ASID/AICA position statement – Infection control guidelines for patients with Clostridium difficile infection in healthcare settings
Rhonda L. Stuart A I , Caroline Marshall B , Mary-Louise McLaws C , Claire Boardman D , Philip L. Russo E , Glenys Harrington F and John K. Ferguson G HA Department of Infectious Diseases, Monash Medical Centre, Southern Health, Melbourne, Vic., Australia.
B Victorian Infectious Diseases Service, Royal Melbourne Hospital and Department of Medicine, University of Melbourne, Melbourne, Vic., Australia.
C Epidemiology, Hospital Infection and Infectious Diseases Control, School of Public Health and Community Medicine, The University of New South Wales, Sydney, NSW, Australia.
D Australian Infection Control Association, GPO Box 3254, Brisbane, QLD 4001, Australia.
E National Program Manager, Hand Hygiene Australia, Austin Hospital, Melbourne, Vic., Australia.
F Infection Control Consultancy (ICC), Melbourne, Vic., Australia.
G John Hunter Hospital, Newcastle, University of Newcastle, Newcastle, NSW, Australia.
H ASID Healthcare Infection Control Special Interest Group (HICSIG).
I Corresponding author. Email: Rhonda.stuart@southernhealth.org.au
Healthcare Infection 16(1) 33-39 https://doi.org/10.1071/HI11011
Submitted: 28 February 2011 Accepted: 28 February 2011 Published: 28 March 2011
Journal Compilation © Australian Infection Control Association 2011
Abstract
Since 2000 there has been an increase in the rates of Clostridium difficile infection (CDI) in many healthcare facilities in the United States, Canada and Europe. This increase is associated with an epidemic strain of C. difficile and this strain (PCR ribotype 027) has recently been identified in Australia. All healthcare services should have in place an optimal evidence-based program for CDI prevention and control. Management principles include the following.
• All healthcare organisations, including residential aged care facilities, must give CDI prevention and control the highest priority, even if the prevailing incidence of CDI is low.
• Surveillance should be integrated into quality improvement programs to optimise prevention, control and clinical care of CDI.
• Antimicrobial stewardship programs aimed at minimising the frequency and duration of antibiotic use and promoting a narrow spectrum antibiotic policy should be implemented.
• Emphasis should be placed on compliance with hand disinfection using alcohol-based hand rub and glove use for CDI patient care to minimise spore contamination.
• Contact precautions should be in place for symptomatic CDI patients, including the donning of gowns/aprons and gloves on entry to patient rooms.
• The use of sporocidal environmental cleaning and disinfection in high-risk areas such as toilets, bathrooms, and CDI patient rooms should be implemented. There should be the limination of other potential fomites by either using disposable equipment or ensuring that equipment is adequately cleaned and disinfected before re-use.
• Education of all healthcare staff, patients and visitors about C. difficile disease, its prevention and management should be implemented.
Introduction
Process of position statement development
The main authors reviewed the literature and current guidelines and new guidelines were formulated. Substantial input was made from members of the Australasian Society for Infectious Diseases (ASID) and the Australian Infection Control Association (AICA). The authors responded to all comments received.
Clostridium difficile is a Gram-positive, anaerobic, spore-forming, potentially toxigenic bacterium that is the most common infectious cause of healthcare-associated diarrhoea.1 In the United States, C. difficile now rivals methicillin-resistant Staphylococcus aureus (MRSA) as the most common healthcare-associated infection (HAI), accounting for $3.2 billion in excess costs annually.1–3
Clostridium difficile infection (CDI) may present with varying severity, from mild diarrhoea to pseudomembranous colitis, toxic megacolon and death. Since 2000 there has been an increase in the rates of CDI in some healthcare facilities in the United States, Canada and Europe that has been associated with an epidemic strain of C. difficile. This strain (B1/NAP1/027, toxinotype III or PCR ribotype 027) is characterised by increased toxin production (toxins A, B and binary toxin) and increased sporulation.1,4 Other virulent strains associated with severe disease in Europe have also emerged, including PCR ribotypes 015, 018 and 056.5
Risk factors for CDI include antimicrobial exposure, gastric acid suppressive therapy, advanced age, prolonged hospitalisation, cancer chemotherapy and immunosuppression (see Table 1). Most cases have been in hospitalised individuals; however, increasing numbers of community-associated cases are being reported in the United States and Europe.6,7 A 2009 French nation-wide study determined that 28% of CDI events were community-associated, with significant rates among long-term residential aged-care residents.8

|
Surveillance and patient identification
McDonald et al. developed interim surveillance definitions and recommendations that have been endorsed in Europe and the United States.9,10 The Australian Commission for Safety and Quality in Healthcare has gained jurisdictional agreement to implement surveillance for CDI that is consistent with this international approach.
Currently, surveillance for C. difficile is inconsistent across Australia, and therefore nationwide rates are unknown. Some states have reported rates between 1.27 and 2.3/10 000 bed days.11,12 This contrasts with rates between 3.8 and 9.5/10 000 bed days in Canada in 1997 and 2005.10 In Quebec rates of CDI increased four-fold with the appearance of the PCR ribotype 027.13 This strain has recently been detected in Australia, in Western Australia14 and Victoria.15
International recommendations support the implementation of CDI surveillance programs as a means of reducing infection rates.10,11 Surveillance should be integrated into quality improvement efforts that aim to optimise prevention and control of CDI. All patients with hospital onset of diarrhoea (>48 h after admission) should be screened for CDI. The case definition should include the presence of symptoms (usually diarrhoea) and a stool test positive for toxigenic C. difficile or its toxins or colonoscopic and/or histological findings of pseudomembranous colitis.10 Infection control (IC) professionals and clinicians must be informed of CDI cases in a timely fashion in order to implement effective IC precautions. Routine identification of asymptomatic carriers is not recommended.10
Hospitals should also be alert to the possibility of CDI presenting in patients from the community or from residential aged-care facilities. It is reasonable to consider testing outpatients >60 years presenting with diarrhoea and those patients with one or more risk factors for CDI (see Table 1).
Paediatric carriage of C. difficile is highest in infants and declines markedly after the first year.16 However, disease is uncommon at this age and therefore national surveillance data usually do not include children <2 years of age.
Microbiology
A review of laboratory practices in Australia and New Zealand with recommendations for testing is currently being performed (J. Ferguson, pers. comm.).
Key points for infection prevention and control include the following.
-
Testing for C. difficile or its toxins should be performed only on unformed stools unless ileus due to C. difficile is suspected (unformed stools being either liquid or soft and adopting the shape of the container).
-
Provided that a sensitive and specific diagnostic test is being used, the predictive value of a negative test is very high and repeat testing of the same patient is not usually recommended.
-
Repeat testing during the same episode of CDI is of limited value and should be discouraged. Recovery from CDI occurs in response to treatment and acquisition of immunity. It is not usually associated with clearance of toxigenic C. difficile from the stool, which may remain detectable for several weeks.10,17
-
Laboratories should provide a daily C. difficile testing service and notify identified cases immediately to IC and treating units.
Routes of transmission
The period between exposure to C. difficile and the occurrence of symptoms has been estimated to be a median of 2–3 days.18 The primary mode of transmission of C. difficile is person-to-person via the faecal-oral route. C. difficile can exist in a vegetative or spore form. These spores can persist in an environmental reservoir for several months and place patients at risk by contaminating healthcare workers’ (HCWs) hands and fomites.18–21 In heavily contaminated environments spores may be aerosolised by movement of HCWs and patients, allowing widespread dissemination.22,23 Spores are resistant to the bactericidal effects of alcohol and most hospital disinfectants.24,25
Infection prevention and control precautions
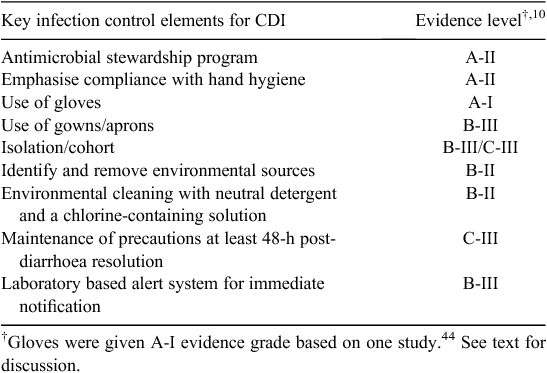
It is likely that patients identified with CDI represent the tip of the iceberg,21,26,27 making targeted control strategies relatively less important than generic ones, such as hand hygiene and antimicrobial stewardship. This has been borne out by the available evidence (Table 2) but there are few good-quality studies evaluating other strategies, such as use of gloves, gowns, contact isolation and environmental disinfection.
|
Antimicrobial stewardship
Receipt of an antibiotic is the major predisposing factor for CDI and most affected patients have received antimicrobials. Virtually all antibiotics have been associated with CDI,28 with certain agents having higher risks, including clindamycin,29,30 cephalosporins,31 and quinolones such as gatifloxacin32 and moxifloxacin.33
Interventional trials examining the impact of antimicrobial restriction on CDI have been recently summarised.34 These have involved restriction of clindamycin, cephalosporins and multiple agents. Two of the trials also included changes to IC practice, with almost all demonstrating significant reductions in CDI rates. Seven studies restricted multiple agents, including quinolones,34,35 making it impossible to determine the impact of isolated quinolone restriction.
Prevention and control of CDI should include an antimicrobial stewardship program aimed at minimising the frequency and duration of antibiotic use and promoting a narrow-spectrum antibiotic policy that particularly restricts use of cephalosporins, lincosamides and quinolones.36
Hand hygiene
Hand hygiene is a crucial measure for prevention of HAI. Point-of-care hand hygiene with alcohol-based hand rub (ABHR) combined with educational programs, has revolutionised practice. Increased compliance has been associated with reductions in HAIs with MRSA.37–39 The pivotal role of hand hygiene in preventing the transmission of C. difficile is also accepted40 but the optimal method remains contentious. Concerns that the widespread use of ABHR could lead to increased rates of CDI have been refuted by recent studies.41–43
Because there are few studies showing the effectiveness of hand washing over use of ABHR and glove use for CDI control,44,45 this Position Statement recommends the primary use of ABHR in accordance with the WHO ‘5 Moments for Hand Hygiene’ when caring for patients with CDI.46 The rationale for this is given as follows.
Asymptomatic carriage of toxigenic C. difficile is common in hospitalised patients and skin contamination can be detected on 50% of patients with CDI up to 7 days after resolution of diarrhoea.47 The vegetative state of C. difficile has been found to be highly sensitive to the action of ABHR, with the spore form being resistant.48 Recovery of the vegetative forms has been shown to be as high as 10-fold compared with that of spore forms in the faeces of 26 patients with CDI,49 suggesting that reducing transmission of vegetative forms may be crucial in CDI control. The importance of vegetative forms may also be supported by studies showing that exposure to gastric acid suppressive therapy is an independent risk factor for CDI.49,50
Published recommendations for use of hand washing for patients with CDI, rather than use of ABHR, are based on the knowledge that spore forms are resistant to alcohol.10,46 More recently, this has been examined in the laboratory setting where the hands of 10 volunteers were experimentally seeded with spores. This study showed superiority of hand washing; however, an artificially heavy inoculum of spores was used, the efficacy against vegetative forms was not assessed and the ABHR preparations trialled had ethanol concentrations of 60%, 62% and 70%, lower than seen in WHO publications.44 There are no clinical studies published that support an additional need for hand washing with soap and water for CDI control even in outbreak settings.
Based on precautionary principles, glove use is recommended in this statement to minimise the level of contamination of spores on the hands of clinicians when caring for patients with known CDI. However, only one observational study has addressed this issue.45 Several important potential confounders were not controlled for in this study, including the level of hand hygiene compliance, which was not reported. Although healthcare-associated CDI rates in the ward where gloves were introduced declined from 7.7 to 1.5 per 1000 patient discharges (relative risk = 0.16, P = 0.015), the overall difference in CDI incidence compared with the control wards was not statistically significant due to the small number of cases (P = 0.14).
Given the risk of spread of C. difficile will be much larger than estimates based only on passive case detection,27 there is unlikely to be a marked additional impact provided by hand washing during care of recognised CDI cases. Importantly, confusing HCWs with mixed messages about the use of ABHR may be detrimental to hand hygiene compliance programs.
In summary, ABHR remains the agent of choice for hand hygiene. Gloves should be used during the care of patients with CDI, to minimise spore contamination. If hands become soiled, or gloves have not been used, then hands must be washed with soap (or an antimicrobial soap) and water. Hands should then be dried thoroughly with paper towels.
Contact precautions
Studies of the efficacy of patient isolation and cohorting only exist in the outbreak setting. There are no studies that examine the efficacy of these measures for containing endemic CDI.34
Patients with three or more loose stools within a 24-h period should be placed in a single room with dedicated toileting facilities. If single rooms are not available, patients should be cohorted with other patients with the same demonstrated cause of diarrhoea, with each area having a dedicated toilet area or commode.10,51
Clear signage indicating that contact precautions are in place should be used. Contact precautions include hand hygiene and the donning of gowns/aprons and gloves on entry to patient rooms. Although there is ample evidence for contamination of HCW hands and clothing with C. difficile when caring for patients with CDI,10,18,45,51,52 there are no data to show that gown/apron use reduces CDI transmission.34 However, because reduction in contamination of clothes is likely via the use of gowns/aprons, this is still recommended. Gloves should be used for all contact with the patient and their environment. Gloves must be changed and hand hygiene performed, when moving from dirty to clean tasks for the same patient. All gloves and gowns/aprons should be removed and hand hygiene performed upon exit from the room.
Dedicated equipment (blood pressure cuffs, wheelchairs, stethoscopes) should be provided for each patient. If rectal thermometers are used they should be disposable.10,19,25 Patient movement should be kept to a minimum. If patients require transport to another clinical area, ensure that the receiving area is aware of the transfer and that wheelchairs, stretchers and patient areas are appropriately cleaned with neutral detergent and disinfected with a suitable sporicidal agent such as hypochlorite (see below).
Ceasing contact precautions
As C. difficile may still be shed from patients despite symptom resolution, contact precautions should be continued until at least 48 h after diarrhoea has ceased.47,53 Retesting for C. difficile is not necessary.
Environmental cleaning and disinfection
The environment is an important source of healthcare-associated CDI.52 C. difficile spores can be found on multiple surfaces in the healthcare setting and can survive for months.18–20,34 In C. difficile-contaminated patient care areas, all horizontal surfaces and frequently touched items within patient reach should be cleaned daily with neutral detergent followed by disinfection with a chlorine-containing solution such as hypochlorite (minimum concentration of 1000 ppm) or another sporicidal solution.54 Higher concentrations of hypochlorite are more reliably sporicidal but may have disadvantages (e.g. odour, caustic effects).10 A one-step process using a combined detergent/sporicide product is also acceptable.
On patient discharge or transfer, terminal cleaning of the room should be thorough. All areas should be cleaned as described above. All curtains need to be laundered and disposable items should be removed and discarded (e.g. paper towels, toilet paper). Particular attention should be paid to the cleaning of patient-specific items such as bed rails, telephones, call bells, light switches and door handles. Special attention should be paid to toilets and commodes.
In healthcare settings where C. difficile is endemic, strong consideration should be given to the routine use of sporicidal cleaning and disinfection for all high-risk areas, including toilets and bathrooms, to reduce the environmental burden.
There is some evidence that the use of vaporised hydrogen peroxide or UV light provides improved environmental disinfection. Specific protocols are available for their safe use.55,56 Routine environmental screening for C. difficile is not recommended.10
Education and quality improvement
All healthcare organisations, including residential aged-care facilities, should give CDI prevention and control the highest priority, even when the incidence of CDI is low. Analysis of individual cases of CDI can provide useful guidance for quality improvement and educational processes.
Hospital staff (including administration, cleaning staff, food services and maintenance staff) should be provided with information on CDI and the measures to prevent and control transmission. Cleaning staff, in particular, require training, feedback and encouragement to ensure that environmental hygiene is optimised.57
Education programs should also focus on antibiotic prescribing as the primary preventative strategy for CDI (see above).
Patients and their visitors
Patients and their visitors should be educated about CDI, contact precautions and hand hygiene. If the visitor is providing care for the patient, gowns/aprons and gloves should be worn and hands disinfected after removal. Visitors should be advised not to use the patient’s bathroom or visit other patients’ rooms.
Cluster investigation and outbreak management
An increase in patients with CDI, above the usual number in any healthcare institution, should prompt an epidemiological investigation.58 A case definition, including clinical and laboratory components, should be used. Additional cases of CDI should be sought by actively seeking new cases of diarrhoea. An epidemic curve by time of onset of symptoms, a bar chart by age and sex and a Gant chart indicating time and location of patients should be generated.58 From this analysis a population at risk can be determined to enable specifically targeted measures. In the case of an outbreak, supplemental control measures that may be considered include:
-
re-doubled efforts to reduce/modify antimicrobial use and improve hand hygiene compliance;
-
universal glove use in units with high CDI rates;59
-
maintaining additional contact precautions until hospital discharge;53 and
-
use of environmental markers (such as fluorescent markers) to assess adequacy of environmental cleaning.57
Compliance with these procedures should be audited immediately and at suitable intervals until the number of cases returns to pre-outbreak levels.
Conclusion
The increasing rates of CDI that have occurred in the United States, Canada and Europe, and the recent identification of the hypervirulent PCR ribotype 027 strain in Australia, are of concern. All healthcare services should have in place an optimal evidence-based program for antimicrobial stewardship, CDI surveillance, infection prevention and control. Further research is required to determine the most effective combination of control strategies.
References
[1] McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis 2006; 12 409–15.| 16704777PubMed |
[2] Dubberke ER, Gerding DN, Classen D, Arias KM, Podgorny K, Anderson DJ, et al Strategies to prevent Clostridium difficile infections in acute care hospitals. Infect Control Hosp Epidemiol 2008; 29 S81–92.
| Strategies to prevent Clostridium difficile infections in acute care hospitals.Crossref | GoogleScholarGoogle Scholar | 18840091PubMed |
[3] O’Brien J, Lahue B, Caro J, Davidson D. The emerging infectious challenge of Clostridium difficile-associated disease in non-surgical patients. Infect Control Hosp Epidemiol 2007; 28 1219–27.
| 17926270PubMed |
[4] Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med 2006; 145 758–64.
| 17116920PubMed |
[5] Bauer M, Notermans D, van Benthem B, et al. Final results of the first pan-European Clostridium difficile infection survey. ECCMID. Vol. O157. Vienna, 2010.
[6] Bauer M, Goorhuis A, Koster T. Two case reports with review of the changing epidemiology of Clostridium difficle-associated diarrhoea. J Med 2008; 66 207–11.
[7] Severe Clostridium difficile-associated disease in populations previously at low risk–four states, 2005. MMWR Morb Mortal Wkly Rep 2005; 54 1201–5.
| 16319813PubMed |
[8] Coignard B, Hebert M, Eckert D, et al. Epidemiological and microbiological characteristics of Clostridium difficile infections, France, 2009: a national, multicentre, prospective survey. ECCMID. Vol. 0158. Vienna, 2010.
[9] McDonald LC, Coignard B, Dubberke E, Song X, Horan T, Kutty PK. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol 2007; 28 140–5.
| Recommendations for surveillance of Clostridium difficile-associated disease.Crossref | GoogleScholarGoogle Scholar | 17265394PubMed |
[10] Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31 431–55.
| Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA).Crossref | GoogleScholarGoogle Scholar | 20307191PubMed |
[11] McGregor A, Riley TV, Van Gessel H. Clostridium difficile associated disease. Reducing Harm to Patients from Healthcare Associated Infection: The Role of Surveillance. Sydney: Australian Commission on Safety & Quality in Healthcare, 2008.
[12] McGregor A, Mitchell B. Tasmanian Acute Public Hospitals Healthcare Associated Infection Surveillance Report. Hobart: Department of Health and Human Services, 2009.
[13] Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ 2005; 173 1037–42.
| Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec.Crossref | GoogleScholarGoogle Scholar | 16179431PubMed |
[14] Riley TV, Thean S, Hool G, Golledge CL. First Australian isolation of epidemic Clostridium difficile PCR ribotype 027. Med J Aust 2009; 190 706–8.
| 19527210PubMed |
[15] Richards M, Know J, Elliot B, Mackin K, Lyras D, Waring LJ, Riley TV, et al Severe infection with Clostridium difficile PCR riotype 027 acquired in Melbourne, Australia. Med J Aust 2011; 194 in print
[16] Viscidi R, Willey S, Bartlett JG. Isolation rates and toxigenic potential of Clostridium difficile isolates from various patient populations. Gastroenterology 1981; 81 5–9.
| 7239125PubMed |
[17] Crobach MJ, Dekkers OM, Wilcox MH, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): data review and recommendations for diagnosing Clostridium difficile-infection (CDI). Clin Microbiol Infect 2009; 15 1053–66.
| European Society of Clinical Microbiology and Infectious Diseases (ESCMID): data review and recommendations for diagnosing Clostridium difficile-infection (CDI).Crossref | GoogleScholarGoogle Scholar | 19929972PubMed |
[18] McFarland L, Mulligan M, Kwok R, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med 1989; 320 204–10.
| Nosocomial acquisition of Clostridium difficile infection.Crossref | GoogleScholarGoogle Scholar | 2911306PubMed |
[19] Mayfield JL, Leet T, Miller J, Mundy LM. Environmental control to reduce transmission of Clostridium difficile. Clin Infect Dis 2000; 31 995–1000.
| Environmental control to reduce transmission of Clostridium difficile.Crossref | GoogleScholarGoogle Scholar | 11049782PubMed |
[20] Brooks SE, Veal RO, Kramer M, Dore L, Schupf N, Adachi M. Reduction in the incidence of Clostridium difficile-associated diarrhea in an acute care hospital and a skilled nursing facility following replacement of electronic thermometers with single-use disposables. Infect Control Hosp Epidemiol 1992; 13 98–103.
| Reduction in the incidence of Clostridium difficile-associated diarrhea in an acute care hospital and a skilled nursing facility following replacement of electronic thermometers with single-use disposables.Crossref | GoogleScholarGoogle Scholar | 1541811PubMed |
[21] Dumford DM, Nerandzic MM, Eckstein BC, Donskey CJ. What is on that keyboard? Detecting hidden environmental reservoirs of Clostridium difficile during an outbreak associated with North American pulsed-field gel electrophoresis type 1 strains. Am J Infect Control 2009; 37 15–9.
| What is on that keyboard? Detecting hidden environmental reservoirs of Clostridium difficile during an outbreak associated with North American pulsed-field gel electrophoresis type 1 strains.Crossref | GoogleScholarGoogle Scholar | 19171247PubMed |
[22] Best EL, Fawley WN, Parnell P, Wilcox MH. The potential for airborne dispersal of Clostridium difficile from symptomatic patients. Clin Infect Dis 2010; 50 1450–7.
| The potential for airborne dispersal of Clostridium difficile from symptomatic patients.Crossref | GoogleScholarGoogle Scholar | 20415567PubMed |
[23] Roberts K, Smith CF, Snelling AM, Kerr KG, Banfield KR, Sleigh PA, et al Aerial dissemination of Clostridium difficile spores. BMC Infect Dis 2008; 8 7
| Aerial dissemination of Clostridium difficile spores.Crossref | GoogleScholarGoogle Scholar | 18218089PubMed |
[24] Wilcox MH, Fawley WN. Hospital disinfectants and spore formation by Clostridium difficile. Lancet 2000; 356 1324
| Hospital disinfectants and spore formation by Clostridium difficile.Crossref | GoogleScholarGoogle Scholar | 11073024PubMed |
[25] Wilcox MH, Fawley WN, Wigglesworth N, Parnell P, Verity P, Freeman J. Comparison of the effect of detergent versus hypochlorite cleaning on environmental contamination and incidence of Clostridium difficile infection. J Hosp Infect 2003; 54 109–14.
| Comparison of the effect of detergent versus hypochlorite cleaning on environmental contamination and incidence of Clostridium difficile infection.Crossref | GoogleScholarGoogle Scholar | 12818583PubMed |
[26] Mutters R, Nonnenmacher C, Susin C, Albrecht U, Kropatsch R, Schumacher S. Quantitative detection of Clostridium difficile in hospital environmental samples by real-time polymerase chain reaction. J Hosp Infect 2009; 71 43–8.
| Quantitative detection of Clostridium difficile in hospital environmental samples by real-time polymerase chain reaction.Crossref | GoogleScholarGoogle Scholar | 19041162PubMed |
[27] Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RL, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis 2007; 45 992–8.
| Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents.Crossref | GoogleScholarGoogle Scholar | 17879913PubMed |
[28] Thomas C, Stevenson M, Riley TV. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: a systematic review. J Antimicrob Chemother 2003; 51 1339–50.
| Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: a systematic review.Crossref | GoogleScholarGoogle Scholar | 12746372PubMed |
[29] Climo MW, Israel DS, Wong ES, Williams D, Coudron P, Markowitz SM. Hospital-wide restriction of clindamycin: effect on the incidence of Clostridium difficile-associated diarrhea and cost. Ann Intern Med 1998; 128 989–95.
| 9625685PubMed |
[30] Pear SM, Williamson TH, Bettin KM, Gerding DN, Galgiani JN. Decrease in nosocomial Clostridium difficile-associated diarrhea by restricting clindamycin use. Ann Intern Med 1994; 120 272–7.
| 8080497PubMed |
[31] Carling P, Fung T, Killion A, Terrin N, Barza M. Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol 2003; 24 699–706.
| Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years.Crossref | GoogleScholarGoogle Scholar | 14510254PubMed |
[32] Gaynes R, Rimland D, Killum E, Lowery KH, Johnson TM, Killgore G, et al Outbreak of Clostridium difficile infection in a long-term care facility: association with gatifloxacin use. Clin Infect Dis 2004; 38 640–5.
| Outbreak of Clostridium difficile infection in a long-term care facility: association with gatifloxacin use.Crossref | GoogleScholarGoogle Scholar | 14986246PubMed |
[33] Biller P, Shank B, Lind L, Brennan M, Tkatch L, Killgore G, et al Moxifloxacin therapy as a risk factor for Clostridium difficile-associated disease during an outbreak: attempts to control a new epidemic strain. Infect Control Hosp Epidemiol 2007; 28 198–201.
| Moxifloxacin therapy as a risk factor for Clostridium difficile-associated disease during an outbreak: attempts to control a new epidemic strain.Crossref | GoogleScholarGoogle Scholar | 17265402PubMed |
[34] Hsu J, Abad C, Dinh M, Safdar N. Prevention of endemic healthcare-associated Clostridium difficile infection: Reviewing the evidence. Am J Gastroenterol 2010; 105 2327–39.
| Prevention of endemic healthcare-associated Clostridium difficile infection: Reviewing the evidence.Crossref | GoogleScholarGoogle Scholar | 20606676PubMed |
[35] Price J, Cheek E, Lippett S, Cubbon M, Gerding DN, Sambol SP, et al Impact of an intervention to control Clostridium difficile infection on hospital- and community-onset disease; an interrupted time series analysis. Clin Microbiol Infect 2010; 16 1297–302.
| Impact of an intervention to control Clostridium difficile infection on hospital- and community-onset disease; an interrupted time series analysis.Crossref | GoogleScholarGoogle Scholar | 19832710PubMed |
[36] Fowler S, Webber A, Cooper BS, Phimister A, Price K, Carter Y, et al Successful use of feedback to improve antibiotic prescribing and reduce Clostridium difficile infection: a controlled interrupted time series. J Antimicrob Chemother 2007; 59 990–5.
| Successful use of feedback to improve antibiotic prescribing and reduce Clostridium difficile infection: a controlled interrupted time series.Crossref | GoogleScholarGoogle Scholar | 17387117PubMed |
[37] Grayson ML, Jarvie LJ, Martin R, Johnson PD, Jodoin ME, McMullan C, et al Significant reductions in methicillin-resistant Staphylococcus aureus bacteraemia and clinical isolates associated with a multisite, hand hygiene culture-change program and subsequent successful statewide roll-out. Med J Aust 2008; 188 633–40.
| 18513171PubMed |
[38] Pittet D, Allegranzi B, Boyce J. The World Health Organization Guidelines on Hand Hygiene in Health Care and their consensus recommendations. Infect Control Hosp Epidemiol 2009; 30 611–22.
| The World Health Organization Guidelines on Hand Hygiene in Health Care and their consensus recommendations.Crossref | GoogleScholarGoogle Scholar | 19508124PubMed |
[39] McLaws ML, Pantle AC, Fitzpatrick KR, Hughes CF. More than hand hygiene is needed to affect methicillin-resistant Staphylococcus aureus clinical indicator rates: clean hands save lives, part IV. Med J Aust 2009; 191 S26–31.
| 19835528PubMed |
[40] Gerding DN, Muto CA, Owens RC Jr. Measures to control and prevent Clostridium difficile infection. Clin Infect Dis 2008; 46 S43–9.
| Measures to control and prevent Clostridium difficile infection.Crossref | GoogleScholarGoogle Scholar | 18177221PubMed |
[41] Boyce JM, Ligi C, Kohan C, Dumigan D, Havill NL. Lack of association between the increased incidence of Clostridium difficile-associated disease and the increasing use of alcohol-based hand rubs. Infect Control Hosp Epidemiol 2006; 27 479–83.
| Lack of association between the increased incidence of Clostridium difficile-associated disease and the increasing use of alcohol-based hand rubs.Crossref | GoogleScholarGoogle Scholar | 16671029PubMed |
[42] Vernaz N, Hill K, Leggeat S, Nathwani D, Philips G, Bonnabry P, et al Temporal effects of antibiotic use and Clostridium difficile infections. J Antimicrob Chemother 2009; 63 1272–5.
| Temporal effects of antibiotic use and Clostridium difficile infections.Crossref | GoogleScholarGoogle Scholar | 19372170PubMed |
[43] Gordin FM, Schultz ME, Huber RA, Gill JA. Reduction in nosocomial transmission of drug-resistant bacteria after introduction of an alcohol-based handrub. Infect Control Hosp Epidemiol 2005; 26 650–3.
| Reduction in nosocomial transmission of drug-resistant bacteria after introduction of an alcohol-based handrub.Crossref | GoogleScholarGoogle Scholar | 16092747PubMed |
[44] Jabbar U, Leischner J, Kasper D, Gerber R, Sambol SP, Parada JP, et al Effectiveness of alcohol-based hand rubs for removal of Clostridium dificile spores from hands. Infect Control Hosp Epidemiol 2010; 31 565–70.
| Effectiveness of alcohol-based hand rubs for removal of Clostridium dificile spores from hands.Crossref | GoogleScholarGoogle Scholar | 20429659PubMed |
[45] Johnson S, Gerding DN, Olson MM, Weller MD, Hughes RA, Clabots CR, et al Prospective, controlled study of vinyl glove use to interrupt Clostridium difficile nosocomial transmission. Am J Med 1990; 88 137–40.
| Prospective, controlled study of vinyl glove use to interrupt Clostridium difficile nosocomial transmission.Crossref | GoogleScholarGoogle Scholar | 2301439PubMed |
[46] Organisation WH. WHO guidelines on Hand Hygiene in Health Care. Vol. 2010, 2009.
[47] Bobulsky GS, Al-Nassir WN, Riggs MM, Sethi AK, Donskey CJ. Clostridium difficile skin contamination in patients with C. difficile-associated disease. Clin Infect Dis 2008; 46 447–50.
| Clostridium difficile skin contamination in patients with C. difficile-associated disease.Crossref | GoogleScholarGoogle Scholar | 18181742PubMed |
[48] Wullt M, Odenholt I, Walder M. Activity of three disinfectants and acidified nitrite against Clostridium difficile spores. Infect Control Hosp Epidemiol 2003; 24 765–8.
| Activity of three disinfectants and acidified nitrite against Clostridium difficile spores.Crossref | GoogleScholarGoogle Scholar | 14587940PubMed |
[49] Jump RL, Pultz MJ, Donskey CJ. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile-associated diarrhea? Antimicrob Agents Chemother 2007; 51 2883–7.
| Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile-associated diarrhea?Crossref | GoogleScholarGoogle Scholar | 17562803PubMed |
[50] Howell MD, Novack V, Grgurich P, Souillard D, Novack L, Pencina M, et al Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170 784–90.
| Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection.Crossref | GoogleScholarGoogle Scholar | 20458086PubMed |
[51] Siegel J, Rhinehart E, Jackson M, Chiarello L, HICPA Committe. 2007 Guideline for isolation precautions : preventing transmission of infectious agents in healthcare settings. Am J Infect Control 2007; 35 S65–164.
| 18068815PubMed |
[52] Weber D, Rutala W, Miller MA, Huslage K, Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: Norovirus, Clostridium difficile and Acenetobacter species. Am J Infect Control 2010; 38 S25–33.
| Role of hospital surfaces in the transmission of emerging health care-associated pathogens: Norovirus, Clostridium difficile and Acenetobacter species.Crossref | GoogleScholarGoogle Scholar | 20569853PubMed |
[53] Sethi AK, Al-Nassir WN, Nerandzic MM, Bobulsky GS, Donskey CJ. Persistence of skin contamination and environmental shedding of Clostridium difficile during and after treatment of C. difficile infection. Infect Control Hosp Epidemiol 2010; 31 21–7.
| Persistence of skin contamination and environmental shedding of Clostridium difficile during and after treatment of C. difficile infection.Crossref | GoogleScholarGoogle Scholar | 19929371PubMed |
[54] Sehulster L, Chinn RY. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 2003; 52 1–42.
| 12836624PubMed |
[55] Boyce JM, Havill NL, Otter JA, McDonald C, Adams NMT, Cooper T, et al Impact of hydrogen peroxide vapor room decontamination on Clostridium difficile environmental contamination and transmission in a healthcare setting. Infect Control Hosp Epidemiol 2008; 29 723–9.
| Impact of hydrogen peroxide vapor room decontamination on Clostridium difficile environmental contamination and transmission in a healthcare setting.Crossref | GoogleScholarGoogle Scholar | 18636950PubMed |
[56] Andersen BM, Banrud H, Boe E, Bjordal O, Drangsholt F. Comparison of UV C light and chemicals for disinfection of surfaces in hospital isolation units. Infect Control Hosp Epidemiol 2006; 27 729–34.
| Comparison of UV C light and chemicals for disinfection of surfaces in hospital isolation units.Crossref | GoogleScholarGoogle Scholar | 16807849PubMed |
[57] Carling PC, Briggs JL, Perkins J, Highlander D. Improved cleaning of patient rooms using a new targeting method. Clin Infect Dis 2006; 42 385–8.
| Improved cleaning of patient rooms using a new targeting method.Crossref | GoogleScholarGoogle Scholar | 16392086PubMed |
[58] Gregg M, editor. Field Epidemiology. 2nd ed., 2003.
[59] CDC. Clostridium difficile (CDI) Infections Toolkit. Vol. 2010, 2009.

