations. The colony (7.6 ± 1.3%) had twice the percentage of bare ground of the heath (3.8 ± 1.5%; paired t-test, t4 = 4.0, P = 0.016).
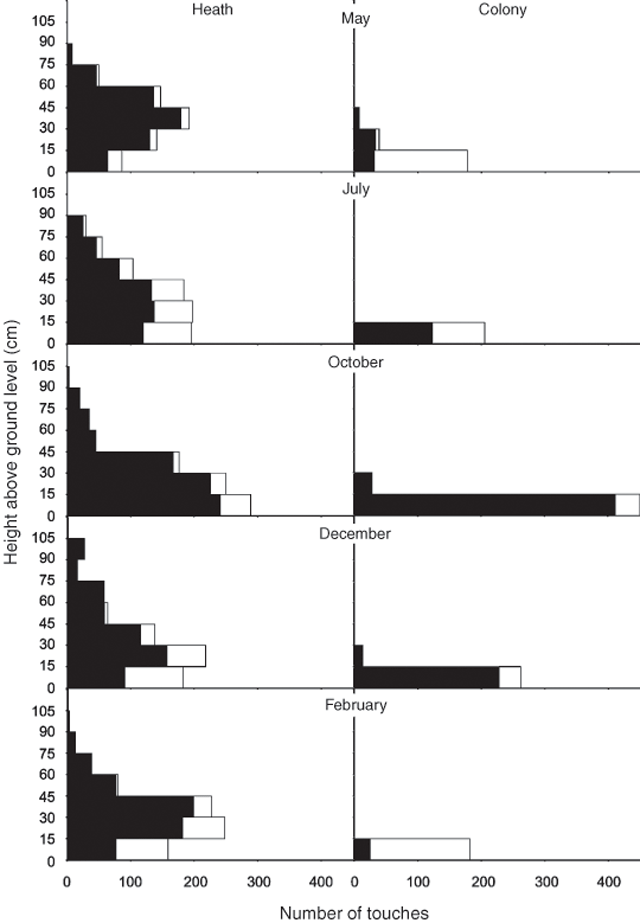
|
Plant productivity
Soil type had a significant effect on plant productivity, with colony soil favouring higher levels of productivity (Figs 4 and 5). The number of leaves on R. baccata cuttings grown in colony soil increased nearly 3.5-fold in a 3-week period of the glasshouse trial, compared with the 1.3-fold increase on plants grown in heath soil (Fig. 4; RM-ANOVA, F1,58 = 81.6, P < 0.001). At Day 14 of the glasshouse trial, there was no difference in the mean number of leaves on plants grown in the colony and heath soil (SNK, P > 0.500), but by Day 35 colony-soil plants had significantly (SNK, P < 0.010) increased their leaf number and had significantly (SNK, P < 0.005) more leaves than heath-soil plants. There was no change in the number of leaves on heath-soil plants (SNK, P > 0.500). At the end of the trial, the dry biomass of plants grown in the two soil types differed significantly (MANOVA, F2,57 = 106.3, P < 0.001; Fig. 5;), with the mass of roots (ANOVA, F1,58 = 60.0, P < 0.001) and foliage (ANOVA, F1,58 = 213.4, P < 0.001) from colony-soil plants 3.4 and 5.4 times, respectively, that of plants grown in heath soil. Field trials agreed with the growth patterns in the glasshouse plants. In the 4 months between September and February, colony R. baccata increased the number of nodes at the apical tip of their stems by 1.8 ± 0.2 s.e., and stem length by 38.8 mm ± 3.9 s.e. Heath plants added significantly fewer nodes (0.6 ± 0.1 s.e.; MANOVA, F1,58 = 22.1, P < 0.001) and less stem length (15.7 mm ± 2.2 s.e.; MANOVA, F1,58 = 27.0, P < 0.001).
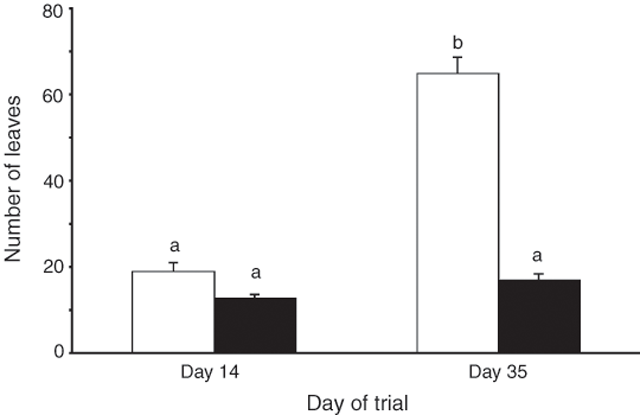
|
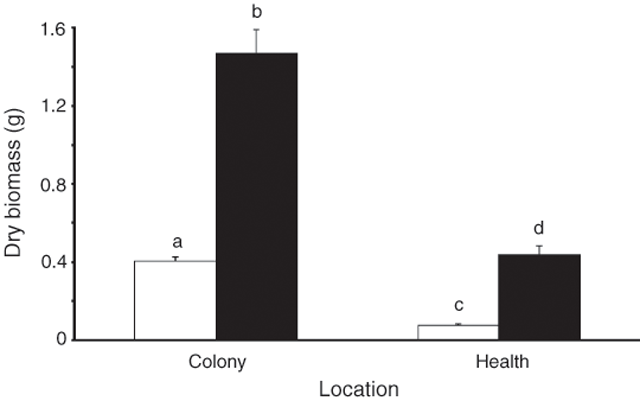
|
Seeds germinated from the natural seed bed in all pots containing colony soil in the glasshouse trial (range 7–38), whereas germination occurred only in 30% of heath pots (range 0–3). The mean number of seedlings that sprouted per pot during the glasshouse trials was far greater in the colony (21.0 ± 1.7 s.e.) than the heath (0.5 ± 0.2 s.e.; t-test, t29.5(adj) = 11.9, P < 0.001). These equate to a potential germination of 2674 seedlings m–2 in the colony and 59 seedlings m–2 in the heath. Most seedlings in the colony pots were M. crystallinum.
Discussion
Community composition
The wedge-tailed shearwater breeding colonies on Rottnest Island support a vegetation community that strongly differs from surrounding non-colony areas. Shearwaters not only deposit considerable quantities of nutrients in their guano, but they also physically disturb the soil environment, and these combined actions have previously been shown to affect a range of physical and chemical soil properties on Rottnest Island (Bancroft et al. 2004a). Soil temperature, bulk density, water content, strength, water repellency, hydraulic conductivity, nutrient composition, organic matter, pH and conductivity are all significantly altered by shearwater biopedturbation. Here we have demonstrated that these edaphic disturbances translate into compositional and structural changes to the colony flora.
The three locations investigated in this study (heath, edge and colony) appear to represent three zones along a continuum of vegetation change. The generalised pattern of species occurrence as one walked out of the heath and into the colony from the landward boundary was as follows (see also the change in species and their proportions from heath to edge to colony shown in Table 2). New burrows constructed in virgin Olearia–Leucopogon–Acanthocarpus–Westringia heath were almost always located at the base of woody shrubs. Burrowing and guano deposition at the base of these plants are the likely mechanisms for the plants to die off (as was reported by Gillham 1960c). The most susceptible of these were Leucopogon and Westringia, with few of these shrubs persisting well into the ecotone. Olearia has been previously shown to withstand a low level of burrowing but dies off at higher burrow intensities (Gillham 1963a) and, as a result, it persisted into areas of modest burrow density. Rhagodia, Threlkeldia and Tetragonia were dominant in the absence of the woody shrubs. Of these, Tetragonia, a ‘bower-former’ (see Gillham 1961), persisted for longer. The presence of dead trunks and stems of Olearia, Leucopogon and Westringia are evidence that the outer- to mid-colony areas were once vegetated by heath. Further into the colony, the burrows were major centres for physical (birds), hydrological and aeolian erosion, and the percentage of bare ground increased. These conditions favoured annual, invasive, ruderal species such as Senecio and Mesembryanthemum. Ultimately, it appears that once Mesembryanthemum became established in the heart of the colony, it dominated to form a monoculture. The succession of colony vegetation is summarised in Fig. 6.
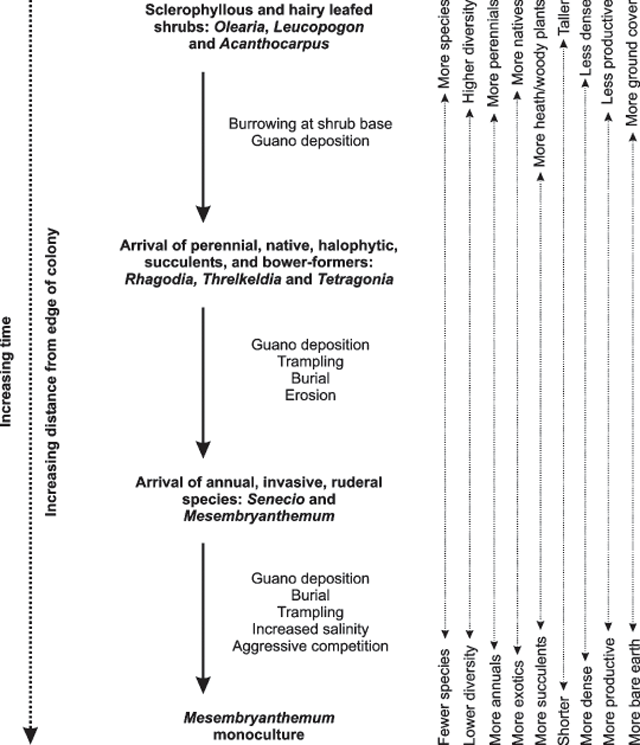
|
Of the three locations studied, the colony was the most different. It shared very little similarity with the edge and heath in terms of both species presence and abundance. Crawley (1986b) noted that abrupt environmental transitions (in this study, the abrupt transition between biopedturbated and undisturbed soils) create distinct plant communities. In comparison to undisturbed, adjacent heath, the vegetation community of the shearwater colony had greatly reduced species richness and a much more uneven species composition, with a few species dominating the overall vegetation cover. In his review of ecosystem-level consequences of ecosystem engineers Crooks (2002) showed that burrowing generally decrease habitat heterogeneity and this drives a reduction in species richness. In addition, he asserted that community complexity should decrease through the replacement of natives by exotic species. This was indeed the case here, since there were fewer native plants in the colony community and it was dominated by exotic species. In particular, large tracts of the colony were covered by a monoculture of the aggressive, invasive, coprophilic, annual Mesembryanthemum crystallinum. The short life cycle and rapid germination of M. crystallinum is advantageous in the highly disturbed soil environment of the colony (Vivrette and Muller 1977). Patches of bare ground, which result from shearwater digging, are particularly favourable to the settlement of this species (Vivrette and Muller 1977). A similar pattern of invasion was observed on Carnac Island, 13 km to the south of Rottnest, where M. crystallinum was first recorded in 1975, in association with seabird colonies, and today dominates half of the island to the exclusion of native species (Abbott et al. 2000). Biopedturbation favours colonisation by smaller, annual plants that can rapidly reproduce (Grime 1979). Habitat modification by seabirds in Western Australia generally favours exotic plant species because only 5% of island natives are annuals, compared with 60% of exotic species (Rippey and Rowland 1995). Highly pelagic seabirds, such as shearwaters, are unlikely to be the transmission vector for exotic seed (Gillham 1960a). Instead, other avian fauna or, more likely, humans are the probable agents for the introduction of exotic vegetation (Gillham 1960a).
The association of exotic species, such as M. crystallinum, with seabird colonies may compound the ecological impact of the shearwaters. M. crystallinum concentrates sodium chloride in its tissues (Vivrette and Muller 1977) and this elevates soil salinity (as was recorded in Rottnest shearwater colonies by Bancroft et al. 2004a). This can inhibit less salt-tolerant species, and encourage the spread of halophiles, as evidenced by the prevalence of the halophytic T. implexicoma and R. baccata in the colony in this study (Brown et al. 1993; Abbott et al. 2000). Succulents such as these are able to draw water into their foliage to dilute the salts taken up by their root systems (Low 2003).
There was a short ecotone between the true heath community and the colony areas, the ‘edge’ location. An ecotone was observed by Gillham (1960b) and a zone of niche overlap between two contrasting chenopod species was also recorded by Garcia et al. (2002) in Mediterranean seagull colonies. Community comparisons in our study showed that this transition zone had features of both community extremes, as would be expected, but also supported the lowest incidence of exotic species. It is generally considered that exotic species will occur more frequently in ecotones, and that species richness in these zones will be higher than in adjacent communities (Risser 1995). Our findings, however, support Walker et al. (2003) who argued that empirical evidence for these statements is weak, and that they should be viewed with some caution.
The edge ecotone may also be a spatial representation of a temporal pattern: the succession of plants following invasion by burrowing seabirds. Rottnest Island colonies have expanded rapidly over the past few decades (Bancroft et al. 2004b). Although it is likely that new burrows are added to the centre of the colonies, there is also a gradual, landward progression of the colony boundaries as they grow (Bancroft et al. 2004b). In particular, young or inexperienced birds burrow near the landward colony boundary, extending the colony into the adjacent heath. There is obviously a graded impact across the colony, with burrow density and historic presence lower at the landward edges. These edges may be representative of the early stages of vegetation succession, following initial disturbance. The patterns observed here (Fig. 6) closely resemble the model of vegetation succession proposed by Gillham (1961) for shearwater and petrel colonies on sandy soils. Gillham (1961) suggested that the major mechanisms for vegetation change were guano deposition, arrival of invasive species, competition, trampling and sand mobility and erosion. Brown et al. (1993) also suggested that the incorporation of organic material into the soil, soil compaction and fire were important determinants of the shearwater-colony vegetation. These authors also tested and dismissed any effect of soil salinity; however, an increase in the sodium chloride content of the soil, as a result of invasion of M. crystallinum, almost certainly plays a major role in the successional process of the Rottnest Island colonies (Fig. 6).
The vegetation communities of established seabird colonies exist in an unstable state of equilibrium, with many species at the limit of their biochemical and physical tolerances (Gillham 1961). It appears that, for now, the climax species of the Rottnest Island shearwater colonies is M. crystallinum. Burrow-nesting seabirds are less hostile to vegetation than surface nesters, and their physical presence seems not to completely destroy all vegetation to leave only bare earth (as is the case with gulls and cormorants; Gillham 1961; Rippey et al. 2002a). This can be explained by their relative size and breeding ecology. Adult shearwaters are present on colonies only for about one-third of a day, between dusk and dawn, and both adults and chicks spend the greater proportion of their colony time below ground. Surface-nesting species such as gulls and cormorants have a much greater body mass (pied cormorants, Phalacrocorax varius, have up to six times the mass of wedge-tailed shearwaters; Dunning 1993), have a surface presence at all times, and, as a result, deposit much greater amounts of surface guano. Surface-nesting seabirds destroy vegetation from the foliage down by trampling, whereas burrowers appear to destroy plants from the roots up.
Vertical vegetation structure
Our study is one of the first to quantitatively measure vertical vegetation structure of seabird colonies (Abbott 1976). The colony vegetation was lower than heath vegetation, with the maximum recorded vegetation height slightly over one third that of the heath. Of the species that co-occurred in both, the colony plants were significantly smaller than those of the heath. It has been previously noted that burrowing causes dwarfism in plants by reducing the water content of soil overlaying the burrows (Gillham 1956). Rottnest Island colony soil is drier than heath soil (Bancroft et al. 2004a) and lack of water may be the mechanism by which plants become stunted. Smaller colony plants may also be the result of reduced competition for light in the absence of the taller shrubs (e.g. O. axillaris, L. parviflorus, W. dampieri; Table 2; Fig. 3; Tilman 1986).
Plant productivity
The sea-to-land transfer of allochthonous nutrients by seabirds has been shown to increase the nitrogen and phosphorous content of colony plants by up to 2.4-fold (Anderson and Polis 1999). Given controlled conditions, and an adequate supply of water, this should translate into increased plant productivity (Chapman 1986). We observed this in our glasshouse trial. Plants grown in biopedturbated soil increased both their root and foliage mass, and had almost 300% more leaves after a 3-week period. Significantly, we were able to reproduce the glasshouse results in the field, with increased biomass and leaf gain by colony-growing plants.
The colony soils that promoted the greatest plant biomass (in our phytometer) also had the densest plant growth and the lowest species richness. Stevens and Carson (1999a, 1999b) proposed that assemblage-level thinning was the mechanism responsible for this pattern. They state that, along an increasing fertility gradient, all plants become larger and, hence, denser, and that density-dependent mortality will result equally among all species. Rare species will be lost through stochastic extinction. Although the biomass of some species, such as R. baccata, increase through nutrient supplementation and this lends some support to this hypothesis, we consider that it may be a simple case of the better adapted species surviving. Wedge-tailed shearwater guano is nitrogen-, phosphorous- and potassium-rich (Bancroft et al. 2004a). Western Australian soils, in particular the sands, are notoriously depauperate in these elements (Milewski 1981; Pate and Beard 1984). Many species have adapted to cope with these limitations (Lamont 1984), and high nutrient levels, as occur in guano deposits, may be phytotoxic (Brown 1987). This effect may be exacerbated in soils that have a low moisture content (Gillham 1960a) as was reported in shearwater colonies by Gillham (1956) and Bancroft et al. (2004a).
Our controlled experiments demonstrated higher productivity for a native perennial in guano-rich colony soils, but field data suggest that nutrient availability alone will not predict the community structure of an expanding colony. Invasion by ruderal species is perhaps the dominant variable that will determine the functional outcome for ecosystems that are subjected to the expansion of ecosystem engineers such as burrowing seabirds.
Summary
The arrival of wedge-tailed shearwaters to Rottnest Island was a natural phenomenon, and these burrowing seabirds have significantly modified the vegetation of their colonies. As predicted a priori, the seabird colonies supported fewer plant species and these were disproportionately represented, with exotic, short-lived succulents (in particular M. crystallinum) dominating. Vegetation was shorter and denser, and the colony soils increased plant productivity significantly.
Acknowledgments
This study was funded by the School of Animal Biology, University of Western Australia. Glasshouse experiments were conducted at Murdoch University and we are grateful for the technical support of Max Dawson, Ian McKearnen, Kim Tan and Jose Minetto. We thank the Rottnest Island Authority for their co-operation. We are indebted to Elizabeth Rippey for her assistance in plant identification and her comments on the manuscript. Neil Gibson also provided helpful comments on an earlier draft of the manuscript. We gratefully acknowledge the contributions of the two anonymous referees who reviewed our manuscript.
Abbott I
(1976) Comparisons of habitat structure and plant, arthropod and bird diversity between mainland and island sites near Perth, Western Australia. Australian Journal of Ecology 1, 275–280.

Abbott I,
Marchant N, Cranfield R
(2000) Long-term changes in the floristic composition and vegetation structure of Carnac Island, Western Australia. Journal of Biogeography 27, 333–346.
| Crossref | GoogleScholarGoogle Scholar |

Anderson, FO ,
and
Kristensen, E (1991). Effects of burrowing macrofauna on organic matter decomposition in coastal marine sediments. In ‘Symposia of the Zoological Society of London—the environmental impact of burrowing animals and animal burrows’. pp. 69–88. (Clarendon Press: London)
Anderson WB, Polis GA
(1999) Nutrient fluxes from water to land: seabirds affect plant nutrient status on Gulf of California islands. Oecologia 118, 324–332.
| Crossref | GoogleScholarGoogle Scholar |

Bancroft WJ,
Garkaklis MJ, Roberts JD
(2004a) Burrow building in seabird colonies: a soil forming process in island ecosystems. Pedobiologia 49, 149–165.

Bancroft WJ,
Garkaklis MJ, Roberts JD
(2004b) Continued expansion of the wedge-tailed shearwater, Puffinus pacificus, nesting colonies on Rottnest Island, Western Australia. Emu 104, 79–82.
| Crossref | GoogleScholarGoogle Scholar |

Bancroft WJ,
Hill D, Roberts JD
(2004c) A new method for calculating volume of excavated burrows: the geomorphic impact of wedge-tailed shearwater burrows on Rottnest Island. Functional Ecology 18, 752–759.
| Crossref | GoogleScholarGoogle Scholar |

Brown, JR (1987).
Brown MJ,
Maruyama N, Williams KJ
(1993) Ecological studies of vegetation in short-tailed shearwater colonies in Tasmania. Papers and Proceedings of the Royal Society of Tasmania 127, 11–16.

Bureau of Flora and Fauna (2002).
Chaffey CJ, Grant CD
(2000) Fire management implications of fuel loads and vegetation structure in rehabilitated sand mines near Newcastle, Australia. Forest Ecology and Management 129, 269–278.
| Crossref | GoogleScholarGoogle Scholar |

Chapman, SB (1986). Production ecology and nutrient budgets. In ‘Methods in plant ecology’. pp. 1–60. (Blackwell Scientific Publications: Oxford, UK)
Crawley, MJ (1986a).
Crawley, MJ (1986b). The structure of plant communities. In ‘Plant ecology’. b. pp. 1–50. (Blackwell Scientific Publications: Oxford, UK)
Crooks JA
(2002) Characterizing ecosystem-level consequences of biological invasions: the role of ecosystem engineers. Oikos 97, 153–166.
| Crossref | GoogleScholarGoogle Scholar |

Dunning, JB (1993).
Garcia LV,
Maranon T,
Ojeca F,
Clemente L, Redondo R
(2002) Seagull influence on soil properties, chenopod shrub distribution, and leaf nutrient status in semi-arid Mediterranean islands. Oikos 98, 75–86.
| Crossref | GoogleScholarGoogle Scholar |

Gillham ME
(1956) Ecology of the Pembrokeshire Islands: IV. Effects of treading and burrowing by birds and mammals. Journal of Ecology 44, 51–82.

Gillham ME
(1960a) Destruction of indigenous heath vegetation in Victorian sea-bird colonies. Australian Journal of Botany 8, 278–317.

Gillham ME
(1960b) Plants and seabirds of granite islands in south-east Victoria. Proceedings of the Royal Society of Victoria 74, 21–35.

Gillham ME
(1960c) Vegetation of New Zealand shag colonies. Transactions of the Royal Society of New Zealand 88, 363–380.

Gillham ME
(1961) Alteration of the breeding habitat by sea-birds and seals in Western Australia. Journal of Ecology 49, 289–300.

Gillham ME
(1962) Granite islands of south-east Victoria as a seabird habitat. Proceedings of the Royal Society of Victoria 75, 45–63.

Gillham ME
(1963a) Association of nesting seabirds and vegetation types on islands off Cape Leeuwin, South-western Australia. Western Australian Naturalist 9, 29–46.

Gillham ME
(1963b) Coral cay vegetation Heron Island, Great Barrier Reef. Proceedings of the Royal Society of Queensland 73, 79–92.

Grime, JP (1979).
Jones CG,
Lawton JH, Shachak M
(1994) Organisms as ecosystem engineers. Oikos 69, 373–386.

Krebs, CJ (1994).
Krebs, CJ (1999).
Lamont, BB (1984). Specialised modes of nutrition. In ‘Kwongan, plant life of the sandplain’. pp. 126–145. (University of Western Australia Press: Perth)
Low T
(2003) Growing where birds breed. Nature Australia Winter 2003, 24–25.

Milewski AV
(1981) A comparison of reptile communities in relation to soil fertility in the Mediterranean and adjacent arid parts of Australia and southern Africa. Journal of Biogeography 8, 493–503.

Mulder CPH, Keall SN
(2001) Burrowing seabirds and reptiles: impacts on seeds, seedlings and soils in an island forest in New Zealand. Oecologia 127, 350–360.
| Crossref | GoogleScholarGoogle Scholar |

Pate, JS ,
and
Beard, JS (1984).
Playford PE
(1983) Geological research on Rottnest Island. Journal of the Royal Society of Western Australia 66, 10–15.

Rippey, E ,
and
Rowland, B (1995).
Rippey E,
Rippey JJ,
Dunlop JN,
Durant C,
Green B, Lord J
(1998) The changing flora of the Shoalwater Bay Islands. Western Australian Naturalist 22, 81–103.

Rippey E,
Rippey JJ, Dunlop JN
(2002a) Increasing numbers of pied cormorants breeding on the islands off Perth, Western Australia and consequences for the vegetation. Corella 26, 61–64.

Rippey E,
Rippey JJ,
Green D, Dunlop JN
(2002b) Comparison of the vegetation of the islands in Shoalwater Bay (Rockingham, Western Australia) with that of the coastal bushland. Journal of the Royal Society of Western Australia 85, 169–179.

Rippey E,
Hislop MC, Dodd J
(2003) Reassessment of the vascular flora of Rottnest Island. Journal of the Royal Society of Western Australia 86, 7–23.

Risser PG
(1995) The status of the science examining ecotones. Bioscience 45, 318–325.

Sanchez-Pinero F, Polis GA
(2000) Bottom-up dynamics of allochthonous input: direct and indirect effects of seabirds on islands. Ecology 81, 3117–3132.

Stern H,
de Hoedt G, Ernst J
(2000) Objective classification of Australian climates. Australian Meteorological Magazine 49, 87–96.

Stevens MHH, Carson WP
(1999a) Plant density determines species richness along an experimental fertility gradient. Ecology 80, 455–465.

Stevens MHH, Carson WP
(1999b) The significance of assemblage-level thinning for species richness. Journal of Ecology 87, 490–502.
| Crossref | GoogleScholarGoogle Scholar |

Tilman, D (1986). Resources, competition and the dynamics of plant communities. In ‘Plant ecology’. pp. 51–76. (Blackwell Scientific Publications: Oxford, UK)
Vivrette NJ, Muller CH
(1977) Mechanism of invasion and dominance of coastal grassland by Mesembyranthemum crystallinum. Ecological Monographs 47, 301–318.

Walker S,
Wilson JB,
Steel JB,
Rapson GL,
Smith B,
King WM, Cottam YH
(2003) Properties of ecotones: Evidence from five ecotones objectively determined from a coastal vegetation gradient. Journal of Vegetation Science 14, 579–590.

Walsh D,
Kirkpatrick JB, Skira IJ
(1997) Vegetation patterns, environmental correlates and vegetation change in a Puffinus tenuirostris breeding colony at Cape Queen Elizabeth, Tasmania. Australian Journal of Botany 45, 71–79.
| Crossref | GoogleScholarGoogle Scholar |



