Is leaf pubescence of Cape Proteaceae a xeromorphic or radiation-protective trait?
R. P. Skelton A D , J. J. Midgley A , J. M. Nyaga A , S. D. Johnson B and M. D. Cramer A CA Department of Botany, University of Cape Town, Private Bag XI, Rondebosch 7701, South Africa.
B School of Biological and Conservation Sciences, University of KwaZulu-Natal, Private Bag X01, Scottsville, Pietermaritzburg 3209, South Africa.
C School of Plant Biology, Faculty of Natural and Agricultural Sciences, The University of Western Australia, 35 Stirling Highway, WA 6009, Australia.
D Corresponding author. Email: skelrob@gmail.com
Australian Journal of Botany 60(2) 104-113 https://doi.org/10.1071/BT11231
Submitted: 8 September 2011 Accepted: 13 January 2012 Published: 6 March 2012
Abstract
Although pubescence has traditionally been considered to be related to the water economy of plants, the results are ambivalent and vary between different species. We tested two contrasting hypotheses for the functional significance of leaf pubescence of Proteaceae species from the Cape Floristic Region. First, we hypothesised that pubescence is a xeromorphic trait that conserves water by increasing the boundary layer resistance to diffusion. Water loss was measured in two morphotypes of Leucospermum conocarpodendron (L.) Buek that differ in the degree of leaf pubescence, using both gas exchange and gravimetric techniques. Pubescence contributed less than 5% of total leaf resistance and pubescent leaves transpired at least as rapidly as glabrous leaves due to having larger numbers of small stomata per leaf area. Although pubescence was not associated with drier sites in L. conocarpodendron, there was a weak negative correlation between rainfall and pubescence across 18 other Proteaceae species. We also hypothesised that pubescence is a radiation-protective trait. We assessed the effect of pubescence on light reflectance, leaf temperature, fluorescence and gas exchange characteristics in situ. Pubescent leaves of L. conocarpodendron were 19.2 ± 0.08% more reflective than glabrous leaves and had significantly greater pre-dawn photochemical efficiency. There was a positive association between leaf pubescence and habitat temperature in Proteaceae. We conclude that although pubescence is unlikely to be a xeric adaptation, it could serve a role in reducing photoinhibition and heat loading in Proteaceae species.
Introduction
Leaf hairs of various shapes, sizes and arrangements are found on many plant species throughout a range of environments (Johnson 1975). Pubescence is often assumed to be a structural adaptation that conserves water through reduced leaf transpiration (e.g. Turner 1994; Richardson et al. 1995; Rotondi et al. 2003), with the suggestion that a layer of dense pubescence on the leaf surface increases the thickness of the boundary layer. Evidence has shown that for densely pubescent leaves the increase in boundary layer thickness is related to the thickness of the pubescence layer (Schuepp 1993; Nobel 2005). Since the rate of gas diffusion across the boundary layer is inversely proportional to its thickness, dense pubescence is bound to impose additional resistance (Nobel 2005). However, the boundary layer thickness of leaves is also influenced by wind speed and is greatest in the absence of wind, but may be negligible in windy conditions (Kramer and Boyer 1995). Several studies have shown that pubescence contributes significantly to overall leaf gas exchange resistance in some species (e.g. Wuenscher 1970; Ripley et al. 1999), yet others have found little or no effect in other species (e.g. Ehleringer and Mooney 1978; Benz and Martin 2006; Galmés et al. 2007). The extent to which the additional boundary layer resistance caused by pubescence influences leaf gas exchange rates depends on the additive contributions of leaf stomatal resistance, cuticular resistance and boundary layer resistance (Kramer and Boyer 1995). Relatively large resistance through the overall diffusive pathway ensures that an increase in boundary layer resistance due to pubescence may have little effect on gaseous exchange. Conversely, when the ratio of boundary layer resistance to total resistance is relatively high pubescence may significantly decrease the rate of gas exchange (Martin et al. 1999). In this case leaf pubescence may limit transpiration and be functional in arid environments. There is some evidence for an association between the occurrence of leaf pubescence and aridity. Ehleringer (1984) reported that 14 genera from seven different families display an increase in pubescence with increasing aridity in North America. In contrast, Jordan et al. (2008) showed that pubescence over the stomatal region has evolved frequently in wet environments and that its incidence was not biased to dry environments in the Proteaceae. These disparate results challenge the view that the primary role of pubescence is to conserve water by reducing transpiration. Further, Ehleringer (1984) did not control for temperature, which may confound the relationship between pubescence and rainfall.
Pubescence has also been shown to influence other biophysical processes, such as light reflectance (e.g. Ehleringer et al. 1976), water or solute excretion or absorption (e.g. Mozafar and Goodin 1970; Benzing et al. 1976) and convective heat loss (e.g. Meinzer and Goldstein 1985). Increased reflectance of light from the surface of leaves caused by pubescence decreases absorption of light energy and may have profound consequences for leaf functionality. For example, pubescence has been shown to reduce photoinhibition in several species occurring in high light environments (e.g. Skaltsa et al. 1994; Ripley et al. 1999). Radiation-induced inhibition may be induced by either excess light – through direct photodamage to photosystem II or the formation of reactive oxygen species – or high temperatures – through reduced RuBisCO efficiency (Nishiyama et al. 2006). Consequently, increased reflectance of light caused by pubescence could serve a radiation-protective function by reducing absorption of excess light or by reducing leaf temperatures (LT). Few investigations into the significance of pubescence as a radiation-protective trait have attempted to tease these two potential mechanisms apart. One way to do so is to examine the correlation between increased pubescence and environments that promote radiation-induced inhibition in different manners – such as low nutrient availability, extremely low or high temperatures and high light intensity. However, these relationships may be prone to autocorrelation and by themselves may be insufficient to discern the exact protective mechanism. For example, Jordan et al. (2005) showed that in the Proteaceae pubescence evolved between six and eight times in open environments, a proxy for high light and low productivity environments. Presumably there is a strong relationship between high light and high temperatures and it is unclear whether pubescence functions to reduce light absorption or to reduce LT. One way to distinguish between these effects is to couple correlative examinations into the relationship between pubescence and abiotic conditions with detailed physiological tests.
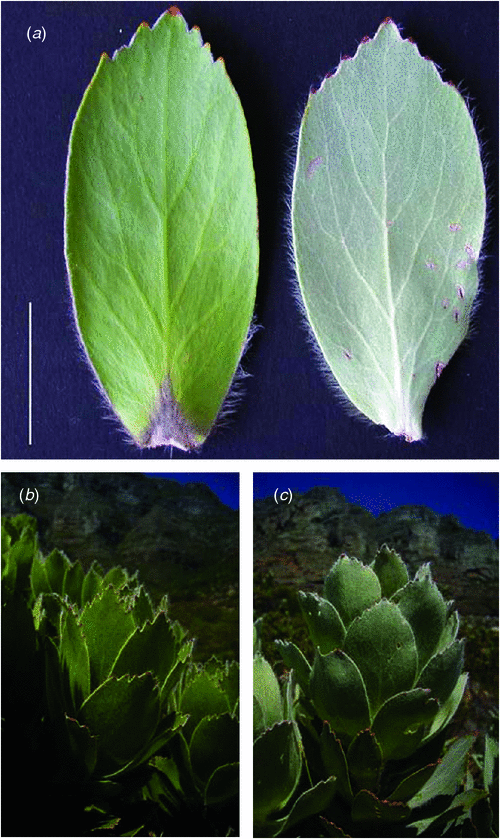
Many Proteaceae species from the Cape Floristic Region (CFR) have waxy cuticles or are covered in dense pubescence layers (Rebelo 2001). For example, ~22 out of 47 species of Leucospermum (Proteaceae) are pubescent at some stage in their life history and several species contain both pubescent and glabrous subspecies (Rourke 1972). However, the functional significance of pubescence in Proteaceae species from the CFR is uncertain. Unique edaphic and climatic factors within the CFR impose constraints on plant functionality and are considered important determinants of plant trait evolution (e.g. Linder 2003; Verboom et al. 2004; Galley et al. 2009). Although the region is characterised by a Mediterranean-type climate and highly oligotrophic soils, both are heterogeneous throughout (Witkowski and Mitchell 1987; Allsopp and Stock 1994; Procheş et al. 2005). This, coupled with high species diversity, makes the CFR an ideal system for testing functional hypotheses for plant traits, such as pubescence. Low water availability in summer could select for traits that conserve water. Alternatively, nutrient impoverishment could impose severe limitation on productivity and select for traits that decrease the amount of excess light incident on the photosystems and reduce photoinhibition and heat loading (Close and McArthur 2002; Jordan et al. 2005). We hypothesised that the functional significance of leaf pubescence in Proteaceae species from the CFR is (1) to increase the boundary layer thickness and reduce water loss and (2) to reflect excess light and reduce photoinhibition and heat loading. We measured gas exchange and fluorescence characteristics and conducted correlation analyses between climatic data and degree of pubescence. Since variation in stomatal traits may impact gas exchange, the relationship between pubescence and stomatal size and density was also assessed. To distinguish between the effects of pubescence on light availability and LT we also measured leaf reflectance and temperature of pubescent and glabrous leaves. Our main study species was Leucospermum conocarpodendron, which is endemic to the CFR and has both relatively pubescent (L. conocarpodendron conocarpodendron) and relatively glabrous (L. conocarpodendron viridum) morphotypes, recognised as subspecies (Rourke 1972; Fig. 1). Comparisons between these morphotypes which share an immediate common ancestor allowed us to control for phylogenetic history and general plant architecture and leaf design in experiments. To assess the generality of our findings we also correlated the degree of pubescence of 18 other Proteaceae species with environmental characteristics.
|
Materials and methods
Study sites
Long-term daily rainfall data from nine weather stations around the Cape Peninsula were obtained from the South African Weather Service (SAWS). Six of these weather stations were situated in areas occupied by the glabrous subspecies and three in areas occupied by pubescent subspecies. Mean monthly and annual rainfall was calculated from daily measurements taken continuously for between 8 and 30 years, depending on the station. Long-term daily maximum and minimum temperature data from four sites along the Cape Peninsula and humidity (at 14 : 00 hours) and wind speed data from three sites were also obtained from SAWS. Mean monthly and annual minimum and maximum daily temperature, wind speed and humidity were calculated from these data. Potential solar radiation was calculated using the solar radiation analysis package implemented in ArcGIS 9.2 (ESRI, Redlands, CA, USA) for 20 sites on the Cape Peninsula where L. conocarpodendron is known to occur (10 sites for each subspecies). Mean daily irradiance (in WH m–2) from the model was converted to units of mean daily photon flux density (μmol m–2 s–1) using conversions of Thimijan and Heins (1983).
Growth conditions
In October 2008 1- to 2-year old seedlings of glabrous and pubescent individuals of L. conocarpodendron were excavated from wild populations occurring in Silvermine (34°05′20″S, 18°25′27″E; 294 m) and Camp’s Bay (33°57′49″S, 18°23′08″E; 230 m), respectively. The seedlings were potted in Fynbos-mix soil obtained from a local nursery and grown in a glasshouse at the University of Cape Town until October 2009. Three days before gas exchange measurements, the plants were transferred to a temperature-controlled growth chamber set to 25°C with a light : dark photoperiod of 14 : 10 h and an irradiance of ~800 μmol m–2 s–1. Here, they were watered every day and, again, immediately before the shoot gas exchange measurements. The youngest fully expanded leaves were used for all experiments and measurements.
Stomatal and pubescence characteristics
Stomatal and pubescence characteristics were determined for pubescent and glabrous individuals of L. conocarpodendron occurring in the wild. Stomatal and hair density were measured by coating the adaxial surface of the leaves with nail varnish, peeling this off, and counting the number of hair or stomatal impressions in a field of view at 400× magnification on a transmission light microscope. Stomatal and hair counts were made on five leaves from 10 individuals at 15 sites. Stomatal size was determined from a single leaf from each of 10 individuals at six sites. Stomatal size was taken as guard cell length multiplied by the width of the guard cell pair (after Franks and Beerling 2009). Pubescence thickness was determined by cutting transverse sections of leaves and measuring the distance from the leaf cuticle to the tip of a trichome using an eye-piece graticule at 100× magnification under a compound microscope. Mean pubescence thickness for each subspecies was measured from leaves collected from 10 individuals at two sites. To establish whether any patterns found in L. conocarpodendron applied more generally, stomatal and hair density were also determined for 18 other Proteaceae species. Seven Leucadendron, five other Leucospermum and six Protea species were selected, encompassing a range of leaf hair densities. Three individuals (~30 cm tall) of each species were bought from Kirstenbosch Botanical Gardens nursery (Cape Town) and grown in a common potting medium in a glasshouse for 2 months. Stomatal and hair counts were made on leaves from three individuals per species.
Hair density was correlated with mean annual rainfall (MAR), mean annual temperature (MAT), mean daily maximum temperature and average pan evaporation (APAN) for each of 19 Proteaceae species (including L. conocarpodendron). The climatic niches of individual species were characterised by querying appropriate databases using distribution data obtained from the Protea Atlas Project (Rebelo 2001). All queries were done using an ARC Geographic Information System, with a 1′ × 1′ grid, giving, for each species, a mean, standard deviation and range. Climatic data were obtained from the South African Atlas of Hydrology and Climatology (Schulze 1997).
Gravimetric water loss
Water loss of glabrous and pubescent shoots and also of intact and shaved pubescent shoots of L. conocarpodendron was measured gravimetrically. Shoots of 20 glabrous and 40 pubescent individuals were collected into water from Red Hill (34°10′50″S, 18°24′59″E; 217 m) and Camp’s Bay sites, respectively, 1 h before measurements. The leaves of 10 shoots were shaved using a Philips 800-series electric shaver (Philips, South Africa), leaving only slight stubble on the leaves with no visible damage to the leaf surface. At the start of the measurements the shoots were re-cut under water and, keeping the cut end immersed, transferred to a beaker of water. These were then placed in a growth chamber maintained at 25°C with an irradiance of ~800 µmol m–2 s–1, relative humidity of ~20–25% and with an effective wind speed of ~1 m s–1. Shoots were weighed periodically and the leaf area of each shoot was measured using a LI-3100 Area Meter (Li-Cor, Lincoln, NE, USA). The rate of water loss remained relatively constant over the duration of the measurements.
Gas exchange measurements
Gas exchange was measured on five pubescent and glabrous individuals using an LI-6400 Portable Photosynthesis System (Li-Cor). The temperature in the cuvette was set at 25°C and CO2 concentration at 400 µmol CO2 mol–1. Irradiance in the cuvette was set at 1000 µmol m–2 s–1. Total leaf resistance to water vapour, rt, was taken as the reciprocal of water vapour conductance (gt, mol m–2 s–1), which was calculated using standard equations (Li-Cor BioSciences Manual). Leaf boundary layer resistance, rbl, was taken as the reciprocal of boundary layer conductance (gbl), which was determined by measuring evaporation from the surface of filter paper placed in darkness in the Li-Cor cuvette. In still air, the additional resistance to water vapour diffusion caused by pubescence is proportional to the thickness of the pubescence layer (Nobel 2005). Resistance through the pubescence layer, rp (mol–1 m2 s1), was calculated as:

(Ehleringer and Mooney 1978; Nobel 2005), where D is the diffusivity of water vapour in air (2.5 × 10−5 m2 s–1), lp is the thickness of the pubescence layer (m), P is the ambient air pressure (atm), R is the universal gas constant (8.205 × 10−5 m3 atm mol–1 K–1) and T is the ambient air temperature (298.15 K). This calculation assumes that the only effect of pubescence on the boundary layer is to increase its thickness and ignores any potential turbulence effects. Stomatal resistance to water vapor, rs, was then calculated as:

The relative contributions of rs, rp and rbl towards rt were then evaluated.
Carbon isotope discrimination
The carbon isotope ratio (δ13C) of glabrous and pubescent leaves was determined and used as a proxy for long-term Ca/Ci values (Farquhar et al. 1989). Two leaves from each of 10 individuals were collected from five pubescent populations and five glabrous populations. Leaves were oven-dried at 60°C for 3 days and subsequently milled to pass through a 1-mm sieve. Samples were weighed into tin cups to an accuracy of 1 µg on a Sartorius micro balance. Samples were combusted in a Flash EA 1112 series elemental analyser (Thermo Finnigan, Italy). The gases were passed to a Delta Plus XP IRMS (isotope ratio mass spectrometer; Thermo Electron, Germany), via a Conflo III gas control unit (Thermo Finnigan, Germany). In-house standards were calibrated against International Atomic Energy Agency standards. Carbon was expressed in terms of its value relative to PDB-belemnite standard.
Leaf reflectance measurements
The youngest, fully-expanded leaves were collected from three sites for each subspecies in March 2010. Reflectance from the adaxial surface of each leaf was measured within 12 h of collection using an ISP-REF Integrating Sphere (Ocean Optics Inc., Netherlands). Reflectance data was binned into wavelength categories and averaged across individuals and populations (sites). Wavelength categories were UV-C (180–280 nm), UV-B (280–320 nm), UV-A (320–400 nm) and photosynthetically active radiation (400–700 nm).
Field measurements of fluorescence and gas exchange
Measures of leaf fluorescence and gas exchange rates were made on 10 co-occurring glabrous and pubescent individuals at Camp’s Bay on 20 February 2010 during a period of hot, dry and clear weather. Glabrous individuals were planted at this normally ‘pubescent’ site during the rehabilitation of a reservoir built in 1984–85 (Smith 1986), creating a unique opportunity to compare performance of the two morphotypes in a common environment. Pre-dawn photochemical efficiency (Fv/Fm) was measured using a PAM-2000 fluorometer and 2030-B leaf-clip holder (Heinz Walz GmbH, Effeltrich, Germany) to provide an indication of sustained photoinhibition (after Close et al. 2007). Midday CO2 assimilation rate (A), transpiration rate (E) and total conductance (gtw) were determined using a LI-6400 IRGA. Light intensity in the IRGA cuvette was set to 2000 µmol m–2 s–1 and temperature was maintained at ~30°C throughout the experiment. Quantum yield of PSII (ΦPSII) was assessed simultaneously on the same individuals using a PAM-2000 fluorometer and 2030-B leaf-clip holder.
Leaf temperature
The effect of pubescence on LT was investigated in two ways. First, the LT of three fully-expanded, sun-exposed leaves from eight glabrous and pubescent individuals was measured using an LS infrared thermometer (Optris, Berlin, Germany). These measurements were taken in situ at midday on a clear, hot summer day (4 February 2010). Second, the temperature of 2-cm2 sections of leaves of pubescent and glabrous individuals was measured under controlled conditions in a glasshouse and a laboratory windowsill. Leaf blocks were fixed at a constant angle to the sun and their temperature and weight were recorded over a period of 1 h. LT was measured when the weight loss per time was constant using an LS infrared thermometer. Energy balance calculations were performed using standard equations (Li-Cor 6400 manual) to ascertain whether leaf temperature readings could be explained by differences in rates of water loss and absorptance.
Statistical analysis
Student’s t-test was used to test for differences in environmental and plant characteristics between glabrous and pubescent populations. Pearson correlation and linear regression analyses were conducted on trait data and trait-environmental data, except where these data violated the assumptions of the tests. In those cases Spearman rank correlation and linear regression analyses were conducted on trait data and trait-environmental data. A one-way ANCOVA analysis was conducted to compare the relationship between hair density and environmental data between each genus. All statistical analyses were performed using Statistica 8.0 (StatSoft Inc., Tulsa, OK, USA).
Results
Leaf traits
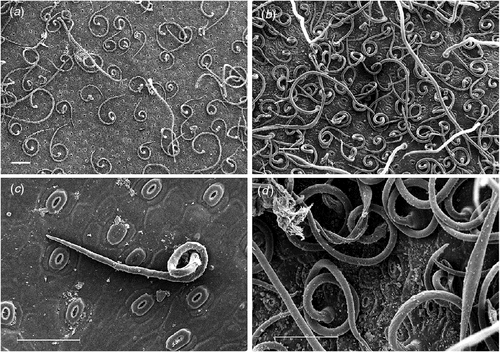
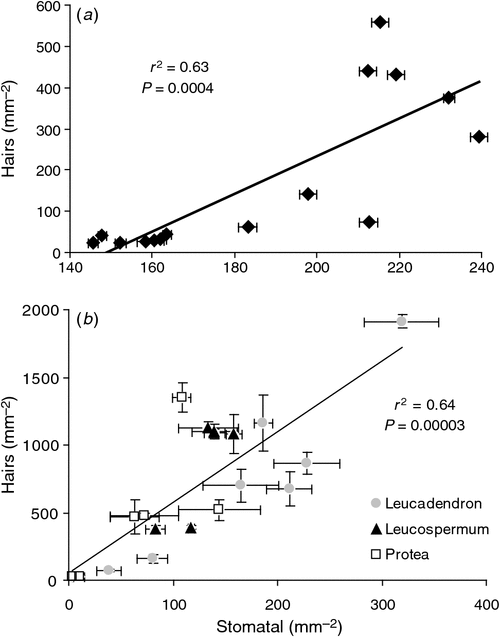
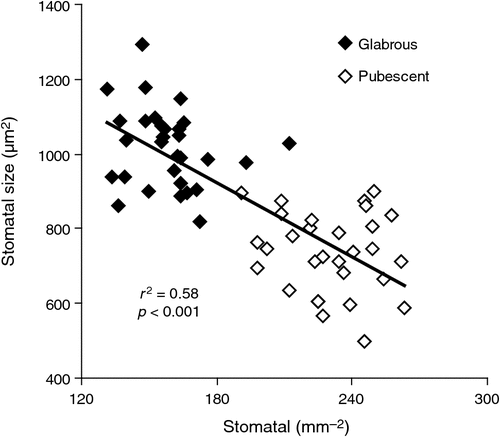
The pubescence layer in L. conocarpodendron is composed of numerous coiled or erect, simple, non-glandular trichomes and is likely to affect leaf physical properties (Fig. 2). L. conocarpodendron conocarpodendron had denser pubescence (mean hair density = 418 ± 16 mm–2) compared with L. conocarpodendron viridum (mean hair density = 50 ± 5 mm–2; t = –20.35, d.f. = 74, P < 0.001). Although leaves of L. conocarpodendron viridum had some hair, we referred to them as being ‘glabrous’. The mean thickness of the pubescence layer on pubescent leaves was found to be 0.18 ± 0.01 mm. A positive linear relationship existed between hairiness and stomatal density for L. conocarpodendron (Fig. 3a) and also more generally for 19 Proteaceae species (Fig. 3b). When the correlation analysis was done within separate genera, significant correlations between hair and stomatal density were found for Leucospermum (r2 = 0.77, P = 0.02, n = 6) and Leucadendron species (r2 = 0.85, P = 0.003, n = 7), but not for Protea species (r2 = 0.50, P = 0.12, n = 6; Fig. 3b). Hair density in the Proteaceae ranged from 23 ± 15 mm–2 for P. repens to 1914 ± 48 mm–2 for Ld. cryptocephalum. Stomatal density ranged from 4 mm–2 for P. cynaroides to 319 ± 35 mm–2 for Ld. cryptocephalum. A negative linear relationship was found between stomatal size and density in L. conocarpodendron (Fig. 4). The mean stomatal size for pubescent leaves was 736 ± 20 µm2 and that of glabrous leaves was 1017 ± 19 µm2. The mean stomatal density for pubescent leaves was 230 ± 4 mm–2 and that of glabrous leaves was 158 ± 3 mm–2. Glabrous and pubescent leaves had statistically indistinguishable foliar total N concentrations, δ13C and leaf mass per area values (Table 1).
|
|
|

|
Leaf trait–environment relationships
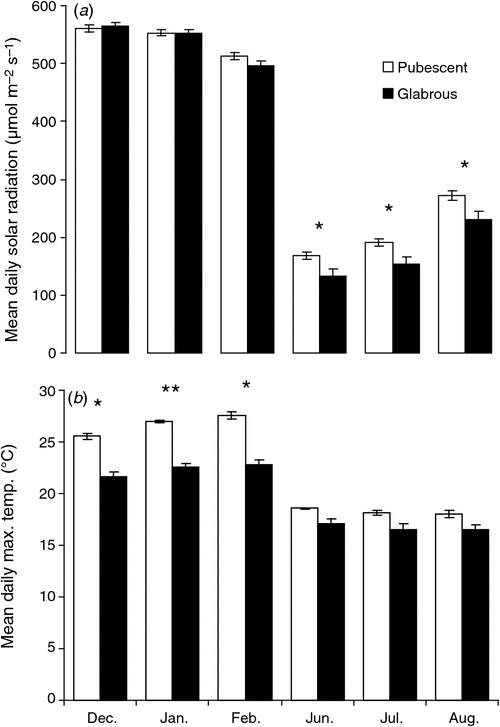
There was no significant difference in MAR between areas occupied by pubescent and glabrous morphotypes of L. conocarpodendron (Supporting information, Table SI). The weather station with the highest reported MAR was Silvermine with 1118 ± 41 mm year–1 and that with the lowest was Cape Point (34°21′10.8″S, 18°29′20.4″E; 231 m) with 355 ± 15 mm year–1. These weather stations were both in areas occupied by glabrous individuals. Rainfall in summer was similar for glabrous and pubescent sites and was slightly higher for pubescent sites compared with glabrous sites in winter (Table SI). Solar radiation was generally similar at sites occupied by the pubescent and glabrous morphotypes in summer (Fig. 5a). Sites with pubescent individuals received ~20% more solar radiation during winter compared with sites with the glabrous individuals (Fig. 5a). Daily maximum temperatures during summer were ~4°C higher for sites occupied by pubescent individuals than sites with glabrous individuals (Fig. 5b).
|
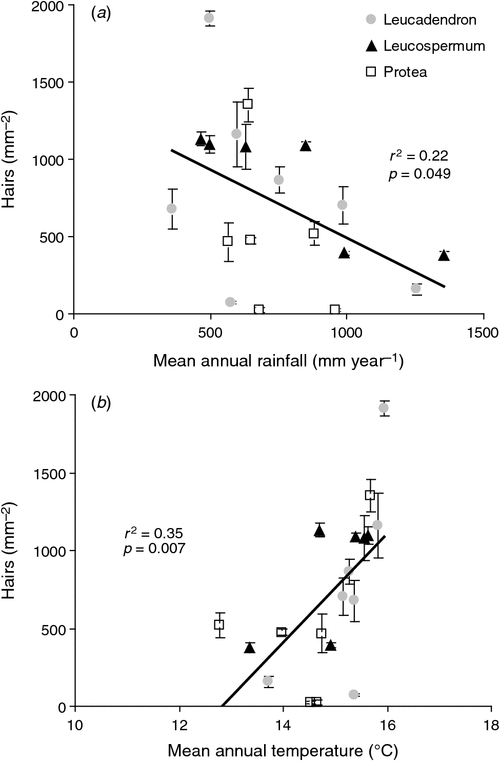
Pubescence was weakly negatively associated with MAR for 19 Proteaceae species (Fig. 6a). When genera were analysed separately, the correlation between hair density and MAR was significant for Leucospermum species (Spearman r = –0.94, P < 0.05, n = 6), but not for Leucadendron (Spearman r = –0.18, P > 0.05, n = 7) or Protea species (Spearman r = –0.31, P > 0.05, n = 6). Neither the slopes of the regression lines (F2,12 = 0.05, P = 0.95), nor the intercepts (F2,14 = 2.63, P = 0.11) were significantly different. Increased hair density was not correlated with either APAN or mean daily maximum temperature for 19 Proteaceae species (data not shown), although it was positively correlated with MAT (Fig. 6b). The correlation between hair density and MAT was not significant within any of the genera when they were analysed separately (Fig. 6b). Neither the slopes of the regression lines (F2,12 = 1.17, P = 0.34), nor the intercepts (F2,12 = 0.37, P = 0.70) were significantly different, suggesting that this was due to a lack of statistical power.
|
Effect of pubescence on leaf gas exchange
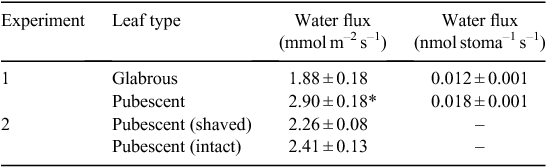
Pubescent and glabrous leaves displayed similar rt values (t = –0.8, d.f. = 8, P = 0.45). The contribution of rs was ~92% of rt for glabrous leaves and ~87% of rt for pubescent leaves. The contribution of rbl to rt was 8 and 9% for glabrous and pubescent leaves, respectively. Pubescence (i.e. rp) contributed only ~4% towards rt. Water loss measured gravimetrically expressed per leaf area was significantly greater for pubescent leaves compared with glabrous leaves (Table 2). When water flux was expressed per stoma there was no significant difference between glabrous and pubescent leaves (Table 2). Shaved and non-shaved pubescent leaves had similar rates of water loss (t = 0.96; d.f. = 18; P = 0.35; Table 2).
|
Reflectance, fluorescence and leaf temperature
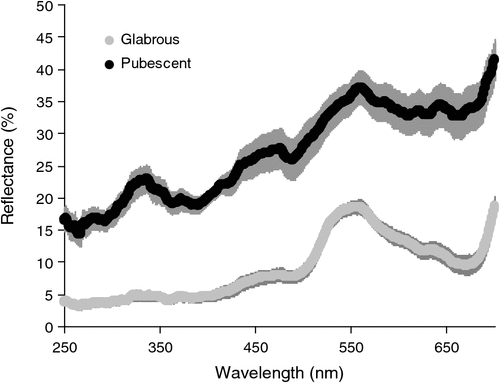
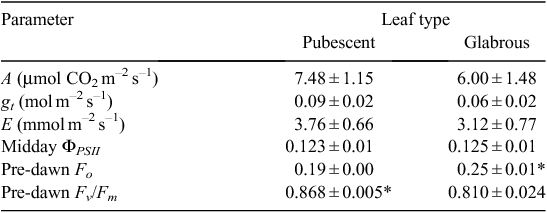
Pubescent leaves reflected significantly more light of wavelength between 250 and 700 nm compared with the glabrous leaves (Fig. 7; Table SII). The reflectance of pubescent leaves averaged across these wavelengths was 9.1 ± 0.1%, while that of glabrous leaves was 27.2 ± 0.1%. Pubescent leaves were relatively more reflective at longer wavelengths compared with glabrous leaves (Fig. 7; Table SII). Mean LT of pubescent leaves was 3.9°C lower than that of glabrous leaves when measured in situ (Table 3). Although the mean temperature of pubescent leaves was consistently lower than that of glabrous leaves when measured in the laboratory or greenhouse, this difference was not statistically significant (Table 3). Pre-dawn Fv/Fm of pubescent leaves was significantly greater than that of glabrous leaves measured at the same site (Table 4). Glabrous leaves had higher pre-dawn Fo values compared with pubescent leaves (Table 4). There was no significant difference between the midday gas exchange parameters or PSII activity of pubescent and glabrous leaves (Table 4).
|
|
Discussion
Gas exchange measurements provided evidence that pubescence in L. conocarpodendron played a minor role in regulating water loss and that instead stomata were the over-riding regulators of leaf diffusive resistance. Even though leaf pubescence in L. conocarpodendron did add to the total resistance to gaseous diffusion, its overall contribution to the diffusive pathway was much less than that of stomata, (which was greater than 85%). Our data also showed that pubescent leaves in L. conocarpodendron are not adapted to have constitutively low transpiration and indeed may display greater gaseous exchange rates compared with glabrous leaves. This finding is in contrast to those reported by Ripley et al. (1999) and Wuenscher (1970) for Arctotheca populifolia and Verbascum thapsus, respectively, which showed significant reductions in transpiration rate in intact pubescent leaves compared with de-haired leaves. It is possible that the nature of the pubescence layer differs between L. conocarpodendron and those two species and that each affects gas exchange differently. Alternatively, since those two studies compared only de-haired leaves with intact leaves their results may reflect a slight change in stomatal properties induced by a change in light intensity or humidity caused by removal of the pubescence layer (Johnson 1975; Brodribb et al. 2009). Although pubescence in L. conocarpodendron was shown not to reduce absolute water loss, is it possible that it serves to increase water-use efficiency (WUE)? Since pubescence increases the boundary layer thickness, it will have a positive effect on WUE if the diffusivity ratio of water vapour to CO2 is lower in the boundary layer compared with the stomatal pore (Hassiotou et al. 2009). Nevertheless, similar carbon δ13C values of pubescent and glabrous leaves (Table 1) suggest that pubescence did not alter WUE in L. conocarpodendron and we conclude that it is unlikely that WUE enhancement is the primary role of pubescence in this species.
Greater capacity for water loss of pubescent leaves of L. conocarpodendron compared with glabrous leaves may be a consequence of them having more, smaller stomata. Smaller stomata allow for greater conductance for a given pore area by reducing the length of the diffusive pathway (Franks and Beerling 2009). The positive relationship between pubescence and stomatal density was also found for 18 other Proteaceae species and suggests a general relationship in the family. There are two possible explanations for this relationship. First, pubescence and stomata may be developmentally linked to each other or to a third leaf trait, such as leaf size. If two traits are developmentally associated, then changes in one due possibly, but not necessarily, to functional reasons may influence the other for reasons unrelated to its function. An alternative explanation is that changes in pubescence have required compensatory changes in stomatal traits. It is intriguing to speculate that a shift to smaller, denser stomata may compensate for the presence of a dense pubescence layer, and the effect that this imposes on gaseous exchange. Smaller, denser stomata would therefore be a functional response to an increase in the boundary layer thickness caused by pubescence, possibly with an unrelated function.
Comparison of rainfall data from various weather stations situated within the current distribution of L. conocarpodendron revealed that pubescence in this species was not associated with lower MAR or summer rainfall, the period when water is most limiting for fynbos species (Table SI). Although MAR is not a direct measure of aridity, it is considered a reliable proxy of water availability (e.g. Lamont et al. 2002). These data seemingly provide further evidence that pubescence is not a xeromorphic trait in L. conocarpodendron. However, it is important to acknowledge that other factors, such as temperature and relative humidity, may play a significant role in determining aridity at a particular site. When data for all 19 species were considered, a significant correlation was found between pubescence and MAR. No relationship was shown to exist between stomatal density and MAR (Fig. S1), suggesting that it is not a reflection of the co-varying relationship between these two leaf traits. There may be several explanations for the contradictory findings regarding the relationship between MAR and pubescence for L. conocarpodendron and for 19 Proteaceae species. First, MAR co-varies with several other factors and consequently the correlation might not reflect a functional relationship. Analysis of the coefficients of a multiple regression showed that MAT was a stronger predictor of hair density than MAR (Table SII). Alternatively, if the relationship is in fact functional, the primary underlying mechanism might not be related to water conservation (see discussion later on).
Pubescence in L. conocarpodendron increased the reflectance of light of all wavelengths from the leaf surface. The increase in reflectance from pubescent compared with glabrous leaves was smaller than that reported by Ehleringer et al. (1976) for Encelia farinosa – the most extreme example of reflectance reported – but was similar to that found in Arctotheca populifolia by Ripley et al. (1999). Increased reflectance from the leaf surface could potentially serve to reduce radiation-inhibition in two ways: either by reducing excess light absorption or by reducing thermal load and reducing LT. Light response curves for individuals of this species grown in a controlled growth chamber showed that leaves are light saturated above light intensity levels of ~1000 μmol m–2 s–1 and display low photochemical efficiency under these conditions (Fig. S1). Individuals of L. conocarpodendron occur where light levels often exceed these intensity levels, although pubescent individuals did not occur in sites with significantly higher solar radiation compared with glabrous leaves. Pubescent individuals did however occur in sites with significantly hotter temperatures compared with glabrous individuals. These conditions are likely to enhance radiation-inhibition and may select for protective traits.
Pubescent leaves of L. conocarpodendron displayed greater pre-dawn photochemical efficiency compared with co-occurring glabrous leaves, suggesting that they maintain greater potential to process light photochemically throughout summer and suffer less from chronic photoinhibition (Maxwell and Johnson 2000; Close et al. 2007). This effect may be caused by several factors, such as cooler LT – caused by higher rates of water loss and reflectance of radiation – or lower chlorophyll content. LT data collected from a site where the two morphotypes co-occurred showed that pubescent leaves were cooler compared with glabrous leaves. Energy balance calculations confirmed that this difference can be explained by pubescent leaves having greater water loss and lower absorptance compared with glabrous leaves (Table SIV). Further, our energy balance calculations showed that reduced absorptance due to pubescence was responsible for up to 2°C of this difference in LT (Table SIV). A large fraction of the remaining difference in LT between the two morphotypes is due to differences in transpiration rate, which provides further explanation as to why pubescent leaves had greater stomatal density compared with glabrous leaves. We suggest that these data together with the negative relationship between MAR and pubescence and the positive relationship between pubescence and MAT in 19 Proteaceae species provides evidence for a role of pubescence in radiation-protection. Decreased moisture availability means that leaves are less able to rely on water loss for leaf cooling, which might result in higher LT. This could reduce a leaf’s ability to process light photochemically and result in it experiencing relatively higher levels of excess solar radiation. Under more arid conditions, increased reflectance from the leaf surface would result in decreased absorptance and LT and reduced radiation-inhibition. Although the contribution of reduced LT and decreased light absorption to radiation-protection remains to be determined, we argue that this is the primary function of pubescence in highly pubescent individuals of the Proteaceae.
Conclusion
Pubescence in L. conocarpodendron is unlikely to be a xeromorphic leaf trait, since it had little potential influence on resistance to gas exchange and was not associated with conditions of greater water stress. Further, pubescent leaves did not conserve water but instead had the capacity to lose as much or more compared with glabrous leaves due to a strong positive relationship between pubescence and stomatal density. This relationship applied more generally across several other Proteaceae species and suggests that the function of stomata and pubescence could possibly be linked. Increased water loss due to greater stomatal density reduces LT, which in turn serves to reduce radiation-inhibition caused by thermal stress and reduced RuBisCO efficiency. When water becomes limiting and the plant is no longer able to reduce temperature through high rates of water loss, increased reflectance from the leaf surface caused by pubescence could become important in reducing light absorption and associated high levels of radiation-inhibition.
Supplementary material
Supplementary material for this article is available on the Journal’s website.
Acknowledgements
The authors wish to thank South African National Parks for granting permission to conduct research in the Table Mountain National Park.
References
Allsopp N, Stock WD (1994) VA mycorrhizal infection in relation to edaphic characteristics and disturbance regime in three lowland plant communities in the south-western Cape, South Africa. Journal of Ecology 82, 271–279.| VA mycorrhizal infection in relation to edaphic characteristics and disturbance regime in three lowland plant communities in the south-western Cape, South Africa.Crossref | GoogleScholarGoogle Scholar |
Benz BW, Martin CE (2006) Foliar trichomes, boundary layers, and gas exchange in 12 species of epiphytic Tillandsia (Bromeliaceae). Journal of Plant Physiology 163, 648–656.
| Foliar trichomes, boundary layers, and gas exchange in 12 species of epiphytic Tillandsia (Bromeliaceae).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xkslalsrs%3D&md5=e1404df96239e12d38da75a10c0b0ec7CAS |
Benzing DH, Henderson K, Kessel B, Sulak J (1976) The absorptive capacities of Bromeliad trichomes. American Journal of Botany 63, 1009–1014.
| The absorptive capacities of Bromeliad trichomes.Crossref | GoogleScholarGoogle Scholar |
Brodribb TJ, McAdam SAM, Jordan GJ, Feild TS (2009) Evolution of stomatal responsiveness to CO2 and optimisation of water-use efficiency among land plants. New Phytologist 183, 839–847.
| Evolution of stomatal responsiveness to CO2 and optimisation of water-use efficiency among land plants.Crossref | GoogleScholarGoogle Scholar |
Close DC, McArthur C (2002) Rethinking the role of many plant phenolics – protection from photodamage not herbivores? Oikos 99, 166–172.
| Rethinking the role of many plant phenolics – protection from photodamage not herbivores?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xosl2ltb0%3D&md5=134940f9a2bae9508fd31d6ccb503745CAS |
Close DC, Davidson NJ, Shields CB, Wiltshire R (2007) Reflectance and phenolics of green and glaucous leaves of Eucalyptus urnigera. Australian Journal of Botany 55, 561–567.
| Reflectance and phenolics of green and glaucous leaves of Eucalyptus urnigera.Crossref | GoogleScholarGoogle Scholar |
Ehleringer J (1984) Ecology and ecophysiology of leaf pubescence in North American desert plants. In ‘Biology and chemistry of plant trichomes’. (Eds E Rodriguez, PL Healey, I Mehta) pp. 113–132. (Plenum Press: New York)
Ehleringer J, Mooney HA (1978) Leaf hairs: effects on physiological activity and adaptive value to a desert shrub. Oecologia 37, 183–200.
| Leaf hairs: effects on physiological activity and adaptive value to a desert shrub.Crossref | GoogleScholarGoogle Scholar |
Ehleringer J, Björkman O, Mooney HA (1976) Leaf pubescence: effects on absorptance and photosynthesis in a desert shrub. Science 192, 376–377.
| Leaf pubescence: effects on absorptance and photosynthesis in a desert shrub.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cvgsVaisg%3D%3D&md5=ba13f2dd5cf5771d78a2869884cebd0fCAS |
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 40, 503–537.
| Carbon isotope discrimination and photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXktlKmu70%3D&md5=81bf4044435e418e817414a44c9935abCAS |
Franks PJ, Beerling DJ (2009) Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proceedings of the National Academy of Sciences of the United States of America 106, 10343–10347.
| Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXot1Giurg%3D&md5=27738e2dc06e61e1ce77aa0747d417dcCAS |
Galley C, Linder HP, Zimmermann NE (2009) Pentaschistis (Poaceae) diversity in the Cape Mediterranean region: habitat heterogeneity and climate stability. Global Ecology and Biogeography 18, 586–595.
| Pentaschistis (Poaceae) diversity in the Cape Mediterranean region: habitat heterogeneity and climate stability.Crossref | GoogleScholarGoogle Scholar |
Galmés J, Medrano H, Flexas J (2007) Photosynthesis and photoinhibition in response to drought in a pubescent (var. minor) and a glabrous (var. palaui) variety of Digitalis minor. Environmental and Experimental Botany 60, 105–111.
| Photosynthesis and photoinhibition in response to drought in a pubescent (var. minor) and a glabrous (var. palaui) variety of Digitalis minor.Crossref | GoogleScholarGoogle Scholar |
Hassiotou F, Evans JR, Ludwig M, Veneklaas EJ (2009) Stomatal crypts may facilitate diffusion of CO2 to adaxial mesophyll cells in thick sclerophylls. Plant, Cell & Environment 32, 1596–1611.
| Stomatal crypts may facilitate diffusion of CO2 to adaxial mesophyll cells in thick sclerophylls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVKhtbbK&md5=3be00a8d0caf39e62c2a7185a7ca67b6CAS |
Johnson HB (1975) Plant pubescence: an ecological perspective. Botanical Review 41, 233–258.
| Plant pubescence: an ecological perspective.Crossref | GoogleScholarGoogle Scholar |
Jordan GJ, Dillon RA, Weston PH (2005) Solar radiation as a factor in the evolution of scleromorphic leaf anatomy in Proteaceae. American Journal of Botany 92, 789–796.
| Solar radiation as a factor in the evolution of scleromorphic leaf anatomy in Proteaceae.Crossref | GoogleScholarGoogle Scholar |
Jordan GJ, Weston PH, Carpenter RJ, Dillon RA, Brodribb TJ (2008) The evolutionary relations of sunken, covered, and encrypted stomata to dry habitats in Proteaceae. American Journal of Botany 95, 521–530.
| The evolutionary relations of sunken, covered, and encrypted stomata to dry habitats in Proteaceae.Crossref | GoogleScholarGoogle Scholar |
Kramer PJ, Boyer JS (1995) ‘Water relations of plants and soils.’ (Academic Press: San Diego)
Lamont BB, Groom PK, Cowling RM (2002) High leaf mass per area of related species assemblages may reflect low rainfall and carbon isotope discrimination rather than low phosphorous and nitrogen concentrations. Functional Ecology 16, 403–412.
| High leaf mass per area of related species assemblages may reflect low rainfall and carbon isotope discrimination rather than low phosphorous and nitrogen concentrations.Crossref | GoogleScholarGoogle Scholar |
Linder HP (2003) The radiation of the Cape flora, southern Africa. Biological Reviews of the Cambridge Philosophical Society 78, 597–638.
| The radiation of the Cape flora, southern Africa.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c%2FgsVWksg%3D%3D&md5=644ff5d0546730cb35d7a6a8719e2efeCAS |
Martin TA, Hinckley TM, Meinzer FC, Sprugel DG (1999) Boundary layer conductance, leaf temperature and transpiration of Abies amabilis branches. Tree Physiology 19, 435–443.
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence – a practical guide. Journal of Experimental Botany 51, 659–668.
| Chlorophyll fluorescence – a practical guide.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjtF2js74%3D&md5=c3b4e4e6530f06ff9c34bf18011bd92aCAS |
Meinzer F, Goldstein G (1985) Some consequences of leaf pubescence in the Andean giant rosette plant Espeletia timotensis. Ecology 66, 512–520.
| Some consequences of leaf pubescence in the Andean giant rosette plant Espeletia timotensis.Crossref | GoogleScholarGoogle Scholar |
Mozafar A, Goodin JR (1970) Vesiculated hairs: a mechanism for salt tolerance in Atriplex halimus L. Plant Physiology 45, 62–65.
| Vesiculated hairs: a mechanism for salt tolerance in Atriplex halimus L.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3cXktlamsr4%3D&md5=a8c840c75cc17516cabf753e20231763CAS |
Nishiyama Y, Allakhverdiev SI, Murata N (2006) A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochimica et Biophysica Acta - Bioenergetics 1757, 742–749.
| A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XotVCrsb4%3D&md5=c678c66959968e2c8b13c2e94c82b0f4CAS |
Nobel PS (2005) ‘Physicochemical and environmental plant physiology.’ (Elsevier Academic Press: Amsterdam)
Procheş Ş, Cowling RM, du Preez DM (2005) Patterns of geophyte diversity and storage organ size in the winter-rainfall region of southern Africa. Diversity & Distributions 11, 101–109.
| Patterns of geophyte diversity and storage organ size in the winter-rainfall region of southern Africa.Crossref | GoogleScholarGoogle Scholar |
Rebelo T (2001) ‘Proteas: a field guide to the proteas of southern Africa.’ (Fernwood Press: Cape Town)
Richardson DM, Cowling RM, Bond WJ, Stock WD, Davis GW (1995) Links between biodiversity and ecosystem function in the Cape Floristic Region. In Mediterranean-type ecosystems: the function of biodiversity (Eds GW Davis, DM Richardson). pp. 285–333. (Springer-Verlag: Berlin)
Ripley BS, Pammenter NW, Smith VR (1999) Function of leaf hairs revisited: the hair layer on leaves of Arctotheca populifolia reduces photoinhibition, but leads to higher leaf temperatures caused by lower transpiration rates. Journal of Plant Physiology 155, 78–85.
| Function of leaf hairs revisited: the hair layer on leaves of Arctotheca populifolia reduces photoinhibition, but leads to higher leaf temperatures caused by lower transpiration rates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXks1SltLg%3D&md5=569c1f695854c072048e6fefa640e587CAS |
Rotondi A, Rossi F, Asunis C, Cesaraccio C (2003) Leaf xeromorphic adaptations of some plants of a coastal Mediterranean macchia ecosystem. Journal of Mediterranean Ecology 4, 25–35.
Rourke JP (1972) Taxonomic studies on Leucospermum R.Br. Journal of South African Botany 8, 1–194.
Schuepp PH (1993) Tansley Review no. 59. Leaf boundary layers. New Phytologist 125, 477–507.
| Tansley Review no. 59. Leaf boundary layers.Crossref | GoogleScholarGoogle Scholar |
Schulze RE (1997) ‘South African atlas of agrohydrology and climatology.’ (Water Research Commission: Pretoria)
Skaltsa H, Verykokidou E, Harvala C, Karabourniotis G, Manetas Y (1994) UV-B protective potential and flavonoid content of leaf hairs of Quercus ilex. Phytochemistry 37, 987–990.
| UV-B protective potential and flavonoid content of leaf hairs of Quercus ilex.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXitFyks74%3D&md5=7979750518b302611e891d50e6f0fbdcCAS |
Smith M (1986) Camp’s Bay reservoir revegetation. Honours Thesis, University of Cape Town.
Thimijan RW, Heins RD (1983) Photometric, radiometric, and quantum light units of measure: a review of procedures for interconversion. HortScience 18, 818–822.
Turner IM (1994) Sclerophylly: primarily protective? Functional Ecology 8, 669–675.
| Sclerophylly: primarily protective?Crossref | GoogleScholarGoogle Scholar |
Verboom GA, Linder HP, Stock WD (2004) Testing the adaptive nature of radiation: growth form and life history divergence in the African grass genus Ehrharta (Poaceae: Ehrhartoideae). American Journal of Botany 91, 1364–1370.
| Testing the adaptive nature of radiation: growth form and life history divergence in the African grass genus Ehrharta (Poaceae: Ehrhartoideae).Crossref | GoogleScholarGoogle Scholar |
Witkowski ETF, Mitchell DT (1987) Variations in soil phosphorous in the Fynbos biome, South Africa. Journal of Ecology 75, 1159–1171.
| Variations in soil phosphorous in the Fynbos biome, South Africa.Crossref | GoogleScholarGoogle Scholar |
Wuenscher JE (1970) The effect of leaf hairs of Verbascum thapsus on leaf energy exchange. New Phytologist 69, 65–73.
| The effect of leaf hairs of Verbascum thapsus on leaf energy exchange.Crossref | GoogleScholarGoogle Scholar |



