ASID (HICSIG)/AICA Position Statement: Preventing catheter-associated urinary tract infections in patients
Brett Mitchell A D E , Chris Ware A , Alistair McGregor A , Saffron Brown A , Anne Wells A , Rhonda L. Stuart B , Fiona Wilson A and Matthew Mason CA Tasmanian Infection Prevention and Control Unit, Department of Health and Human Services, Hobart, Tasmania, Australia.
B Department of Infection Prevention and Epidemiology and Department of Infectious Diseases, Southern Health, Melbourne, Victoria, Australia.
C James Cook University, Thursday Island, Qld, Australia.
D James Cook University, Townsville, Qld, Australia.
E Corresponding author. Tasmanian Infection Prevention & Control Unit, Population Health, GPO Box 125, Hobart 7001, Australia. Email: brett.mitchell@dhhs.tas.gov.au
Healthcare Infection 16(2) 45-52 https://doi.org/10.1071/HI11007
Submitted: 16 February 2011 Accepted: 2 May 2011 Published: 23 June 2011
Journal Compilation © Australian Infection Control Association 2011
Abstract
Catheter-associated urinary tract infections (CAUTIs) occur frequently in healthcare settings. The insertion and maintenance of indwelling urinary catheters is a routine element of healthcare. In order to prevent CAUTI, it is important that healthcare professionals providing catheter care understand the indications for catheter use and the correct procedure for insertion and maintenance of catheters. This paper reviews and summarises three recent key publications on the prevention of CAUTIs and proposes the use of a care bundle and checklist for catheter indications, insertion and maintenance, and quality improvement.
Process of position statement development
The Tasmanian Infection Prevention and Control Unit (TIPCU) examined and synthesised three catheter-associated urinary tract infections (CAUTI) reviews in the current literature. From this process a checklist/care bundle was developed. Substantial input was then received from the Australasian Society for Infectious Disease (ASID) members, including members of the ASID Healthcare Infection Control Special Interest Group (HICSIG), and the Australian Infection Control Association (AICA). The authors responded to all comments received.
Background
Catheter-associated urinary tract infections (CAUTIs) are common in healthcare settings. Around 20% of all healthcare-associated infections are urinary tract infections,1 and most of these are associated with some form of instrumentation of the urinary tract.2
Between 15% and 25% of hospitalised patients receive a short-term indwelling urinary catheter.3–6 The duration of catheterisation is the most important risk factor for development of infection,7 while additional risk factors include female sex, older age and not maintaining a closed drainage system.8
The limitations and heterogeneity of definitions used in various studies present major challenges to defining clinical and surveillance definitions of CAUTI.2 The three articles reviewed in this paper do not provide definitions, but rather defer to National Healthcare Safety Network9 (NHSN) definitions, or in the case of Tenke et al. (2009) definitions are not discussed. NHSN definitions were recently revised and now provide additional clarity and uniformity in the distinguishing of asymptomatic and symptomatic CAUTI and the time period for follow-up surveillance following catheter removal.2
Most microorganisms causing CAUTI derive from the patient’s own colonic and perineal flora or from the hands of healthcare workers.10,11 Bacteria can enter the urinary tract at the time of catheter insertion via the catheter tip or subsequently once the patient is catheterised via the extraluminal or intraluminal route. Common inoculation source-sites include the catheter tip, when the perineum and distal urethra are inadequately cleaned;12 the taps of the drainage bag which afford bacteria access to the catheter and eventually the bladder;7 and residual urine remaining in the collection bag, which acts as a focus for organisms to spread via the intraluminal route.12 While infections may arise via the intraluminal route when a closed system is not maintained or the collection bag is contaminated, it is believed that the majority of infections arise from extraluminal (via the outside of the catheter) bacterial migration.10
To reduce the risk of CAUTI, there is a need to develop and implement a range of prevention measures. Over the past 2 years, three major publications have reviewed the literature and developed recommendations on the prevention of CAUTIs, namely:
-
European and Asian Guidelines on management and prevention of catheter-associated urinary tract infections;7
-
Strategies to Prevent Catheter-Associated Urinary Tract Infections in Acute Care Hospitals Practice Recommendation, Society for Healthcare Epidemiology of America (SHEA) and Infectious Diseases Society of America;8
-
Guidelines for Prevention of Catheter-Associated Urinary Tract Infections 2010, Healthcare Infection Control Practices Advisory Committee (HICPAC).2
The purpose of this paper is to propose a clear approach to the prevention of CAUTIs for adoption across Australia applicable to all healthcare settings including residential and aged care. The approach described is consistent with an Australian context, using recommendations from the publications described above and the NHMRC Australian Guidelines for the Prevention and Control of Infection in Healthcare.13 A suggested checklist has also been developed for the insertion of indwelling urinary catheters.
Recommendations
Practices to minimise the risk of CAUTIs can be summarised into three distinct areas: insertion, maintenance and quality improvement. This paper provides recommendations supported by the literature in each of these areas. A summary of key recommendations, including their grading, are detailed in Tables 1 and 2.

|

|
Insertion
The first and foremost goal of CAUTI prevention is a reduction in the overall use of indwelling catheters, especially in those at high risk of complications from CAUTIs. Those at high risk include women, the elderly and patients with impaired immunity, severe underlying illness, diabetes, renal dysfunction or incontinence.2 Wherever possible, alternatives, such as suprapubic catheters, condom drainage systems or intermittent catheterisation should always be considered where appropriate.2,7 To assist in this process, there is a need to establish clear indications for the use of indwelling catheters, as summarised in Table 3. Low quality evidence suggested a benefit of alternative catherisation methods in selected population groups.2

|
When inserting a urethral catheter, the correct procedure must be followed to mimimise the risk of introducing bacteria into the bladder. Recommendations relating to the insertion of indwelling catheters are summarised as follows.
-
Provide and follow written guidelines for catheter use, insertion and maintenance.8
-
Consider the use of a portable ultrasound device to assess urine volume in patients undergoing intermittent catheterisation.2,8
-
Implement a system for documenting the following information in the patient record: indications for catheter insertion, date and time of catheter insertion, details of the individual who inserted catheter, and date and time of catheter removal.8
-
Ensure that only trained, competent personnel insert urinary catheters.8 This may include hospital personnel, family members, or patients themselves who know the correct technique of aseptic catheter insertion and maintenance.2
-
Perform hand hygiene immediately before insertion of the catheter.2,8
-
Clean (i.e. non-sterile) equipment is appropriate for chronic intermittent catheterisation in a non-acute care setting.7,14,15
-
Insert an indwelling catheter using an aseptic non-touch technique, using sterile equipment. Equipment required includes: sterile gloves, a drape, sterile swabs, a sterile solution for cleaning the urethral meatus, and a single-use packet of sterile lubricant jelly for insertion.2,8,13 Antiseptic lubricants need not be used routinely to prevent CAUTI.13,14 While there is very low-quality evidence suggesting a reduced risk of CAUTI from using lubricants during catheter insertion, several studies have found no significant difference between antiseptic lubricants and non-antiseptic lubricants in preventing CAUTI.2
-
Use the smallest catheter as possible, consistent with proper drainage, to minimise urethral trauma.2,8
-
Do not use antibiotic-impregnated catheters routinely as there is no evidence that they decrease symptomatic infection.7
-
Do not use systemic antimicrobials routinely as prophylaxis in patients requiring either short or long-term catheterisation unless indications exist.2,8 There are studies suggesting the incidence of catheter-associated bacteruria may be reduced by antimicrobial prophylaxis;11,16 however, this protective effect is transient (lasts only a few days) and is associated with the selection of resistant organisms. Prophylaxis is not indicated for patients at low risk for acquired bacteruria and in whom the sequelae of catheter-associated infections are infrequent.16
-
Urinary catheter systems with pre-connected, sealed catheter-tubing junctions may reduce the risk of CAUTI compared with unsealed catheter systems.2
Maintenance
The following suite of strategies relating to the maintenance of a catheter will aid in the reduction of infection risk.
-
Properly secure indwelling catheters after insertion to prevent movement and urethral traction.2,8
-
Perform hand hygiene before and after any manipulation of the catheter site or apparatus,2,8 consistent with the ‘five moments’ of hand hygiene.17
-
Use standard precautions as appropriate during any manipulation of the catheter or collecting system.2
-
The catheter must be maintained as a sterile, continuously closed drainage system, with unobstructed urine flow.2,7,8
-
Do not disconnect the catheter and drainage tube unless the catheter has to be irrigated.
-
Keep the collecting bag below the level of the bladder at all times, but do not rest the bag on the floor.2,8,13
-
If breaks in aseptic non-touch technique, disconnection, or leakage occur, replace the catheter and collecting system using aseptic non-touch technique and sterile equipment.2,8
-
Avoid catheter irrigation if possible, and do not perform continuous irrigation of the bladder with antimicrobials as a routine infection prevention measure. If obstruction is anticipated, closed continuous irrigation may be used to prevent it. To relieve obstruction due to clots, mucus or other causes, an intermittent method of irrigation may be used.8
-
The use of topical antiseptics or antibiotics applied to the catheter, urethra or meatus is not recommended;7 routine hygiene is adequate.13
-
Chronic antibiotic suppressive therapy is generally not recommended.7
-
For examination of fresh urine, a small sample should be collected by aspirating urine from the sampling port with a sterile needle and syringe after cleansing the port with disinfectant.2,8 Obtain larger volumes of urine for special analyses (not culture) aseptically from the drainage bag.2,8
-
Do not screen for asymptomatic bacteruria (ASB) in catheterised patients using a dipstick or a laboratory test (M&C&S) (note that ASB is no longer included in the NHSN surveillance definitions for UTI).2,8
-
Do not treat ASB in catheterised patients except before invasive urologic procedures.8
-
Long-term indwelling catheters should be changed at intervals adapted to the individual patient.7 There is insufficient evidence to support the practice of changing catheters at fixed intervals.7 It is suggested therefore, that the decision to change catheters and drainage bags should be based on clinical indications such as infection, obstruction or a break in the closed system.2
-
Clamping indwelling catheters before removal is unnecessary.2,13
-
Minimise the duration of catheterisation; that is, catheterisation should be only for as long as indications exist.2,7,8
Quality improvement
Positioning CAUTI prevention strategies within an infection prevention and control framework allows for targeted monitoring, assessment and evaluation of interventions. Furthermore, it requires the identification of responsibilities and roles for addressing the prevention of CAUTIs. Under the umbrella of quality improvement, the following recommendations provide guidance to organisations to reduce the risk of CAUTI.
-
A hospital or healthcare organisations’ Chief Executive Officer and senior management should be responsible for ensuring that the healthcare system supports an infection prevention and control program and that this program reduces the risks of CAUTI and the transmission of epidemiologically significant pathogens.8 An infection control program should include the provision of education about CAUTI, other complications of urinary catheterisation and alternatives to indwelling catheters.2,8
-
Healthcare organisations should consider implementing quality improvement (QI) programs to enhance appropriate use of indwelling catheters and to reduce the risk of CAUTI.2 Such QI programs could include the development and implementation of institutional policies requiring continual (daily) review of the need for continued catheterisation.8 Electronic or other types of reminders may be useful in assisting in complying with such a recommendation. Examples of strategies to monitor the continued need for urinary catheters include: automatic stop orders requiring renewal of the order for continuation of the indwelling catheter; standardised reminders placed into the patient record; and daily ward rounds by clinical staff to review all patients with urinary catheters and to ascertain continuing necessity of the catheter.8
-
A surveillance program (either process or outcome) would assist in monitoring the rates of urinary tract infections. Such a program could be developed based on a facility risk assessment.2 Examples of programs related to CAUTI surveillance and QI could include the establishment of a system for analysing and reporting data on catheter use and adverse events from the catheter, including stratification by relevant risk factors (e.g. sex, age, ward and duration).2,8
Unresolved issues
Either a lack of compelling evidence or a limited number of studies make it difficult to form recommendations addressing several clinical and administrative aspects of catheter use. To elicit appropriate guidance and resolve these issues, further work is required in the following areas:
-
Evidence on effect of silver alloy catheters in reducing the risk of symptomatic and asymptomatic bacteruria has been reported in a small number of studies.18–21 However, reviews of catheter materials have concluded that the use of silver alloy impregnated catheters has no significant advantages and these are not recommended for routine use. The question of the benefit of using other types of antimicrobial-coated catheters remains unresolved as does the question of identifying which catheter materials best delay the onset of bacteruria, bacterial adherence and bacterial growth.7,8
-
The benefit of using an antiseptic solution versus the use of sterile saline for meatal cleaning before catheter insertion remains unresolved.2,8
-
The role of periodic (e.g. night time) use of condom catheters in incontinent male patients is unresolved.2,8
-
The use of catheters to prevent skin breakdown is unresolved.2,8
-
There is limited evidence that post-operative intermittent catheterisation reduces the risk of bacteruria compared with an indwelling catheter.7
-
Finally, there are no standardised surveillance measures of CAUTI that would aid in inter-regional assessments and external reporting.8
Suggested care bundle/checklist
To elicit a consistent and pro-active response to the guidelines summarised here, we recommend the adoption of a care bundle/checklist (Figs 1, 2). Primarily, this would serve as a checklist of considerations taken to ensure catheter use is limited. If catheterisation is indicated, the checklist provides evidence-based guidelines to follow to reduce the risk of acquiring a CAUTI. Secondarily, the use of a checklist provides a source of catheter documentation, this being an important step in the monitoring and analysis of CAUTIs. The checklist could exist as a preliminary stand-alone measure before the implementation of a more holistic institutional policy, or as an adjunct to such a policy, such as a clinical care pathway. Regardless of where the checklist is positioned within institutional policy, in order to achieve holistic, systemic improvements to the way catheter care is administered, the checklist should be underpinned by leadership, culture change and measurement.22,23 Further, the checklist could easily be broadened to include post-insertion information such as the onset of bacteruria, any other adverse events resulting from catheter use and the eventual duration of catheterisation. The checklist provided can be added to or be modified according to organisational needs.
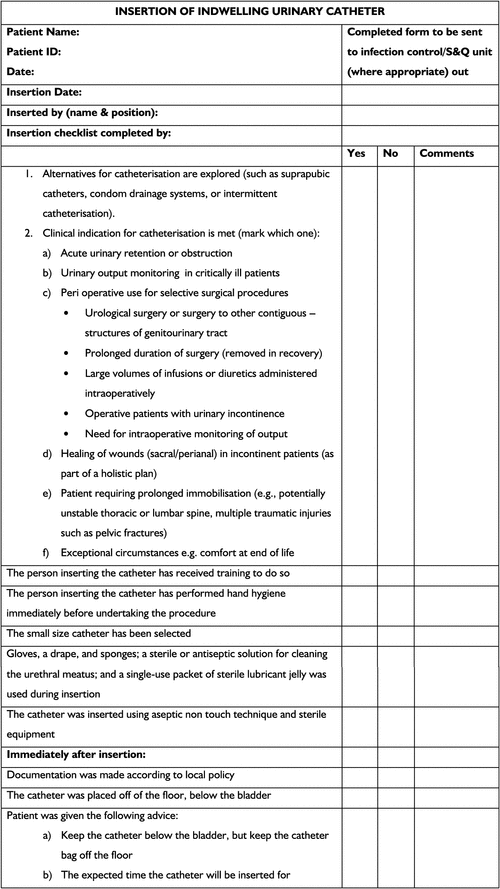
|
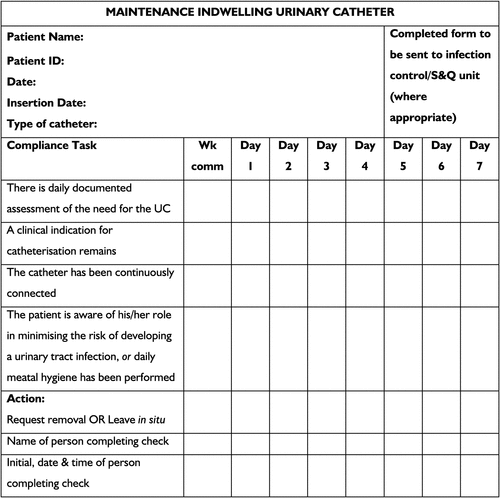
|
Summary and future work
After reviewing key publications on the prevention of CAUTIs, it appears there is consensus on the major recommendations and issues relating to the insertion, maintenance and quality improvement practices needed to reduce the incidence of CAUTIs. This AICA/ASID position statement reflects these recommendations. One of the key principles in the prevention of CAUTIs is to minimise both the number of indwelling catheters inserted and also the duration of catheterisation of individuals. Quality improvement activities form a fundamental part of CAUTI prevention, with one key QI activity being surveillance. The issue of CAUTI surveillance is an area that particularly requires further work and exploration, and the AICA and ASID would encourage this work to occur.
Conflicts of interest
The authors have no conflicts to declare.
Acknowledgements
The authors acknowledge the feedback provided by members from the Australian Infection Control Association and the Australasian Society of Infectious Diseases. The authors would like to particularly acknowledge Claire Boardman and Dr John Ferguson for facilitating this process.
References
[1] Smyth ETM, McIlvenny G, Enstone JE, Emmerson AM, Humphreys H, Fitzpatrick F, et al Four country healthcare associated infection prevalence survey 2006: overview of the results. J Hosp Infect 2008; 69 230–48.| Four country healthcare associated infection prevalence survey 2006: overview of the results.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cngsVWnsA%3D%3D&md5=8f772742e4885cac0ec8f84da1520ca2CAS | 18550218PubMed |
[2] Gould CV, Umscheid Craig A, Agarwal Rajender K, Kuntz G, Pegues David A. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol 2010; 31 319–26.
| Guideline for prevention of catheter-associated urinary tract infections 2009.Crossref | GoogleScholarGoogle Scholar | 20156062PubMed |
[3] Saint S, Wiese J, Amory JK, Bernstein ML, Patel UD, Zemencuk JK, et al Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med 2000; 109 476–80.
| Are physicians aware of which of their patients have indwelling urinary catheters?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M%2FjsVSksw%3D%3D&md5=fb3bad4a6be1f216cbf04e10db50934eCAS | 11042237PubMed |
[4] Warren J. Catheter-associated urinary tract infections. Journal of Antimicrobial Agents 2001; 17 299–303.
| Catheter-associated urinary tract infections.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXisVals7Y%3D&md5=dd1089d4d4d36ef4359f41ca2965cc26CAS |
[5] Munasinghe R, Yazdani H, Siddique M, Hafeez W. Appropriateness of use of indwelling urinary catheters in patients admitted to the medical service. Infect Control Hosp Epidemiol 2001; 22 647–9.
| Appropriateness of use of indwelling urinary catheters in patients admitted to the medical service.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38%2FkvVyitw%3D%3D&md5=bdf0eae8fc62231b61f460d5c0b444b1CAS | 11776352PubMed |
[6] Jain P, Parada JP, David A, Smith LG. Overuse of the indwelling urinary tract catheter in hospitalized medical patients. Arch Intern Med 1995; 155 1425–9.
| Overuse of the indwelling urinary tract catheter in hospitalized medical patients.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2MzhsVCgtA%3D%3D&md5=15b2df1c8d2fe0f891c77b95417bae1dCAS | 7794092PubMed |
[7] Tenke P, Kovacs B, Bjerklund Johansen TE, Matsumoto T, Tambyah PA, Naber KG. European and Asian guidelines on management and prevention of catheter-associated urinary tract infections. Int J Antimicrob Agents 2008; 31 68–78.
| European and Asian guidelines on management and prevention of catheter-associated urinary tract infections.Crossref | GoogleScholarGoogle Scholar |
[8] Lo E, Nicolle L, Classen D, Arias Kathleen M, Podgorny K, Anderson Deverick J, et al Strategies to revent catheter-associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol 2008; 29 S41–50.
| Strategies to revent catheter-associated urinary tract infections in acute care hospitals.Crossref | GoogleScholarGoogle Scholar | 18840088PubMed |
[9] Centres for Disease Control and Prevention. NHSN Patient Safety Component Manual. 2010. Available from http://www.cdcgov/nhsn/TOC_PSCManualhtml (accessed Jan 2011).
[10] Maki DG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis 2001; 7 1–6.
| Engineering out the risk for infection with urinary catheters.Crossref | GoogleScholarGoogle Scholar | 11266288PubMed |
[11] Garibaldi RA, Burke JP, Britt MR, Miller WA, Smith CB. Meatal colonization and catheter-associated bacteriuria. N Engl J Med 1980; 303 316–8.
| Meatal colonization and catheter-associated bacteriuria.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3c3gvF2qtQ%3D%3D&md5=a6e6331ace4844b16fd3d6040d755b1dCAS | 6991947PubMed |
[12] Barford JMT, Coates ARM. The pathogenesis of catheter-associated urinary tract infection. British Journal of Infection Control 2009; 10 50–56.
[13] NHMRC. Australian Guidelines for the Prevention & Control of Infection in Healthcare. Canberra: Commonwealth of Australia; 2010.
[14] Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol 2010; 31 319–26.
| Guideline for prevention of catheter-associated urinary tract infections 2009.Crossref | GoogleScholarGoogle Scholar | 20156062PubMed |
[15] Lo E, Nicolle L, Classen D, Arias KM, Podgorny K, Anderson DJ, et al Strategies to prevent catheter associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol 2008; 29 S41–50.
| Strategies to prevent catheter associated urinary tract infections in acute care hospitals.Crossref | GoogleScholarGoogle Scholar | 18840088PubMed |
[16] Britt MR, Garibaldi RA, Miller WA, Hebertson RM, Burke JP. Antimicrobial prophylaxis for catheter-associated bacteriuria. Antimicrob Agents Chemother 1977; 11 240–243.
| 1:STN:280:DyaE2s7ls1Shuw%3D%3D&md5=a4e3a1040a6bac1b8182feb51d8bb35dCAS | 848927PubMed |
[17] Grayson L, Russo P, Ryan K, Bellis K, Havers S, Heard K, et al. eds. 5 Moments for Hand Hygiene. Canberra: Australian Commission on Safety and Quality in Healthcare, 2010. Available from http://www.hha.org.au/home/5-moments-for-hand-hygiene.aspx (accessed Jan 2011).
[18] Niël-Weise BS, Arend SM, van den Broek PJ. Is there evidence for recommending silver-coated urinary catheters in guidelines? J Hosp Infect 2002; 52 81–7.
| Is there evidence for recommending silver-coated urinary catheters in guidelines?Crossref | GoogleScholarGoogle Scholar | 12392898PubMed |
[19] Brosnahan J, Jull A, Tracy C. Types of urethral catheters for management of short-term voiding problems in hospitalised adults. Cochrane Database of Systematic Reviews 2004 (issue 1).
[20] Jahn P, Preuss M, Kernig A, Seifert-Hühmer A, Langer G. Types of indwelling urinary catheters for long-term bladder drainage in adults. Cochrane Database of Systematic Reviews 2007 (issue 3).
[21] Scott BM. Clinical and cost effectiveness of urethral catheterisation: a review. J Perioper Pract 2010; 20 235–40.
| 20701200PubMed |
[22] Morton A, Cook D, Mengersen K, Waterhouse M. Limiting risk of hospital adverse events: avoiding train wrecks is more important than counting and reporting them. J Hosp Infect 2010; 76 283–6.
| Limiting risk of hospital adverse events: avoiding train wrecks is more important than counting and reporting them.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cbntVeqtA%3D%3D&md5=0050e870325f18376b449b9a6d4b08bcCAS | 20692071PubMed |
[23] Bosk CL, Dixon-Woods M, Goeschel CA, Pronovost PJ. The art of medicine: reality check for checklists. Lancet 2009; 374 444–5.
| The art of medicine: reality check for checklists.Crossref | GoogleScholarGoogle Scholar | 19681190PubMed |
[24] Pratt RJ, Pellowe CM, Wilson JA, Loveday HP, Harper PJ, Jones S, et al epic2: National evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 2007; 65 S1–59.
| epic2: National evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England.Crossref | GoogleScholarGoogle Scholar | 17307562PubMed |
[25] Health Protection Scotland. CAUTI maintenance bundle. 2008. Available from http://www.hpsscotnhsuk/haiic/ic/CAUTIPreventionBundleaspx (accessed Jan 2011).
[26] Institute for Healthcare Improvement. Urinary Tract Infection Bundle Compliance Audit Tool. Institute for Healthcare Improvement 2010 [cited 2010 4th January 2011]. Available from: http://www.ihi.org/IHI/Topics/HealthcareAssociatedInfections/InfectionsGeneral/Tools/UTIBundleComplianceAuditTool.htm

