Fritillaria thumbergii—a new host of Potato leafroll virus in China
S. Tu A C , C. Qin B and C. Chen BA Laboratory of Molecular Biology, College of Life Sciences, Heilongjiang University, 74 Xue-fu Road, Harbin 150080, PRC.
B Fishery College, Huazhong Agricultural University, 1 Lion Street, Hongshan District, Wuhan 430070, PRC.
C Corresponding author. Email: shanjuntufupjv@yahoo.ca
Australasian Plant Disease Notes 1(1) 31-32 https://doi.org/10.1071/DN06014
Submitted: 7 March 2006 Accepted: 8 September 2006 Published: 24 October 2006
Abstract
The medicinal plant Fritillaria thumbergii was confirmed for the first time as a new host of Potato leafroll virus (PLRV) by reverse-transcription polymerase chain reaction (RT-PCR). This is the first report of Fritillaria thumbergii as a host of PLRV in China.
Fritillaria thumbergii (Chinese common name ‘Beimu’) is a medicinal plant belonging to the Liliaceae family. It contains diterpenoid and steroidal alkaloids (Kitajima et al. 1982a, 1982b), which have medicinal usages, acting as anti-tumour and anti-inflammatory agents (Rao and Gurfinkel 2000; Miyata et al. 2005). It is cultivated in many Asian countries, such as Korea, China and Japan, and is grown as an ornamental in many botanical gardens in European countries. In China, it has been cultivated for hundreds of years as a medicinal plant and as a cash crop. The bulb production yield of this species varies each year due to infection by a wide range of unknown pathogens. Field observations by cultivators have indicated that no harvest of F. thumbergii bulbs was made in affected areas measuring several thousands of hectares. Viruses are major pathogens that cause considerable economic losses in worldwide agricultural production including medicinally important plant bulbs. There is no published report of viruses infecting F. thumbergii. In this paper, we report for the first time, a F. thumbergii survey to determine the level of virus infection of the crop using the reverse-transcription polymerase chain reaction (RT-PCR) with specific primers for eight known viruses.
A total of 20 plant samples were randomly collected on three occasions from four areas of Ningbo region, China. This region represented several thousand hectares of cultivated F. thumbergii. One or three samples were taken from each area. Each sample consisted of 10 leaves, which were taken from one plant. In a single area each plant sampled was ~100 m from the next sampled plant.
RNA extraction was performed mainly according to the procedure described by Singh et al. (1995). Primers for the detection of several viruses were synthesised by SSBETS Co., Ltd, China. A total of 9 pairs of specific primers including ones for Potato leafroll virus (PLRV) (Keese et al. 1990) and Tomato spotted wilt virus (TSWV) (Mumford et al. 1996) were used for detection of eight different viruses, respectively. RT-PCR was carried out with a PTC-200 thermocycler (MJ Research, Inc. USA) with reaction conditions described by Singh et al. (1995). Five microlitres of amplified products were analysed by gel electrophoresis on a 1.5% agarose gel which was pre-stained with ethidium bromide and photographed under UV light at 254 nm (Gel Doc EQ, Bio-Rad).
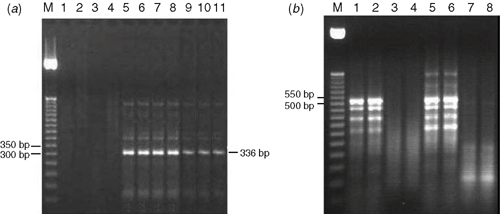
Our results show that most of the samples were negative for the eight viruses for which they were tested. Six viruses, Beet necrotic yellow vein virus, Cucumber mosaic virus, Lily symptomless virus, Potato virus Y, Tobacco mosaic virus and Turnip mosaic virus, were not detected in any samples. Only PLRV and TSWV were detected by RT-PCR. The results shown in Fig. 1 reveal that the size of the fragment amplified using the PLRV primers was 336 bp, as expected (Fig. 1a). However, the amplified fragment (~525 bp) that was obtained using the TSWV primers was different from the published expected fragment size (297 bp) (Fig. 1b). One reason for this may be that the Tospovirus genus has been reported to have several distinct members (Mumford et al. 1996). Therefore, this could be a new strain of the virus. The fact that healthy plants did not give any specific bands indicates that the 525-bp band is indeed related to TSWV. Further studies to clarify this point are now under way. No specific bands were visible in the negative control. Each of these two viruses was always detected alone suggesting that PLRV and TSWV do not co-infect the same plants. The frequency of PLRV infection in F. thumbergii in the field was 40%, whereas the frequency of TSWV infection was 10%.
|
In Europe, North America, North Africa and Australia, as well as many areas of Asia, the two viruses, PLRV and TSWV, are vectored by viruliferous aphids or Western flower thrips and cause important losses on potato, lily and peanut, respectively (Keese et al. 1990; Mumford et al. 1996; Robert et al. 2000; Sharma et al. 2005; Murakami et al. 2006). Results from the present work demonstrate that F. thumbergii is a new host of PLRV. It is highly likely that the poor harvests or serious reductions in both number and size of F. thumbergii progeny bulbs, observed in affected areas in the past, were partly due to infection with these aphid-transmitted viruses. Epidemiological studies of the viruses are being undertaken to enable monitoring in different plant species and in different geographical areas of China. In the present work the rapid and reliable detection method used has detected viruses in F. thumbergii. To our knowledge, this is the first report of F. thumbergii as a new host record of PLRV in China. Further investigation to identify insect vectors is needed for virus control strategies.
Acknowledgements
We are very grateful to Professor Mounir G. Abouhaidar (Laboratory of Virology, University of Toronto, Canada) for his help when preparing the manuscript. Financial support from the Initial Scientific Research Grants of the Heilongjiang University to Shanjun Tu is gratefully acknowledged.
Keese P,
Martin RR,
Kawchuk LM,
Waterhouse PM, Gerlach WL
(1990) Nucleotide sequence of an Australian and a Canadian isolate of potato leafroll luteovirus and their relationships with two European isolates. The Journal of General Virology 71, 719–724.
| PubMed |

Kitajima J,
Komori T,
Kawasaki T, Schulten HR
(1982a) Basic steroid saponins from aerial parts of Fritillaria thunbergii. Phytochemistry 21, 187–192.
| Crossref | GoogleScholarGoogle Scholar |

Kitajima J,
Komori T, Kawasaki T
(1982b) Studies on the constituents of the crude drug “Fritillariae Bulbus” III. On the diterpenoid constituents of fresh bulbs of Fritillaria thunbergii Miq. Chemical & Pharmaceutical Bulletin 30, 3912–3921.

Miyata Y,
Sato T, Ito A
(2005) Triptolide, a diterpenoid triepoxide, induces antitumor proliferation via activation of c-Jun NH2-terminal kinase 1 by decreasing phosphatidylinositol 3-kinase activity in human tumor cells. Biochemical and Biophysical Research Communications 336, 1081–1086.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mumford RA,
Barkerb I, Wood KR
(1996) An improved method for the detection of Tospoviruses using the polymerase chain reaction. Journal of Virological Methods 57, 109–115.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Murakami M,
Gallo-Meagher M,
Gorbet DW, Meagher RL
(2006) Utilizing immunoassays to determine systemic tomato spotted wilt virus infection for elucidating field resistance in peanut. Crop Protection 25, 235–243.
| Crossref | GoogleScholarGoogle Scholar |

Rao AV, Gurfinkel DM
(2000) The bioactivity of saponins: triterpenoid and steroidal glycosides. Drug Metabolism and Drug Interactions 17, 211–235.
| PubMed |

Robert Y,
Trefor Woodford JA, Ducray-Bourdin DG
(2000) Some epidemiological approaches to the control of aphid-borne virus diseases in seed potato crops in northern Europe. Virus Research 71, 33–47.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sharma A,
Mahinghara BK,
Singh AK,
Kulshrestha S,
Raikhy G,
Singh L,
Verma N,
Hallan V,
Ram R, Zaidi AA
(2005) Identification, detection and frequency of lily viruses in Northern India. Scientia Horticulturae 106, 213–227.
| Crossref | GoogleScholarGoogle Scholar |

Singh RP,
Kurz J,
Boiteau G, Bernard G
(1995) Detection of potato leafroll virus in single aphids by the reverse transcription polymerase chain reaction and its potential epidemiological application. Journal of Virological Methods 55, 133–143.
| Crossref | GoogleScholarGoogle Scholar | PubMed |



