Repeated multimodal supervision programs to reduce the central line-associated bloodstream infection rates in an Indian corporate hospital
Namita Jaggi A B and Pushpa Sissodia AA Artemis Health Institute, Sector 51, Gurgaon, Haryana 122001, India.
B Corresponding author. Email: namita@artemishealthsciences.com
Healthcare Infection 19(2) 53-58 https://doi.org/10.1071/HI13030
Submitted: 2 July 2012 Accepted: 16 October 2013 Published: 19 December 2013
Abstract
Background: Central line-associated bloodstream infections (CLABSI) are associated with significant morbidity, mortality and costs. Multimodal intervention programs are effective in bringing down the rates of CLABSI, but they are difficult to sustain. In an attempt to improve sustainability, we implemented two multimodal intervention programs focusing on high-yield measures and assessed their effect on monthly CLABSI rates over a period of 42 months.
Methods: The CLABSI rates were tracked on a monthly basis in a 300-bed Indian Corporate hospital and an analysis of the various contributing variables was done. The first intervention program in July 2009 put into practice the central line bundle. The second program went beyond the bundle and introduced high-yield measures like dedicated central line team and trolley, involved the senior management and promoted the ‘Scrub the Hub’ campaign while rectifying deficiencies observed in the first intervention program. The rates of CLABSI were statistically analysed in both the pre- and post-intervention periods.
Results: The CLABSI rates varied between 0 to 9.8 infections per 1000 catheter days in the 42 months period, the mean being 2.9. The difference in mean CLABSI rates before and after the first intervention program was not significant (5.2 versus 4.4 infections per 1000 catheter days (P > 0.05)). However, the next intervention program saw a significant decrease in the mean rates of CLABSI in the subsequent 24 months (0.7 infections per 1000 catheter days (P < 0.05)). An overall 86.3% reduction in CLABSI rates in the entire study period was observed.
Conclusions: Repeated multimodal intervention programs with a focus on high-yield measures resulted in a sustained reduction in CLABSI rates (86.3%).
Implications
-
Successive intervention programs bring about a reduction in CLABSI rates.
-
High-yield measures (hand hygiene, daily review of line necessity and needleless connectors) may prove effective.
-
Involvement of senior management is an effective strategy to facilitate a sense of responsibility and a safety culture within the organisation.
Background
Central line-associated bloodstream infections (CLABSIs) are the first or second most common hospital-acquired infections (HAI) in intensive care units (ICU) (30 to 40%)1 and a predominant infection in chemotherapy units and dialysis. They are costly and potentially lethal with a mortality rate of 12 to 25%.2 CLABSI rates in ICUs range from 0.0 to 5.4 throughout the world:3 1.5 to 2.9 in developed countries4 and 7.7 to 17.6 in developing countries5.
Centers for Disease Control and Prevention6 (CDC) defines CLABSI as bacteraemia and/or fungaemia in a patient with an intravascular catheter with at least one positive blood culture obtained from a peripheral vein, clinical manifestations of infections (i.e. fever, chills, and/or hypotension), and no apparent source for the bloodstream infection except the catheter. CLABSI has been identified by CDC as one of its seven healthcare safety challenges with a goal to reduce such complications by 50% in 5 years.7
Many factors influence CLABSI,8 including catheter-related factors such as the material of the lines, patient-related factors9 and pathogenic mechanisms of the microbe.10 An intervention by Pronovost et al.11 demonstrated that the incidence of CLABSI could be significantly reduced by skin antisepsis with chlorhexidine (2%), maximal sterile precautions, hand hygiene, optimal catheter site selection and daily review of line necessity.6,11
Traditionally in the western world, Gram-positive isolates predominate in CLABSIs (80%) with coagulase-negative Staphylococci (CONS), Staphylococcus aureus, and Enterococci being the commonly isolated ones.12 However, some studies indicate a Gram-negative preponderance.13 The Gram-negatives include Pseudomonas spp., Enterobacter spp., Serratia spp., Klebsiella spp., E. coli and Acinetobacter baumannii. Candida spp., especially Candida albicans and Candida glabrata are the common fungal pathogens in patients receiving parenteral nutrition fluids.12
To study the impact of repeated multimodal intervention programs involving bundled interventions on CLABSI rates, we undertook this study for a period of 42 months (January 2009 to June 2012) in the form of a 6-month pre-intervention period (January 2009 to June 2009) followed by two interventions: Phase I (July 2009 to June 2010) and Phase II (July 2010 to June 2011) in a tertiary-care hospital in India. A 1-year post-intervention period (July 2011 to June 2012) was also included in the study. In our interventions we moved beyond the bundle and looked at other factors which could positively impact CLABSI.
Methods
Setting
A 300-bed tertiary-care private hospital in India.
Study design
The present study was carried out for a 3.5-year period (January 2009 to June 2012) in patients from the ICU and wards (admitted under cardiology, neurology, oncology and nephrology specialities) which involved retrospective analysis for the first 6 months followed by the prospective analysis of the CLABSI rates. We also introduced multimodal intervention programs in the form of CLABSI prevention bundle and observed its impact on reduction of CLABSI in the subsequent months.
The study was divided into the following phases.
Pre-intervention
The study began with a retrospective analysis of CLABSI rates for a period of 6 months (January 2009 to June 2009). Diagnosis of a CLABSI case was done by utilising the appropriate clinical and laboratory diagnostic criteria as recommended by CDC- NNIS (National Nosocomial Infections Surveillance System) and NHSN (National Healthcare Safety Network).
CLABSI rates were calculated as:

Intervention
Phase I: first intervention program (July 2009). The first intervention program introduced the ‘Central line bundle’– a set of evidence-based interventions for patients with central lines that, when implemented together have been shown to result in better outcomes than when implemented individually.14 Implementing the bundle involved establishing a culture of safety among clinicians, ensuring access to resources, knowledge and competence among all healthcare workers associated with central line.
Bundle components:
-
use of chlorhexidine skin antisepsis (2% chlorhexidine w/v in 70% alcohol)
-
maximal barrier precautions (patient: sterile drape covering from head to toe with a small opening and operator: cap, mask, gown and gloves)
-
hand hygiene (WHO 5 moments of hand hygiene were used as standards: particularly at the point-of-care and during high-risk procedures (before and after contact with central line)).
-
optimal catheter site selection (subclavian site preferred)
-
daily review of line necessity
-
introduction of needleless connectors (neutral displacement, luer-activated, mechanical valve with internal blunt cannula offering a closed system)
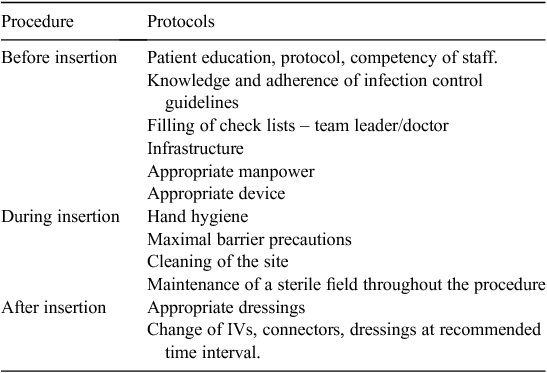
Protocols were established before, during and after insertion of the central line (Table 1) based on the Society for Healthcare Epidemiology of America (SHEA) and Infectious Diseases Society of America (IDSA) guidelines15 and incorporated into the CLABSI checklist (attached as Appendix).
|
Process surveillance was conducted to see the compliance achieved to the bundle components. The impact of the intervention on the CLABSI rates was prospectively analysed in the subsequent 1-year period (July 2009 to June 2010) and deficiencies noted.
Phase II: second intervention program (June 2010). The second intervention program in June 2010 saw us moving beyond the bundle and the following interventions were included:
-
Rectification of the deficiencies observed in the first intervention program and reiteration of all the components of bundle
-
Introduction of an active, dedicated and trained central line team. This team involved a group of personnel trained in central line insertion and maintenance to reduce the chances of repeated manipulation of the line by the untrained staff leading to reduced contamination and overall CLABSIs.
-
Dedicated central line trolley with supplies. This trolley contained all the essential items for central line insertion and maintenance procedure in order to prevent the occurrence of CLABSI due to contaminated trolleys being used for other purposes and also to decrease movement of personnel and instruments outside of the aseptic field.
-
‘Scrub the Hub’ campaign: It involved cleaning of the hub of needleless connectors with an alcohol swab at least for 15 s every time the hub was accessed to reduce contamination.
-
Teamwork building and development of communication skills
-
Involvement of senior doctors and management in the programs by highlighting the data
The impact of the second intervention on CLABSI rates was analysed in the intervention period phase II (July 2010 to June 2011).
Post-intervention
The reduction in CLABSI rates was again observed in the post intervention period (July 2011 to June 2012) in order to observe the sustaining effects of the interventions.
Data collection. The compliance of the individual bundle was checked from the checklists filled by the staff for each central line. Each checklist was collected and the data was compiled for a month. The mean monthly compliance with the checklist was calculated.
Data analysis. The data was analysed statistically for the pre-intervention, intervention and the post-intervention periods using SPSS software version 20.0 (IBM Corp., Armonk, NY, USA). The CLABSI rates followed a normal distribution and therefore the mean values of the data were used for analysis. A paired t-test was applied to the dataset and P-values were obtained. The significance (P < 0.05) of each bundle element in contributing CLABSI reduction was calculated statistically.
Ethics waiver was granted by ethics committee as this was an observational study.
Results
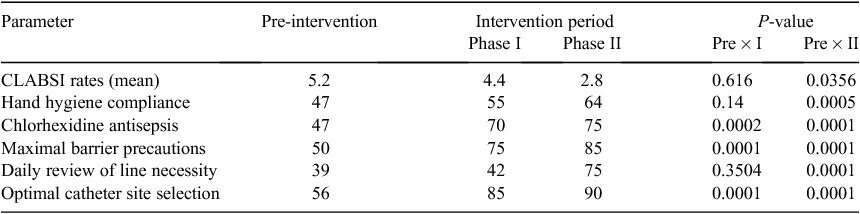
The CLABSI rates varied between 0.0 to 9.8 infections per 1000 days in the 42 months period showing a normal distribution, the mean being 2.7 (overall 38 CLABSI for 13 459 catheter days). The first 2 months were the observation months with zero CLABSI rates, therefore the actual analysis began from March 2009 when the CLABSI rates started rising from 4.7 in March to 9.8 per 1000 catheter days in June 2009. The mean CLABSI rate for the first 6 months was 5.2 infections per 1000 catheter days.
After the first intervention program introduced in July 2009, the mean CLABSI rates in the intervention period Phase I (July 2009 to June 2010) were 4.4. The percentage reduction in the CLABSI rates in this period was 15.3% (P > 0.05). The bundle components hand hygiene, use of chlorhexidine skin antisepsis, maximal barrier precautions, optimal catheter site selection, daily review of line necessity and use of needleless connectors showed an individual average compliance of 55%, 70%, 75%, 85%, 42% and 41% respectively (Table 2).
The deficiencies detected following the first intervention program included lack of adherence to the prevention bundle components (especially hand hygiene and daily review of line necessity showing low bundle compliance of 55% and 42% respectively), inadequate training of staff regarding insertion, maintenance and removal of central line, lack of dedicated central line trolley and low acceptance of needleless connectors.
The CLABSI rates also increased in the last few months of this period. This indicated the need of a second intervention program to fill in the gaps left by the first one.
The second intervention program introduced in June 2010 showed a significant decrease of 36.9% in the mean rates of CLABSI in the subsequent year reducing the rate to 2.8 per 1000 catheter days (P < 0.05). The compliance to the bundle components was reviewed and an increase of 16.3% and 78.6% was observed in hand hygiene and daily review of line necessity from the previous figures, raising their individual compliance rates to 64% and 75% respectively. There was a further reduction of 74.6% in the mean CLABSI rates (0.7 per 1000 catheter days) in the post intervention period (July 2011 to June 2012).
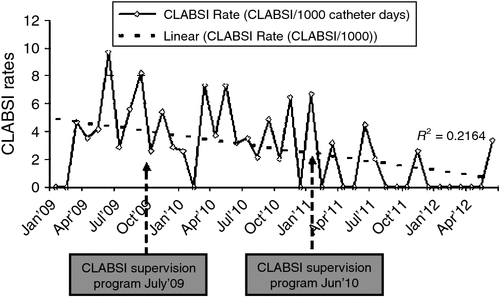
An overall 86.3% reduction was observed in the CLABSI rates in the 3.5-year study period as the mean CLABSI rate reduced from 5.2 per 1000 catheter days (pre intervention period) to 0.7 per 1000 catheter days (post intervention period) (Fig. 1).
|
The organisms isolated from CLABSI cases were studied and showed the predominance of Gram-negative isolates 79.3% over the others (Gram-positive 17.2% and yeasts 3.5%) (Table 3).

|
Discussion
Intravascular catheters are indispensable in modern-day medical practice, particularly in ICUs. Although such catheters provide necessary vascular access, their use puts patients at risk for local and systemic infectious complications, including local site infection, CLABSI, septic thrombophlebitis, endocarditis, and other metastatic infections (e.g. lung abscess, brain abscess, osteomyelitis, and endophthalmitis). However, the majority of serious catheter-related infections are associated with central venous catheters (CVCs). In the ICU, central venous access might be needed for extended periods of time and the catheter can be manipulated multiple times per day for the administration of fluids, drugs, and blood products, allowing patients to be colonised with hospital-acquired organisms.4
The magnitude of CLABSIs in our study ranged from 0.0 to 9.7 infections per 1000 catheter days and the mean being 2.7. According to the NHSN system of the CDC, the pooled mean of CVC-associated bloodstream infection is 4.5 per 1000 catheter days in the ICU16 and the median rate of CLABSI ranges from 1.8 to 5.2 per 1000 catheter days.6,17 Though our rates are below the pooled mean of NHSN, our target is always to minimise CLABSI to a lowest possible level with the aspirational target being zero.
A multimodal intervention program has been shown to effectively reduce the rate of CLABSIs.14,18 Based on this premise, we introduced a multimodal intervention program in the month of July 2009 incorporating bundle components and assessed its effect on CLABSI rates in the subsequent months. The lowest compliance was observed with hand hygiene (55%) and daily review of line necessity (42%). Appropriate hand hygiene before catheter insertion or maintenance, combined with proper aseptic technique during catheter manipulation, provides protection against CLABSI.6 The reason for low compliance rates in hand hygiene may be high attrition rate of staff and work overload. Daily review of line necessity showed a low compliance due to lack of knowledge and inadequate training regarding its importance in preventing infections. Use of chlorhexidine skin antisepsis, maximal barrier precautions and optimal site of catheter insertion were, however, adhered to by the staff. Use of needleless connectors with closed ports was also encouraged to reduce the incidence of sharp injuries and possible decrease of manipulation and contamination,19 thereby reducing CLABSI.
The deficiencies detected in the first intervention program were analysed and rectified in the following intervention program (June 2010). The possible causes of the deficiencies may have been inadequate training of staff regarding insertion, maintenance and removal of central line, low acceptance of needleless connectors initially, lack of proper documentation and lack of adherence to checklists and inadequate audits.
While reiterating the bundle components in the second intervention program the hand hygiene compliance rose from 55% to 64% and daily review of line necessity from 42% to 75% in the following year. All this resulted in further reduction of CLABSI rates in the intervention period from 4.4 per 1000 catheter days in phase I to 2.8 per 1000 catheter days in phase II and 0.7 per 1000 catheter days in the post-intervention period (overall reduction: 84.1%). The other interventions in the second intervention program were introduction of an active, dedicated and trained central line team, a dedicated central line trolley with supplies, ‘Scrub the Hub’ campaign, teamwork building and involvement of the senior management in the programs by highlighting the data. All these interventions were complied to, leading to an overall decrease in CLABSI rates.
The analysis of the repeated multimodal intervention programs showed an overall reduction of 86.3%, as compared with the pre-intervention mean rates from 5.2 to 0.7 CLABSIs per 1000 catheter days in the post-intervention period. Corresponding results were obtained by Pronovost et al.11 where the median rate of infection decreased from 2.7 per 1000 catheter-days at baseline to 0 within the first 3 months after the implementation of the intervention and an overall 66% reduction in the CLABSI rates in 16 to 18 months. Another study by Rosenthal20 showed a reduction in CLABSI rates in three phases (from 45.9 to 11.1 bloodstream infections per1000 catheter days) as a result of two consecutive intervention programs.
The pathogens isolated from cultures included Gram-negative organisms, 79.3% (K. pneumonia: 27.6%, A. baumannii: 24.1% and E. coli: 6.9%), Gram-positive organisms, 17.2% (S. aureus and CONS each contributing 6.9%), and fungal pathogens, 3.5% (only Candida spp. was isolated). A similar study done by Tan et al.13 also reported the Gram-negatives as the most common organisms, 80.5%. In contrast to our findings, studies by CDC-NNIS systems have reported Gram-negative bacilli, 14%, with S. aureus and CONS, 37% and 13% respectively.6
Studies on Candida spp. have revealed its contribution as 8% to catheter-related bloodstream infections.21–23 On the Indian subcontinent, Gram-negative infections take a precedence over Gram-positive and fungal ones, probably because of lack of appropriate hygiene measures adopted at all times and colonisation with gut flora. We recommend more detailed studies to understand the pathogenic microorganisms associated with CLABSI.
Conclusions
The present study highlighted the role of repeated multimodal intervention programs (involving a root cause analysis followed by a corrective action) in keeping the CLABSI rates low. This was evidenced by the sustained reduction in CLABSI rates after the second intervention program in phase II (36.9%) and the post intervention period (74.9%) as compared with the first one (15.3%). Hand hygiene and daily review of line necessity have proved to be the high-yield measures in reducing CLABSIs. Other factors like introduction of needleless connectors, a dedicated and active central line team and central line trolley and regular training and auditing have also contributed in bringing the CLABSI rates down. We have been successful in sustaining the positive impact of the intervention programs and inculcating a natural culture of safety in the organisation.
Conflict of interests
None existed.
Funding
The only source of funding for the present study was Artemis Health Institute. No external grants or funds have been obtained for the study.
Acknowledgement
We owe our great thanks to Artemis Health Institute for extending their kind support and encouraging us in pursuing this study.
References
[1] Rello J, Ochagavia A, Sabanes E, Roque M, Mariscal D, Reynaga E, et al Evaluation of outcome of intravenous catheter-related infections in critically ill patients. Am J Respir Crit Care Med 2000; 162 1027–30.| Evaluation of outcome of intravenous catheter-related infections in critically ill patients.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3cvotFajsQ%3D%3D&md5=bf4b3a470c170cd24c3b319bac17088bCAS | 10988125PubMed |
[2] Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med 2000; 132 391–402. [Erratum, Ann Intern Med 2000;133 : 5.]
| Prevention of intravascular catheter-related infections.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c7ktlSlsA%3D%3D&md5=9459145940b50d1637f7825e79317e04CAS | 10691590PubMed |
[3] Dudeck MA, Horan TC, Peterson KD, Bridson KA, Morrell GC, Pollock DA, et al. National Healthcare Safety Network (NHSN) Report, Data Summary for 2009. Device-associated Module. Atlanta, GA: Centers for Disease Control and Prevention; 2010.
[4] Edwards JR, Peterson KD, Andrus ML,, Tolson J, Goulding J, Dudeck M, et al National Healthcare Safety Network (NHSN) Report, Data Summary for 2006, issued June 2007. Am J Infect Control 2007; 35 290–301.
| National Healthcare Safety Network (NHSN) Report, Data Summary for 2006, issued June 2007.Crossref | GoogleScholarGoogle Scholar | 17577475PubMed |
[5] Rosenthal VD. Central Line-Associated Bloodstream Infections in Limited resource Countries: A Review of Literature. Clin Infect Dis 2009; 49 1899–907.
| Central Line-Associated Bloodstream Infections in Limited resource Countries: A Review of Literature.Crossref | GoogleScholarGoogle Scholar | 19911941PubMed |
[6] CDC Guidelines for the prevention of intravascular catheter-related infections. Morb Mortal Wkly Rep 2002; 51 1–29.
[7] CDC Vital Signs: Central Line-Associated Blood Stream Infections — United States, 2001, 2008, and 2009. Morb Mortal Wkly Rep 2011; 60 1–60.
[8] Sheth NK, Franson TR, Rose HD, Buckmire FL, Cooper JA, Sohnle PG. Colonization of bacteria on polyvinyl chloride and Teflon intravascular catheters in hospitalized patients. J Clin Microbiol 1983; 18 1061–3.
| 1:STN:280:DyaL2c%2Fmt1Wqtw%3D%3D&md5=ecf6c21b800f2e21b19be12a6e6457e2CAS | 6643657PubMed |
[9] Herrmann M, Lai QJ, Albrecht RM, Mosher DF, Proctor RA. Adhesion of Staphylococcus aureus to surface-bound platelets: role of fibrinogen/fibrin and platelet integrins. J Infect Dis 1993; 167 312–22.
| Adhesion of Staphylococcus aureus to surface-bound platelets: role of fibrinogen/fibrin and platelet integrins.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s7jt1ersg%3D%3D&md5=92ec3295d5bc5399aeb01a986889a163CAS | 8421166PubMed |
[10] Gray ED, Peters G, Verstegen M, Regelmann WE. Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response. Lancet 1984; 323 365–7.
| Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response.Crossref | GoogleScholarGoogle Scholar |
[11] Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al An Intervention to Decrease Catheter-Related Bloodstream Infections in the ICU. N Engl J Med 2006; 355 2725–32.
| An Intervention to Decrease Catheter-Related Bloodstream Infections in the ICU.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhslSmtA%3D%3D&md5=537496f03dac4abb5fd133551e49b1afCAS | 17192537PubMed |
[12] O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections. Atlanta, GA: Centers for Disease Control and Prevention; 2011.
[13] Tan CC, Zanariah Y, Lim KI, Balan S. Central Venous Catheter-Related Blood Stream Infections: Incidence and an Analysis of Risk Factors. Med J Malaysia 2007; 62 370–4.
| 1:STN:280:DC%2BD1critlSjug%3D%3D&md5=fa098345aa2c42960e36804751ca01d4CAS | 18705468PubMed |
[14] Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C, et al Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med 2009; 37 2167–73.
| Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections.Crossref | GoogleScholarGoogle Scholar | 19487942PubMed |
[15] Marschall J, Mermel LA, Classen D,, Arias K, Podgorny K, Anderson D, et al Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol 2008; 29 S22–30.
| Strategies to prevent central line-associated bloodstream infections in acute care hospitals.Crossref | GoogleScholarGoogle Scholar | 18840085PubMed |
[16] CDC National Health and Safety Network (NHSN) System report, data summary from October 1986–April 1998, issued June 1998. Am J Infect Control 1998; 26 522–33.
| National Health and Safety Network (NHSN) System report, data summary from October 1986–April 1998, issued June 1998.Crossref | GoogleScholarGoogle Scholar | 9795682PubMed |
[17] CDC National Health and Safety Network (NHSN) System report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004; 32 470–85.
| National Health and Safety Network (NHSN) System report, data summary from January 1992 through June 2004, issued October 2004.Crossref | GoogleScholarGoogle Scholar | 15573054PubMed |
[18] Burrell AR, McLaws M, Murgo M, Calabria E, Pantle AC, Herkes R. Aseptic insertion of central venous lines to reduce bacteraemia The Central Line Associated Bacteraemia in NSW Intensive Care Units (CLAB ICU) Collaborative. Med J Aust 2011; 194 583–7.
| 21644871PubMed |
[19] Bouza E, Munoz P, Lopez-Rodriguez J,, Perez M, Rincon C, Rabadan P, et al A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect 2003; 54 279–87.
| A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization: a prospective randomized study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3svislOgsA%3D%3D&md5=6d166c6fce010b94d371156a1819595eCAS | 12919758PubMed |
[20] Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device- associated bloodstream infections in intensive care units in Argentina. Am J Infect Control 2003; 31 405–9.
| Effect of an infection control program using education and performance feedback on rates of intravascular device- associated bloodstream infections in intensive care units in Argentina.Crossref | GoogleScholarGoogle Scholar | 14639436PubMed |
[21] CDC National Health and Safety Network (NHSN) System report, data summary from January 1990–May 1999, issued June 1999. Am J Infect Control 1999; 27 520–32.
| National Health and Safety Network (NHSN) System report, data summary from January 1990–May 1999, issued June 1999.Crossref | GoogleScholarGoogle Scholar | 10586157PubMed |
[22] Pfaller MA, Jones RN, Messer SA, Edmond MB, Wenzel RP. National surveillance of nosocomial blood stream infection due to Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn Microbiol Infect Dis 1998; 31 327–32.
| National surveillance of nosocomial blood stream infection due to Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjtFWgsb8%3D&md5=4ab15e484db75b5175f0f0574c64accbCAS | 9597393PubMed |
[23] Pfaller MA, Jones RN, Messer SA, Edmond MB, Wenzel RP. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn Microbiol Infect Dis 1998; 30 121–9.
| National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c3hsVOqug%3D%3D&md5=1f389c0a26c35eb737288d20497e1193CAS | 9554180PubMed |