Educational pamphlets for improving uptake of cancer screening: a systematic review
Boon See Teo 1 2 3 4 5 , Esther Li 1 , Clara Tan 2 , Yasmin Lynda Munro 41 Camry Medical Centre, Block 95 Toa Payoh Lorong 4, #01-66, Singapore
2 Yong Loo Lin School of Medicine, National University of Singapore, Singapore
3 Duke-NUS Medical School, Singapore
4 Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore
5 Corresponding author. Email: drteobs@gmail.com
Journal of Primary Health Care 11(3) 207-216 https://doi.org/10.1071/HC18093
Published: 30 September 2019
Journal Compilation © Royal New Zealand College of General Practitioners 2019 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
INTRODUCTION: The effectiveness of cancer screening programmes is highly dependent on screening uptake. Many interventions have been tested to increase screening uptake.
AIM: The goal of this study was to evaluate the effectiveness of cancer screening pamphlets as a standalone intervention. The outcome of interest was uptake of cancer screening tests.
METHODS: A systematic review was performed on the effectiveness of pamphlets compared to usual care without pamphlets. We searched five databases for research papers in English from 2000 up to May 2019. Randomised controlled trials were included. This research group independently selected studies, extracted data, assessed risk of bias and then compared the information as a group.
RESULTS: A total of nine trials involving 4912 participants met our inclusion criteria, of which five were about colorectal cancer screening, three were about prostate cancer screening and one was about lung cancer screening. Five of the nine trials showed that pamphlets alone increased uptake significantly, while the remaining four trials did not show significant effects.
DISCUSSION: There is some evidence that pamphlets increase uptake for cancer screenings, especially for colorectal cancer. Due to the small number of studies in this area, generalisability could be limited.
KEYWORDS: screening; health care education; health literacy; non-communicable diseases; randomized trials
Introduction
Cancer screenings are tests conducted on asymptomatic individuals to determine whether cancer might be present. They are targeted screenings, usually given to individuals of the age or sex at risk for the cancer.1 The aim of screening is early detection and treatment to reduce cancer mortality and other serious consequences.2
Certain forms of cancer screening have been shown to reduce cancer mortality considerably. Since the 1960s, most developed countries have implemented population-based screening programmes, and reductions in mortality of 25–31% for breast cancer, 16% for colorectal cancer and 50–80% for cervical cancer have been attributed to screening.3 These three cancers are among the seven most common cancers worldwide, together accounting for 25.3% of all cancer cases.4
A screening programme’s effectiveness is highly dependent on its uptake.5 There is no universal approach to improving uptake, but many interventions have been tested.6 Some are provider-oriented, such as physician reminders,7 while others are patient-oriented, such as telephone counselling,8 one-on-one education, group education, patient reminder letters, financial incentives and small media9 (flyers, posters and brochures).
Pamphlets have been used to promote uptake of cancer screening for more than two decades.10 The objective of this review was to evaluate the effectiveness of pamphlets as a standalone intervention for increasing cancer screening uptake among asymptomatic patients, when compared to usual care without pamphlets in randomised controlled trials.
Methods
Eligibility criteria
Our search was for studies published in English in or after the year 2000. We included randomised controlled trials (RCTs) in which an intervention using pamphlets only was compared to usual care without pamphlets. Outcomes measured had to include cancer screening uptake.
We excluded studies in which the intervention did not consist of pamphlets alone as we wanted to see the effect of pamphlets as a standalone intervention. We also excluded studies where the control group did not receive pamphlets, or in which the ‘usual care’ that the control group received was not defined. This was to exclude the possibility of the control group receiving some other form of pamphlet that was unreported.
Databases and search strategy
Electronic searches
We devised a core search strategy in Ovid MEDLINE and adapted it for other databases using the appropriate syntax (see Supplementary Material file S1). We limited our searches to RCTs only using pre-defined RCT filters, with the publication date from January 2000 to the current day.
On 10 May 2019, we searched the following five electronic databases: Ovid MEDLINE® and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Daily and Versions® 1946 to 10 May 2019; Embase 1974 to 10 May 2019 (Ovid); The Cochrane Library (Wiley); PsycInfo (EBSCOHost); CINAHL (EBSCOHost).
Searching other resources
A search for grey literature was carried out in May 2019 with the following resources, focusing on clinical trial registries: ClinicalTrials.gov (https://clinicaltrials.gov/); UK Clinical Trials Gateway (https://bepartofresearch.nihr.ac.uk/); UK Clinical Trials Gateway (https://bepartofresearch.nihr.ac.uk/); EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/); International Clinical Trials Registry Platform (ICTRP) (https://www.who.int/ictrp/en/); and Australian Clinical Trials (https://www.australianclinicaltrials.gov.au/).
Keywords such as ‘booklet’, ‘booklets’, ‘brochure’, ‘brochures’, ‘pamphlet’, ‘pamphlets’, ‘leaflet’, ‘leaflets’, ‘handout’, ‘handouts’, ‘information sheet’ and ‘information sheets’ were combined using the ‘OR’ boolean operator and used for searching the registries. Where possible, they are combined using the ‘AND’ boolean operator with the keywords ‘cancer’ and ‘carcinoma’, to retrieve relevant search results.
For more details on the grey literature search, please refer to Supplementary Material table S2.
Study selection and data collection process
The search results for the electronic searches were combined in Covidence (www.covidence.org), the standard production platform for Cochrane reviews, headquartered in Australia. Each title and abstract were independently screened by at least two reviewers. If there were disagreements after the two reviewers had screened, a third reviewer would have the deciding vote.
In the next phase, full-text versions of the shortlisted papers were retrieved and each was independently assessed against eligibility criteria by two out of the three reviewers. Disagreements were resolved by consensus among all three reviewers.
Grey literature titles were screened by all three reviewers. All were excluded.
All relevant data were extracted using a structured form. Duplicates of the form were made, and for each study, at least two reviewers independently extracted the data using the form. The reviewers then compared the data extracted and differences were resolved through discussion with the third reviewer.
Data items
Information was extracted from each study on:
-
Country where the study was conducted
-
Site(s) of study
-
Sample size
-
Type of participants
-
Type of screening
-
Different study arms
-
Details of study intervention (or not) received by each arm
-
Uptake-related outcome that was measured
-
Magnitude of outcome for each arm (% uptake)
-
Effect size for pamphlet versus no pamphlet
Risk of bias in individual studies
To assess risk of bias in this review, we adapted the Scottish Intercollegiate Guidelines Network 50 (SIGN 50) checklist for controlled trials.11 The papers were scored on validity and sample size. Some modifications were made to the SIGN 50 criteria as pamphlets were educational and not therapeutic interventions.
A structured form was created using the modified SIGN 50 criteria. Duplicates of the form were made and for each study two reviewers independently assessed risk of bias using the form. The authors then compared their scores and differences were resolved through discussion with the third reviewer.
Summary measures
We report the effect size for pamphlets as difference in screening rate, which we calculated as the percentage outcomes in the pamphlets arm minus the percentage outcomes in the control arm. If P < 0.05 for the outcomes, we characterised a 0–10% difference as small, >10–20% as moderate and >20% as large.
Results
Included studies
Our searches yielded a total of 2621 citations, of which nine were included in the review. The PRISMA flowchart in Figure 1 shows the selection of articles. The characteristics of the included studies are summarised in Table 1.
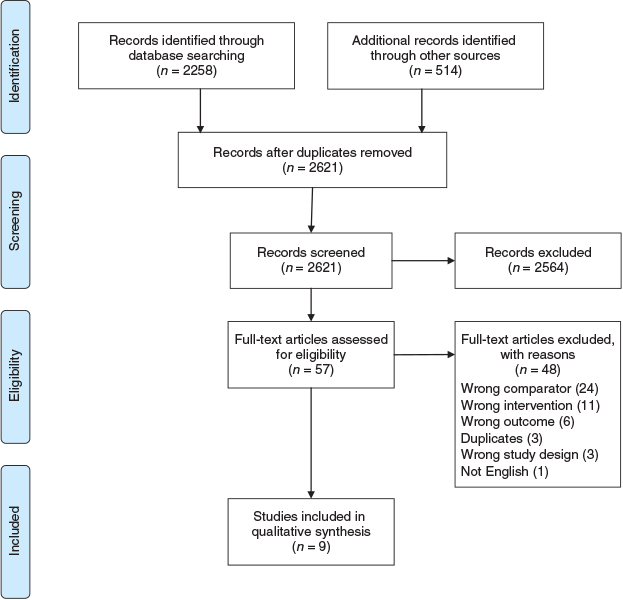
|

|
Although many RCTs were found about interventions to increase screening uptake, only nine examined whether pamphlets actually increase uptake compared to usual care without pamphlets. Many of the excluded RCTs either studied the effectiveness of pamphlets relative to other interventions, or combined pamphlets with another intervention, or measured an outcome other than uptake, such as patient knowledge or inclination to take up the test.
Types of studies
All included studies were published in peer-reviewed journals and had an RCT design.
Five of the included studies were conducted in the USA, two were conducted in Australia, one in Greece and one in Japan. Five of the studies were for colorectal cancer screening, three were for prostate cancer screening and one was for lung cancer screening. No cervical cancer or breast cancer screening studies met our search criteria.
Three of the studies were conducted in primary care settings, two were conducted in internal medicine clinics and one was conducted across both a family medicine clinic and an internal medicine clinic. Two were conducted only in hospitals or institutions, and one was city-wide.
Effects of interventions
Five studies reported that pamphlets significantly increased screening uptake (P < 0.05). Of these, two studies (Stamatiou et al. 200819; Le 201417) showed a large increase in uptake (>20%) and three studies (Denberg et al. 200613; Harris et al. 200014; Lee et al. 200918) showed a moderate increase (10–20%). The remaining four studies reported that the pamphlets did not have a significant effect on uptake.
In the studies by Le (2014)17 and Harris et al. (2000)14, pamphlets were distributed before physician encounters, while in the studies by Denberg et al. (2006)13 and Lee et al. (2009)18, pamphlets were distributed a period of time after physician encounters. Stamatiou et al. (2008)19 did not specify when the pamphlet was distributed. The effectiveness of the pamphlets was not limited to distribution at a single timepoint.
Colorectal cancer screening
Five studies were about colorectal cancer screening. Of these, four (Denberg et al. 200613; Harris et al. 200014; Le 201417; Lee et al. 200918) showed a moderate (10–20%) or large (>20%) increase in uptake with the pamphlets and their risk-of-bias scores ranged from four to eight out of eight. The remaining study, which did not show a significant difference, was Stephens and Moore (2008).20 Compared to the other studies, it had a smaller sample size of 91 and a lower risk-of-bias score of three out of eight.
All four studies showing a significant effect involved patients’ encounters with their primary care physician. In Denberg et al. (2006),13 the leaflets were personalised with the patient’s primary care physician’s name. Denberg et al.13 reported a relative risk of 1.20 for brochure uptake versus usual care uptake (95% CI = 1.09–1.33). The study by Harris et al. (2000)14 involved 26 general practices and the pamphlet was given to relatives on enrolment, when they attended the practice. Harris et al. (2000)14 showed a significant increase in screening tests. It found that the odds ratio between intervention and control groups was 4.7 in favour of the pamphlet (P = 0.01, 95% CI = 1.4–18.7).
Like Harris et al. (2000),14 the study by Stephens and Moore (2008)20 was conducted in Australia and targeted first-degree relatives of colorectal cancer patients. The study by Stephens and Moore (2008)20 was conducted in a hospital surgical setting and did not involve encounters with primary care physicians.
Prostate cancer screening
Three studies addressed prostate cancer screening. Of these, two showed no significant difference with the pamphlets. In both studies, the pamphlets were decision aids that covered, among other things, the risks of screening (Landrey et al. 201316) and current uncertainties (Krist et al. 2007).15
The remaining study (Stamatiou et al. 2008)19 found a large increase in prostate-specific antigen (PSA) test uptake in the informed group (38.6% control vs. 80% intervention). However, we observed large differences in baseline characteristics of the two study groups. Stamatiou et al. (2008)19 found that PSA testing was well accepted in the informed group, while digital rectal examination (DRE) was not. This was despite the pamphlet encouraging the patient to agree to a DRE together with PSA tests. Reasons are unclear; the authors’ view is that this result is probably due to a prejudice of the male population or that when given information about its low sensitivity and specificity, patients considered DRE ‘worthless’.
Lung cancer screening
One study focused on lung cancer screening. It showed an increase in the pamphlets arm (18% control vs. 29% intervention), but this effect was of unknown significance.
Risk of bias across studies
Our risk-of-bias assessment of the studies is shown in Table 2. One study (Lee et al. 2009)18 scored the full eight marks on our risk-of-bias assessment (48.4% control vs. 64.6% intervention). This study reported a 15.2% increase in uptake. Four studies scored five to seven marks and the remaining four studies scored four or less.

|
Discussion
The aim of this review was to evaluate the effectiveness of cancer screening pamphlets as a standalone intervention to increase screening uptake, in comparison with usual care. There is some evidence that pamphlets increase uptake for colorectal cancer screening when used in primary care. As for prostate cancer and lung cancer screening, we found very few studies, so generalisability is limited. We were unable to find data on pamphlets as a standalone intervention for breast and cervical cancer screening.
Limitations
When people participate in research and are aware of being studied, there is a possible effect on their behaviour, often termed the Hawthorne effect.22 Three of the nine identified studies (Denberg et al. 200613, Landrey et al. 201316 and Le 201417) used strategies to avoid the Hawthorne effect – the former two by obtaining waiver of consent, the latter one through randomisation by day of clinic rather than by patient. The other six studies did not use such strategies and therefore may be influenced by the Hawthorne effect.
The small number of studies retrieved limits the generalisability of our conclusions. The narrow scope of the review does not allow us to explore the effectiveness of pamphlets combined with or compared to other interventions. We are also unable to make an assessment of publication bias and there may have been relevant studies that were not written in English.
Conclusion
There is some evidence that pamphlets increase uptake for cancer screenings, especially for colorectal cancer. These studies involved primary care physicians. Future research could explore whether primary care physician involvement makes a difference to the effect, and the reasons for this.
Funding statement
This research did not receive any specific funding.
Competing interests
The authors do not have any competing interests.
Acknowledgements
The authors wish to thank Ms Caroline Pang, Director of Heritage Centre & Medical Library, Lee Kong Chian School of Medicine, Nanyang Technological University for her inputs and access to the library’s resources, and Professor Gerald Koh and Professor Helen Smith for their advice.
References
[1] Brawley OW, Kramer BS. Cancer screening in theory and in practice. J Clin Oncol. 2005; 23 293–300.| Cancer screening in theory and in practice.Crossref | GoogleScholarGoogle Scholar | 15637392PubMed |
[2] Bonita R, Beaglehole R, Kjellström T. Basic epidemiology, 2nd edn. Chapter 6: Epidemiology and prevention: chronic noncommunicable diseases. Geneva: World Health Organization; 2006. pp. 110–114.
[3] Stewart BW, Wild CP. 4.8. Screening – implementation. World Cancer Report 2014. Lyon: International Agency for Research on Cancer; 2014. pp. 330–335.
[4] Forman D, Forlay J. 1.1. The global and regional burden of cancer. World Cancer Report 2014. BW Stewart, CP Wild, eds. Lyon: International Agency for Research on Cancer; 2014.
[5] Weller DP, Campbell C. Uptake in cancer screening programmes: a priority in cancer control. Br J Cancer. 2009; 101 S55–9.
| Uptake in cancer screening programmes: a priority in cancer control.Crossref | GoogleScholarGoogle Scholar | 19956164PubMed |
[6] Weller DP, Patnick J, McIntosh HM, Dietrich AJ. Uptake in cancer screening programmes. Lancet Oncol. 2009; 10 693–9.
| Uptake in cancer screening programmes.Crossref | GoogleScholarGoogle Scholar | 19573798PubMed |
[7] Sequist TD, Zaslavsky AM, Marshall R, et al. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2009; 169 364–71.
| Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar | 19237720PubMed |
[8] Champion V, Skinner CS, Hui S, et al. The effect of telephone v. print tailoring for mammography adherence. Patient Educ Couns. 2012; 100 130–4.
[9] Maxwell AE, Hannon PA, Escoffery C, et al. Promotion and provision of colorectal cancer screening: a comparison of Colorectal Cancer Control Program grantees and nongrantees 2011–2012. Prev Chronic Dis. 2014; 11 140183
| Promotion and provision of colorectal cancer screening: a comparison of Colorectal Cancer Control Program grantees and nongrantees 2011–2012.Crossref | GoogleScholarGoogle Scholar | 25275807PubMed |
[10] Mahon SM. Using brochures to educate the public about the early detection of prostate and colorectal cancer. Oncol Nurs Forum. 1995; 22 1413–20.
| 8539182PubMed |
[11] Scottish Intercollegiate Guidelines Network. Methodology Checklist 2: Controlled Trials. [cited 2018 May 26]. Available from: https://www.sign.ac.uk/sign-50.html
[12] Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol. 2009; 62(10):1006–12. [cited 2017 April 17]. Available from: http://www.equator-network.org/reporting-guidelines/prisma/.
[13] Denberg TD, Coombes JM, Byers TE, et al. Effect of a mailed brochure on appointment-keeping for screening colonoscopy. Ann Intern Med. 2006; 145 895–900.
| Effect of a mailed brochure on appointment-keeping for screening colonoscopy.Crossref | GoogleScholarGoogle Scholar | 17179058PubMed |
[14] Harris MA, Byles JE, Cockburn J, D’Este C. A general practice-based recruitment strategy for colorectal cancer screening. Aust N Z J Public Health. 2000; 24 441–3.
| A general practice-based recruitment strategy for colorectal cancer screening.Crossref | GoogleScholarGoogle Scholar | 11011475PubMed |
[15] Krist AH, Woolf SH, Johnson RE, William Kerns J. Patient education on prostate cancer screening and involvement in decision making. Ann Fam Med. 2007; 5 112–9.
| Patient education on prostate cancer screening and involvement in decision making.Crossref | GoogleScholarGoogle Scholar | 17389534PubMed |
[16] Landrey AR, Matlock DD, Andrews L, et al. Shared decision making in prostate-specific antigen testing. J Prim Care Community Health. 2013; 4 67–74.
| Shared decision making in prostate-specific antigen testing.Crossref | GoogleScholarGoogle Scholar | 23799692PubMed |
[17] Le V. Patient prompting of their physician resulted in increased colon cancer screening referrals. World J Gastrointest Oncol. 2014; 6 257–62.
| Patient prompting of their physician resulted in increased colon cancer screening referrals.Crossref | GoogleScholarGoogle Scholar | 25024817PubMed |
[18] Lee JK, Reis V, Liu S, et al. Improving fecal occult blood testing compliance using a mailed educational reminder. J Gen Intern Med. 2009; 24 1192–7.
| Improving fecal occult blood testing compliance using a mailed educational reminder.Crossref | GoogleScholarGoogle Scholar | 19774423PubMed |
[19] Stamatiou K, Skolarikos A, Heretis I, et al. Does educational printed material manage to change compliance with prostate cancer screening? World J Urol. 2008; 26 365–73.
| Does educational printed material manage to change compliance with prostate cancer screening?Crossref | GoogleScholarGoogle Scholar | 18421460PubMed |
[20] Stephens JH, Moore JW. Can targeted intervention in CRC patients’ relatives influence screening behaviour? A pilot study. Colorectal Dis. 2008; 10 179–86.
| 17459064PubMed |
[21] Yoshida M, Kondo K, Nakanishi C, Tada T. Interventional study for improvement of lung cancer screening rate. J Med Invest. 2012; 59 127–35.
| Interventional study for improvement of lung cancer screening rate.Crossref | GoogleScholarGoogle Scholar | 22450001PubMed |
[22] McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014; 67 267–77.
| Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects.Crossref | GoogleScholarGoogle Scholar | 24275499PubMed |


