New Zealand women’s experience during their first year of Jadelle® contraceptive implant
Christine Roke 1 4 , Helen Roberts 2 , Anna Whitehead 31 National Medical Advisor, Family Planning, Auckland, New Zealand
2 Associate Professor, Women’s Health, Faculty of Medical and Health Sciences, University of Auckland, New Zealand
3 Locality Medical Advisor, Family Planning, Hamilton, New Zealand
4 Correspondence to: Dr Christine Roke, National Medical Advisor, Family Planning, Private Bag 99929, Newmarket, Auckland 1149, New Zealand. Email: christine.roke@familyplanning.org.nz
Journal of Primary Health Care 8(1) 13-19 https://doi.org/10.1071/HC15040
Published: 31 March 2016
Journal Compilation © Royal New Zealand College of General Practitioners 2016.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
INTRODUCTION: Subsidisation of the levonorgestrel-releasing Jadelle® contraceptive implant in 2010 resulted in a rapid uptake. Clinicians had little prior experience of client satisfaction, side effect profile, and removal rate of this contraceptive method.
AIM: To obtain information on satisfaction, bleeding patterns, continuation rates and reasons for removal for New Zealand women during their first year of use of a subsidised contraceptive implant, Jadelle®.
METHODS: Women having a Jadelle® implant inserted in New Zealand Family Planning clinics were recruited to be followed up by phone, text or email at 1, 3, 6, 9 and 12 months. They were asked about their bleeding pattern, satisfaction and their views on benefits of, or problems with, implant use.
RESULTS: 252 women were recruited. The three common bleeding patterns in the cohort were regular periods, amenorrhoea and irregular bleeding. Eighteen percent had their implant removed within the first year with more than half of those being unhappy with their bleeding pattern. This was usually prolonged bleeding. Otherwise satisfaction rates were high throughout the year.
DISCUSSION: The majority of New Zealand women using Jadelle® were satisfied with this method of contraception during their first year of use. Implant removals were most likely to be related to prolonged bleeding. However the commonest bleeding pattern was regular periods.
KEYWORDS: Contraceptive implant; progestin; bleeding; satisfaction; continuation rate; reducing reproductive health inequalities
| WHAT GAP THIS FILLS |
| What we already know: Contraceptive implants are extremely effective long acting reversible contraceptives. Irregular and prolonged bleeding is commonly reported with this method and can lead to premature discontinuation. |
| What this study adds: New Zealand women’s experience. The three common bleeding patterns in the first year of use were regular periods, amenorrhoea and irregular bleeding. Satisfaction rates remained high throughout the first year in spite of 18% removal rate with the most common reason being prolonged bleeding. |
Introduction
Jadelle® is a two rod contraceptive implant with each rod containing 75 mg of levonorgestrel which is licenced for 5 years.1
There is an increased emphasis on the use of long-acting methods of reversible contraception (LARC) because of their high efficacy and ‘fit and forget’ nature. Compliance and continuation are the main factors determining contraceptive effectiveness, and discontinuation rates are higher with the contraceptive pill than with LARCs.2 Failure rates for pills can be 5% or higher for adolescent women compared to one in a thousand per year for Jadelle® in the first 4 years of use and 1% in the fifth year of use, an average 20-fold difference between long acting and short acting contraceptives.1,2
Prior to subsidisation of a contraceptive implant in New Zealand, there was a very low uptake of this contraception method because of the cost relative to other subsidised methods. From August 2010, Jadelle® was subsidised by the Ministry of Health and there was a rapid increase in uptake so that Family Planning clinics were inserting ~3 500 implants per year.
After insertion of a Jadelle®, women who have no problems with it may not be seen at a Family Planning clinic until they need a different service such as a cervical smear or until they request removal of the implant. So most implant-using women that clinicians see are experiencing a problem. Consequently, clinicians may get an unbalanced view of implant side effects and removal rates.
There is limited and quite variable information on women’s experience with Jadelle® internationally3 and none relating to New Zealand which has a significant indigenous population. Ethnic and age differences in continuation of implant use will influence expected reductions in unplanned pregnancy and abortions.
This study was undertaken to obtain New Zealand data on satisfaction, bleeding patterns, continuation rates and reasons for removal during the first year of use of a subsidised contraceptive implant, Jadelle®. This information could then be used by clinicians when counselling women considering an implant as a method of contraception or women who are experiencing problems in the early months of its use.
Methods
We asked Family Planning staff in clinics throughout New Zealand to invite all women having Jadelle® inserted to participate in this study until 250 women had been recruited. A study size of 250 was chosen as it was thought to be a manageable number of clients to contact within the timeframe, and would provide sufficient numbers for bleeding patterns and satisfaction levels to be determined.
Participants were contacted by one of two nurses one, three, six, nine and 12 months after insertion, usually by phone but sometimes by text or email if they preferred. Using a standardised form, the nurse asked each woman about her experience since the last contact. They were asked about their bleeding pattern, any problems or benefits, their satisfaction with the method and reason for removal if relevant. Bleeding patterns were grouped into 6 categories: amenorrhoea, infrequent/occasional spotting, regular/similar to period, two weekly bleeding, irregular bleeding and prolonged episodes/bleeding most of the time. Women were asked to rate satisfaction on a five point scale: ‘great’, ‘OK’, ‘neither good nor bad’, ‘no good’, or ‘awful’.
Women were excluded if they were under 16 years old, would find it difficult to converse in English during a telephone conversation or would not be available for follow up by telephone.
Women were enrolled between July and September 2012. At the end of the year, the Family Planning clinical records of women who were unable to be contacted throughout the whole year and women who had the implant removed at a Family Planning clinic were reviewed for any other implant-related consultations. This information was added to the study data.
Ethics Committee approval was obtained from the New Zealand Multi-region Ethics Committee (file number MEC/12/EXP/070).
Results
252 women consented to be involved in the study. By the end of the first year, 45 women (18%) had their implant removed and 54 (21%) were lost to follow up.
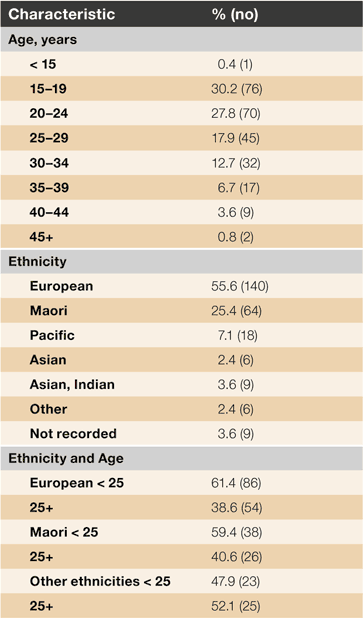
Ages ranged from 13 to 50 years old with a mean of 24.5 (Table 1). Self-identified ethnicities and age versus ethnicity are presented in Table 1. There were similar proportions of young Māori and European women (60% and 61% aged < 25 years, respectively) while a lower percentage of younger women (48%) were of other ethnicities.
|
In the first year, 14% (21/147) of women aged < 25 years had the implant removed compared to 22% (23/105) of women aged = 25 years. Twenty eight percent (41/147) of the women aged < 25 years were lost to follow up by the end of the year compared to 12% (13/105) of the women aged = 25 years. Subtracting known implant removal and loss to follow up figures from the total provides study continuation rates. There was no statistically significant difference in study continuation rates between women aged < 25 years of age and older women.
A comparison between study continuation rates for the three ethnic groupings (European, Māori and other) showed no statistically significant difference. However the implant removal rate was 23% (32/140) for Europeans, 8% (5/64) for Māori and 17% (8/48) for other ethnicities. Loss to follow up rates were 19% (26/140) for Europeans, 30% (19/64) for Māori and 19% (9/48) for other ethnicities.
Patterns of bleeding became more stable from six months (Fig. 1). The three most common bleeding patterns were regular periods, irregular bleeding and amenorrhoea. The most frequent pattern was regular period-like bleeding with 34%, 36%, and 34% of women reporting this at six, nine, and 12 months (one in three women). Irregular bleeding was reported by 27%, 29%, and 27% at six, nine, and 12 months (one in four women). Amenorrhoea was reported by 22%, 19%, and 23% at six, nine, and 12 months (one in five women). Other less common bleeding patterns are shown in Fig. 1. Medication to control bleeding was reported at each time interval through the year. It varied from 2% at one and three months of implant use to 8%, 9% and 6% at six, nine, and 12 months of use. Women increasingly reported that their bleeding pattern was problematic as the year went by, reaching 15% by 12 months.
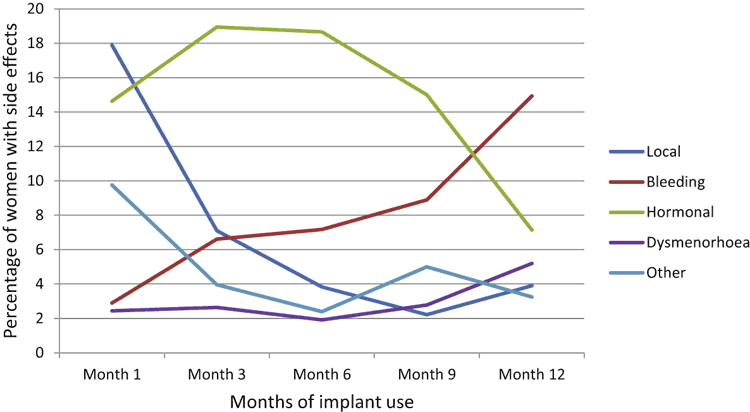
When asked whether they had experienced any problems with the Jadelle®, 59% said ‘no’ by one month, 63% by three months, 73% by six months, 66% by nine months and 65% by one year. The most commonly reported side effects initially were local soreness, bruising or tingling at the insertion site or in that arm (Fig. 2). Eighteen percent noted this at one month, but the rate dropped to 7% at three months, 4% at six months, 2% at nine months, and 4% at one year.
Hormonal side effects including mood changes, weight, headaches, or acne were consistently common for the first 9 months of use with 15% of women reporting one or more of these side effects at one month, 20% at three months, 19% at six months and 15% at nine months. At 12 months the rate had dropped to 7%. Women increasingly reported that their bleeding pattern was problematic as the year went by, reaching 15% by 12 months. Other side effects such as tiredness, nausea and dizziness were reported initially.
Forty five women (18%) had their implant removed within the first year of use (Figs. 3 and 4). The commonest reason for removal was bleeding. The first removal for this reason was 5 months after insertion. More than half of the women requesting removal because of bleeding problems had experienced prolonged episodes of bleeding. Hormonal side effects for which women requested removal included headaches, moodiness and weight gain. Some women requested removal for both bleeding and hormonal problems. Initially a few women had their implant removed because of local effects such as tingling and pain. Several women no longer required contraception; one found she had become pregnant around the time of insertion and one requested removal because she was diagnosed with breast cancer.
At least 80% of women who were contacted gave a positive response at each time point. Women commented that they liked the ‘fit and forget’ nature of the contraceptive implant so that they did not have to remember to take a pill each day. Some had even forgotten they had it and others felt that they had fewer hormonal side effects compared to their experience with previous hormonal contraceptive methods. Even women with irregular bleeding often expressed satisfaction with the method.
Discussion
Overall there was a high rate of satisfaction with Jadelle®, sometimes in spite of an experience of minor side effects.
No significant differences have been found in bleeding patterns of levonorgestrel-releasing implants Norplant® and Jadelle®.4 This follow-up study, like other studies, showed that bleeding patterns tended to stabilise with contraceptive implant use from 6 months onwards.5 The commonest reported bleeding pattern was monthly bleeds similar to a normal period. There is a common perception that irregular bleeding is the most common pattern although studies acknowledge that women may settle into a pattern of regular period-like bleeding.4,5 The one in five amenorrhoea rate is higher than in other studies that report 10% in the first year of use.5,6
Study participants who continued to experience prolonged or irregular bleeding became increasingly dissatisfied over time. As with other studies,5,7,8 bleeding problems were the most common reason for removal. Ten percent of participants stated that bleeding problems, either alone or with hormonal side effects, were the reason they sought removal of their implant. The Jadelle® data sheet cites a figure of 14% of users having their implant removed prematurely because of bleeding problems.1
Up to 10% of women used medication to control irregular bleeding. With ongoing bleeding problems, investigations such as a sexually transmitted infection (STI) check and pregnancy test should be carried out to exclude other causes.9 There appears to be a ‘therapeutic window’ for some form of intervention with implant bleeding problems, with one study showing that women reached a ‘tipping point’ at which intervention was no longer possible.10 Clinicians need to demonstrate a proactive approach to the management of bleeding problems by recommending medication as soon as a woman indicates there is a problem.11 The best approach is to prescribe the combined oral contraceptive pill.12 Women with no contraindications to oestrogen can take a pill containing ethinylestradiol 30 mg and levonorgestrel 150 mg (Ava 30®) either cyclically or continuously, for a few months or throughout the lifetime of the implant. This allows them the contraceptive benefit of an implant while controlling bleeding disturbances.
Initially many women noticed bruising and discomfort at the insertion site. Women who experienced hormonal side effects such as changes in mood, weight, headaches and skin noticed these early on and in most cases these settled as the initial higher progestin levels decreased. The UK Faculty of Sexual and Reproductive Healthcare’s guideline on progestin-only contraceptive implants notes its reliance on data from non-comparative studies and refers to the UK National Institute for Health and Clinical Excellence’s guideline on the topic. The Faculty concludes that ‘although some women do report changes in weight, mood and libido when using the progestogen-only implant, there is no evidence of a causal relationship’.13
Eighteen per cent of the cohort had their implant removed within 1 year of insertion with the most common reason being for bleeding problems. Earlier studies have shown Jadelle® continuation rates to be 88–94% at one year.14,15 A study of women using Norplant® found a 14% discontinuation at two years of use for bleeding problems.5 At five years, 55% of women were still using the Jadelle® implant, giving a cumulative 5-year life table discontinuation rates for menstrual problems of 16.4/100.7 More recent studies such as the Choice project had similar discontinuation rates to those of this study with 17% at 1 year for Implanon® users.16
Total study discontinuation rates, made up of both known removals and loss to follow up rates, showed no difference between ethnicities and age. However known removals were less likely for Māori and younger women and loss to follow up rates were more likely in these two groups.
Māori women made up a quarter of the cohort who registered for this study. During the three months of study enrolment, 15.3% of Family Planning consultations throughout the country were for Māori 64.8% were for Europeans and 19.9% for other ethnicities. The 2013 New Zealand Census found that 14.9% of the population usually living in New Zealand were of Māori ethnicity so Māori were over represented in this study. New Zealand Māori are also over-represented in low income and socio-economic groups and have higher rates of abortion.17
Cost is not likely to be a barrier to the use of an implant at Family Planning clinics because of the low cost for young women and women with a Community Services Card. Age has been shown to impact on implant continuation with younger women having higher rates of discontinuation.8,14,18 This study found that known removals were less likely for younger women than older women. However, the higher loss to follow up rate of younger women may signal more Jadelle® removals in this group. There were no reports of pregnancies while using Jadelle®, although there was one conception immediately before insertion.
A strength of this research was that the study group represented the average population of women accessing Family Planning clinics and using a contraceptive implant. There were few exclusions and there was no change in management – just follow up contacts. Because there were no exclusions relating to menstrual bleeding or previous contraception, some women entering the study were amenorrhoeic because they were breast feeding or had been using Depo Provera. Therefore their bleeding pattern in the initial months of the follow up may have been more influenced by their previous menstrual state than the contraceptive implant. A weakness was the high loss to follow up rate of 21% by the end of the first year.
Jadelle® has proven to be an effective method of long acting, reversible contraception for New Zealand women with high levels of satisfaction in the first year of use. Common bleeding patterns were regular periods, irregular bleeding and amenorrhoea. Eighteen per cent of participants were known to have their implant removed with 58% of these removals for bleeding problems such as prolonged bleeding. Therefore, 10% of the participants in this study had their implant removed within the first year because of an unsatisfactory bleeding pattern, compared to 14% cited in the data sheet for five years.1 Possible bleeding patterns and their management are an important topic for discussion before Jadelle® insertion. Medication, such as a combined oral contraceptive pill used cyclically or continuously, should be offered as soon as women seek help for bleeding disturbances. This can be used throughout the life of the implant so women can have both very effective contraception and a satisfactory bleeding pattern.
Acknowledgement
Rebecca Mackenzie-Proctor for collation of data.
Margaret Sparrow Research grant funded by Family Planning.
None declared.
References
[1] http://www.medsafe.govt.nz/profs/datasheet/j/Jadelleimplant.pdf. Accessed 12.12.15.[2] Winner B, Peipert JF, Zhao Q, et al. Effectiveness of Long-Acting Reversible Contraception. N Engl J Med 2012; 366 1998–2007.
| Effectiveness of Long-Acting Reversible Contraception.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XotVynsL4%3D&md5=7f8bbc623501f595987f16dd446ad1aeCAS | 22621627PubMed |
[3] Meirik O, Fraser IS. d’Argangues C for the WHO Consultation on Implantable contraceptives for women. Implantable contraceptives for women. Hum Reprod Update 2003; 9 49–59.
| d’Argangues C for the WHO Consultation on Implantable contraceptives for women. Implantable contraceptives for women.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjtVelt7c%3D&md5=0738db131329f39073c95dfc5790361fCAS | 12638781PubMed |
[4] Hickey M, d’Arcangues C. Vaginal bleeding disturbances and implantable contraceptives. Contraception 2002; 65 75–84.
| Vaginal bleeding disturbances and implantable contraceptives.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhtlGgsbo%3D&md5=ecec6d03a91101f9bed3d7db8aea0a00CAS | 11861057PubMed |
[5] Zheng SR, Zheng HM, Qian SZ, et al. A randomized multicenter study comparing the efficacy and bleeding pattern of a single-rod (Implanon) and a six-capsule (Norplant) hormonal contraceptive implant. Contraception 1999; 60 1–8.
| A randomized multicenter study comparing the efficacy and bleeding pattern of a single-rod (Implanon) and a six-capsule (Norplant) hormonal contraceptive implant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXntVCgsLk%3D&md5=4c08a85fe26b972f8c7e6c5ad30aa79eCAS | 10549446PubMed |
[6] Fraser IS, Titinen A, Affandi B, et al. Norplant® Consensus and background review. Contraception 1998; 57 1–9.
| Norplant® Consensus and background review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c3hsVylsQ%3D%3D&md5=f0403e10678d25bb7ebdf2f772005935CAS | 9554244PubMed |
[7] Sivin I, Campodonico I, Kiriwat O, et al. The performance of levonorgestrel rod and Norplant contraceptive implants: a 5-year randomized study. Hum Reprod 1998; 13 3371–8.
| The performance of levonorgestrel rod and Norplant contraceptive implants: a 5-year randomized study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXltFOltg%3D%3D&md5=658931bcc95b5394d823809c758b6b43CAS | 9886517PubMed |
[8] Sivin I, Mishell DR, Darney P, et al. Levonorgestrel capsule implant in the United States: a 5-year study. Obstet Gynecol 1998; 92 337–44.
| Levonorgestrel capsule implant in the United States: a 5-year study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlvVylsbg%3D&md5=c31278e9c2ea24f1bd80c7dc3e6cc43cCAS | 9721766PubMed |
[9] http://www.fsrh.org/pdfs/CEUGuidanceProblematicBleedingHormonalContraception.pdf 2012. Accessed 13.12.15.
[10] Hoggart L, Newton VL, Dickson J. ‘I think it depends on the body, with mine it didn’t work’: explaining young women’s decisions to request subdermal contraceptive implant removal. Contraception 2013; 88 636–40.
| ‘I think it depends on the body, with mine it didn’t work’: explaining young women’s decisions to request subdermal contraceptive implant removal.Crossref | GoogleScholarGoogle Scholar | 23829976PubMed |
[11] Dickson J, Hoggart L, Newton VL. Unanticipated bleeding with the etonogestrel implant: advice and therapeutic interventions. J Fam Plann Reprod Health Care 2014; 40 158–60.
| Unanticipated bleeding with the etonogestrel implant: advice and therapeutic interventions.Crossref | GoogleScholarGoogle Scholar | 24939479PubMed |
[12] Mansour D, Bahamondes L, Critchley H, et al. The management of unacceptable bleeding patterns in etonogestrel-releasing contraceptive implant users. Contraception 2011; 83 202–10.
| The management of unacceptable bleeding patterns in etonogestrel-releasing contraceptive implant users.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhvVyrsbk%3D&md5=1033d1838676fb6ee439543a07351e7aCAS | 21310280PubMed |
[13] Progestogen-only implants 2014. http://www.fsrh.org/pdfs/CEUGuidanceProgestogenOnlyImplants.pdf. Accessed 13.12.15.
[14] O’Neil-Callahan M, Peipert JF, Zhao Q, et al. Twenty-Four–Month Continuation of Reversible Contraception. Obstet Gynecol 2013; 122 1083–91.
| Twenty-Four–Month Continuation of Reversible Contraception.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1Oku7bI&md5=edd418cb7064121bbd35f9d3bd5f48c4CAS | 24104781PubMed |
[15] Sivin I. Risks and benefits, advantages and disadvantages of levonorgestrel releasing contraceptive implants. Drug Saf 2003; 26 303–35.
| Risks and benefits, advantages and disadvantages of levonorgestrel releasing contraceptive implants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjsFyiurs%3D&md5=de8dae73b6291698071c7bfa4c3c4c7dCAS | 12650633PubMed |
[16] http://www.choiceproject.wustl.edu/ accessed 1 March 2015.
[17] http://www.justice.govt.nz/tribunals/abortion-supervisory-committee/annual-reports/asc-annual-report-2014. Accessed 13.12.15.
[18] Vekemans M, Delvigan A, Paesmans M. Continuation rates with levonorgestrel-releasing contraceptive implant (Norplant). A retrospective study in Belgium. Contraception 1997; 56 291–9.
| Continuation rates with levonorgestrel-releasing contraceptive implant (Norplant). A retrospective study in Belgium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXkvFOrtw%3D%3D&md5=04bfae7422be6340ba5b486cced1d9d8CAS | 9437557PubMed |