Influence of seed dimorphism and provenance on seed morphology, dispersal, germination and seedling growth of Brachyscome ciliaris (Asteraceae)
Rina Aleman A F , Manfred Jusaitis B C , Joan Gibbs A , Phillip Ainsley B D , Fleur Tiver E and Sophie Petit AA Sustainable Environments Research Group, School of Natural and Built Environments, University of South Australia, Mawson Lakes, SA 5095, Australia.
B South Australian Seed Conservation Centre, Botanic Gardens of Adelaide, North Terrace, Adelaide, SA 5000, Australia.
C Department of Ecology & Environmental Science, School of Biological Sciences, University of Adelaide, SA 5005, Australia.
D Zoos SA, Frome Road, Adelaide, SA 5000, Australia.
E PO Box 158, Norton Summit, SA 5136, Australia.
F Corresponding author. Email: rina.aleman@yahoo.com
Australian Journal of Botany 63(8) 705-713 https://doi.org/10.1071/BT15142
Submitted: 18 June 2015 Accepted: 20 October 2015 Published: 9 December 2015
Abstract
Brachyscome ciliaris is a floriferous Australian native daisy, with potential for use as a horticultural species. The species is hardy and seeds are relatively easy to germinate, but it is unique within the Brachyscome genus in that seeds are distinctly dimorphic. Within a fruiting capitulum, ray seeds are smooth and narrow with a minute pappus, whereas disc seeds have broad flat wings with curled hairs and a longer pappus than that of ray seeds. Both seed morphs, collected from five populations of the species, were tested to determine differences in their morphology, germination speed and percentage, seedling growth and wind-dispersal characteristics. Ray seeds were generally lighter and smaller than disc seeds and their length varied significantly with provenance. Dormancy levels of the two seed morphs and growth of ray- and disc-derived seedlings did not differ significantly, but differences were significant among the five populations tested. Seeds germinated readily, and germination was optimal under winter or summer conditions and lower in spring or autumn. Seed production by plants raised from ray or disc seeds was identical, but Noora-sourced plants yielded more seed than did plants sourced from the other provenances tested. Seed size, germination and plant growth of B. ciliaris varied significantly among populations. Winged disc seeds were dispersed slightly further by wind than were wingless ray seeds. We concluded that dormancy, germination and seed-yield characteristics of B. ciliaris were all influenced more by seed provenance than by seed morph (ray or disc).
Additional keywords: Brachycome, dimorphic, disc seed, disk seed, dormancy, ray seed, wind dispersal.
Introduction
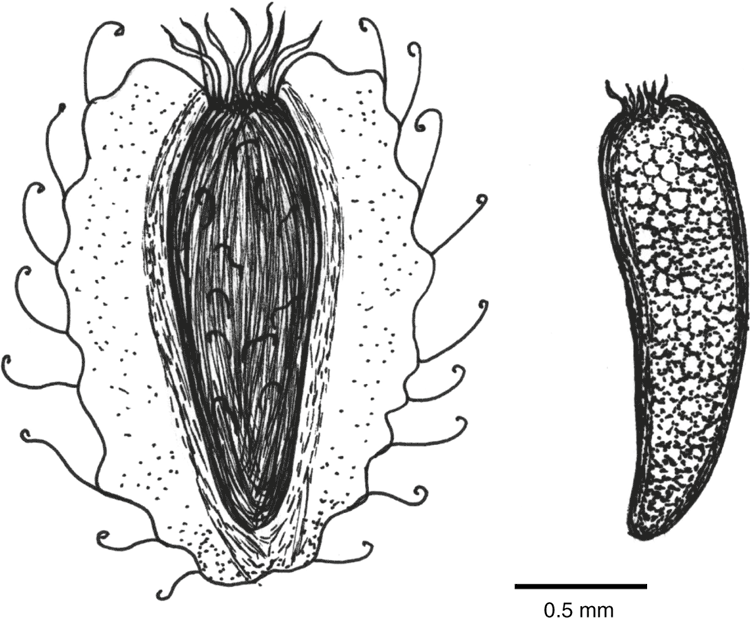
Brachyscome ciliaris (Labill.) Less. (Asteraceae) has the following five recognised varieties: var. brachyglossa Gauba, var. ciliaris, var. lanuginosa (Steetz) Benth., var. lyrifolia (J.M.Black) G.L.R.Davis and var. subintegrifolia G.L.R.Davis (State Herbarium of South Australia 2015). Brachyscome ciliaris var. ciliaris was the focus of the present study and, for the remainder of the paper, will be referred to as Brachyscome ciliaris. This variety is distinguished from the other varieties on the basis of leaf shape, type of hairs and length of ray floret (Salkin et al. 1995) and is distributed throughout Australia, except for the northern tropics. Brachyscome ciliaris grows in a diverse range of habitats (woodlands, mallee, sand hills, gibber plains and clay pans) and growth forms may vary from perennial to annual. Plants are usually branched with an erect, bushy habit, and are 20–40 cm high and 10–40 cm wide with purple or white flowers throughout the year in the wild (Salkin et al. 1995). Profuse flowering and tolerance to a wide range of environmental conditions suggest good horticultural potential for the species. However, little is known about the inherent achene (hereinafter referred to as seed) dimorphism in the species, specifically as it relates to germination requirements, dormancy and seedling growth. Ray seeds of B. ciliaris are smooth and narrow with flattened tuberculate faces and a minute pappus. Disc seeds have broad flat wings with curled hairs and a longer pappus (Fig. 1).
The Asteraceae are predisposed to seed polymorphism because of inherent structural differences between ray and disc florets (Silvertown 1984). For example, the centripetal development of the flower head provides more time for outer florets to develop, allowing them to reach a larger size (McEvoy 1984). Ray seeds often have a thicker pericarp than do disc seeds, lack dispersal structures and are dormant, whereas disc seeds (inner portion of the capitulum) are usually lighter than ray seeds, have dispersal structures and germinate readily (Venable and Levin 1985; Gibson 2001; El-Keblawy 2003). Seed polymorphism is thought to be an adaptation to environmental variation, improving survival and reproduction over a range of environments, thus avoiding unfavourable conditions by escaping in time (dormancy) or space (dispersal) (Evans and Dennehy 2005).
Species with seed dimorphism may display differences in seed germination between the two seed types (Venable and Levin 1985; Gibson 2001; van Mölken et al. 2005). Tanowitz et al. (1987) suggested that for Hemizonia increscens (Asteraceae), lower germination of ray seed than disc seed may be due to the thicker pericarp of the former. Some Asteraceae, such as Packera tomentosa, exhibit cryptic seed polymorphism, where ray and disc seeds have very similar morphologies but disc seeds have a larger embryo and germinate faster and in higher proportion than do ray seeds (Leverett and Jolls 2014). In the Chenopodiaceae, it is common for some species of Atriplex and Chenopodium to produce non-dormant brown seeds and dormant black seeds on the same plant (Harper et al. 1970). Likewise, in Anthemis chrysantha (Asteraceae), non-dormant white seeds are produced, as well as dormant dark seeds (Aguado et al. 2011). These variations in dormancy within a species are interpreted to be a form of bet-hedging against future catastrophes (Evans and Dennehy 2005). A high-risk–low-risk seed dimorphism occurs when conditions for germination are similar for the two seed morphs, but one morph is more dormant than the other. The dormant seed morphs are at lower risk because germination can be spread over time. Alternatively, a high-risk–high-risk dimorphism occurs when germination of one seed morph is controlled by one environmental factor and germination of the other is controlled by a different environmental factor, but both seed types germinate readily (Venable 1985).
In some species, seed mass was found to affect growth and vigour of plants derived from dimorphic seeds (Zhang 1993; Imbert et al. 1996; Susko and Lovett-Doust 2000). Heavy ray seeds of Hedypnois cretica and Crepis aspera (Asteraceae) produced larger seedlings than did the lighter disc seeds (El-Keblawy 2003). Seed provenance may also influence germination and growth behaviour of seeds. Imbert et al. (1996) examined three different populations of the dimorphic Crepis sancta (Asteraceae) and showed that differences in germination and seedling growth observed between morphs from one population did not necessarily extend to the others.
In the Asteraceae, wings or pappus structures that assist with wind dispersal are often found on disc rather than ray seeds. The two main characteristics that affect primary wind-dispersal distance are the height of seed release and the terminal velocity of the dispersule, which can be influenced by pappus or achene diameter (Fenner and Thompson 2004). For example, Heterotheca latifolia (Asteraceae) disc achenes (with pappus) were dispersed further by wind than were ray achenes (no pappus; Venable and Levin 1985). Secondary dispersal includes subsequent movement by animals, water, gravity or wind, and is more difficult to measure than primary dispersal. Potentially time consuming and expensive, suitable techniques include radioactive or fluorescent marking, or stable isotope analyses coupled with molecular genetic markers to trace seeds and offspring to their parent plant (Wang and Smith 2002).
The ecological advantages of a long-distance dispersal strategy include a widespread distribution of populations, establishment of new populations and less competition within a population. Conversely, seeds without a dispersal mechanism have the advantage of a greater chance of germinating in their originally suitable habitat and becoming locally abundant. Species such as B. ciliaris, that produce two morphologically different seed types, may have the advantage of both dispersal strategies, ensuring that some seeds germinate in their original habitat, whereas others may populate new areas.
We aimed to study the effects of seed dimorphism of B. ciliaris on seed characteristics and dormancy strategies, germination requirements and seedling growth, and primary seed dispersal by wind. The influence of seed provenance was also examined by comparing seed characteristics and germination of seeds collected from five populations.
Materials and methods
Seed collection
Mature seeds of Brachyscome ciliaris var. ciliaris were collected from five locations in South Australia in 2011 (location; mean annual rainfall (Bureau of Meteorology 2015); collection month): North Haven (34.786°S, 138.499°E; 444 mm; October), Monarto (35.163°S, 139.113°E; 394 mm; November), Glendambo (30.969°S, 135.750°E; 175 mm; August), Yankaninna (30.363°S, 139.059°E; 258 mm; December) and Noora Evaporation Basin (EB) (34.430°S, 140.869°E; 267 mm; November). Rainfall at Yankaninna and Glendambo peaks during the summer months (December to March), whereas the remaining locations receive heavy winter rainfall (June to August). Yankaninna and Glendambo also experience warmer summer temperatures than do the other locations. After collection, seeds were sieved through consecutive 1-mm and 0.6-mm sieves to separate ray and disc seeds. Seeds were kept under controlled storage conditions (15°C and 20% RH in darkness) until used in experiments in June 2012.
Seed characteristics and viability
For each collection location and each seed morph (ray and disc), a sample of 50 seeds was tested for viability after imbibing seeds on moist filter paper overnight, then soaking in tetrazolium stain (0.1% w/v) for 24 h. After removal of the pericarp, embryos showing an even red stain were assessed as viable. Seed mass was calculated by weighing a sample of 250 seeds of each seed type from each locality, and determining the mean individual seed mass by division. Internal seed length (excluding pericarp) of at least 25 seeds of each seed type from each locality was measured using a dissecting microscope fitted with an eyepiece graticule. Standard errors of the means were calculated, two-way ANOVA was used to analyse the effects of seed type and provenance on seed length and Fisher’s protected least significant difference was used to separate means.
Seed germination
Four replicates of 25 seeds of each morph (ray and disc) from each collection location were plated onto water agar (1% w/v). Seeds were germinated under average summer (14 h day–1 photoperiod, 30°C/15°C day/night temperature), spring or autumn (hereafter spring/autumn; 12 h day–1 photoperiod, 22°C/10°C day/night temperature) or winter (10 h day–1 photoperiod, 15°C/5°C day/night temperature with a 20/4 h thermoperiod) conditions for South Australia. Germination was recorded at weekly intervals over 8 weeks. To determine differences in germination among seasons and between seed types, a generalised linear model analysis (binomial family) with logit link function and robust error structure was performed (Schütz and Rave 1999; Turner et al. 2009). Results are reported as an odds ratio (OR), which is a relative measure of effect and represents the odds of germination occurring.
Seedling growth
Seeds that germinated under spring/autumn incubation (the season that produced healthiest-looking germinants) were transferred to Osmocote (Scotts, Bella Vista, NSW, Australia) seed-raising mix in seedling trays, and hardened in a glasshouse maintained at 15−28°C under natural light conditions and irrigated with overhead mister irrigation (5 min day–1). After 4–6 weeks, enough plants were available from the Glendambo and Noora EB seed lots to study their subsequent growth. Subsamples of 10 ray-derived and 10 disc-derived seedlings from each of these provenances were transferred to pots of seed-raising mix, topped with a layer of propagating sand and 8–10 slow-release fertiliser balls (Macracote, CRF Grey Plus, Wangara, WA, Australia). Plants were placed into the same glasshouse conditions as before, in a randomised block design with 10 blocks, each with one ray- and one disc-produced plant from each of the two provenances. Each block contained plants of a similar size to minimise variation within blocks. Blocks were rotated in the glasshouse every week to minimise the effects of small temperature and light variations. Plant growth was monitored weekly for 6 weeks by recording plant height (to the nearest 0.5 cm, measured from soil surface to the tallest leaf or flower in natural position), plant diameter (to the nearest 0.5 cm, measured horizontally across the widest section of foliage, not including dead or shriveled leaves), number of living leaves, number of buds (including open flowers) and plant health rating, assessed using a scale of 1–5, as follows: 5 = very healthy, 4 = healthy, 3 = average, 2 = unhealthy, 1 = very poor, with ranks corresponding to ≤20%, 21–40%, 41–60%, 61–80% or 81–100% of the plant showing chlorosis and/or necrosis, respectively. For each growth attribute, data at 6 weeks were subjected to two-way ANOVA, followed by mean separation using Fisher’s protected least significant difference test.
Seed production
After plants produced from both ray and disc seeds from Noora EB, Yankaninna and Glendambo had flowered and set seed, the numbers of ray and disc seeds per capitulum were counted on a sample of 10 flowers. A paired Student’s t-test was used to determine any difference in the number of ray and disc seeds produced per capitulum, tested at individual levels of provenance and plant origin (plant originating from a ray or disc seed). A two-way ANOVA was used to determine any difference in the overall number of seeds produced per seed head between plants originating from ray and disc seeds, and among the three provenances. A post hoc Fisher’s protected least significant difference test was used to separate the means.
Seed dispersal by wind
An experiment was conducted to determine dispersal distances of ray and disc seeds in response to wind. Five trials were conducted in a laboratory by using an electric fan set up to simulate low (~10 km h−1) or high (~20 km h−1) wind speed. Wind speed was measured with a hand-held portable wind meter (Dwyer Instruments, Michigan, IN, USA). In each trial, at least 10 ray and 10 disc seeds were released individually at a height of 30 cm, immediately in front of the fan. The distance of travel from point of release to final resting place was measured for each seed individually. Two-way ANOVA was used to examine the effects of wind speed and seed type on dispersal distance and Fisher’s protected least significant difference test was used post hoc for pairwise comparison of the means.
Statistical analysis
Data from the seed-germination experiment were analysed using IBM SPSS Statistics. All other statistical tests were performed using Stata 12 software.
Results
Seed characteristics and viability
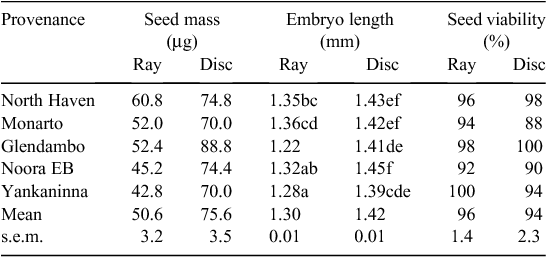
Ray seeds were lighter than disc seeds at each collection site, with an overall mean mass of 50.6 ± 3.2 µg for ray seeds, and 75.6 ± 3.5 µg for disc seeds (Table 1). Ray seeds were also slightly shorter than disc seeds, with a mean embryo length of 1.30 ± 0.01 mm for ray seeds and 1.42 ± 0.01 mm for disc seeds (Table 1). Both provenance (F4,269 = 8.97, P < 0.001) and seed morph (F1,269 = 114.66, P < 0.001) significantly influenced embryo length, with a significant interaction effect (F4,269 = 4.57, P = 0.0014). Glendambo produced the shortest ray seeds and Noora EB produced the longest disc seeds. North Haven and Monarto produced the longest ray seeds, which were significantly longer than those from Glendambo and Yankaninna. Both ray and disc seeds from all provenances showed high viability (88–100%) with tetrazolium staining (Table 1).
|
Seed germination
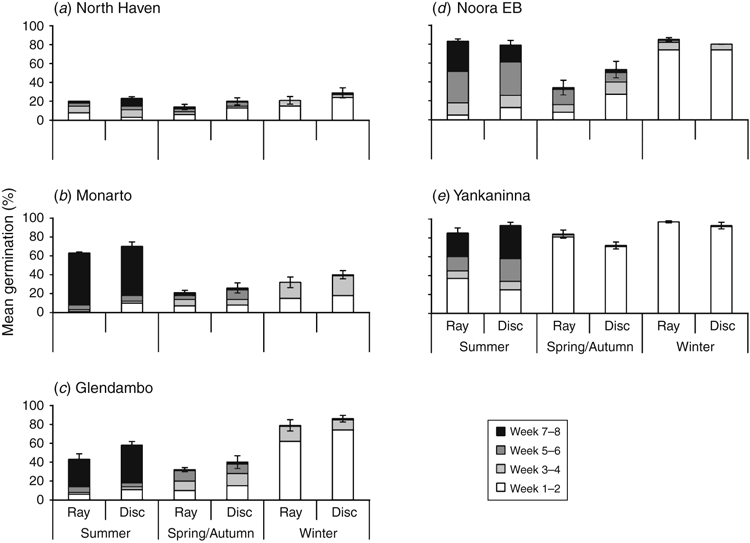
Differences in germination were observed among the three seasonal treatments and the five provenances, but ray and disc seeds were consistent in their germination responses (Fig. 2). A statistically significant difference was observed between seed morphs from Noora EB, where disc germination (53%) was significantly higher than ray germination (34%) under spring/autumn conditions (OR = 2.19). Also, Yankaninna disc seed germination (93%) was significantly higher than ray germination (85%) under summer conditions (OR = 2.34), and ray germination (84%) was higher than disc germination (72%) under spring/autumn (OR = 2.04) and also winter conditions (ray 97%, disc 93%; OR = 2.43).
|
Seeds collected from North Haven had poor germination (<30%) compared with the other provenances (Fig. 2). All other provenances showed consistent germination responses to season, with high but slow germination in summer and rapid germination under winter conditions. More Monarto seeds germinated under summer than under winter conditions, although, realistically, summer germination would be unsustainable in situ because of low summer rainfalls. Seeds collected from Yankaninna displayed high germination (>70%) under all seasonal conditions.
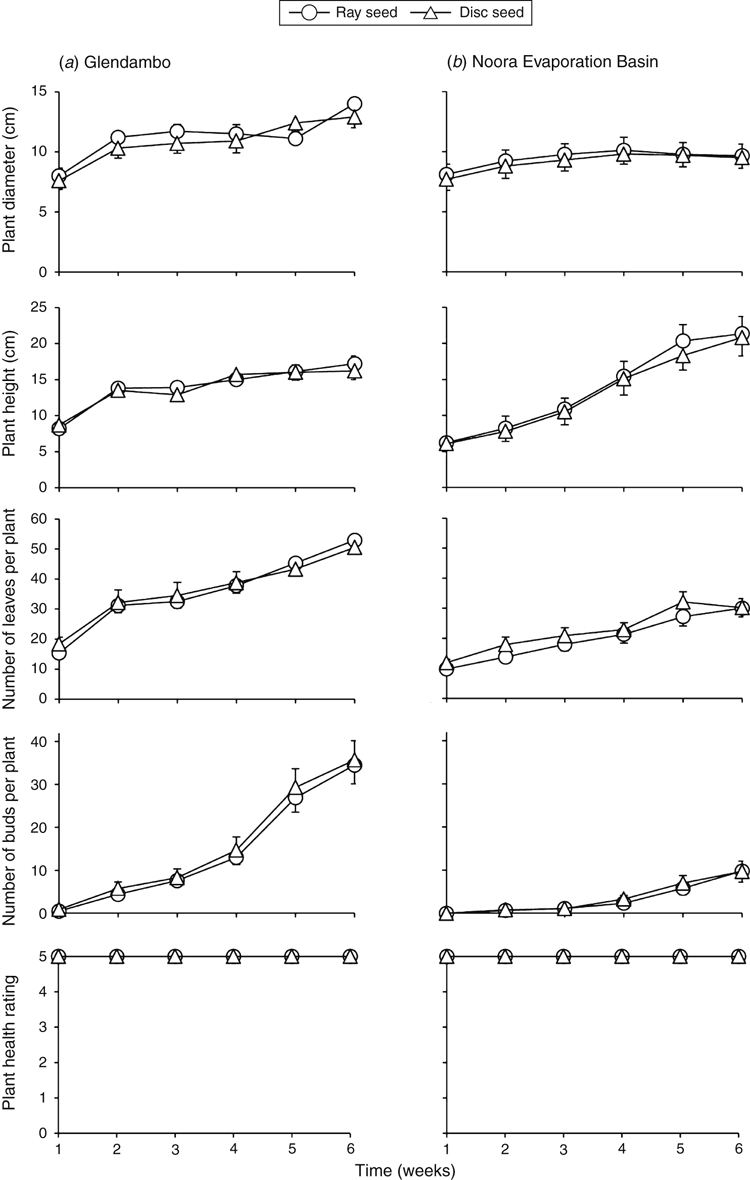
Seedling growth
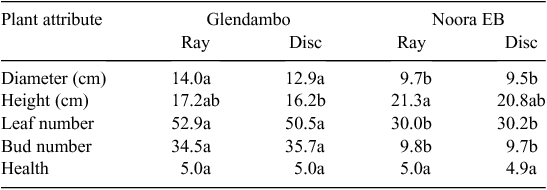
Plant size (diameter and height), leaf and bud number, and plant health did not differ significantly between ray-derived and disc-derived plants sourced from Glendambo and Noora EB (Fig. 3). However, some differences in plant growth were observed between the two provenances tested. After 6 weeks, plants originating from seed sourced from Glendambo were slightly larger in diameter and had more leaves and flower buds than did plants originating from Noora EB (Table 2).
|
Seed production
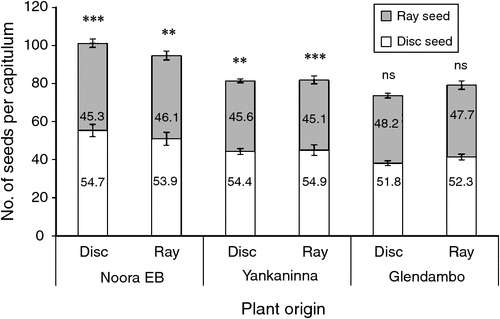
At all levels of provenance and plant derivation (from ray or disc seeds), cultivated plants generally produced more disc seeds than ray seeds, with ~52–55% of seeds on a capitulum being disc (Fig. 4). However, a paired Student’s t-test indicated that only Noora EB and Yankaninna plants produced significantly more disc seeds on both disc-derived and ray-derived plants. Results of a two-way ANOVA comparing the total number of seeds produced per capitulum indicated no difference in seed numbers between plants originating from ray and disc seeds, but provenance significantly influenced the number of seeds per seed head (F2,44 = 16.8, P < 0.001). Noora EB-derived plants produced significantly more seeds per capitulum (97.9) than did those derived from Yankaninna (81.7) or Glendambo (76.4) seeds. No significant interaction between the origin of the plant (whether from ray or disc seed) and provenance was evident.
|
Seed dispersal by wind
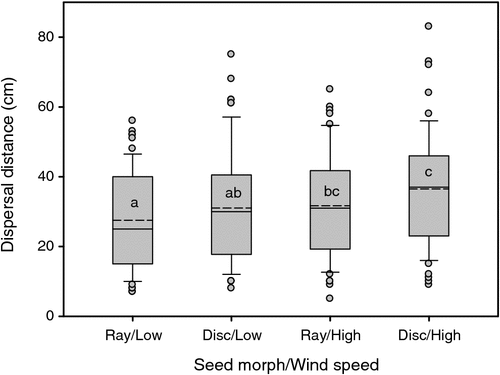
Dispersal of B. ciliaris seeds was significantly affected by both wind speed (F1,229 = 8.81, P = 0.003) and seed morph (F1,229 = 4.96, P = 0.027). Disc seeds, on average, dispersed further than did ray seeds by ~4.5 cm. Whereas differences between seed morphs within each wind speed were not significant, clear differences were evident between ray seeds subjected to low wind speed and disc seeds under high wind speed (Fig. 5). Under our test conditions, overall dispersal distance was <1 m for all seeds.
|
Discussion
Seed dimorphism in Brachyscome ciliaris distinguishes this species from most other Brachyscome species (Short 2014). In the present study, seed mass and seed length were greater for disc seeds than for ray seeds in all B. ciliaris populations examined. A larger seed mass was expected in disc seeds because of their winged structure. The larger internal (embryo) size of disc seeds than ray seeds was unexpected because centripetal development of the flower head usually provides ray achenes with a longer growing period, thereby predisposing them to a larger size (Harper et al. 1970; McEvoy 1984). However, larger seeds are produced by increasing either the growing period or the speed of growth, so it appears that disc seeds of B. ciliaris may grow at a faster rate than ray seeds to produce the observed difference in seed size.
Although we found clear differences in size and structure between ray and disc seeds, their viability and germination did not differ greatly. Differences in germination between ray and disc seeds were minor, and neither significant nor consistent enough to conclude that either ray or disc seed had better germinability in any population studied here. Viability (assessed by tetrazolium staining) was equally high (>88%) for ray and disc seeds from all collection localities. In many Asteraceae with seed dimorphism, the disc or central seeds tend to germinate more readily than do ray seeds. Examples include Heterotheca latifolia (Venable and Levin 1985), Prionopsis ciliata (Gibson 2001) and Hedypnois cretica and Crepis aspera (El-Keblawy 2003). Exceptions have been recorded, as in Bidens frondosa (Asteraceae) where peripheral seeds are less dormant and germinate faster than the central seeds (Brãndel 2004). Brachyscome ciliaris has a high-risk germination strategy, in that both seed morphs have similar germination requirements and both germinate readily regardless of provenance. Species with a high-risk germination strategy expose the population to a high risk of decline if conditions suddenly become unfavourable. For example, rapid germination with the first rain, if followed by a period of drought, could prove ruinous for the population (Evans and Dennehy 2005).
Differences in germination were more notable among seasonal treatments (summer, spring/autumn or winter) than between seed types. Brachyscome ciliaris has a distinct preference for winter germination. Germination occurred rapidly under winter conditions, when temperature and rainfall are most favourable for seedling survival. However, summer conditions also supported germination of B. ciliaris, albeit at a much slower rate. In particular, more Monarto seeds germinated under summer conditions than during other seasons, although the speed of germination was reduced considerably. However, under natural conditions in southern Australia, it is unlikely that soil would remain sufficiently moist over 8 weeks during summer to enable this level of germination to occur. Lower germination under winter and spring/autumn conditions, despite high seed viability, suggested that Monarto seeds may be dormant, and that a short period of warm after-ripening (summer conditions) may be required to release such dormancy. Because seeds used in these experiments were stored for 6–10 months before use, it is possible that some after-ripening may have occurred during this time, although we did not test it. The other partially dormant seed lot (North Haven) responded poorly to all temperature regimes, suggesting that these seeds may have a deeper dormancy requiring further amelioration. For all seed lots, lowest germination occurred under spring/autumn conditions. The ability of B. ciliaris to germinate quickly in winter is advantageous for a hardy, widespread plant adapted to growing under relatively dry conditions and on most soil types.
Seed dormancy was clearly related to provenance and the environment from which seeds were collected. Seeds collected from North Haven had very low germination (<30%) regardless of season, even though viability was high, whereas seeds from Yankaninna germinated readily over all seasons. North Haven seeds originated from a small population (<20 plants) in a fenced-off remnant conservation zone in the urban area of Adelaide, whereas all other collections were from larger populations across wide-spread natural habitats. Small populations are at greater risk of genetic erosion, which may not affect germination in some species (Ouborg and Van Treuren 1995), whereas in others (e.g. Silene regia), it has been linked to reduced germination, possibly owing to inbreeding depression or reduced visitation by pollinators (Menges 1991). Other factors that can influence germination within a species include year of collection and the vigour of the mother plants from which seeds were collected (Andersson and Milberg 1998). Additionally, environmental factors such as temperature, daylength, light conditions, drought and nitrogen concentrations may be associated with variations in germinability (Fenner 1991). In Beta vulgaris (Amaranthaceae), for example, seed dormancy varied geographically and appeared to be selected by climate. Wagmann et al. (2012) studied a large number of populations of Beta vulgaris and observed a pattern of increasing dormancy with decreasing latitude, which was likely to be due to dormancy being released by periods of drought. It is unknown which of the many environmental or genetic variables produce differences in dormancy among populations of B. ciliaris.
Plants originating from ray or disc seeds of B. ciliaris did not differ significantly in the attributes of growth measured. In species that demonstrate differences in plant growth between dimorphic seed types, it is often the larger seed that produces larger, more vigorous seedlings (Zhang 1993; Imbert et al. 1996; El-Keblawy 2003). Although ray and disc seeds of B. ciliaris did vary in size, this did not result in effects on seedling size or reproductive attributes. However, differences in plant growth were observed between the two provenances tested in the growth experiment. Glendambo-derived plants were slightly larger with more leaves and flower buds than were Noora EB plants, even though seed size was slightly smaller at Glendambo. We know that in the wild, the habit of B. ciliaris varies from dwarf to more upright forms among populations (Salkin et al. 1995). Thus, differences in plant-growth attributes between provenances in the glasshouse may possibly have resulted from different naturally occurring plant forms.
We found a greater number of disc seeds (52–55%) than ray seeds produced per capitulum. The number of seeds of each morph and total number of seeds produced were not affected by whether the parent plant was raised from ray or disc seed. Interestingly, in Suaeda corniculata (Amaranthaceae), germination timing, rather than seed morph from which the parent plant originated, was a determinant of resulting seed morph ratios, so that the proportion of brown seeds produced on a plant increased if germination was delayed (Yang et al. 2015). Whether germination timing influences any seed characteristics in B. ciliaris could not be determined, because all seeds were germinated at approximately the same time under laboratory conditions. Seed provenance did affect the total number of seeds produced per seed head in B. ciliaris; however, the proportion of ray to disc seeds was similar among all provenances. Noora EB produced significantly more seeds per seed head than did Yankaninna and Glendambo. This result was possibly due to the slight genetic differences in naturally occurring plant forms among populations.
For many Asteraceae with seed dimorphism, the disc or central seeds produce the main dispersal structures (Imbert et al. 1996; Ruiz de Clavijo and Jiménez 1998). The structure of disc seeds increases their likelihood of dispersal, enabling enlarged populations or encroachment into new sites, whereas ray seeds are more likely to germinate close to the parent plant where conditions are historically suitable for survival. Dimorphic species that produce seeds with different dispersal mechanisms are able to escape in space from unfavourable locations (Evans and Dennehy 2005). A further advantage of spatial rather than temporal (dormancy) dispersal is increased genetic variation within populations and reduced inbreeding depression (Buoro and Carlson 2014). In B. ciliaris, the chance of spatial dispersal is improved by the fact that more disc seeds than ray seeds are produced on plants. Wingless ray seeds may also be more likely to disperse and germinate in clusters, resulting in greater stress and competition for nutrients and moisture. For example, in Catananche lutea (Asteraceae), seed type was associated with vegetative biomass, not because of biological differences between seed types, but because the seed without a dispersal mechanism produced plants in dense clusters, resulting in density stress and competition (Ruiz de Clavijo and Jiménez 1998).
The present study demonstrated that ray and disc seeds of B. ciliaris differ in seed morphology, with potential effects on seed-dispersal strategy. The wind-dispersal experiment showed that a proportion of disc seeds disperses further than ray seeds with a strong wind, although no seeds were recorded at a dispersal distance greater than 1 m. The low height of flowers of this plant (~20 cm) limits the distance over which seeds will be carried, because wind-dispersal distance is strongly correlated with seed-release height (Thomson et al. 2011). Other forms of secondary dispersal were not measured here, but could well be significant. The winged structures of disc seeds may increase their secondary dispersal by wind and water, particularly on slopes. Although we have observed that both ray and disc seeds float in water, their movements and ability to adhere to the soil may differ because of their shape and size. For example, seed shape can influence burial depth (Weiher et al. 1999) and lodgment in microsites where seedlings are able to establish (Harper et al. 1970). It is also possible that different seed morphs are released at different times from the parent plant, as in Anthemis chrysantha (Asteraceae), where white non-dormant seeds are released first, and the dark dormant seeds remain on plants until dispersed by rainfall (usually in autumn; Aguado et al. 2012).
Secondary seed dispersal potentially plays an important role in the seed ecology of B. ciliaris. Many of the environs where this plant occurs are subject to heavy summer rains, which would occur shortly after primary seed dispersal, potentially resulting in short periods of localised flooding. Such flooding may provide a key secondary dispersal mechanism for this species, further substantiating a requirement for seed dimorphism. Additional research on dispersal movements of ray and disc seeds in their natural environment is required to clarify the influence of seed morphology on aspects such as seed movement, persistence in the soil and microsite preference.
Acknowledgements
We are grateful to the late John Petkov for his generous assistance with statistical analyses. Thanks go to Cathy Offord and Carol Baskin for evaluating an earlier version of the manuscript. Thanks also go to Jenny Guerin and Dan Duval for assistance with seed collections. This research was supported in part by a Lirabenda Endowment Fund Research Grant.
References
Aguado M, Martínez-Sánchez JJ, Reig-Armiñana J, García-Breijo FJ, Franco JA, Vicente MJ (2011) Morphology, anatomy and germination response of heteromorphic achenes of Anthemis chrysantha J.Gay (Asteraceae), a critically endangered species. Seed Science Research 21, 283–294.| Morphology, anatomy and germination response of heteromorphic achenes of Anthemis chrysantha J.Gay (Asteraceae), a critically endangered species.Crossref | GoogleScholarGoogle Scholar |
Aguado M, Vicente MJ, Miralles J, Franco JA, Martínez-Sánchez JJ (2012) Aerial seed bank and dispersal traits in Anthemis chrysantha (Asteraceae), a critically endangered species. Flora 207, 275–282.
| Aerial seed bank and dispersal traits in Anthemis chrysantha (Asteraceae), a critically endangered species.Crossref | GoogleScholarGoogle Scholar |
Andersson L, Milberg P (1998) Variation in seed dormancy among mother plants, populations and years of seed collection. Seed Science Research 8, 29–38.
| Variation in seed dormancy among mother plants, populations and years of seed collection.Crossref | GoogleScholarGoogle Scholar |
Brãndel M (2004) Dormancy and germination of the heteromorphic achenes of Bidens frondosa. Flora 199, 228–233.
| Dormancy and germination of the heteromorphic achenes of Bidens frondosa.Crossref | GoogleScholarGoogle Scholar |
Buoro M, Carlson SM (2014) Life-history syndromes: integrating dispersal through space and time. Ecology Letters 17, 756–767.
| Life-history syndromes: integrating dispersal through space and time.Crossref | GoogleScholarGoogle Scholar | 24690406PubMed |
Bureau of Meteorology (2015) ‘Australian Bureau of Meteorology climate data online.’ Available at http://www.bom.gov.au/climate/data/ [accessed 7 October 2015].
El-Keblawy A (2003) Effects of achene dimorphism on dormancy and progeny traits in the two ephemerals Hedypnois cretica and Crepis aspera (Asteraceae). Canadian Journal of Botany 81, 550–559.
| Effects of achene dimorphism on dormancy and progeny traits in the two ephemerals Hedypnois cretica and Crepis aspera (Asteraceae).Crossref | GoogleScholarGoogle Scholar |
Evans MEK, Dennehy JJ (2005) Germ banking: bet-hedging and variable release from egg and seed dormancy. The Quarterly Review of Biology 80, 431–451.
| Germ banking: bet-hedging and variable release from egg and seed dormancy.Crossref | GoogleScholarGoogle Scholar |
Fenner M (1991) The effects of the parent environment on seed germinability. Seed Science Research 1, 75–84.
| The effects of the parent environment on seed germinability.Crossref | GoogleScholarGoogle Scholar |
Fenner M, Thompson K (2004) ‘The ecology of seeds.’ (Cambridge University Press: Cambridge, UK)
Gibson JP (2001) Ecological and genetic comparison between ray and disc achene pools of the heteromorphic species Prionopsis ciliata (Asteraceae). International Journal of Plant Sciences 162, 137–145.
| Ecological and genetic comparison between ray and disc achene pools of the heteromorphic species Prionopsis ciliata (Asteraceae).Crossref | GoogleScholarGoogle Scholar |
Harper JL, Lovell PH, Moore KG (1970) The shapes and sizes of seeds. Annual Review of Ecology and Systematics 1, 327–356.
| The shapes and sizes of seeds.Crossref | GoogleScholarGoogle Scholar |
Imbert E, Escarre J, Lepart J (1996) Achene dimorphism and among-population variation in Crepis sancta (Asteraceae). International Journal of Plant Sciences 157, 309–315.
| Achene dimorphism and among-population variation in Crepis sancta (Asteraceae).Crossref | GoogleScholarGoogle Scholar |
Leverett LD, Jolls CL (2014) Cryptic seed heteromorphism in Packera tomentosa (Asteraceae): differences in mass and germination. Plant Species Biology 29, 169–180.
| Cryptic seed heteromorphism in Packera tomentosa (Asteraceae): differences in mass and germination.Crossref | GoogleScholarGoogle Scholar |
McEvoy PB (1984) Dormancy and dispersal in dimorphic achenes of tansy ragwort, Senecio jacobaea L. (Compositae). Oecologia 61, 160–168.
| Dormancy and dispersal in dimorphic achenes of tansy ragwort, Senecio jacobaea L. (Compositae).Crossref | GoogleScholarGoogle Scholar |
Menges ES (1991) Seed germination percentage increases with population size in a fragmented prairie species. Conservation Biology 5, 158–164.
| Seed germination percentage increases with population size in a fragmented prairie species.Crossref | GoogleScholarGoogle Scholar |
Ouborg NJ, Van Treuren R (1995) Variation in fitness-related characters among small and large populations of Salvia pratensis. Journal of Ecology 83, 369–380.
| Variation in fitness-related characters among small and large populations of Salvia pratensis.Crossref | GoogleScholarGoogle Scholar |
Ruiz de Clavijo E, Jiménez MJ (1998) The influence of achene type and plant density on growth and biomass allocation in the heterocarpic annual Catananche lutea (Asteraceae). International Journal of Plant Sciences 159, 637–647.
| The influence of achene type and plant density on growth and biomass allocation in the heterocarpic annual Catananche lutea (Asteraceae).Crossref | GoogleScholarGoogle Scholar |
Salkin E, Thomlinson G, Armstrong B, Courtney B, Schaumann M (1995) ‘Australian Brachyscomes.’ (The Australian Daisy Study Group: Melbourne)
Schütz W, Rave G (1999) The effect of cold stratification and light on the seed germination of temperate sedges (Carex) from various habitats and implications for regenerative strategies. Plant Ecology 144, 215–230.
| The effect of cold stratification and light on the seed germination of temperate sedges (Carex) from various habitats and implications for regenerative strategies.Crossref | GoogleScholarGoogle Scholar |
Short PS (2014) A taxonomic review of Brachyscome Cass. s.lat. (Asteraceae: Astereae), including descriptions of a new genus, Roebuckia, new species and new infraspecific taxa. Journal of the Adelaide Botanic Gardens 28, 1–219.
Silvertown JW (1984) Phenotypic variety in seed germination behavior: the ontogeny and evolution of somatic polymorphism in seeds. American Naturalist 124, 1–16.
| Phenotypic variety in seed germination behavior: the ontogeny and evolution of somatic polymorphism in seeds.Crossref | GoogleScholarGoogle Scholar |
Susko DJ, Lovett-Doust L (2000) Patterns of seed mass variation and their effects on seedling traits in Alliaria petiolata. American Journal of Botany 87, 56–66.
| Patterns of seed mass variation and their effects on seedling traits in Alliaria petiolata.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mngs1GqsQ%3D%3D&md5=e8ba638661f157cd92ab00f502562693CAS | 10636830PubMed |
State Herbarium of South Australia (2015) ‘Census of South Australian plants, algae and fungi.’ (Department of Environment, Water and Natural Resources: Adelaide). Available at http://www.flora.sa.gov.au/census.shtml [Verified 10 November 2015]
Tanowitz BD, Salopek PF, Mahall BE (1987) Differential germination of ray and disc achenes in Hemizonia increscens (Asteraceae). American Journal of Botany 74, 303–312.
| Differential germination of ray and disc achenes in Hemizonia increscens (Asteraceae).Crossref | GoogleScholarGoogle Scholar |
Thomson FJ, Moles AT, Auld TD, Kingsford RT (2011) Seed dispersal distance is more strongly correlated with plant height than with seed mass. Journal of Ecology 99, 1299–1307.
| Seed dispersal distance is more strongly correlated with plant height than with seed mass.Crossref | GoogleScholarGoogle Scholar |
Turner SR, Merritt DJ, Renton MS, Dixon KW (2009) Seed moisture content affects afterripening and smoke responsiveness in three sympatric Australian native species from fire-prone environments. Austral Ecology 34, 866–877.
| Seed moisture content affects afterripening and smoke responsiveness in three sympatric Australian native species from fire-prone environments.Crossref | GoogleScholarGoogle Scholar |
van Mölken T, Jorritsma-Wienk LD, van Hoek PHW, de Kroon H (2005) Only seed size matters for germination in different populations of the dimorphic Tragopogon pratensis subsp. pratensis (Asteraceae). American Journal of Botany 92, 432–437.
| Only seed size matters for germination in different populations of the dimorphic Tragopogon pratensis subsp. pratensis (Asteraceae).Crossref | GoogleScholarGoogle Scholar | 21652419PubMed |
Venable DL (1985) The evolutionary ecology of seed heteromorphism. The American Naturalist 126, 577–595.
| The evolutionary ecology of seed heteromorphism.Crossref | GoogleScholarGoogle Scholar |
Venable DL, Levin DA (1985) Ecology of achene dimorphism in Heterotheca latifolia. Journal of Ecology 73, 743–755.
| Ecology of achene dimorphism in Heterotheca latifolia.Crossref | GoogleScholarGoogle Scholar |
Wagmann K, Hautekèete N, Piquot Y, Meunier C, Schmitt SE, Van Dijk H (2012) Seed dormancy distribution: explanatory ecological factors. Annals of Botany 110, 1205–1219.
| Seed dormancy distribution: explanatory ecological factors.Crossref | GoogleScholarGoogle Scholar | 22952378PubMed |
Wang BC, Smith TB (2002) Closing the seed dispersal loop. Trends in Ecology & Evolution 17, 379–385.
| Closing the seed dispersal loop.Crossref | GoogleScholarGoogle Scholar |
Weiher E, van der Werf A, Thompson K, Roderick M, Gardiner E, Eriksson O (1999) Challenging Theophrastus: a common core list of plant traits for functional ecology. Journal of Vegetation Science 10, 609–620.
| Challenging Theophrastus: a common core list of plant traits for functional ecology.Crossref | GoogleScholarGoogle Scholar |
Yang F, Baskin JM, Baskin CC, Yang X, Cao D, Huang Z (2015) Effects of germination time on seed morph ratio in a seed-dimorphic species and possible ecological significance. Annals of Botany 115, 137–145.
| Effects of germination time on seed morph ratio in a seed-dimorphic species and possible ecological significance.Crossref | GoogleScholarGoogle Scholar | 25395107PubMed |
Zhang J (1993) Seed dimorphism in relation to germination and growth of Cakile edentula. Canadian Journal of Botany 71, 1231–1235.
| Seed dimorphism in relation to germination and growth of Cakile edentula.Crossref | GoogleScholarGoogle Scholar |