Topical corticosteroid addiction and withdrawal in a 6 year old
Belinda ShearyCorrespondence to: Belinda Sheary, 70/73-115 Belmore Road, Randwick, NSW 2031, Australia. Email: belinda.sheary@ipn.com.au
Journal of Primary Health Care 9(1) 90-93 https://doi.org/10.1071/HC16049
Published: 10 February 2017
Journal Compilation © Royal New Zealand College of General Practitioners 2017.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Topical corticosteroid (TCS) addiction and withdrawal is a distinct adverse effect of TCS overuse.1 This case describes the history of a six-year-old girl with intractable eczema who was diagnosed with TCS addiction after inadvertent TCS withdrawal. She had used a potent TCS daily for over two years. On ceasing TCS she developed widespread erythema, ankle oedema, skin exfoliation, pain and severe itch. The patient appeared to complete the withdrawal process in 14 months and she was left with mild residual eczema symptoms. TCS addiction and withdrawal can occur in children, and may be considered in patients with eczema who fail to improve despite appropriate medical therapy, especially if there has been prolonged and frequent use of moderate to high potency TCS.
Case report
The patient first developed typical eczema to her limbs at 10 months of age, and this was treated with topical methylprednisolone 0.1% ointment. Table 1 summarises her subsequent eczema management until TCS were ceased. Her skin became progressively worse from age 4 with the development of left upper lid symptoms. Use of a moisturiser became a daily ritual and soap was avoided. At age 5 two dermatologists and a paediatric immunologist were consulted and subsequently topical tacrolimus and oral montelukast were trialled and dust measures were implemented (see Table 1). The patient failed to improve and at age 6 TCSs were no longer managing her symptoms.
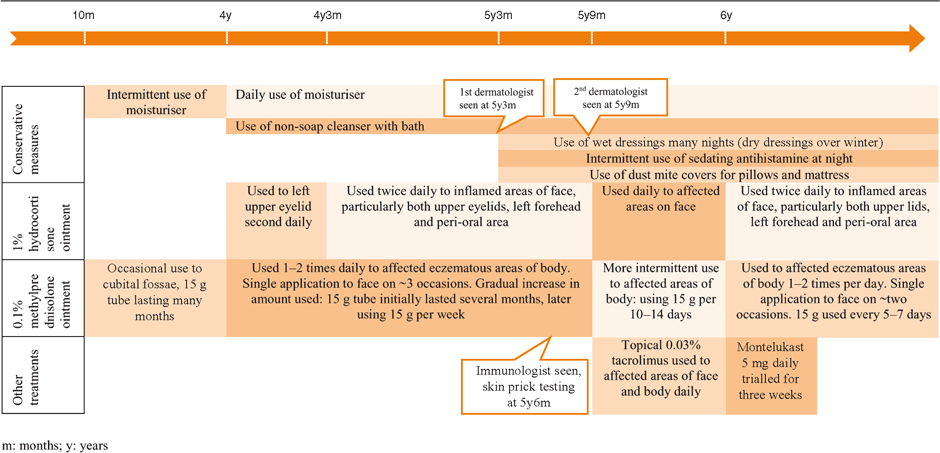
|
At this point, 0.1% methylprednisone was ceased, while 1% hydrocortisone continued to be applied to the face once daily. Within weeks the patient developed widespread red skin, excessive exfoliation, temperature dysregulation (reported feeling cold on warm summer nights), worsening lymphadenopathy and a ‘blue tinge’ (severe vasodilatation) to her popliteal fossae (similar to Figure 1 taken at a later date). The popliteal fossa skin changes resolved with the reintroduction of topical 0.1% methylprednisone to the area twice daily for three days. An ultrasound of the axilla and groin demonstrated numerous reactive nodes, but a 10 day course of cephalexin made no difference to either the patient’s skin or the lymphadenopathy.

|
TCS withdrawal was diagnosed on the basis of history and examination. The patient had used TCS to her face daily for over two years, her eczema had progressively deteriorated and her use of a potent TCS had escalated over time. When she underwent inadvertent partial TCS withdrawal by ceasing 0.1% methylprednisone she developed some of the signs and symptoms described in TCS withdrawal, and upon absolute TCS cessation further features arose including oedema to her ankles, the ‘headlight sign’2 (Figure 2) and ‘elephant wrinkles’3 (Figure 3). To manage her symptoms, various distraction techniques were employed with varying success. 250 g of emollient was applied daily during the first two weeks; after this it was ceased at the patient’s request and not used again. On completion of the process at around 14 months she was left with mild eczema to her cubital fossae. On review at 24 months her eczema symptoms remained mild and she was noted to have telangiectasia to her cheeks.
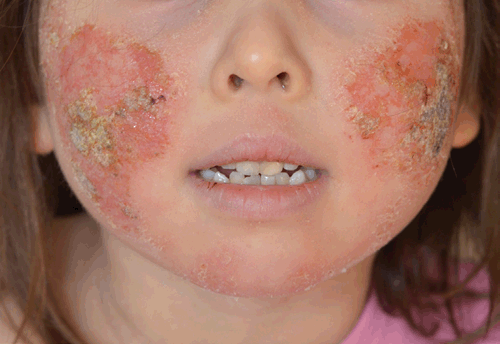
|
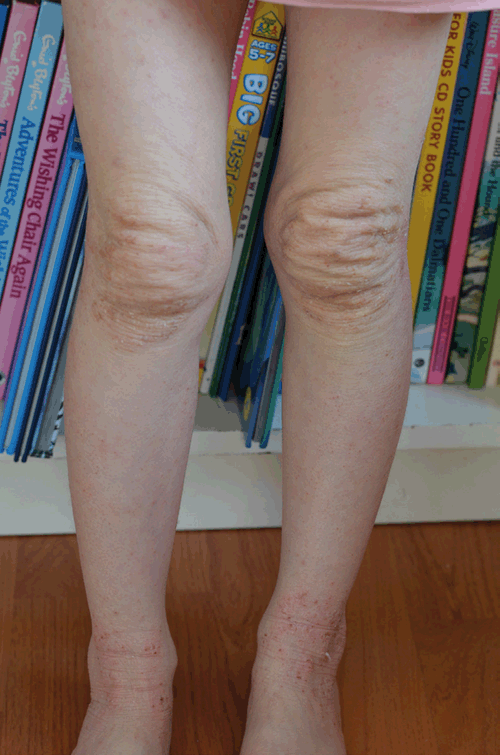
|
Differential diagnoses were considered. An eczematous flare was unlikely – TCS had failed to clear her skin for several years and there had been neither a change to her diet or environment around the time of ceasing TCS. Her skin did not improve with oral antibiotics, reducing the likelihood of an infectious exacerbation. Patch testing to exclude allergic contact dermatitis was not possible due to widespread red skin (see Figure 4) and once this (and other symptoms) abated and then later resolved, testing was no longer deemed necessary.
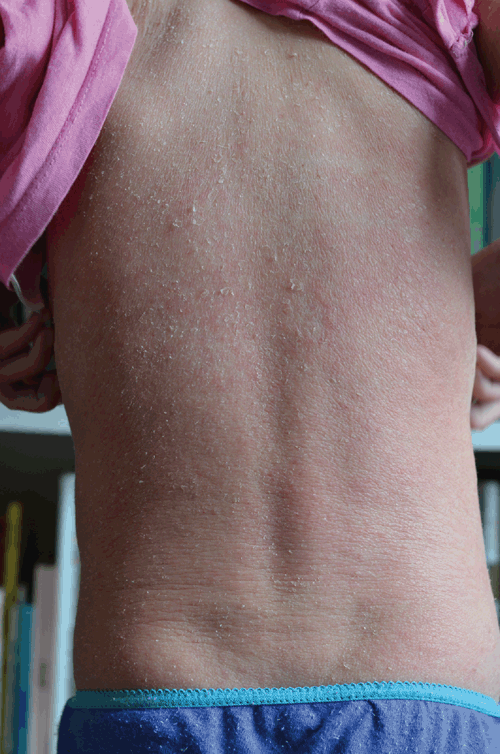
|
Discussion
This case may be considered atypical as TCS use to the face was almost exclusively of a low potency and the patient is a child. While TCS withdrawal is most often seen where there has been long-term inappropriate use of moderate to potent TCS to the face or genital area,1 low potency TCSs have been reported to cause TCS addiction and withdrawal in 5.9% of erythematoedematous cases.1 It is suspected that the long-term daily use of a potent TCS to large areas of the body would have also contributed to this patient’s TCS addiction. There are very few paediatric case reports of TCS addiction and withdrawal, and it is unclear whether this is due to under-reporting in this population or if children are less likely to develop this complication.1 It would seem counter-intuitive that they would be at lower risk as children have a proportionately greater body surface area to weight ratio than adults, and hence a higher degree of TCS absorption for the same amount applied.4 However, due to limited recognition of this condition in the medical community,5 it is possible that TCS addiction and withdrawal is under-diagnosed in the paediatric population.
Were there signs of TCS overuse in this patient?
The patient’s development of peri-ocular dermatitis may have been the first sign of skin damage from TCS. A recognised side effect of TCS use, it has been compared to peri-oral dermatitis.6,7 The patient’s first dermatologist commented on several lower limb bruises. Easy bruising is an adverse effect of TCS attributed to skin atrophy or thinning.8 The third side effect, hypopigmentation,8 was not recognised until ~10 months post cessation of TCS when a pigmented macule, first noted to the patient’s forehead at the age of six months, reappeared.
What is safe use of TCS?
The authors of a recent Australasian consensus statement on the safety of topical corticosteroids in children recommend using TCS once or twice a day, as per the product information, to all the inflamed skin until the eczema clears.9 The US National Eczema Association, in their education announcement10 on topical corticosteroids for eczema and TCS withdrawal, have advised: ‘Do not use TCS continuously for more than two to four weeks – then frequency should be tapered to twice weekly use. Your provider should strive to help create a safe and effective long term treatment plan that does not include daily use of TCS, especially on more sensitive areas. Close follow up and careful monitoring with good communication will help ensure this.’
References
[1] Hajar T, Leshem YA, Hanifin JM, et al. A systematic review of topical corticosteroid withdrawal (“steroid addiction”) in patients with atopic dermatitis and other dermatoses. J Am Acad Dermatol 2015; 72 541–9.| A systematic review of topical corticosteroid withdrawal (“steroid addiction”) in patients with atopic dermatitis and other dermatoses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXosFKiug%3D%3D&md5=1ad27d93fbf446c00acbb5f215a38423CAS |
[2] Rapaport M, Rapaport V. The red skin syndromes: corticosteroid addiction and withdrawal. Expert Rev Dermatol 2006; 1 547–61.
| The red skin syndromes: corticosteroid addiction and withdrawal.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFGhtb3P&md5=81c1bd4de97baf65e5ad7afde1c6c9acCAS |
[3] Sheary B. Topical corticosteroid addiction and withdrawal – An overview for GPs. Aust Fam Physician 2016; 45 386–8.
[4] Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis. J Am Acad Dermatol 2014; 71 116–32.
| Guidelines of care for the management of atopic dermatitis.Crossref | GoogleScholarGoogle Scholar |
[5] Fukaya M, Sato K, Sato M, et al Topical steroid addiction in atopic dermatitis. Drug Healthc Patient Saf 2014; 6 131–8.
| Topical steroid addiction in atopic dermatitis.Crossref | GoogleScholarGoogle Scholar |
[6] Fiona LM, Tucker W. Peri-orbital dermatitis – a variant of peri-oral dermatitis. J Am Acad Dermatol 2004; 50 P37
| Peri-orbital dermatitis – a variant of peri-oral dermatitis.Crossref | GoogleScholarGoogle Scholar |
[7] Fisher AA. Periocular dermatitis akin to perioral variety. J Am Acad Dermatol 1986; 15 642–4.
| Periocular dermatitis akin to perioral variety.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s%2FjsVCgsQ%3D%3D&md5=bb83554fff9df4ab6a03fd2b945cc00dCAS |
[8] Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical corticosteroids. J Am Acad Dermatol 2006; 54 1–15.
| Adverse effects of topical corticosteroids.Crossref | GoogleScholarGoogle Scholar |
[9] Mooney E, Rademaker M, Dailey R, et al Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol 2015; 56 241–51.
| Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement.Crossref | GoogleScholarGoogle Scholar |
[10] National Eczema Association. Education announcement: Use of topical corticosteroids for eczema. San Rafael, CA: NEA, 2015. [cited 2016 October 18] Available from: https://nationaleczema.org/educationannouncement-topical-corticosteroids-eczema


