Contrasting water-use strategies in two sympatric cool-temperate rainforest species, Nothofagus cunninghamii (Nothofagaceae) and Atherosperma moschatum (Atherospermataceae)
Katy E. Sommerville A B and Jennifer Read A CA School of Biological Sciences, Monash University, Vic. 3800, Australia.
B Present address: Ecosystem Dynamics Group, Research School of Biological Sciences, The Australian National University, Canberra, ACT 0200, Australia.
C Corresponding author. Email: jenny.read@sci.monash.edu.au
Australian Journal of Botany 56(2) 109-118 https://doi.org/10.1071/BT07138
Submitted: 29 July 2007 Accepted: 22 October 2007 Published: 19 March 2008
Abstract
Nothofagus cunninghamii (Hook.) Oerst. and Atherosperma moschatum Labill. co-occur in cool-temperate rainforest across the wetter parts of Tasmania and Victoria, Australia, but A. moschatum extends to drier areas than N. cunninghamii. Possible reasons include differential tolerance of drought and fire or dispersal capacity. Here, we compare these species in their responses to water deficits. Differences in seedling survival, leaf tissue damage, shoot water relations, stomatal sensitivity, allocation of biomass and the long-term water-use efficiency of each species in response to water stress were investigated. N. cunninghamii showed traits typical of a high-water-use species, such as high stomatal conductance, a strategy that is not surprising in a rainforest species. However, it also displayed an exceptional ability to draw water from the soil and longer seedling roots, allowing replacement of water lost, at least in the short term. A. moschatum showed a more conservative water-use strategy, surviving greater internal dehydration with less damage, and displaying greater stomatal sensitivity to drought and long-term water-use efficiency in trees. The apparently superior long-term drought resistance of A. moschatum may in part explain its more common occurrence in drier regions than N. cunninghamii, at least in Tasmania, while the capacity of N. cunninghamii to survive short but severe periods of water stress correlates well with its higher position in the canopy and greater exposure to sunlight and desiccating winds. However, there is little evidence to suggest that the absence of N. cunninghamii from the rainforests of eastern Victoria is due to drought. We also suggest that the water-use strategy of N. cunninghamii may relate not just to surviving water deficits, but to maximising annual carbon gain in a temperate climate that is, on average, driest during the warmest time of the year.
Introduction
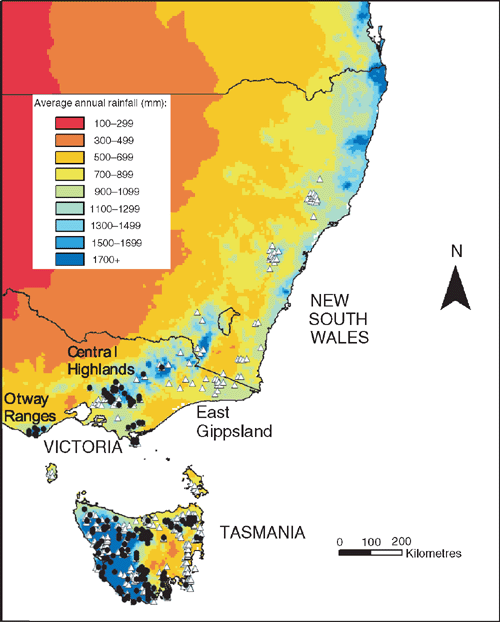
Coexistence of tree species may be facilitated by contrasting resource-use strategies. The cool temperate rainforest trees Nothofagus cunninghamii (Hook.) Oerst. and Atherosperma moschatum Labill. co-occur throughout much of their range in south-eastern Australia (Read 1999). Although both are canopy species, N. cunninghamii most often dominates the upper portion of the rainforest canopy, where desiccating forces are greater. Foliage of A. moschatum is mostly lower in the canopy. Given this tendency, N. cunninghamii could be expected to have a greater drought resistance than A. moschatum. However, in an apparent contradiction, N. cunninghamii is conspicuously absent from drier sites in south-eastern Australia where A. moschatum is common (Busby 1986; Neyland 1991; Neyland and Brown 1993) (Fig. 1). In this context, the extent and manner in which water is used by these two species is of great interest.
|
Comparisons of the minimum annual precipitation experienced by each species indicate that A. moschatum extends to areas of markedly lower annual precipitation than does N. cunninghamii (5-percentile values of 890 mm per year as compared with 1153 mm) (Read and Busby 1990). Indeed, summer precipitation appears to be an important factor in limiting the occurrence of N. cunninghamii (Busby 1986; Read and Busby 1990; Lindenmayer et al. 2000). These analyses support the notion that differences in the capacity to survive water deficits may explain differences in the distribution of these two species. It should be noted that this pattern could also be the result of a causal relationship between summer rainfall and fire regime, or of a greater capacity of A. moschatum propagules to disperse into peripheral but drier areas (Neyland and Brown 1993; Read and Brown 1996; Read 1999). However, the present study focuses on the response of these species to drought as early support for this hypothesis appears promising.
Preliminary studies suggest that N. cunninghamii has a greater ability than A. moschatum to develop low shoot water potentials at a given water content (Read 1999). This would potentially allow N. cunninghamii to draw water from the soil at a greater rate than A. moschatum, at least during the initial stages of dehydration. N. cunninghamii appears to keep its stomata open at larger tissue water deficits than some co-occurring species, including A. moschatum (Read and Brown 1996; Cunningham 2004). This would potentially allow greater carbon gain for N. cunninghamii than for A. moschatum, providing a possible growth advantage during periods of water deficit. However, the occurrence of A. moschatum in areas receiving less precipitation suggests a more complex picture. Hence, further detail of the relative water-use strategies and the comparative survival and growth of each species during periods of water stress is required.
The present study investigates differences in the relative drought resistance of N. cunninghamii and A. moschatum and the possible implications for their co-existence and differences in relative distribution. The focus is primarily on the seedling stage due to the importance of the seedling stage in determining the eventual position and abundance of a species, and the difficulty of imposing water stress on mature trees. However, some comparisons between trees of each species are also included.
Materials and methods
Plant material and growth conditions
Seedlings were collected from Cement Creek at Mount Donna Buang in the Central Highlands of Victoria, Australia (37°43′S, 145°42′E, 800 m asl) in February 2001 and transferred to pots of sandy loam in glasshouses at Monash University, Melbourne, for experimentation. Investigations were also undertaken in March 2001 on mature trees at Cement Creek. Seedlings used in the survival experiment were ~8–12 months old and 5–10 cm in height. Plants were selected so that size and leaf development was as similar as possible between species; the difficulty in finding large numbers of similarly sized seedlings restricted the degree of replication for some experiments.
Glasshouse temperature ranged between a mean daily minimum of 18.6°C and a mean maximum of 29.6°C and average glasshouse humidity was 65% (data recorded every 30 min with OTML Tinytag data loggers (Gemini Data Loggers (UK) Ltd, Chichester, UK)). A 16/8 h light/dark cycle was established with a halogen lamp with ambient daylight. Plant position in the glasshouse was randomised every 1–2 weeks to minimise the effect of localised environmental differences. Volumetric soil water content at 6-cm depth was measured with a Thetaprobe ML2 soil moisture sensor (Delta-T Devices, Cambridge, UK). Field capacity of the soil was 0.250 m3 m–3 (the volume of water per volume of soil). A pilot study indicated that the soil water content necessary to impose sublethal water stress on seedlings grown in the glasshouse was ~0.035 m3 m–3 and this level was used to impose water stress in drought experiments.
Survival with decreasing soil moisture
The capacity of N. cunninghamii and A. moschatum to survive water deficits was determined by estimating their critical drought-avoidance point, i.e. the soil water content below which a rewatered plant will not recover (Levitt 1980; Hale and Orcutt 1987). Twenty seedlings of each species were deprived of water until reaching a set of progressively lower soil moisture levels (0.103, 0.046, 0.029, 0.018 and 0.007 m3 m–3), following Penka (1956) (in Slavík 1974). At each designated soil moisture level, four replicate plants of each species were rewatered to field capacity and maintained in this state for 2 weeks, at which time survival was assessed. A three-parameter Gompertz curve (Eqn 1) was used to model the relationship between soil moisture content (x) and the proportion of plants alive (y) as follows:

The soil moisture content at which 50% of plants of each species died was derived and used as the critical drought-avoidance point (Levitt 1980).
Comparative responses of seedlings to well watered and drought conditions
Ten seedlings of each species were randomly allocated to well watered and drought treatments. Plants in the drought treatment were deprived of water for ~8 weeks until reaching sublethal levels of moisture stress (~0.035 m3 m–3), at which point they received 25 mL of water. Plants in the well watered treatment received 100 mL of water as often as was necessary to maintain soil moisture at ~0.159 m3 m–3 (just under field capacity).
Shoot water potential
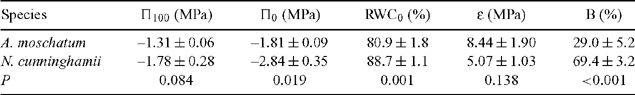
The effect of dehydration on shoot water potential of seedlings under experimental conditions and trees at Cement Creek was determined by the use of a pressure chamber (Model 1000, PMS Instrument Co., Albany, OR, USA) (Tyree and Hammel 1972; Lamont 1999). The bench-drying technique described by Koide et al. (1989) was used, except that shoots were dried at 24°C in a controlled-environment cabinet with PPFD of 50 μmol m–2 s–1. Previous studies have shown that water potential responses of plants in the field are highly correlated with low water potentials measured from cut shoots in the laboratory (Bannister and Kissel 1986). All tree shoots were collected at dawn (maximising the degree of hydration) from the outer edges of the canopy to reduce any variation in leaf physiological responses to water stress owing to position in the canopy (Myers et al. 1987). Shoots were cut under water and kept moist for transport to the laboratory. Saturated mass was determined after placing the cut ends of plant shoots in water at room temperature, enclosing the shoots in plastic bags and allowing them to take up water overnight. At dawn the following morning, shoots were blotted dry and weighed to give the saturated mass. Fresh mass and water potential were recorded for a shoot from each plant. These shoots were then allowed to dehydrate and fresh mass and water potential were remeasured at 30–120-min intervals, the time interval depending on the rate of water loss. Relative water content (RWC) was determined as 100 × (fresh mass – dry mass) / (saturated mass – dry mass). The pressure–volume curve for each shoot was derived by the software Template (Radford and Lamont 1992), with estimation of osmotic potential at full turgor (Π100), the osmotic potential at the turgor loss point (Π0), the RWC at the turgor loss point (RWC0) and the bulk modulus of elasticity adjusted to exclude apoplastic water (ϵ), the amount of bound water (B) (water adhering to cell wall polysaccharides) in each treatment for each replicate plant. At the conclusion of the experiment, leaves were rehydrated in water for 24 h and turgid leaf area was determined for each plant by image analysis (Bioscan, Monash University, Melbourne).
Biomass allocation
Seedlings were harvested and partitioned into roots, stems and leaves. Leaf area was measured by image analysis, and then all parts were dried to a constant mass at 80°C. The ratios of root dry mass to shoot dry mass, root dry mass to leaf area, leaf area to leaf dry mass, and leaf area to total plant dry mass were calculated.
Long-term water-use efficiency
The long-term water-use efficiency of seedlings in each experimental treatment and trees at Cement Creek was estimated from discrimination against 13C (Farquhar et al. 1989). The leaves of five seedlings of each species in each treatment and five mature trees of each species were ground to a fine powder with a Spex Freezer Mill (SPEX CertiPrep, Metuchen, NJ, USA). These samples were analysed for 13C : 12C by mass spectrometry (School of Geosciences, Monash University). Discrimination (Δ) was calculated as Δ = (δa – δp)/(1 + δp) following (Farquhar et al. 1989), where a refers to air and p refers to plant and δ was computed with reference to the standard (s), PDB. δa = Ra/(Rs – 1) and δp = Rp/(Rs – 1), where R is the molar abundance of the ratio 13C/12C.
Leaf tissue tolerance of dehydration
Comparative tolerance of dehydration was assessed in leaves from well watered N. cunninghamii and A. moschatum seedlings. Six to eight mature leaves growing in exposed positions were excised from each of five replicate plants and their petioles sealed with vaseline. The leaves were dehydrated under lights at 20°C and 55% relative humidity in a controlled-environment cabinet to ~100, 80, 60, 40, 20 and 15% water content (measured as 100 × fresh mass / saturated mass). Following dehydration, leaves were wrapped in wet paper towel, sealed in plastic bags and stored for 1 week in a cabinet at 10°C and 25–30 µmol quanta m–2 s–1. The degree of damage was determined on the basis of the percentage area of leaf visibly changed in colour (from green to yellow, brown or black) and/or texture (tissue becoming translucent, pocked or brittle). The relationship between leaf damage and water content was modelled by Eqn 1, allowing determination of the water content at which 20 and 50% of leaf area was damaged. Curves fitted to derive these values produced R2 (observed v. predicted) ≥0.93.
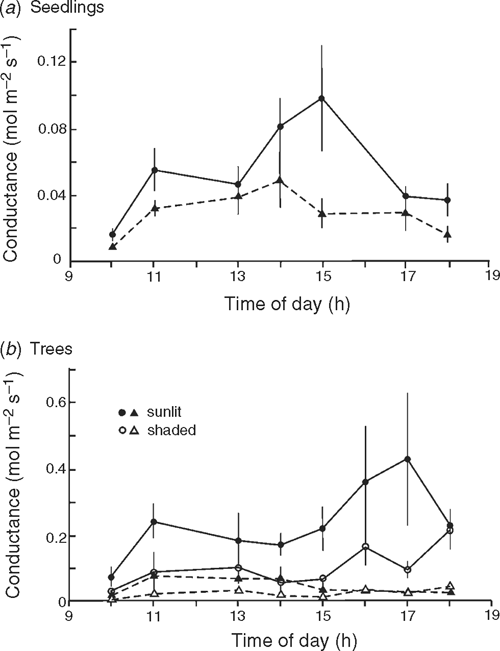
Conductance over the course of a day
Stomatal conductance was measured at 1–2-h intervals during the course of a day on six mature trees of both species at Cement Creek and on five well watered seedlings in the glasshouse with a Delta-T Porometer (Delta-T Devices Ltd, Cambridge, UK) (readings calibrated for temperature). The order in which plants were assessed at each reading was randomised. Of the trees selected, two were in sunny positions, two in positions of dappled shade and two were coppice shoots in sunny positions. These differences were chosen to reflect possible variations in leaf microclimate and growth history. Measurements were taken during warm to hot dry conditions (33°C maximum temperature within the glasshouse and 26°C maximum temperature in the field).
Water potential of trees at Cement Creek
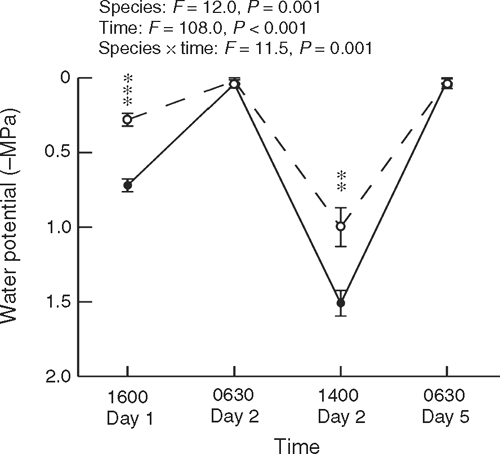
Shoot water potential was measured in trees at dawn and mid-afternoon during a 5-day hot period (maximum temperatures exceeding 30°C on three days) at Cement Creek with a pressure chamber. Measurements were undertaken on shoots collected at 2–3 m above ground in sunlit trees near a road edge (five trees per species, randomly selected on each occasion), and some measurements were also undertaken on N. cunninghamii foliage ~15 m above ground (accessed by a canopy walkway).
Statistical analysis
In the seedling-survival experiment, the frequency of surviving seedlings was compared between species at each of the designated soil moisture contents by analysis of contingency tables (SPSS). One-way ANOVA was used to compare water relations variables between species (SYSTAT v. 10, Systat Software Inc., San Jose, CA, USA). Conductance data of trees at Cement Creek were analysed by using one-way repeated-measures REMLs (Genstat v. 10.1.71, VSN International Ltd, Hemel Hempstead, UK). Two-way ANOVA was used to compare variables between species and treatments, followed by tests of simple main effects if interaction terms were significant (SYSTAT v. 10). Data assumptions were checked before analysis and a critical level of α = 0.05 was used for hypothesis tests.
Results
Survival with decreasing soil moisture
More N. cunninghamii plants survived in drier soil than did A. moschatum, with N. cunninghamii showing higher survival at a soil moisture content of 0.018 m3 m–3 (Pearson chi-square = 8.00, P = 0.029). The critical drought-avoidance point of N. cunninghamii was considerably lower (0.008 m3 m–3) (b = –29.1, c = –3700.5) than that of A. moschatum (0.029 m3 m–3) (b = –20.1, c = –694.1). However, roots of N. cunninghamii extended to the bottom of the pot, whereas those of A. moschatum did not, and so it is possible that N. cunninghamii had access to more water for longer periods than did A. moschatum.
Shoot water potential and pressure–volume curves
Nothofagus cunninghamii seedlings showed a larger decrease in water potential per unit decrease in RWC than did A. moschatum seedlings. However, A. moschatum shoots survived for longer (18.6 ± 12.6 h cf. 4.2 ± 1.7 h) and to a lower RWC (~56% cf. ~79%) than did N. cunninghamii shoots. Π0 was significantly lower in N. cunninghamii than in A. moschatum, but there was no effect of drought treatment (Table 1). B was considerably higher in N. cunninghamii than in A. moschatum (Table 1), and high in N. cunninghamii compared with values normally recorded (Turner 1981). ϵ differed between species and drought treatments, with higher values in drought than in well watered plants and in A. moschatum than in N. cunninghamii (Table 1). There was no significant difference in Π100 or RWC0 between species or drought treatments (Table 1).
As in the seedling experiment, N. cunninghamii tree shoots showed a larger decrease in water potential per unit RWC, and a lower Π0 than did A. moschatum tree shoots (Table 2). Again, shoots of A. moschatum tree survived for longer (50.4 ± 20.9 h) and to a lower RWC (~54%), than did those of N. cunninghamii (6.2 ± 1.4 h and ~75% RWC). A. moschatum tree shoots also had significantly lower RWC0 (Table 2). B was again high in N. Cunninghamii, compared with A. moschatum (Table 2) and with values normally recorded (Turner 1981). The trend in ϵ was the same as recorded in seedlings (higher in A. moschatum than N. cunninghamii), but without significant differences.
|
Investigation of trees at Cement Creek during a hot period showed that N. cunninghamii developed significantly lower water potentials than did A. moschatum during the afternoons, with both species rehydrating overnight to give dawn readings near zero (Fig. 2).
|
Growth and allocation
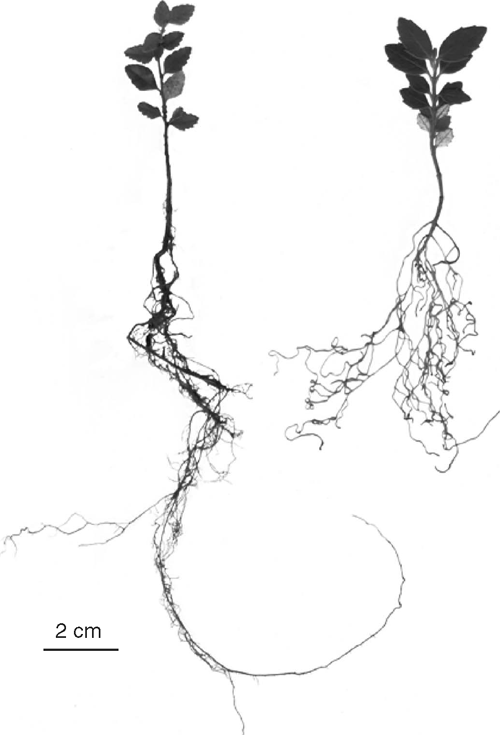
A higher root : shoot ratio was recorded in seedlings of A. moschatum than in those of N. cunninghamii (Table 3). However, roots appeared finer and were longer in N. cunninghamii seedlings (Fig. 3). No difference in leaf area : leaf mass was recorded between species or treatments. There was a significant interaction between species and treatments for leaf area per root mass and total plant mass (Table 3). Tests of simple main effects indicated that droughted N. cunninghamii had a significantly higher leaf area per unit of root mass (F = 10.2, P = 0.022) and per total plant mass (F = 16.8, P = 0.006) than did well watered N. cunninghamii. These variables did not differ between treatments in A. moschatum.
|
Long-term water-use efficiency
Mature A. moschatum trees showed higher long-term water-use efficiency (less discrimination against 13C) (Δ = 21.0 ± 0.3‰) than did N. cunninghamii trees (Δ = 22.1 ± 0.2‰) (t = 2.50, P = 0.037). There was no difference between species or treatments in long-term water-use efficiency of seedlings (Δ = 23.2–23.6‰).
Leaf tissue tolerance of dehydration
Detached A. moschatum leaves showed less damage with increasing dehydration than did N. cunninghamii leaves; 20% damage occurred at a RWC of 47% in A. moschatum and at 68% in N. cunninghamii (P = 0.002) and 50% damage occurred at a RWC of 36% in A. moschatum and at 58% in N. cunninghamii (P = 0.001).
Conductance during the course of a day
Mean conductance was significantly higher in N. cunninghamii seedlings (F = 5.25, P = 0.033) and trees (F = 10.28, P = 0.009) than in A. moschatum (Fig. 4). For the trees, variations in conductance within species appeared to be associated with variation in the intensity of sunlight.
|
Discussion
Nothofagus cunninghamii and A. moschatum have contrasting water-use strategies. Seedlings of N. cunninghamii survived in drier soil than did those of A. moschatum. This was apparently due largely (since A. moschatum can better survive tissue dehydration) to a superior capacity to extract water from the soil via osmotic adjustment, although N. cunninghamii may also have had access to moister soil because of its longer roots. In addition, N. cunninghamii seedlings maintained high rates of conductance during warm to hot conditions. Similar patterns were observed in trees at Cement Creek, with higher conductance and lower water potentials developed in N. cunninghamii than in A. moschatum on warm to hot days. N. cunninghamii also had a greater quantity of bound water (B) in both trees and seedlings than did A. moschatum, but a lower bulk modulus of elasticity (ϵ). Higher values of B and ϵ are common in drought-tolerant plants because cells are often smaller, with thicker and denser cell walls that are less likely to collapse with desiccation, and so more water is apoplasmic (Vertucci and Leopold 1987; Rascio et al. 1999). The decoupling of B and ϵ in the present study requires further investigation. N. cunninghamii also showed a higher seedling leaf area per root mass and total plant mass. The lower root : shoot ratio in N. cunninghamii than in A. moschatum, particularly in combination with the higher conductance recorded in N. cunninghamii, suggests its root function is more efficient. This efficiency could result either from osmotic adjustment, allowing a stronger gradient of plant–soil water potential to develop, or from differences in root architecture (longer and finer roots with potentially more root tips) observed but not quantified in the present study. If the latter observations do hold true, this architecture allows more absorptive area and greater effectiveness via greater depth achieved for the same root mass, i.e. increasing absorptive power without necessarily compromising allocation to leaf area. So long as drought stress is not severe, this strategy, together with the maintenance of high rates of conductance during warm dry periods, should allow N. cunninghamii juveniles (and adults) to maintain photosynthetic rates, grow rapidly (relative to co-occurring species), and compete successfully for access to resources, including soil water.
In contrast, A. moschatum survived to lower tissue RWC, with less damage to leaves, and showed greater long-term water-use efficiency in mature trees. A. moschatum also displayed a greater increase in ϵ with drought. However, despite attempts to impose water stress on seedlings in a rigorous accountable fashion, the drought treatment appeared insufficient to induce stress in plants. It seems likely that moisture was retained at the bottom of the pot since seedlings in the drought treatment showed only slight indication of stress or acclimation to stress. As such, we will focus on differences between species rather than the effect of treatments.
Atherosperma moschatum also had a greater mass of roots per unit of shoot mass than did N. cunninghamii. Even though the higher relative allocation to roots might be viewed as an adaptation to enhance water uptake, it may be a less efficient strategy in the long term than that of N. cunninghamii because of the diversion of mass away from the foliage and consequent impact on photosynthesis and ultimately on growth. While A. moschatum appears less able to withdraw water from the soil at a given RWC, it loses less water through transpiration. This more conservative strategy in terms of water expenditure is paired with a greater tolerance of tissue water deficits, as has been recorded elsewhere (Bannister and Kissel 1986).
Water relations in the differential distribution of Nothofagus cunninghamii and Atherosperma moschatum
From these and other data (Howard 1973a; Read and Brown 1996; Read 1999), we suggest that N. cunninghamii and A. moschatum are adapted to different regimes of water deficits. N. cunninghamii appears to maximise physiological access to water (particularly via osmotic adjustment) to enhance growth. Its ability to draw water from the soil allows some delay in foliar desiccation, while potentially maintaining high rates of photosynthesis in the short term. However, given its lower tolerance of internal dehydration than is the case for A. moschatum, the high water use of N. cunninghamii must increase the likelihood of its exposure to critical levels of tissue water deficit. Its high rate of water use must also increase the risk of depleting limited soil reserves of water more quickly. Hence, N. cunninghamii is predicted to be less common on shallow soils or in areas with lower or less reliable summer rainfall than A. moschatum.
By contrast, A. moschatum appears to adopt a strategy of water conservation in the face of water deficits. As it is relatively shade-tolerant (Read and Hill 1985; Olesen 1997), photosynthetic rates are lower and stomatal closure may limit photosynthesis less, while allowing greater survival during longer periods of drought; furthermore, rapid growth may be of less importance in maintaining the abundance of this species in the rainforest (Read 2001). The greater survival time of detached A. moschatum shoots, combined with higher tolerance of internal dehydration, is consistent with an hypothesis of greater resistance during longer periods of drought, i.e. where severe soil water deficits limit the effectiveness of the water-uptake strategy of N. cunninghamii.
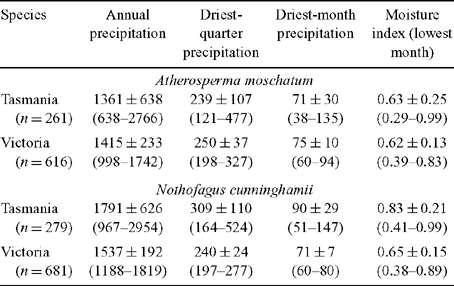
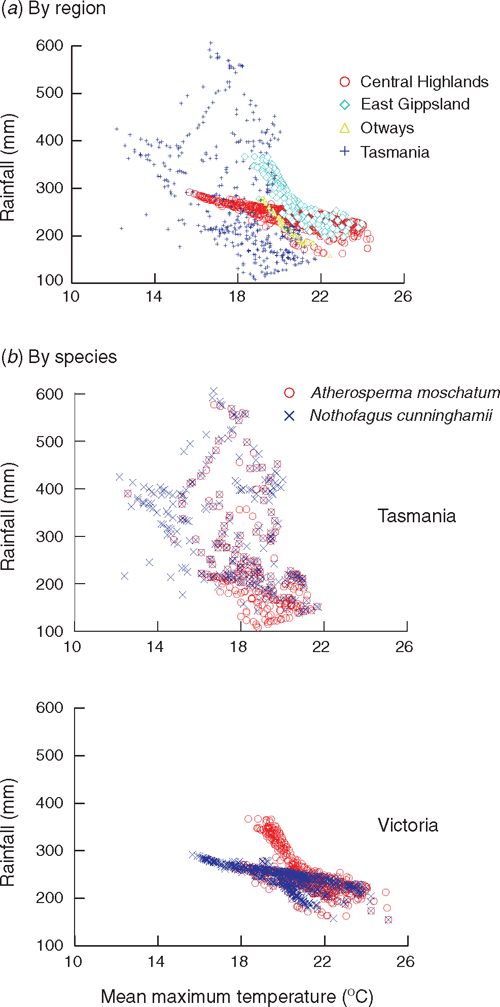
The hypothesis that A. moschatum, once established, is better at surviving long-term drought is consistent with its occurrence at drier sites than N. cunninghamii in some regions. However, a more detailed analysis of the climate profile of these species showed a more complex pattern with respect to rainfall (Table 4, Fig. 5). In Tasmania, A. moschatum is more common than N. cunninghamii at drier sites (in terms of mean annual precipitation, precipitation of the driest periods and moisture index), and both species extend to drier sites in terms of annual precipitation, than is the case in Victoria (Table 4), where temperatures are higher on average. However, in Victoria, although A. moschatum is more common at sites with a lower annual precipitation than is N. cunninghamii, there is no evidence of it occurring at sites that have lower precipitation at the driest time of the year (Table 4). Summer rainfall is normally seen as being critical to the distribution of these species, either directly via water deficits or indirectly by the influence of climate on fire regime (Jackson 1968; Busby 1986; Busby and Brown 1994; Read 1999). Considering only summer rainfall (January to March) in conjunction with summer maximum temperatures suggests that although A. moschatum is more common at drier, warmer sites in Tasmania than is N. cunninghamii, the same is not true for Victoria (Fig. 5). In Victoria, mean annual rainfall across the distribution of N. cunninghamii, adjusted to summer maximum temperatures (by analysis of covariance) is 1512 ± 6 mm, and for A. moschatum it is 1446 ± 7 mm; however, mean summer rainfall adjusted to summer temperature is 236 ± 1 mm for N. cunninghamii, and for A. moschatum it is 257 ± 1 mm. In particular, in the cool-temperate rainforest in East Gippsland, where N. cunninghamii is absent, summer rainfall for many sites is similar to that of sites where N. cunninghamii occurs in the Central Highlands (Fig. 5a). The main difference in the summer-rainfall regime between East Gippsland and Victorian regions where N. cunninghamii does occur is in the high summer rainfall of the cooler East Gippsland sites. We suggest that for Victoria, at least, the absence of N. cunninghamii from regions such as East Gippsland is more strongly related to factors such as difficulty in dispersing across intervening dry corridors than to the precipitation regime per se, as suggested by Busby (1986). There is at least little evidence that N. cunninghamii is excluded by a drier climate, although there may be subtle differences among regions in water availability that are not apparent from these climate estimates, e.g. owing to cloud-lie, drainage patterns, soil characteristics and the occurrence of drought years (with effects on fire regimes). Such differences could directly or indirectly (via competition with other canopy species) contribute to the absence of N. cunninghamii from East Gippsland.
|
|
Occupation of different niches at the same site
Plants growing in the uppermost layers of vegetation are exposed to greater desiccation than those growing below (Nagy et al. 1993). The greater drought resistance of N. cunninghamii in periods of short-term drought is consistent with its higher position in the rainforest canopy where strong desiccating forces can occur daily but are short-lived (Chiariello 1984; Shuttleworth et al. 1985). The conservative water-use strategy shown by A. moschatum may have relatively little impact on its assimilation rates for two reasons. First, since the bulk of its foliage is usually lower in the canopy of mixed rainforests, it is more protected from desiccating forces, and so is less likely to experience strong leaf–air vapour-pressure gradients. However, it does occur as the canopy dominant in some rainforests (Neyland and Brown 1993; Olesen 1997), and in these forests may be more exposed to desiccating forces, depending on site conditions. Second, as noted above, A. moschatum is a shade-tolerant species, with lower maximum rates of carbon assimilation, than for N. cunninghamii. Consequently, stomatal closure in A. moschatum may have less impact on rates of carbon gain than in N. cunninghamii.
Coexistence of these species may be less likely in regions that experience summer drought. Since N. cunninghamii has a high rate of water use, it potentially accesses the soil water that A. moschatum has ‘conserved’. Hence, a rainforest of A. moschatum may be able to grow at drier sites than a mixed rainforest of A. moschatum and N. cunninghamii. However, coexistence of A. moschatum and N. cunninghamii may be enhanced by differences in rooting depth and thus partitioning of available water resources. Jackson et al. (1995) found that species accessing deeper and more abundant water resources had higher rates of water use, and observations suggest that N. cunninghamii roots extend proportionately deeper than those of A. moschatum. In addition, both species are mycorrhizal, with N. cunninghamii forming ectomycorrhizal associations (Howard 1973b; Bougher et al. 1994) and A. moschatum forming arbuscular mycorrhizae, as indicated by the presence of intracellular hyphal coils (T. Cavagnaro, J. Read and S. Kerr, unpubl. data from seedlings at Cement Creek). Although mycorrhizae were not detected in seedlings in the glasshouse experiments, they may have been present and contributing to the trends observed. Mycorrhizae may contribute significantly to water uptake (Augé 2001) by seedlings and trees in the field, with the different mycorrhizal associations possibly contributing to differences in water-use strategies of these two tree species. Rooting depth, soil water changes with depth, root interactions and water partitioning differences between these two species require further investigation.
Comparisons with trees from other forest types
These cool-temperate rainforest species share water-use strategies with trees adapted to much drier climates. The mean maximum stomatal conductance of N. cunninghamii trees in full sun (0.42 mol m–2 s–1) is well above the upper limit suggested by Körner et al. (1979) for evergreen woody plants (0.2 mol m–2 s–1). Such high rates of conductance have also been observed in other (non-rainforest) Australian tree species, such as the widely distributed Eucalyptus camaldulensis and the monsoonal species E. miniata (Farrell et al. 1996; Myers et al. 1997), and so are not surprising in rainforest trees that typically inhabit wetter areas. The mean maximum conductance of A. moschatum trees in full sun (0.08 mol m–2 s–1) appears more consistent with the lower limit suggested by Körner et al. (1979) for evergreen woody plants (0.04 mol m–2 s–1) and the lower conductance is consistent with its shade tolerance.
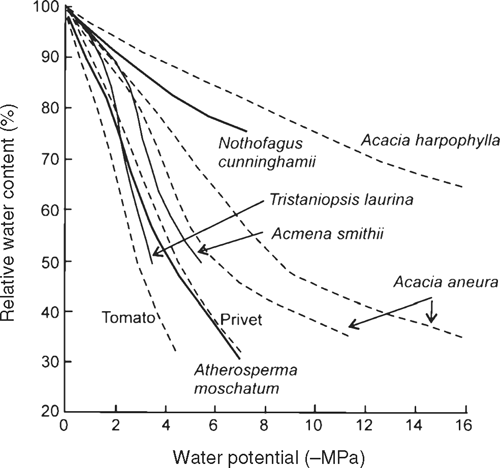
What is more surprising for a species restricted to regions of high rainfall is the low osmotic potential at the turgor loss point of N. cunninghamii, indicative of a superior capacity to draw water from the soil (Hsiao 1973), even by comparison with some species from drier habitats (Fig. 6). For example, N. cunninghamii trees had an osmotic potential of –2.8 MPa at the turgor loss point (Π0), the same as that found for the ground creeper Banksia petiolaris which grows in the dry scrub–heath of Western Australia (Witkowski et al. 1992) and lower than that of Eucalyptus regnans (–1.9 MPa) (Ashton and Sandiford 1988) with which N. cunninghamii is commonly found. However, N. cunninghamii is not able to survive to such low RWC and water potentials as species native to drier habitats (Fig. 6). The behaviour of N. cunninghamii differs from some related Nothofagus species, that tend instead to close their stomata at higher water contents (Körner and Bannister 1985), and do not appear to develop such low osmotic potentials (Bannister 1986).
|
There has been relatively little study of the water relations of co-occurring trees from evergreen rainforests, perhaps because it is assumed that species confined to such moist climates will have similar water-use strategies. The contrasting responses of these two species show first that co-occurring rainforest trees can differ markedly in responses to water deficits, and second, that they can share some water-use strategies with trees adapted to much drier climates, although without the same tolerance of extreme drought. Differences in water-use strategies have also been noted between two co-occurring warm-temperate rainforest trees by Melick (1990), these contributing to differences in distribution in the warm-temperate rainforests of eastern Victoria. Water-use strategies are important not only in survival of drought, but in their influence on rates of carbon gain and subsequently on growth rates and competitive interactions. Hence, to understand the adaptive significance of water-use strategies, it is important to consider more than just drought conditions. These cool-temperate rainforests occur in regions experiencing uniformly high rainfall (on average) across the year, but also in regions in which summer rainfall drops to ~50 mm on average in the driest month. Photosynthetic rates are low in the cold winters and it may be particularly important for these species to maximise opportunities for photosynthesis during the warmer months (Read and Farquhar 1991). However, the warmer months are also the driest, on average. In addition, the averaging of rainfalls across years masks the dry extremes to which these species may be exposed. A conservative water-use strategy may be sufficient for shade-tolerant species in the lower canopy. However, for a light-demanding canopy species to maximise photosynthesis in a summer-dry climate, there is likely to be strong selection pressure to maximise water uptake rather than relying on minimisation of water loss which reduces uptake of CO2. Hence, we predict trends in water relations in other rainforest species growing in temperate summer-dry climates similar to those we have recorded in the present study.
Acknowledgements
We thank Parks Victoria and the Department of Sustainability and Environment for permission to undertake research in the Yarra Ranges National Park and the Parks and Wildlife Service Tasmania, Royal Botanic Gardens Melbourne and Tasmanian Herbarium for supplying location data. We also thank Dr Terry Neeman, Australian National University, for advice regarding analysis of conductance data, although any errors are entirely our own.
Ashton DH, Sandiford EM
(1988) Natural hybridisation between Eucalyptus regnans F.Muell. and E. macrorhyncha F.Muell. in the Cathedral Range, Victoria. Australian Journal of Botany 36, 1–22.
| Crossref | GoogleScholarGoogle Scholar |

Augé RM
(2001) Water relations, drought and vesicular–arbuscular mycorrhizal symbiosis. Mycorrhiza 11, 3–42.
| Crossref | GoogleScholarGoogle Scholar |

Bannister P
(1986) Drought resistance, water potential and water content in some New Zealand plants. Flora 178, 23–40.

Bannister P, Kissel RM
(1986) The daily course of water potential and leaf conductance in some New Zealand plants. Flora 178, 329–342.

Bougher NL,
Fuhrer BA, Horak E
(1994) Taxonomy and biogeography of Australian Rozites species mycorrhizal with Nothofagus and Myrtaceae. Australian Systematic Botany 7, 353–375.
| Crossref | GoogleScholarGoogle Scholar |

Busby JR
(1986) A biogeoclimatic analysis of Nothofagus cunninghamii (Hook.) Oerst. in south eastern Australia. Australian Journal of Ecology 11, 1–7.
| Crossref | GoogleScholarGoogle Scholar |

Cunningham SC
(2004) Stomatal sensitivity to vapour pressure deficit of temperate and tropical evergreen rainforest trees of Australia. Trees—Structure and Function 18, 399–407.

Farquhar GD,
Ehleringer JR, Hubick KT
(1989) Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 40, 503–537.
| Crossref | GoogleScholarGoogle Scholar |

Farrell RCC,
Bell DT,
Akilan K, Marshall JK
(1996) Morphological and physiological comparisons of clonal lines of Eucalyptus camaldulensis.I. Responses to drought and waterlogging. Australian Journal of Plant Physiology 23, 497–507.

Howard TM
(1973a) Studies in ecology of Nothofagus cunninghamii Oerst. III. Two limiting factors: light intensity and water stress. Australian Journal of Botany 21, 93–102.
| Crossref | GoogleScholarGoogle Scholar |

Howard TM
(1973b) Studies in the ecology of Nothofagus cunninghamii Oerst. II. Phenology. Australian Journal of Botany 21, 79–92.
| Crossref | GoogleScholarGoogle Scholar |

Hsiao TC
(1973) Plant responses to water stress. Annual Review of Plant Physiology 24, 519–570.
| Crossref |

Jackson PC,
Cavelier J,
Goldstein G,
Meinzer FC, Holbrook NM
(1995) Partitioning of water resources among plants of a lowland tropical forest. Oecologia 101, 197–203.
| Crossref | GoogleScholarGoogle Scholar |

Körner C, Bannister P
(1985) Stomatal responses to humidity in Nothofagus menziesii. New Zealand Journal of Botany 23, 425–429.

Körner C,
Scheel JA, Bauer H
(1979) Maximum leaf diffusive conductance in vascular plants. Photosynthetica 13, 45–82.

Lindenmayer DB,
Mackey BG,
Cunningham RB,
Donnelly CF,
Mullen IC,
McCarthy MA, Gill AM
(2000) Factors affecting the presence of the cool temperate rain forest tree myrtle beech (Nothofagus cunninghamii) in southern Australia: integrating climatic, terrain and disturbance predictors of distribution patterns. Journal of Biogeography 27, 1001–1009.
| Crossref | GoogleScholarGoogle Scholar |

Melick DR
(1990) Relative drought resistance of Tristaniopsis laurina and Acmena smithii from riparian warm temperate rainforest in Victoria. Australian Journal of Botany 38, 361–370.
| Crossref | GoogleScholarGoogle Scholar |

Myers BA,
Duff GA,
Eamus D,
Fordyce IR,
O’Grady A, Williams RJ
(1997) Seasonal variation in water relations of trees of differing leaf phenology in a wet–dry tropical savanna near Darwin, northern Australia. Australian Journal of Botany 45, 225–240.
| Crossref | GoogleScholarGoogle Scholar |

Myers BJ,
Robichaux RH,
Unwin GL, Craig IE
(1987) Leaf water relations and anatomy of a tropical rain forest tree species vary with crown position. Oecologia 74, 81–85.
| Crossref | GoogleScholarGoogle Scholar |

Nagy Z,
Tuba Z, Csintalan Z
(1993) Ecophysiological responses of different density maize stands under drought stress and during recovery. Photosynthetica 28, 351–359.

Neyland MG, Brown MJ
(1993) Rainforest in eastern Tasmania—floristics and conservation. Papers and Proceedings of the Royal Society of Tasmania 127, 23–32.

Olesen T
(1997) The relative shade-tolerance of Atherosperma moschatum and Elaeocarpus holopetalus. Australian Journal of Ecology 22, 113–116.
| Crossref | GoogleScholarGoogle Scholar |

Penka M
(1956) Hodnocení půdní vody s biolického hlediska metodou vysychacích křivek. Československa Biolgie 5, 105–116.

Rascio A,
Russo M,
Platani C,
Ronga G, Di Fonzo N
(1999) Mutants of durum wheat with alterations in tissue affinity for strongly bound water. Plant Science 144, 29–34.
| Crossref | GoogleScholarGoogle Scholar |

Read J
(2001) Soil and rainforest composition in Tasmania: correlations of soil characteristics with canopy composition and growth rates in Nothofagus cunninghamii associations. Australian Journal of Botany 49, 121–135.
| Crossref | GoogleScholarGoogle Scholar |

Read J, Busby JR
(1990) Comparative responses to temperature of the major canopy species of Tasmanian cool temperate rainforest and their ecological significance. II. Net photosynthesis and climate analysis. Australian Journal of Botany 38, 185–205.
| Crossref | GoogleScholarGoogle Scholar |

Read J, Farquhar GD
(1991) Comparative studies in Nothofagus (Fagaceae). I. Leaf carbon isotope discrimination. Functional Ecology 5, 684–695.
| Crossref | GoogleScholarGoogle Scholar |

Read J, Hill RS
(1985) Dynamics of Nothofagus-dominated rainforest on mainland Australia and lowland Tasmania. Vegetatio 63, 67–78.

Shuttleworth WJ,
Gash JHC,
Lloyd CR,
Moore CJ,
Roberts J,
Marques A de O,
Fisch G,
Silva V de P,
Ribeiro MNG,
Molion LCB,
de Sa LDA,
Nobre JC,
Cabral OMR,
Patel SR, de Moraes JC
(1985) Daily variations of temperature and humidity within and above Amazonian rainforest. Weather 40, 102–108.

Turner NC
(1981) Techniques and experimental approaches for the measurement of plant water status. Plant and Soil 58, 339–366.
| Crossref |

Tyree MT, Hammel HT
(1972) The measurement of the turgor pressure and the water relations of plants by the pressure-bomb technique. Journal of Experimental Botany 23, 267.
| Crossref | GoogleScholarGoogle Scholar |

Vertucci CW, Leopold AC
(1987) The relationship between water binding and desiccation tolerance in tissues. Plant Physiology 85, 232–238.
| PubMed |

Witkowski ETF,
Lamont BB,
Walton CS, Radford S
(1992) Leaf demography, sclerophylly and ecophysiology of two Banksias with contrasting leaf life spans. Australian Journal of Botany 40, 849–862.
| Crossref | GoogleScholarGoogle Scholar |





