Healthcare associated urinary tract infections: a protocol for a national point prevalence study
Brett Mitchell A B D , Anne Gardner B , Wendy Beckingham C and Oyebola Fasugba BA Faculty of Nursing and Health, Avondale College of Higher Education, Cooranbong, NSW 2265, Australia.
B School of Nursing, Midwifery and Paramedicine, Australian Catholic University, Fitzroy, Vic. 3065, Australia.
C Canberra Hospital and Health Services, Garran, ACT 2605, Australia.
D Corresponding author. Email: brett.mitchell@avondale.edu.au
Healthcare Infection 19(1) 26-31 https://doi.org/10.1071/HI13037
Submitted: 5 October 2013 Accepted: 20 November 2013 Published: 10 December 2013
Journal Compilation © Australasian College for Infection Prevention and Control 2014
Abstract
Background: Urinary tract infections account for ~30% of healthcare-associated infections reported by hospitals. Virtually all healthcare-associated urinary tract infections (HAUTIs) are caused by instrumentation of the urinary tract, creating an opportunity to prevent a large proportion of HAUTIs, including catheter-associated urinary tract infections (CAUTIs). In Australia, there is no specific national strategy and surveillance system in place to address HAUTIs or CAUTIs. To determine the need for prospective surveillance of HAUTIs, we propose undertaking a national point prevalence study. This paper describes the methods that could be used to undertake such a study.
Methods: A cross-sectional point prevalence design is proposed. The population is all patients hospitalised overnight in Australian hospitals, with the sample to exclude outpatients and those in emergency departments. The proposed operational definition is that used by the Health Protection Agency. A standardised training package for data collectors is recommended with standardised data collection and analysis processes described. Individual patient consent should be waived.
Discussion: Explanation of aspects of the proposed methods are provided, primarily based on findings from a pilot study that informed the development of the proposed protocol. This included development and delivery of training for data collectors and use of the Health Protection Agency HAUTI surveillance definition, rather than the Centers for Disease Control definition.
Conclusion: Conducting a national point prevalence study on HAUTIs including CAUTIs will provide evidence that can be subsequently used to debate the cost effectiveness and value of prospective surveillance. By conducting a pilot study and critically evaluating that process, we have been able to propose a method that could be used for a single hospital or national study.
Implications
-
In Australia, there is no specific national strategy and surveillance system in place to address urinary tract infection surveillance
-
A consistent methodology for a point prevalence study on urinary tract infections is required
-
We propose a method for that could be used for a single hospital or national study
Background
Healthcare associated infections (HAIs) are a relatively common but unintended consequence of receiving healthcare, particularly in hospitals. The effects of HAIs are not only felt by individual patients through increased morbidity and mortality, but also by a health service through higher costs associated with infections. The magnitude of the effect of HAIs on patients is evidenced by several point prevalence studies1–3 and a report from the World Health Organization.4 These studies suggest the prevalence of HAIs in hospitals to be ~8%, with urinary tract infections (UTIs) being one of the leading types of infection, accounting for ~30% of infections reported by acute care hospitals.5 In addition, virtually all healthcare-associated urinary tract infections (HAUTIs) are caused by instrumentation of the urinary tract, with ~80% traced to the use of an indwelling urinary catheter (catheter-associated urinary tract infections or CAUTIs).6 This creates an opportunity to prevent a large proportion of HAUTIs.
Calculation of how many CAUTIs may be preventable varies considerably, with estimates from unpublished data ranging from 17% to 69%.7 The first Australian national prevalence survey for nosocomial and community-acquired infections was conducted in 1984, with reports of a 6.3% prevalence of HAIs and 22% of these infections being UTIs.8 More recently, an incidence of 1.66% for HAUTIs specifically has been reported from two hospitals in Queensland.9 A recent point prevalence survey in the United States of America reported a prevalence of 6.0% for HAIs, with 15.5% arising from urinary tract infections.10
To date, in Australia there is no specific national strategy and surveillance system in place to address HAUTIs and CAUTIs.11 To determine the need for prospective surveillance of HAUTIs, we proposed undertaking a national point prevalence study. To provide a foundation for this study and for future prospective interventional studies, we conducted a preliminary study in six Australian hospitals (three public and three private) in two Australian States/Territories. In this pilot study, we examined not only the prevalence of HAUTI and CAUTI, but also compared two HAUTI surveillance definitions for their positive predictive value and useability. These findings will be published elsewhere. In addition, we evaluated our approach to conducting a point prevalence study through reflection and surveys of the research assistants who undertook this. Through this process, we have proposed this protocol, to provide a suitable methodological approach for conducting prevalence surveys for HAUTIs in Australia.
A consistent methodology for conducting prevalence surveys in Australia is required to facilitate hospitals undertaking such a study and allow data to be collected in a manner which permits comparison and aggregation. The proposed protocol can be used by infection control personnel to evaluate the burden of HAUTI in their hospital and provides a framework for policy makers to determine the feasibility of a national point prevalence study in this area. In this paper we describe a suitable methodological approach for conducting point prevalence surveys for HAUTI in Australia.
Methods
Aim
The aim for a study using this protocol is to determine the point prevalence of HAUTIs in Australian hospitals.
Design
A cross-sectional study.
Study setting and participants (national)
All patients hospitalised in an overnight bed in an Australian hospital. Units where the risk of UTI is deemed very low, for example those with no catheter usage, such as mental health units, can be excluded. Emergency department and outpatient department patients may also be excluded as these fluctuating numbers will greatly affect the calculation of denominators. Outpatients and patients categorised as maintenance care type (awaiting nursing home placement) should also be excluded.
Data collection
Definitions
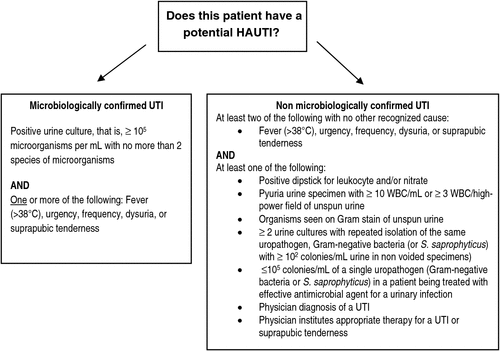
There are two methods that can be used to determine whether a person has a UTI – a microbiological or non-microbiological approach.1 A person has a UTI if they meet the criteria for one or both of these approaches.1 Symptoms used to define a case of microbiologically or non-microbiologically confirmed UTI must be documented in the medical or nursing notes. Verbal communication is not acceptable. The definitions of both these approaches are provided below and summarised in Fig. 1.
-
A microbiologically confirmed UTI is when a patient has at least one of the following signs or symptoms with no other recognised cause: fever (>38°C), urgency, frequency, dysuria, or suprapubic tenderness and the patient has a positive urine culture, that is, ≥105 microorganisms per mL of urine with no more than two species of microorganisms.1
-
A non-microbiologically confirmed UTI is when a patient has at least two of the following with no other recognised cause: fever (>38°C), urgency, frequency, dysuria, or suprapubic tenderness and at least one of the following: positive dipstick for leukocyte esterase and/or nitrate; pyuria urine specimen with ≥10 WBC mL–1 or ≥3 WBC/high-power field of unspun urine; organisms seen on Gram stain of unspun urine; at least two urine cultures with repeated isolation of the same uropathogen (Gram-negative bacteria or S. saprophyticus) with ≥ 102 colonies mL–1 urine in non-voided specimens; ≤105 colonies mL–1 of a single uropathogen (Gram-negative bacteria or S. saprophyticus) in a patient being treated with effective antimicrobial agent for a urinary infection; physician diagnosis of a urinary tract infection; or physician institutes appropriate therapy for a urinary infection.1 Bloodstream infections secondary to asymptomatic bacteriuria are not included in this definition.
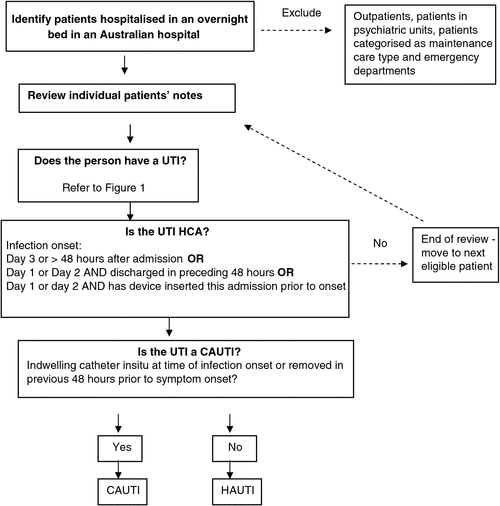
A patient has a CAUTI if they meet the definition of a having a UTI and if an indwelling catheter was in situ at time of infection onset or removed in previous 48 h before symptom onset.
A person with a UTI, including HAUTI, is defined as ‘healthcare associated’ when infection onset is on day 3 of admission onwards (or greater than 48 h if this can be easily identified). In addition, cases are healthcare associated if infection onset occurs on day 1 or day 2 and patient was discharged from a hospital in the preceding 48 h or if infection onset was on day 1 or day 2 and patient has a relevant device inserted on this admission before onset.1
Data items to be collected
For each patient, the following data items are to be collected: date of survey, hospital, ward/location, sex, date of birth, date of admission, whether the person has a HAUTI and if so whether it was microbiologically or non-microbiologically confirmed. For those persons with a UTI, the symptoms should be recorded to assist with validation or further analysis at a later date.
Training
Persons responsible for data collection (research assistants) should be trained and assessed in their knowledge and application of surveillance definitions. This is an essential set in improving the reliability of study findings. A standardised training package should be developed and be available for use by participating hospitals. An assumption is that research assistants will have a healthcare professional background.
Procedure
Data collection will occur on a given day in all participating hospitals. Research assistants will collect data on all in-patients in each ward/unit in the hospital at the beginning of the day from the hospital patient administration system or equivalent. In larger hospitals or where there are insufficient researchers to complete the survey on one day, there may be queries regarding inclusion criteria or follow up of patients. In such circumstances, the following principles are to be applied:
-
If patient notes are available to enable data collection after the study day, this is permissible. The researcher must only review data from the actual study day and the lead up. For example, if the study occurred on 1 January, but the researcher was unable to review the notes until 4 January, the assistant will be looking to determine whether the person had an infection on 1 January and cannot use information available after this date.
-
If the patient has been transferred to another ward during data collection, every attempt should be made to follow that person up.
-
If the patient was discharged on the study day, every attempt should be made to review the notes of that patient and include them in the study where possible.
-
If the patient notes or laboratory results were unavailable at the time of data collected, e.g. in theatre, an attempt should be made to follow that person up.
-
Where data are not available, that patient should not be included in the calculation of prevalence. However, a statement of limitation should be made about the number of patients for which data are not available.
Fig. 2 summarises the procedure for determining cases of HAUTI.
|
Data management and analysis
In clinical settings where Wi-Fi is available, an online data collection form should be used for data entry. Alternatively, data can be collected in a hard copy form and subsequently entered into an online database. The online database should be developed and managed by one organisation. Descriptive analysis such as counts and percentages for categorical data and measures of central tendency and dispersion for continuous data will be performed. The HAUTI and CAUTI point prevalence will be calculated using the total eligible patient population surveyed on the day (excluding patients for whom data are not available) as the denominator.
Ethical considerations
Participating organisations may require ethics approval from their Human Research Ethics Committee. Advice should be sought locally. For organisations requiring ethics approval, this research protocol can be used to assist in the process. No individual patient will be contacted. Information is obtained from laboratory results and from patient notes recorded as part of their routine care. Consent from individual patients will not be obtained. Section 2.3.1 of the National Statement on Ethical Conduct outlines the principles for wavering consent.12 To assist constructing an ethics application for organisations that require ethics approval, we will now provide some key principles for the justification for wavering consent.
-
The research is low risk. There are no interventions and no harm or discomfort as a result.
-
The benefits of the research justify any risk of harm associated with not obtaining consent. No harm from not obtaining consent is envisaged. Results of the research are not individualised or indeed patient identifiable.
-
There is no known or likely reason for thinking that patients would not have consented if they had been asked. The study requires no direct involvement of patients; rather it collates existing information obtained during their hospitalisation.
-
The privacy and confidentiality of patients and patient data will be maintained by ensuring that no re-identifiable information will be stored or held by the researcher after the conclusion of data collection and no re-identifiable data will be passed onto the national database for analysis.
-
Obtaining consent would also necessitate the researchers having access to a greater level of personal information.
-
No new information will be obtained about individual patients; therefore results will have no significance for the individual welfare of patients.
-
The study will not result in depriving patients of any financial benefits.
-
The potential benefits to the public outweigh the risks or harm associated with no obtaining consent. There are ~175 000 healthcare-associated infections (HAIs) in Australian acute healthcare facilities per year, making HAIs the most common complication affecting patients in hospital.13
-
Finally, obtaining patient consent would greatly increase the time and cost of the surveillance.
Discussion
The focus of this discussion is in defence of the protocol outlined in this paper. There were important lessons learnt from the pilot study conducted in six Australian hospitals informing this research protocol. The pilot study examined the development and delivery of training for data collection, an evaluation of different surveillance definitions, time taken to collect data and the trial and modification of a data collection instrument.
During the pilot of this research project, a training package was developed for data collectors explaining all necessary procedures. In developing this package, it was assumed that all data collectors have some prior clinical and infection control knowledge, for example registered nurses. The training package was developed based on the Health Protection Scotland Education and Training Events resources.14 The package included a paper-based manual and electronic presentation which took approximately 1 hour to deliver. It was mandatory for all data collectors to undertake this training. The outcome of the training was evaluated by post training case study assessments and participants were allowed to proceed with data collection based on achieving a minimum score of 80% in the assessments. Such a process enhanced inter-rater reliability and we propose that any national point prevalence study include the development and delivery of a robust training package, conducted well before the data collection day. During our pilot, we provided research assistants with a manual and a one page surveillance definition, as described in Fig. 1.
After completing our HAUTI point prevalence study in Australian hospitals, 10 of the 11 research assistants (data collectors) involved in the study completed an online anonymous survey designed to gain valuable feedback for the development of this protocol paper. Sixty-three per cent of respondents indicated it took between 6 and 10 h per 100 patients to complete data collection. It is important to note, however, that during the pilot additional information was being collected, including an audit of all indwelling catheter usage and documentation from a patient’s entire admission, and that two different surveillance definitions to determine the HAUTI status were being used. The research assistants reported that the most time-consuming element of the data collection process was reviewing patients’ notes to collect information on catheter usage – something that is not proposed in a national point prevalence study.
Sixty per cent of respondents suggested that it took minimal time to determine the HAUTI and CAUTI status of patients, whilst 20% indicated it took a long time. For most of patients, the data collected suggested 40% of the time it took less than 5 min per patient to determine whether they had a HAUTI or CAUTI. From the data collected and our experience of the pilot, we estimate it would take ~3–6 h per 100 patients. This time would be reduced further with direct online data entry at the point of data collection.
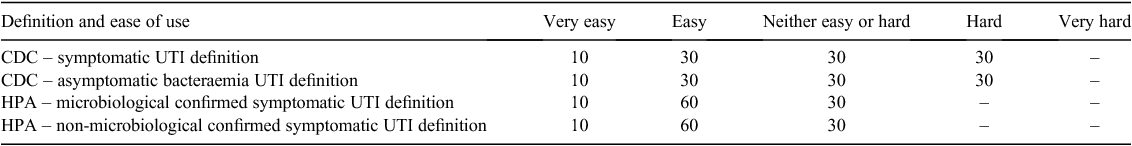
In our pilot study, we also evaluated the data collection form used and the utility of two surveillance definitions, namely the Health Protection Agency1 and Centers for Disease Control and Prevention5 definitions. An evaluation of the positive predictive value of the two surveillance definitions is published elsewhere finding little difference. However, in this paper, we report the evaluation of research assistants (Table 1). These data, coupled with the positive predictive value of the HPA surveillance definition, are the main reasons why we have proposed the Health Protection Agency surveillance definitions for use in the Australian context, as summarised in Figs 1 and 2.
|
Communication is also a key element of conducting any study, including a point prevalence study. In the hospitals where we conducted the pilot, ward manager and senior nurses were notified well in advance of the study date. In the lead up to the study, reminders were sent and written information about the study was available for interested staff. We did not encounter any challenges by ward or clinical staff in conducting our pilot.
Conclusion
Conducting a national point prevalence study on HAUTIs including CAUTIs will provide evidence that can be subsequently used to debate the cost effectiveness and value in prospective surveillance. By conducting a pilot study and critically evaluating that process, we have been able to propose a method that could be used for any national point prevalence study and or for individual hospitals to conduct their own prevalence study.
Funding
The pilot study informing this protocol paper received funding from Covidien and Australian Catholic University.
Conflict of interest
Three of the authors have editorial affiliations with Healthcare Infection. They played no role in peer review or editorial processes related to this paper.
References
[1] Health Protection Agency. Fourth National Point Prevalence Survey on Healthcare Associated Infections and First National Point Prevalence Survey on Antimicrobial Use and Quality Indicators in England. London: HPA; 2011. Available from: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1296687778967 [verified November 2013][2] Smyth ETM, McIlvenny G, Enstone JE, Emmerson AM, Humphreys H, Fitzpatrick F, et al Four Country Healthcare Associated Infection Prevalence Survey 2006: overview of the results. The Journal of Hospital Infection 2008; 69 230–248.
| Four Country Healthcare Associated Infection Prevalence Survey 2006: overview of the results.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cngsVWnsA%3D%3D&md5=0fa22f31f44354a0c1a3f2bbb4c10569CAS |
[3] Lanini S, Jarvis WR, Nicastri E, Privitera G, Gesu G, Marchetti F, et al Healthcare‐Associated Infection in Italy: Annual Point‐Prevalence Surveys, 2002–2004. Infection Control and Hospital Epidemiology 2009; 30 659–665.
| Healthcare‐Associated Infection in Italy: Annual Point‐Prevalence Surveys, 2002–2004.Crossref | GoogleScholarGoogle Scholar | 19496645PubMed |
[4] World Health Organization. Report on the Burden of Endemic Health Care-Associated Infection Worldwide. Geneva: WHO; 2011.
[5] Centers for Disease Control and Prevention. NHSN Patient Safety Component Manual. Atlanta, GA: CDC; 2012. Available from: http://www.cdc.gov/nhsn/pdfs/pscManual/7pscCAUTIcurrent.pdf [verified November 2013]
[6] Elvy J, Colville A. Catheter associated urinary tract infection: what is it, what causes it and how can we prevent it? Journal of Infection Prevention 2009; 10 36–41.
| Catheter associated urinary tract infection: what is it, what causes it and how can we prevent it?Crossref | GoogleScholarGoogle Scholar |
[7] Umscheid CA, Mitchell MD, Agarwal R, Williams K, Brennan PJ. Mortality from Reasonably-Preventable Hospital Acquired Infections. Arlington, VA: The Society for Healthcare Epidemology of America; 2008. Available from: http://www.shea-online.org/assets/files/0408_penn_study.pdf [verified November 2013]
[8] McLaws M-L, Gold J, King K, Irwig LM, Berry G. The prevalence of nosocomial and community-acquired infections in Australian hospitals. The Medical Journal of Australia 1988; 149 582–590.
| 1:STN:280:DyaL1M%2FntVemuw%3D%3D&md5=30896b3aa0416f1277228bcb5542934eCAS | 3143900PubMed |
[9] Graves N, Tong E, Morton AP, Halton K, Curtis M, Lairson D, et al Factors associated with health care-acquired urinary tract infection. American Journal of Infection Control 2007; 35 387–392.
| Factors associated with health care-acquired urinary tract infection.Crossref | GoogleScholarGoogle Scholar | 17660009PubMed |
[10] Magill SS, Hellinger W, Cohen J, Kay R, Bailey C, Boland B, et al Prevalence of healthcare-associated infections in acute care hospitals in Jacksonville, Florida. Infection Control and Hospital Epidemiology 2012; 33 283–291.
| Prevalence of healthcare-associated infections in acute care hospitals in Jacksonville, Florida.Crossref | GoogleScholarGoogle Scholar | 22314066PubMed |
[11] Cruickshank M, Murphy C, eds. Reducing Harm to Patients from Healthcare Associated Infections: An Australian Infection Prevention and Control Model for Acute Hospitals. Sydney: Australian Commission on Safety and Quality in Health Care; 2009.
[12] The National Health and Medical Research Council, The Australian Research Council, The Australian Vice-Chancellors’ Committee. National Statement on Ethical Conduct in Human Research 2007 – Updated May 2013. Canberra: Commonwealth of Australia; 2013.
[13] Graves N, Halton K, Paterson D, Whitby M. Economic rationale for infection control in Australian hospitals. Healthcare Infection 2009; 14 81–88.
| Economic rationale for infection control in Australian hospitals.Crossref | GoogleScholarGoogle Scholar |
[14] Scottish Surveillance of Healthcare Associated Infection Programme. Catheter Associated Urinary Tract Infection Surveillance for Acute Settings. Glasgow: Health Protection Scotland; 2012.