Specifications for temperature and humidity in sterile storage environments – Where’s the evidence?
Terry McAuleySTEAM Consulting, Melbourne, Victoria, PO Box 100, Endeavour Hills, Vic. 3802, Australia. Email: terry@steamconsulting.com.au
Healthcare Infection 14(4) 131-137 https://doi.org/10.1071/HI09020
Published: 21 December 2009
Abstract
The concept of event-related sterility for storage of medical devices is widely applied in Australian healthcare facilities. This paper reviews available literature, international standards and guidance documents with respect to the concept of event-related sterility in order to determine: (i) why commercially produced sterile medical devices may withstand adverse temperature and humidity conditions better than items produced in a Sterile Services Department; (ii) what effects temperature and humidity have on maintenance of sterility; and (iii) whether temperature and humidity conditions specified in AS/NZS4187:2003 are evidence-based and whether changes to these could be made in any future revisions. The literature review revealed that manufacturers of commercially available sterile medical devices are subject to stringent medical device regulations that require them to ensure that the devices remain both fit for purpose and sterile until use. This necessitates that manufacturers conduct accelerated ageing and environmental challenge tests to establish and justify the expiry dates given on the package label. The review also found a variety of specifications for temperature and humidity and the guidance documents gave no indication of evidence used as a basis for these requirements. In addition, it was apparent that many published studies on event-related sterility did not include consideration of the effect of temperature and humidity on the duration of sterility. After examination of a range of published material, it was concluded that the specifications in any future edition of AS/NZS4187 could be expanded to a temperature range of 16–25°C and a relative humidity range of 30–75%.
Introduction
In Australia, reprocessing of reusable medical devices takes place in accordance with the Australian and New Zealand Standard Cleaning, disinfecting and sterilising reusable medical and surgical instruments and equipment and maintenance of the associated environment in healthcare facilities [AS/NZS4187:2003].
This Standard recognises the concept of event-related sterility and includes specifications for storage environments for sterile medical devices [SMD] irrespective of whether these are produced by a Sterile Services Department [SSD] or a commercial manufacturer. Conditions for SMD storage require temperatures between 18–22°C and relative humidity [RH] between 35–68%.1
Extreme weather conditions experienced in Australia over the past several years, in conjunction with ageing air-handling systems in many healthcare facilities, has highlighted issues regarding the ability to maintain these conditions. Thus SSD managers are questioning the temperature and humidity specifications in AS/NZS4187:2003, as commercially manufactured SMD are often labelled with acceptable storage conditions outside those written in the Standard.
The questions asked include:
-
Are commercially manufactured SMD able to withstand adverse temperature and humidity conditions more readily than those SMD produced by SSD and if so why?
-
What are the effects of elevated temperature and/or humidity in terms of sterility maintenance?
-
Is there any safe duration of exposure to these conditions and are there any simple method(s) to ensure sterility maintenance under adverse conditions?
-
What are recommended temperature and humidity ranges for storage of SMD in other published standards and guidance documents?
-
What evidence has been the basis for these conditions?
-
In light of the above, what changes should be considered during future revisions of AS/NZS4187?
A literature review was conducted to locate published studies on event-related sterility to determine if these included consideration of the effects of temperature and humidity on sterility maintenance. In addition, published guidance from a variety of sources was examined to identify temperature and humidity specifications for storage of SMD and compare these with AS/NZS4187:2003.
The anticipated outcomes of the literature review were:
-
Finding answers to the SSD manager’s questions, and
-
Identification of evidence-based recommendations for temperature and humidity ranges for sterile storage environments which could, if necessary, be used in a future revision of AS/NZS4187:2003.
This paper will discuss the findings, using the questions posed by SSD managers as subject headings.
Background
Although temperature and humidity monitoring in sterile storage environments is currently not required by AS/NZS4187:2003, several Australian SSD managers implemented monitoring systems during extreme weather conditions experienced over the past several years.
Monitoring revealed that deviations from specified conditions occur at various intervals and for varying lengths of time, raising concerns for patient safety, as increased humidity should be considered an adverse event.2,3
The usual practice upon identification of deviations from acceptable temperature and/or humidity is to reprocess SSD-produced items. However, some commercially produced SMD have specified limits for temperature and humidity on the packaging which allow exposure to conditions outside those specified by AS/NZS4187:2003. As long as these conditions have not been exceeded, these items can be retained. However, all commercially produced SMD with no specified storage conditions, or those showing signs of moisture, should be discarded.
In some hospitals, problems with humidity pre-existed and/or persisted even after extreme weather conditions passed. Costs to fix or replace air-conditioning systems are prohibitive and with some facilities planned for refurbishment over the next 5–10 years, there is a reluctance to invest significant capital in solving these problems.
In situations where ongoing exposure to adverse conditions has been experienced, SSD managers are resorting to sealing SMD in impervious plastic dust covers in order to mitigate the exposure to increased humidity and minimise the cost and impact of reprocessing or discarding SMD. However, these practices also incur costs in both time and resources.
Clearly, evidence-based guidance is required to inform practice and to ensure patient safety is maintained.
Literature review
Due to a limited timeframe for conduct of the literature review, research was limited to sources readily available through the internet or already on hand in the author’s personal library.
The author had on file several older articles on event-related sterility in addition to copies of various international standards and guidance documents pertaining to the areas of sterilisation, packaging and sterile barrier systems. In addition, ProQuest, PubMed, Google™ and Google Scholar™ search engines were used to identify and locate peer-reviewed and non-peer reviewed literature available on the internet.
The search terms included event-related sterility, shelf-life, humidity, storage, packaging, sterile barrier systems and surgical instruments. Key words were also typed into the search engines as separate items to identify other possible sources of material.
Search results were scanned to eliminate:
-
duplications,
-
items that were facility-specific guidelines or policies,
-
patent applications,
-
product-specific literature with limited exceptions,
-
articles which cited no references, and
-
book results.
Book results were not included due to lack of ready access.
Materials were considered for inclusion based on their content and contribution to developing an understanding of event-related sterility and the possible basis for temperature and humidity specifications in sterile storage environments.
Reference lists were examined to identify further relevant literature and where possible, were accessed online. However, difficulties were experienced in locating some articles due to their age, changes in publishers or discontinuation of the journals. In addition, the author did not pursue the purchase of several expensive resources, such as testing standards for ageing of SMD and related guidance that may have assisted in developing a broader appreciation of the medical device industry context for specifying storage conditions. Both of these factors are recognised limitations of this review.
After conclusion of the search, over 45 documents had been selected for further examination in order to determine whether relevant information pertaining to the effect of temperature and humidity on maintenance or duration of sterility was presented.
Discussion
Are commercially manufactured SMD able to withstand adverse temperature and humidity conditions more readily than those SMD produced by SSD and if so, why?
Very simply, the answer is frequently yes, because the methods of sterilisation employed by SMD manufacturers allow more robust packaging systems to be used in comparison to those available for use in SSD.4
Annex 1 of the Medical Devices Directive [MDD] 93/42 EEC requires medical devices be designed and manufactured so sterility will be maintained during storage or transport, providing manufacturer’s specified storage and handling instructions are followed.5 While the MDD is not directly applicable in Australia, Australian medical device legislation is very similar. In addition, many products compliant with the European MDD are used in Australia.
ISO11607–1 requires manufacturers of packaging systems to ensure the products are able to perform as a microbial barrier and ‘maintain sterility until the point of use or until the expiry date’ and should provide information on ‘known restrictions on handling and use’ which presumably includes ‘any shelf-life limitations…for post-sterilisation storage’ and recognises that ‘loss of sterility is regarded as event-related’, therefore accelerated and real-time ageing studies, stability and biocompatibility tests are required.6
Consequently significant progress has been made in improving packaging systems and developing procedures for accelerated ageing and environmental challenge studies that include consideration of the effects of temperature and humidity, allowing more precise labelling of acceptable storage conditions.7,8
Nonetheless, it should be noted that the Sterilisation Packaging Manufacturers Council (SPMC) advocates shelf-life testing should be distinct from ‘the environmental impact of temperature and humidity’ and there is still no universally agreed method for performance of microbial barrier testing.9,10
Rutala and Weber11 advise that when selecting a sterilisation wrap, SSD managers should ask the manufacturer for evidence of independent laboratory testing on the time and event-related performance of their product as a microbial barrier.
The author contacted several manufacturers of sterile barrier systems sold for use in SSD applications to identify if their product literature contained any specifications for storage conditions to maintain sterility of items until use. At the time of writing, limited responses had been received from two manufacturers, indicating this information was not currently available, but may be in the future.
It is therefore feasible to expect manufacturers of packaging materials for use in SSD to undertake post-sterilisation validation studies of the performance of their products under extreme temperature and humidity conditions, utilising the testing protocols used by SMD manufacturers and thus provide recommendations for storage conditions suitable for event-related sterility programs.
Such testing programs would be advantageous because, to date, the published studies on event-related sterility all use vastly different approaches in experimental design, making comparison of the results challenging.
In the author’s opinion, testing of packaging systems under assumed ‘worst-case’ conditions currently used by commercial manufacturers of SMD could be applied to manufacturers of packaging products for use in SSD. This would provide SSD managers with guidance as to acceptable storage conditions to ensure effective maintenance of sterility for SSD-produced SMD.
What are the effects of elevated temperature and humidity in terms of sterility maintenance?
Dunkelberg and Rohmann12 state: ‘Studies of the sterile integrity of sterilised packages, conducted under hospital conditions and various different environmental conditions (e.g., level of cleanliness, humidity, and temperature), have been hampered by technical and statistical difficulties.’ This is also true for commercially produced SMD, for example SMPC8 indicates that the effect of temperature on ageing of SMD is well understood, although the effect of humidity requires further study.
Some SMD manufacturers indicate higher temperatures adversely affect the stability of some products.13 Phillips13 also states that minor variation of 1–2°C should have little effect on the product, while temperatures in excess of 5–6°C higher could significantly reduce shelf life, although no mention is made if this is loss of sterility.
During email correspondence between the author, Dr F. McGain and Professor Tallentire, Tallentire14 wrote:
‘My colleague and I are not aware of any evidence to suggest a storage temperature of 27 degrees poses any greater risk to loss of sterility compared with a temperature of 20 degrees.’
However, the Sterile Processing University15 state that microbes prefer warmer temperatures; therefore, controlling temperatures below the range preferred by microorganisms is a method to reduce environmental bioburden.
Several studies on event-related sterility mention temperature and humidity were monitored or controlled during experiments; although few indicate what the ranges were. One exception is the study by Widmer, Houston, Bollinger and Wenzel16 who stated temperatures were in the range of 20–22°C and RH fluctuated between 30–80% during the experiment; however the impact of this on study results were not discussed.
Other studies indicated temperature and/or humidity were not controlled or monitored during storage periods and results of sterility testing did not indicate higher levels of contamination.17,18 Webster et al.19 attempted to examine the effect of a variety of controlled and uncontrolled storage conditions in their study over a 2-year period. One interesting finding was from testing articles stored in a box in the rear of a car for 9 years. Although these devices were subjected to extremes of temperature and humidity in a sub-tropical climate, no contamination was found.
There is wide recognition microbes require ‘vehicles’ for transportation such as lint, dust and air currents and moisture allows microorganisms to penetrate through packaging materials.20 Only one article on event-related sterility indicated high humidity promotes fungal growth.21 The Canadian Standards Association3 advises exposure to high humidity and moisture could allow ‘wicking’ of microbes through packaging and facilitate microbial proliferation, therefore this must be considered an event. Clearly, high humidity has an effect on sterility maintenance.
Several recent studies led by Dunkelberg demonstrate that the number of atmospheric pressure changes, increasing microbial load with consideration of humidity, resulted in increased levels of contamination of sterile packages.12,22,23
While studies by Rutala17 and Webster et al. 19 provide some assurance most packaging materials in use in healthcare facilities can withstand the rigours of storage and handling in uncontrolled environments while maintaining an effective microbial barrier, there is no clear evidence in the available literature that definitively demonstrates the impact of known levels of elevated temperature and humidity on sterility maintenance and links these to specified conditions for storage environments.
Is there any safe duration of exposure to these conditions and are there any simple method(s) to ensure sterility maintenance under adverse conditions?
AS/NZS4187:2003 makes no recommendations for monitoring temperature and humidity in sterile storage environments, nor does guidance available from the United Kingdom. However, ANSI/AAMI ST79:200624 and CSA3 recommend that temperature and humidity in storage areas be monitored and recorded daily. However, ANSI/AAMI24 gives no indication what action is required if limits are exceeded.
In many cases, commercially prepared SMD have specifications for storage conditions on package labels, thereby making judgement of whether sterility of the device has been compromised more straightforward. However, there is much less certainty in the case of SMD produced within SSDs.
A consensus statement developed by an ad hoc committee of sterilisation experts regarding high relative humidity advises that humidity in excess of 70% is considered an event, although no evidence is provided to support the selection of this critical variable.3 CSA recommends that where humidity >70% is detected, packs should be assessed and if no moisture or other effects are noted the packs may still be used. In addition, they recommend that corrective action be taken to rectify the problem, and items be transferred to an alternative location while humidity exceeds specification.3
If the humidity reading is still high 24 h later, any packs remaining in the storage area should be inspected again and a risk assessment conducted. Packs showing wetness, moisture or other adverse effects must be discarded; however, items packaged in impervious materials, such as plastic may still be able to be used. However, it should be noted that no evidence is cited for these recommendations.3
The USA Food and Drug Administration stated in an advisory notice that a strategy to protect sterile items from high humidity is to ‘enclose them in plastic containers to keep them dry’.25 This approach was first advocated by Mayworm.20 Therefore the strategies being practiced by SSD managers in Australian healthcare facilities are in keeping with those recommended elsewhere.
What are the specified temperature and humidity ranges in other guidance documents?
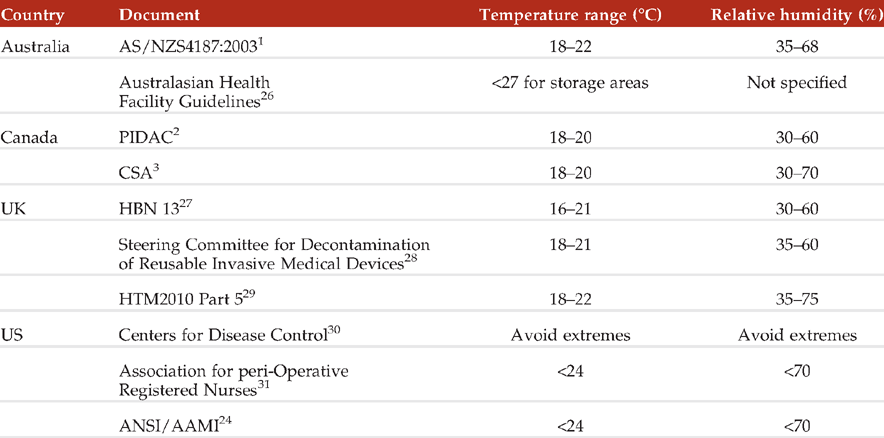
Review of guidance documents and standards revealed specifications for temperature and humidity were similar in range, although inexplicably different as seen in Table 1.
|
What evidence has been the basis for these conditions?
None of the published guidance cited evidence-based sources for specified temperature and humidity conditions. Where a source was indicated, tracing back to the original references showed brief mention of temperature and humidity being a factor influencing sterility maintenance, without any scientific evidence for this or indication of actual limits determined through an experimental process. ANSI/AAMI24 indicated the specified ranges have been adapted from the American Institute of Architects and not from published literature or studies on sterility maintenance.
Phillips13 indicates that commonly accepted definition for room temperature in various pharmacopoeias is 15–25°C and this definition is often used by medical device manufacturers. The adoption of room temperature conditions for storage of SMD by default was confirmed in email correspondence between the author, Dr F. McGain and Professor Tallentire.14
Therefore, it appears that specified conditions for temperature and humidity in sterile storage environments may have been derived from pharmacopoeias and guides to ‘good manufacturing practice’ rather than actual evidence of the effect of temperature and humidity on sterility maintenance, or at least this has been true in the past.13
What changes should be considered during future revisions of AS/NZS4187?
Based on information in Table 1, it appears published guidance supports storage conditions with upper and lower limits for temperature and humidity being between 16–27°C and RH 30–75%, respectively.
However, the article written by Phillips13 indicated many SMD manufacturers specify <25°C for storage of their SMD and labelling indicates items should be stored in ‘a dry place’. Some SMD manufacturers specify exact storage conditions for their products. For example Vallelylab32 indicates that RH >75% can affect the water-based adhesive on Tyvek® packaged products, while Cook33 indicates products can withstand RH up to 90% and temperatures <40°C.
Therefore, at face-value, it appears a storage temperature of 16–25°C and RH between 30% and 70–75% should be suitable for most SMD whether produced in an SSD or by a commercial manufacturer, unless the manufacturer specifies alternative conditions.
Providing these conditions are appropriate for those sterile barrier systems used in SSD applications, and subject to further confirmation, the author recommends that specified ranges for temperature and humidity in sterile storage environments according to AS/NZS4187 be changed to 16–25°C and RH 30–75%.
Conclusion
This paper has explored the effect of increased temperature and humidity in sterile storage environments and the possible impact on duration of sterility through examination of published literature and guidance. No scientific studies were identified that definitively examined this issue. Further, existing guidance documents are not consistent in specifying temperature and humidity conditions for storage of SMD and no indication is given as to evidence upon which conditions were based.
However, the literature review, while somewhat limited in scope, clearly demonstrates that requirements of the MDD and ISO11607 have led to developments in testing the performance of packaging systems used for commercially produced SMD and these concepts for package testing could possibly be applied to validation of packaging materials used in SSD.
Such testing would improve understanding of the temperature and humidity conditions able to be tolerated by packaging systems used in SSD and allow evidence-based specifications to be included in published guidance documents in the future.
Recommendations
-
Manufacturers of packaging systems used in SSD applications should consider validating the post-sterilisation performance of their products utilising accelerated ageing and environmental challenge testing protocols, in order to provide evidence-based instructions for acceptable temperature and humidity ranges in sterile storage environments.
-
The specified temperature and humidity conditions in AS/NZS4187 should be changed to a temperature range of 16–25°C and a RH of 30–75% during the next revision process, and make reference to following manufacturer’s instructions.
-
A requirement for daily monitoring of temperature and humidity in sterile storage areas should be included in the next edition of AS/NZS4187 along with recommendations for preventative and corrective action when conditions deviate from specified limits or those documented in manufacturer’s instructions.
Conflict of Interest
No conflict of interest has occurred in the development of this paper.
Acknowledgement
With thanks and in recognition of the Sul Stuart Fraser Scholarship awarded me by the College of Nursing, facilitating my participation in the Master of Science Medical Device Decontamination program offered by the University of Highlands and Islands, Scotland.
[1]
[2]
[3]
[4]
[5]
[6]
[7]
[8]
[9]
[10]
[11]
[12] Dunkelberg H, Rohmann S. Test to Determine Sterile Integrity of Wrapped Medical Products at a Probability of Recontamination of 1 : 1,000,000. Infect Control Hosp Epidemiol 2006; 27(4): 367–71.
| Crossref | GoogleScholarGoogle Scholar | PubMed | last accessed 27 April 2009
[14]
[15]
[16] Widmer AF, Houston A, Bollinger E, Wenzel RP. A new standard for sterility testing of autoclaved surgical trays. J Hosp Infect 1992; 21 253–60.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed | last accessed 16 April 2009
[22] Dunkelberg H, Fleitmann-Glende F. ‘Measurement of the microbial barrier effectiveness of sterilization containers in terms of the log reduction value for prevention of nosocomial infections.’ Am J Infect Control 2006; 34 285–9.
| Crossref | GoogleScholarGoogle Scholar | PubMed | last accessed 26 April 2009
[26]
[27]
[28]
[29]
[30]
[31] Association of peri-Operating Registered Nurses [AORN] ‘Recommended Practices for Selection and Use of Packaging Systems for Sterilization.’ AORN J 2007; 85(4): 801–12.
| Crossref | GoogleScholarGoogle Scholar | PubMed | last accessed 17 April 2009
[33]

