Patient awareness, knowledge and use of colchicine: an exploratory qualitative study in the Counties Manukau region, Auckland, New Zealand
Caraliese Rebello 1 , Maree Thomson 1 , Deborah Bassett-Clarke 2 , Nataly Martini 3 41 School of Pharmacy, Faculty of Medical and Health Sciences, The University of Auckland, Private Bag 92019, Auckland, New Zealand
2 Teacher-Practitioner, School of Pharmacy, Faculty of Medical and Health Sciences, The University of Auckland & Counties Manukau Health, Private Bag 92019, Auckland, New Zealand
3 Senior Lecturer, School of Pharmacy, Faculty of Medical and Health Sciences, The University of Auckland, Auckland, New Zealand
4 Correspondence to: Nataly Martini School of Pharmacy, Faculty of Medical and Health Sciences, The University of Auckland, Private Bag 92019, Auckland, New Zealand n.martini@auckland.ac.nz
Journal of Primary Health Care 8(2) 140-148 https://doi.org/10.1071/HC15023
Published: 30 June 2016
Journal Compilation © Royal New Zealand College of General Practitioners 2016.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
INTRODUCTION: Treatment of gout, specifically with colchicine, varies globally. Colchicine can be fatal due to its narrow therapeutic index and potential for interactions. In New Zealand, cases of intentional and unintentional colchicine overdose have been documented.
AIMS: To explore patients’ knowledge on the use of gout medicines, and in particular their awareness of the maximum dose of colchicine, the dangers of colchicine overdose, and their opinions on restricting colchicine dispensing. The study also investigates where patients receive gout information.
METHODS: Thirty people with gout presenting to their regular gout clinic in Auckland currently or previously taking colchicine were invited to participate in a 30-min semi-structured interview. Data were analysed using a general inductive thematic approach.
FINDINGS: Overall, participants had a lack of knowledge regarding colchicine and used variable doses during an acute gout attack. Participants were unsure of the maximum dose of colchicine and several took more than prescribed. The prophylactic use of colchicine and allopurinol varied from 3 weeks to 15 years. Mixed views were reported on restricting colchicine supply. Most participants received gout information from their general practitioner (GP).
CONCLUSION: Poor understanding of colchicine contributed to inappropriate use and highlights the need for targeted patient education. Considerable inter-patient variability exists in the use of colchicine for acute gout, suggesting the efficacy of low dose regimens be explored. The length of adjunctive colchicine use, as part of a prophylaxis regimen, needs to be regularly reviewed and tailored to each patient. Further research is required on limiting the amount of colchicine dispensed.
KEYwords: Gout; New Zealand; colchicine; patient perspectives
| WHAT GAP THIS FILLS |
| What we already know: Gout is highly prevalent in NZ and colchicine is a commonly prescribed and clinically useful treatment. Due to wide patient inter-variability and a narrow therapeutic index, if colchicine is taken incorrectly, dire consequences can result. |
| What this study adds: The study highlights how patients take colchicine and the need for colchicine prescribing to be explored with regards to low dose colchicine and prophylactic use. Targeted patient education regarding colchicine is required in order to provide ongoing safe and effective use. |
Introduction
The prevalence of gout has risen in the past 30 years in the United States (US), New Zealand (NZ) and Taiwan, with the greatest increase seen in elderly men.1,2 In 2009, a NZ study reported a nationwide prevalence of gout of 2.89% but the rate among Māori and Pacific men was at least double the national average (6.06% and 7.63%, respectively).3 The greater prevalence among certain ethnic groups is due to genetic variation in uric acid excretion combined with factors such as lower socioeconomic status, barriers to healthcare, lower health literacy, medication non-adherence and risk averseness to treatment.3,4
Despite longstanding, clinically useful pharmacotherapeutic agents like colchicine and allopurinol, gout remains poorly managed.5 Patient views, and those of the wider community, that gout is a minor condition caused by overindulgence of food or drink and only requires medication during the acute phase, has normalized the condition and allowed it to be tolerated. Patients do not seek treatment for a variety of reasons including shame at having the condition, previous poor experiences with medication, having a short-term view of treatment options or underestimating the benefit of long-term pharmaceutical agents.5,6
Worldwide, different prescribing practices and guidelines for the treatment of gout exist, in particular and of concern is the variability of suggested doses of colchicine used during an acute gout attack (Table 1). The AGREE trial demonstrated that low-dose colchicine (1.2 mg, followed by 0.6 mg one hour later) for an acute gout flare was as effective as the higher dose regimen, with a lower incidence of gastrointestinal adverse effects.7 Although this trial was small and conducted in patients with acute gout of less than 12 h duration, this study caused Australia to change its prescribing guidelines.8 Already in place in Australia is the stipulation that the maximum quantity able to be dispensed is 30 tablets, with a maximum of five repeats i.e. 6 months in total.8 In NZ the traditional dose regimen remains as 1 mg initially, followed by 0.5 mg every 6 h and patients can have three-months supply i.e. 90 tablets.9 The risk potential with colchicine is considerable. Although healthcare practitioners are mindful of the care and understanding required to keep this medicine safe, colchicine should still be considered a high-risk medicine.10

|
Examples of risk include a 90 percent fatality rate with doses greater or equal to 0.8 mg/kg, with some as low as 0.125 mg/kg.11–13 In a major NZ hospital, 15 case reports of intentional and unintentional colchicine overdoses were reported over a 10-year period.14 Unintentional overdoses are most likely attributed to interacting medicines, as reported by study in a US hospital where 46% of colchicine fatalities were attributed to a likely interaction.15 Interactions occur most often with clarithromycin,15–18 and statins19–21 which requires routine creatinine kinase levels to gauge the incidence and severity of these rare complications.
Existing research worldwide has unanimously highlighted the clinically significant impact of gout and the need for better understanding in order to optimise care,5,22,23 but few studies have described the patient experience with colchicine.22–24 There is limited information available on the use and knowledge of gout medications in NZ. This study aims to explore patients’ knowledge on the use of medicines, in particular colchicine, for the management of both acute and chronic gout. Patients’ awareness of the maximum dosage of colchicine, the dangers of a colchicine overdose, and opinions on restricting the amount of colchicine tablets dispensed at a time, will be explored. The sources of medicine information and advice about gout given to patients will also be explored.
Methods
Study participants
Participants were recruited from a rheumatology clinic at Manukau Super Clinic, an offsite health centre comprising multiple clinics and two elective surgical wards under the supervision of Counties Manukau Health (CMH) in South Auckland, an area where gout is extremely prevalent.
Potential participants were identified by CMH staff (pharmacists, rheumatologists, rheumatology nurses, clinic staff) from a list of patients attending a routinely scheduled gout clinic appointment and therefore deemed to have a diagnosis of gout, as shown by their previous attendance at the same clinic, and evident from clinic letters. Inclusion criteria for the study were participants currently taking recognised gout treatment(s), primarily responsible for their medications, and could communicate well in English. If they were not taking colchicine currently, they had to have discontinued it within the past two years, otherwise recall of the drug and benefits versus side effects would be difficult. All patients in the study taking colchicine (28/30 participants) were using it both for the treatment of acute gout and adjunctive prophylaxis, usually alongside allopurinol. The most common prophylactic dose of colchicine was 0.5 mg twice daily.
Participants were approached in the clinic waiting area, provided with a participant information sheet and after a period of reading and contemplation, invited to participate in the study. Of 81 patients scheduled to attend their three monthly gout clinic appointment in July 2013, 22 did not attend their appointment. Applying the inclusion criteria to the remaining 59 potential participants, 43 were invited to participate in the study. Thirteen declined due to time pressure of family and/or work commitments or were unwilling to participate. The remaining 30 participants were interviewed individually in a designated clinic room by two student researchers and participants were encouraged to have a support person (usually a family member) present at the interview. Eleven had family members present at the interview. It was explained at the time that the support person could not contribute to the interview process.
Study design
Qualitative methodology was considered the best approach to gain a deeper understanding of participant experiences and perspectives on gout and their medicines.30 Semi-structured interviews were chosen as specific questions needed to be addressed to meet the study’s aims, although a semi-structured interview still allowed for flexibility of responses.
A semi-structured interview (Box 1) was developed jointly by the authors. The questionnaire was based on previous literature and anecdotal accounts in clinical practice and allowed exploration of participants’ experience in dealing with acute gout attacks, their knowledge and level of medication use (particularly colchicine) in managing chronic gout and the types or sources of medicines information they received. Consistency of questioning style and appropriateness was ensured through a training session with external researchers, and the process was piloted with senior academics at the University of Auckland and lay people. After participants had read the Participant Information Sheet and agreed to participate, they signed a consent form and permission was sought for the interview to be tape recorded. At least two researchers were present during participant interviews (one as questioner and one as observer/note taker). A debriefing with co-researchers took place after each interview to ensure consistency of the interview process.
| Box 1. Semi-structured Interview Questions |
|
Analysis and coding
Interview recordings were transcribed verbatim by CR and MT, and through discussion with four other final year Pharmacy students the data were imported into NVivo version 10 for coding and analysis. We developed a coding frame based on participant responses to the interviews and other key themes and concepts that subsequently emerged from the data. All members of the study team contributed to developing the coding frame; a draft was discussed within the research team and refined, and further modifications were made after applying codes to the first two transcripts. The research team discussed the coding process as it progressed and to ensure inter-coder reliability a consensus was reached where there were uncertainties about how to code particular data items. A thematic approach was used to analyse the data.
Ethics approval
Ethics approval was obtained from The University of Auckland Human Participants Ethics Committee (# 9641) and a locality agreement with the CMH Ethics group was obtained. Permission to conduct participant interviews onsite at Manukau Super clinic was obtained from the General Manager of the facility. A staff-specific participant information sheet and consent form were also provided for clinic staff.
FINDINGS
Participant analysis
Of the 30 interviewees, 24 (80%) were male and 6 (20%) female, ranging in age from 28 to 76 years. The main ethnicities represented were Samoan (n = 8; 27%), Māori (n = 7; 23%) and NZ European (n = 7; 23%).
The time of gout diagnosis ranged from 2 weeks to one participant having established disease of more than 50 years. Sixteen (64%) participants reported having a first-degree relative with gout. Twenty-five (83%) participants had pre-existing co-morbidities, 17 (68%) with multiple co-morbidities. The most common co-morbidities were hypertension, cardiovascular disease and/or diabetes.
Qualitative results
The five key themes that resulted from the interview were gout treatment; colchicine use; colchicine knowledge, effectiveness and adherence; colchicine supply, and gout medicines information.
Gout treatment
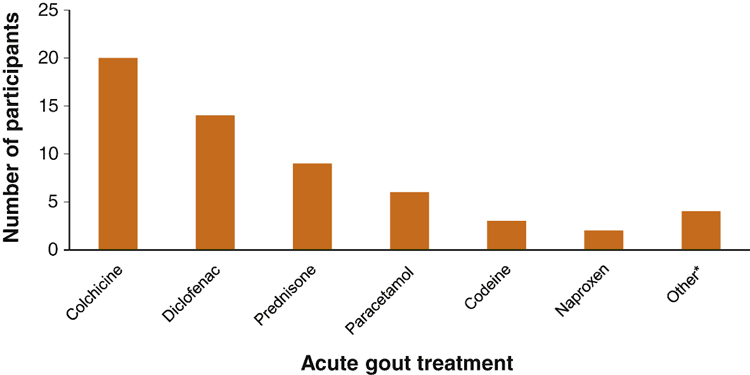
Colchicine was the most commonly used medication for treatment of acute flare (see Figure 1). However, a few discontinued it after 1–2 days due to gastrointestinal side effects, like diarrhoea; whereas others persisted with colchicine and self-medicated with anti-diarrhoeal bought over the counter.
|
Most participants were taking colchicine and allopurinol concurrently; some took allopurinol alone; one or two used colchicine alone; and several used probenecid adjunctively as well. Two participants had stopped using colchicine and allopurinol due to renal impairment and another stopped allopurinol because of developing a rash. These three changed to medication with benzbromarone.
About half took complementary remedies as well, such as tart cherry juice, MonaVieTM (açai berry) health juice, celery pills or indigenous herbal medicines.
‘I take it because it’s herbal… there’s no chemicals in the herbal tablets’ [P11].
‘I think they should cut down on drugs…I don’t like the medications which we’re taking, they’re poisons!’ [P16].
Some participants used homemade remedies such as boiled black pepper, soda water with milk and puha water (the juice reserved after boiling native NZ sow thistle). However, most participants said these remedies ‘didn’t seem to help’ [P25] and quickly discontinued them. Non-pharmacological remedies such as ice packs, acupuncture and massage were given mixed reviews by participants. Two participants reported an improvement in their gout symptoms after undergoing heart surgery and subsequent cardiovascular medication use.
Colchicine use
The most common regimen for colchicine prescribed in acute gout flares was either 0.5 mg or 1 mg daily, but higher dose regimens were used by some patients during an attack.
‘…initially just one…if I had to take more I’d probably just take two [tablets] max… and then one after that every four hours’ [P28].
‘...8 or 10? Over the whole attack [just] as prescribed’ [P17].
Just over half of participants reported taking colchicine prophylactically as well with a dose of 0.5 mg twice daily being the most common dose regimen.
Several participants reported varying their doses and exceeding the maximum dose of colchicine allowed per day to get relief from an acute attack. One would take ‘an overdose of it’ [P9] and another admitted to taking four (2 mg) at once then four again later after a meal, knowing it was contrary to what the GP advised.
‘I think it’s [the maximum you can take] twelve isn’t it? Twelve? Eight or twelve…’ [P14].
When asked about concurrent medications, half took five or more medications and a few participants took eight. Several participants took medicines that could potentially worsen gout such as low dose aspirin or thiazide diuretics. Two participants took a potentially interacting medicine such as a statin or roxithromycin.
Colchicine knowledge, effectiveness and adherence
The majority of the participants viewed colchicine as a ‘painkiller’ for use only during an acute flare. However, a few knew that low dose colchicine was effective in preventing or reducing gout attacks or reducing uric acid levels, and two understood that colchicine was to be taken until allopurinol was established.
‘If you take the allopurinol by itself it has a different effect... If you take it with colchicine it sort of makes it the softener for the allopurinol. That’s why the [uric acid] level has dropped. For some people... it’s like a pain killer’ [P18].
‘…allopurinol is the main one that I need so if I can get my uric acid levels down I might not need colchicine at all’ [P3].
Almost half of the participants thought colchicine was effective in controlling their gout but others did not find it effective stating they would ‘double the dose’ [P9] to eliminate the gout attack.
‘I’ve had double or triple [the dose]…especially when there was no diclofenac around. And I can take six... in that day... and it didn’t help…’ [P22].
Many participants experienced gastrointestinal symptoms with colchicine, but few connected the diarrhoea to colchicine. Most taking colchicine experienced diarrhoea immediately, but some developed it only after a few days of taking colchicine.
Very few participants admitted to non-adherence. One stopped their medications because they believed they were taking ‘too many pills’ [P15] and another stopped because they did not ‘like drinking tablets’ [P3].
Some participants admitted to sharing their gout medication with others if needed.
Colchicine supply
Among participants prescribed colchicine, some preferred three monthly dispensing while others preferred restricted dispensing of 30 tablets per month. One participant stated that they preferred ‘to be controlled’ [P10]. However, most disagreed and were annoyed at the idea of limiting dispensing, saying ‘it would be a nuisance’ or ‘incur additional costs’.
Medicines information
Most of the participants received most of their information about gout and gout medicines from their GP, while a few downloaded digital references or printed material themselves. Only a couple of participants mentioned their pharmacist as an information resource.
The medicines information received was mostly about dietary triggers, causes of gout, indications for colchicine and its side effects and the complications or consequences of leaving gout untreated. Most participants asked the researchers for extra information about gout because they found the information they had previously received was contradictory and difficult to understand. Participants with English as a second language usually found it difficult to comprehend medical jargon spoken by physicians.
‘‘It’s very hard for us [Pacific Island] people to understand the terminologies…but it’s good…if they can…print it out… and give us information especially in our own language so our elders can understand…’ [P11].
‘…less words more pictures…I know a picture…it tells me a lot. But little extra information; not too much; highlight; bullet point maybe…’ [P8].
‘…they said what not to eat on the book...on the sheet said I can eat it… so it was confusing… Feel annoyed like I had no idea’ [P3].
Discussion
Key findings from this study suggest that although colchicine is a common and valuable treatment for both acute flare and chronic management of gout, patients have limited knowledge regarding safe doses and adverse effects. Some participants reported inadequate pain relief when taking prescribed amounts of colchicine, suggesting not only wide inter-patient variability and therefore increased likelihood of overdose, but also indicating that the mechanism of action was poorly understood because many perceived it as a pain killer. Previous work has shown that purpose or indication, side effects and mechanism of action are the three key pieces of information, participants require in order to use medication safely.31 Given the poor safety profile of colchicine, it is of concern that participants did not know the maximum dosage of colchicine, giving rise to risky behaviour and increasing the potential for overdose.12–14
Sharing of medicines was not widespread in this study, but this practice increases the risk of a potential overdose. Colchicine can be fatal in overdose and treatment options for overdose remain limited.14 The results highlight the necessity of patient–focused education, both verbal and written. In addition, healthcare professionals need to emphasise the maximum dosage of colchicine to take, and the risks and consequences of overdose.
Participants who reported side effects of colchicine, especially diarrhoea, raised safety concerns because discontinuation is usually recommended if symptoms of diarrhoea, abdominal pain, nausea or vomiting occur, even if gout relief has not been fully achieved.32 Despite this, participants persisted with colchicine, presumably because they found it effective and instead self-medicated with anti-diarrhoeal medications.
These results of this study revealed the necessity of regular reviews of gout regimens, particularly colchicine use. While the optimal duration of prophylactic use of colchicine alongside urate-lowering agents is not conclusive, most authors recommend that adjunctive colchicine use be reviewed within 12 months, once urate levels normalise.8,33,34
Current co-morbidities affect or limit the choice of therapeutic agents.18 Several participants reported one or more co-morbidities such as hypertension, cardiovascular disease, diabetes and/or renal impairment, all known risk factors for exacerbating gout and increasing the risk of tophi development.18,35 Healthcare professionals need to be mindful of medication safety aspects in complex patients.
Similarly, multiple medications (known as ‘polypharmacy’) increase the risk of drug-drug interactions. The combination of macrolides plus colchicine, especially, has caused fatalities.15,16,36 Clarithromycin requires a dosage reduction of colchicine of 33–66%.15 In our study, one participant was taking roxithromycin which, arguably is deemed to be the safest macrolide and does not normally require a dosage reduction but monitoring of potential toxicity is still recommended.8 The combination of colchicine and a statin poses a theoretical increased risk of myopathy and rhabdomyolysis.18,19 Some participants in this study were taking this drug combination, and although clinicians are expected to regularly monitor creatinine kinase, another NZ study claimed that a quarter of all patients with renal impairment who are at high risk of colchicine-induced myopathy did not actually receive adequate safety monitoring.37
Other drugs of concern taken by the participants in this study were thiazide diuretics and low dose aspirin, both known to elevate serum urate levels and increase the risk of an acute gouty attack.17,38 Overall, we would like to signal ongoing pharmacovigilance and appropriate monitoring is necessary, when gout medication is prescribed for patients with complex health issues.
Previous literature has identified that Polynesian peoples are more commonly concerned about side effects of medication and ‘toxic build up’ in their bodies, leading to self-investigation and use of herbal remedies alongside conventional gout medication.31 This study highlighted that some patients preferred to use herbal remedies to treat gout but we did not explore further in this study the underpinning reasons as to why this was the case.
We found that most participants did not support the idea of restricting quantities of colchicine to monthly dispensings, citing increased cost of collecting prescriptions and inconvenience. Limiting the amount of colchicine dispensed at one time could possibly promote safer and more optimal use of colchicine, and requires further investigation.8,39
Previous gout studies in NZ concluded that better educational support, visual aids, and clear written instructions in English were important for effective gout management.40,41 Healthcare professionals need to check patients’ understanding of gout and attendant medications to avoid confusion, especially with patients for whom language is a barrier. Participants in this study stated they would like simple concise information in English and pictures would further improve their understanding. Directing patients to other reputable sources of information (eg reliable peer-reviewed websites) and available support groups might help to minimise the risk of contradictory information being accessed.
A limitation of this study was that the small sample size did not allow data saturation to be reached. Themes emerging from the interviews were largely dictated by the design of the semi-structured interview, and did not allow for a deeper exploration of concerns emerging during interviews, such as colchicine sharing or self-medicating with anti-diarrhoeals for colchicine related gastrointestinal side-effects. These issues, together with the use of high-dose colchicine during an acute attack by many of the participants in this study, highlight the need for investigation into how healthcare professionals can better communicate the risks of colchicine while promoting the benefits of this agent in the management of gout.
Conclusion
Wide inter-patient variability in colchicine efficacy is well known, suggesting that prescribing adjunctive colchicine as part of a chronic gout regimen needs ongoing and periodic review.
With reports of intentional and unintentional colchicine overdoses in NZ and elsewhere, this study has identified important safety concerns regarding colchicine, including patient lack of awareness of maximum colchicine doses and possible sharing of colchicine between family and friends. The results have reiterated the need for targeted patient education on the appropriate use of colchicine including the frequency, maximum dose, adverse effects, and signs of toxicity and the risks of overdose. Involving community pharmacists in developing these resources might be a solution to this problem. Further studies are required to investigate if reduced amounts of colchicine dispensed in a stat dose would make a difference to the safety profile of this clinically relevant drug.
COMPETING INTERESTS
The authors have no potential conflicts.
ACKNOWLEDGEMENTS / FUNDING
The authors would like to thank student researchers Shao-Ping Ling, Nandita Kant, Casslyn Tee and Kayla Thomas for their contribution; Dr Peter Gow, Hazra Sahid, the Rheumatology team and nursing staff at Manukau Super Clinic; and Counties Manukau Health Pharmacy Manager, Sanjoy Nand.
References
[1] Roddy E, Zhang W, Doherty M. The changing epidemiology of gout. Nat Clin Pract Rheumatol 2007; 3 443–9.| The changing epidemiology of gout.Crossref | GoogleScholarGoogle Scholar | 17664951PubMed |
[2] Saag KG, Hyon C. Epidemiology, risk factors, and lifestyle modification for gout. Arthritis Res Ther 2006; 8 S2
| Epidemiology, risk factors, and lifestyle modification for gout.Crossref | GoogleScholarGoogle Scholar | 16820041PubMed |
[3] Winnard D, Wright C, Taylor W, et al. National prevalence of gout derived from administrative health data in Aotearoa New Zealand. Rheumatology 2012; 51 901–9.
| National prevalence of gout derived from administrative health data in Aotearoa New Zealand.Crossref | GoogleScholarGoogle Scholar | 22253023PubMed |
[4] Singh JA. Racial and gender disparities in patients with gout. Curr Rheumatol Rep 2013; 15 307
| Racial and gender disparities in patients with gout.Crossref | GoogleScholarGoogle Scholar | 23315156PubMed |
[5] Dalbeth N, Lindsay K. The patients experience of gout: New insights to optimize management. Curr Rheumatol Rep 2012; 14 173–8.
| The patients experience of gout: New insights to optimize management.Crossref | GoogleScholarGoogle Scholar | 22198831PubMed |
[6] Doherty M, Jansen TL, Nuki G, et al. Gout: why is this curable disease so seldom cured? Ann Rheum Dis 2012; 71 1765–70.
| Gout: why is this curable disease so seldom cured?Crossref | GoogleScholarGoogle Scholar | 22863577PubMed |
[7] Terkeltaub RA, Furst DE, Bennett K, et al. High versus low dosing of oral colchicine for early acute gout flare: Twenty four-hour outcome of the first multicenter, randomized, double blind, placebo controlled, parallel group, dose comparison colchicine study. Arthritis Rheum 2010; 62 1060–8.
| High versus low dosing of oral colchicine for early acute gout flare: Twenty four-hour outcome of the first multicenter, randomized, double blind, placebo controlled, parallel group, dose comparison colchicine study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFGnu7rE&md5=99620cb7690da4bc6c9606019fdbfb3aCAS | 20131255PubMed |
[8] Australian Government. Department of Health [internet]. Australia: Pharmaceutical Benefit Schedule, 2013 [cited 23 August 2013]. Available from: www.pbs.gov.au/medicine/item/3410L.
[9] New Zealand Formulary [internet]. Woods, DJ. Updated September 1, 2012 [cited 19 August 2013]. Available from: www.nzf.org.nz/nzf_5674.html?searchterm=colchicine.
[10] Ahern MJ, Reid C, Gordon TP, McCredie M, et al. Does colchicine work? The results of the first controlled study in acute gout. Aust N Z J Med 1987; 17 301–4.
| Does colchicine work? The results of the first controlled study in acute gout.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c%2FkvFegsg%3D%3D&md5=05fb4a416c12c04fe13450fe0923439bCAS | 3314832PubMed |
[11] Hood RL. Colchicine poisoning. J Emerg Med 1994; 12 171–7.
| Colchicine poisoning.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2c3mslWisQ%3D%3D&md5=80cc0abcb5c1765353834e005d03b743CAS | 8207152PubMed |
[12] Flanagan RJ, Jones AL. Fab antibody fragments. Drug Saf 2004; 27 1115–33.
| Fab antibody fragments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXktlemtA%3D%3D&md5=3a7d5ffa1f621d5e460cdd8407898226CAS | 15554746PubMed |
[13] Jarvie D, Park J, Stewart M. Estimation of colchicine in a poisoned patient by using high performance liquid chromatography. Clin Toxicol 1979; 14 375–81.
| Estimation of colchicine in a poisoned patient by using high performance liquid chromatography.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXkvVart7Y%3D&md5=3d6336ec0a9bb8216f790f6ca1db2f37CAS | 466979PubMed |
[14] Jayaprakash V, Ansell G, Galler D. Colchicine overdose: the devil is in the detail. NZ Med J 2007; 120 U2402
[15] Mullins M, Cannarozzi AA, Bailey TC, et al. Unrecognized fatalities related to colchicine in hospitalized patients. Clin Toxicol 2011; 49 648–52.
| Unrecognized fatalities related to colchicine in hospitalized patients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFWks77L&md5=bd70a38fa40230203b06e2e848c7ea68CAS |
[16] Terkeltaub RA, Furst DE, Digiacinto JL, et al. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3a4/P-glycoprotein inhibitors. Arthritis Rheum 2011; 63 2226–37.
| Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3a4/P-glycoprotein inhibitors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsFejsbY%3D&md5=5e38b14f3d317f571495f61e4b2b4cf6CAS | 21480191PubMed |
[17] U.S. Food and Drug Administration [internet]. Information for Healthcare Professionals: New Safety Information for Colchicine (marketed as Colcrys). [cited 18 August 2003]. Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm174315.htm.
[18] Stamp LK, Chapman PT. Gout and its comorbidities: implications for therapy. Rheumatology 2013; 52 34–44.
| Gout and its comorbidities: implications for therapy.Crossref | GoogleScholarGoogle Scholar | 22949727PubMed |
[19] Hsu WC, Chen WH, Chang MT, Chiu HC. Colchicine-induced acute myopathy in a patient with concomitant use of simvastatin. Clin Neuropharmacol 2002; 25 266–8.
| Colchicine-induced acute myopathy in a patient with concomitant use of simvastatin.Crossref | GoogleScholarGoogle Scholar | 12410059PubMed |
[20] Tufan A, Dede DS, Cavus S, et al. Rhabdomyolysis in a patient treated with colchicine and atorvastatin. Ann Pharmacother 2006; 40 1466–9.
| Rhabdomyolysis in a patient treated with colchicine and atorvastatin.Crossref | GoogleScholarGoogle Scholar | 16772404PubMed |
[21] Alayli G, Cengiz K, Canturk F, et al. Acute myopathy in a patient with concomitant use of pravastatin and colchicine. Ann Pharmacother 2005; 39 1358–61.
| Acute myopathy in a patient with concomitant use of pravastatin and colchicine.Crossref | GoogleScholarGoogle Scholar | 15914514PubMed |
[22] Harrold LR, Mazor KM, Peterson D, et al. Patients’ knowledge and beliefs concerning gout and its treatment. BMC Musculoskelet Disord 2012; 13 180
| 22995041PubMed |
[23] Lindsay K, Gow P, Vanderpyl J, et al. The experience and impact of living with gout: a study of men with chronic gout using a qualitative grounded theory approach. J Clin Rheumatol 2011; 17 1–6.
| The experience and impact of living with gout: a study of men with chronic gout using a qualitative grounded theory approach.Crossref | GoogleScholarGoogle Scholar | 21169857PubMed |
[24] Harrold LR, Mazor KM, Velten S, et al. Patients and providers view gout differently. Chronic Illn 2010; 6 263
| Patients and providers view gout differently.Crossref | GoogleScholarGoogle Scholar | 20675361PubMed |
[25] Medsafe [internet]. Wellington: New Zealand Medicines and Medical Devices Safety Authority. Colchicine: Lower doses for greater safety; November 2005 [cited 23 August 2013]. Available from: http://www.medsafe.govt.nz/profs/PUArticles/colchdose.htm.
[26] Peterson G.. Gout: a very old disease with some new approaches. Australian Pharmacist 2013; 31 2430–2.
[27] Khanna D, Khanna P, Fitzgerald JD, et al. American College of Rheumatology guidelines for management of gout. Part 2: Therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res 2012; 64 1447–61.
| American College of Rheumatology guidelines for management of gout. Part 2: Therapy and antiinflammatory prophylaxis of acute gouty arthritis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVWjsLbL&md5=13fa690026bf12714c4317390daecabaCAS |
[28] Rider TG, Jordan KM. The modern management of gout. Rheumatology 2010; 49 5–14.
| The modern management of gout.Crossref | GoogleScholarGoogle Scholar | 19808695PubMed |
[29] Richette P, Bardin T. Successful treatment with rasburicase of tophaceous gout in a patient allergic to allopurinol. Nat Clin Pract Rheumatol 2006; 2 338–42.
| 1:CAS:528:DC%2BD28Xmt1yqsbs%3D&md5=972328a276d2d66df6a37989ab420e73CAS | 16932713PubMed |
[30] Phellas CN. et al. Doing Research: Interviews, Questionnaires and Observation. In: Seale C. Researching Society and Culture. London: Sage Publications. 2011;182–183.
[31] Bassett-Clarke D, Krass I, Bajorek B. Ethnic differences of medicines-taking in older adults: a cross cultural study in New Zealand. Int J Pharm Pract 2012; 20 90–8.
| Ethnic differences of medicines-taking in older adults: a cross cultural study in New Zealand.Crossref | GoogleScholarGoogle Scholar | 22416933PubMed |
[32] National Prescribing Service [internet]. Colchicine for acute gout: updated information about dosing and drug interactions; 14 May 2010 [cited 5 September 2013]. Available from: http://www.nps.org.au/publications/health-professional/nps-radar/2010/may-2010/brief-item-colchicine#fnote16.
[33] Sivera F, Andres M, Carmona L, et al. Multinational evidence-based recommendations for the diagnosis and management of gout: integrating systematic literature review and expert opinion of a broad panel of rheumatologists in the 3e initiative. Ann Rheum Dis 2014; 73 328–35.
| 23868909PubMed |
[34] Borstad GC, Bryant LR, Abel MP, et al. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol 2004; 31 2429–32.
| 1:CAS:528:DC%2BD2MXivFKruw%3D%3D&md5=69b69b6eec588ff88d7ee2a46a0f1e2dCAS | 15570646PubMed |
[35] Choi HK, Atkinson K, Karlson EW, et al. Obesity, weight change, hypertension, diuretic use and risk of gout in men: the health professionals follow-up study. Arch Intern Med 2005; 165 742–8.
| Obesity, weight change, hypertension, diuretic use and risk of gout in men: the health professionals follow-up study.Crossref | GoogleScholarGoogle Scholar | 15824292PubMed |
[36] Hung IFN, Wu AKL, Cheng VCC, et al. Fatal interaction between clarithromycin and colchicine in patients with renal insufficiency: A retrospective study. Clin Infect Dis 2005; 41 291–300.
| Fatal interaction between clarithromycin and colchicine in patients with renal insufficiency: A retrospective study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXosVagurw%3D&md5=6723f0937d34ece5dc528da0997e4e86CAS |
[37] Ly J, Gow P, Dalbeth N. Colchicine prescribing and safety monitoring in patients with gout. N Z Med J 2007; 120 U2808
| 18264188PubMed |
[38] Zhang Y, Neogi T, Chen C, et al. Low-dose aspirin use and recurrent gout attacks. Ann Rheum Dis 2014; 73 385–90.
| 1:CAS:528:DC%2BC2cXltFKhs7o%3D&md5=95d2e6a41513f499088e74d763b9581cCAS | 23345599PubMed |
[39] Gresham C, Utting K, Williams C, et al. Colchicine poisoning: defusing the ticking time bomb. N Z Med J 2013; 126 115–6.
| 23474521PubMed |
[40] Te Karu L, Bryant L, Elley CR. Māori experiences and perceptions of gout and its treatment: a Kaupapa Māori qualitative study. J Prim Health Care 2013; 5 214–22.
| 23998172PubMed |
[41] Merriman T.. Gout: An alarm bell for diabetes and cardiovascular disease. BPJ 2011; 41–3.


