Does our bundle stack up! Innovative nurse-led changes for preventing catheter-associated urinary tract infection (CAUTI)
Michelle Giles A B , Wendy Watts C , Anthony O’Brien A B , Sandy Berenger D , Michelle Paul E , Karen McNeil A and Kamana Bantawa A FA Nursing and Midwifery Research Centre, Hunter New England Local Health District, Newcastle, NSW 2310, Australia.
B School of Nursing and Midwifery, University of Newcastle, Newcastle, NSW 2308, Australia.
C Urology Royal Newcastle Centre, Hunter New England Local Health District, Newcastle, NSW 2310, Australia.
D Infection Prevention and Control (IPCU), Hunter New England Local Health District, Newcastle, NSW 2310, Australia.
E Continence, Hunter New England Local Health District, Newcastle, NSW 2310, Australia.
F Corresponding author. Email: kamana.bantawa@hnehealth.nsw.gov.au
Healthcare Infection 20(2) 62-71 https://doi.org/10.1071/HI14035
Submitted: 22 October 2014 Accepted: 2 March 2015 Published: 21 April 2015
Journal Compilation © Australasian College for Infection Prevention and Control 2015
Abstract
Introduction: The aim of this project was to develop and implement an innovative nurse-led model of care in the use and management of indwelling urinary catheters (IUC) utilising evidence-based ‘bundle interventions’ to reduce the incidence of catheter-associated urinary tract infections (CAUTI).
Design and method: A pre and post intervention study designed to progress in three phases was conducted in the orthopaedic ward and urology ward of a large tertiary referral facility. Phase one involved a clinical data collection pre intervention on all inpatients receiving an IUC over a 3-month period from February to April 2013. A staff survey assessed knowledge and skills and an evidence-based care bundle, nurse-led protocols, and education resources were developed through collaboration with clinicians. Phase two involved implementation and Phase three was an evaluation with the primary outcome targets being reduced IUC usage, days IUC in situ and incidence of CAUTI.
Results: Pre audit data revealed a high rate of IUC use: 31% of all inpatients in the orthopaedic ward and 25% in the urology ward. Compliance with current guidelines was inconsistent and documentation related to IUCs was poor. Overall CAUTI rate was relatively low at 2.2% of all patients with an IUC and was higher in the orthopaedic ward.
Conclusion: The development of a systematic and standardised approach to IUC care for inpatients using bundle care interventions will potentially reduce IUC use, provide a clear pathway for nurse-initiated IUC removal and reduce the incidence of catheter-associated urinary tract infections (CAUTI).
Implications
-
Standardised practice guidelines through the use of a CAUTI care bundle for insertion and management of IUCs and audit tools to monitor practice compliance were developed.
-
Clinicians’ knowledge of CAUTI risks associated with IUC use increased.
-
A clear pathway for nurse-initiated ICU removal that will reduce IUC use and the incidence of CAUTI was provided.
Introduction
Catheter-associated urinary tract infection (CAUTI) has received greater attention in recent years as it accounts for 40% of all Australian healthcare-associated infections (HAI).1 There are an estimated 100 million urinary catheters used annually around the world2 and urinary tract infections (UTI) are estimated to cause one death per 1000 episodes of urinary catheterisation.3 Despite these staggering statistics and a collective agreement that there is a need to reduce the use of indwelling urinary catheters (IUC) to minimise the impact CAUTI incidence, international literature suggests that between 15 and 25% of all hospital inpatients have an IUC inserted during hospitalisation.4,5
It is argued that IUC insertion can be unjustified in up to 50% of cases.6,7 Furthermore, the risk of CAUTI increases with the duration of catheterisation: 26% of patients with an IUC in situ for a period of between 2 and 10 days will develop bacteriuria and 25% of these patients will develop a CAUTI.6 CAUTI risk also increases with catheter care violations and older age.8 Evidence indicates that effective care strategies to reduce the rate of catheter-based infections incorporating staff educational programs, multidisciplinary team involvement, compliance monitoring and feedback, can be effective in reducing CAUTI rates9 but uptake of this evidence into clinical practice has been slow.
This paper outlines a project aimed at reducing the incidence of CAUTI using a coordinated nurse-led interprofessional approach to develop protocols for managing IUC insertion within a large trauma referral centre in New South Wales, Australia. The facility has 600 plus beds and is an adult and paediatric teaching hospital with 75 768 (2011–2012) separations annually; 70% of these are older people over the age of 65.
Search strategy
Using the search terms: ‘CAUTI’, ‘catheter AND urinary tract infections’, and ‘catheter AND bacteriuria’, searches were conducted for the period between 2000 and 2013 in the following electronic databases: Ovid MEDLINE(R), CINAHL Plus, Embase, Wiley Online, Proquest, Oxford Journals Online, Science Direct and EBSCO.
Literature
Historically, CAUTIs were viewed as a normal consequence of hospitalisation, but they are now considered as ‘an unacceptable harm resulting from medical care’ (p. 2783),10 which are in most cases preventable.5 In the United States, hospitals are no longer compensated for the costs of hospital-acquired UTI’s because they are perceived to be preventable.11 Despite the extensive literature on CAUTI prevention, interventions to reduce the placement of inappropriate IUCs have been implemented inconsistently across healthcare settings.1 IUCs have been overused in acute care settings1 and as a consequence CAUTI’s are one of the most frequently occurring nosocomial healthcare infections.12,13 Morbidity and mortality rates resulting from CAUTIs result in a substantial burden of care, and significant hospitalisation costs related to length of stay and infection treatment.5
Most studies however, do not differentiate between symptomatic and asymptomatic CAUTIs (also known as asymptomatic bacteraemic urinary tract infection or ABUTI).4,5,14,15 It is important to note that the presence of bacteria in the urine (bacteriuria) of otherwise healthy patients with an IUC is generally asymptomatic and this typically resolves with the removal of the catheter. ABUTI does not pose an increased risk of symptomatic CAUTI unless the patient is predisposed to developing an infection.1 Nonetheless, antimicrobial treatment is commonly used in patients with ABUTI16 even though it has not been shown to be beneficial.17
The duration of catheterisation is the key risk factor for the development of CAUTIs. Occurrences of short-term catheter-related bacteriuria as stated earlier are generally asymptomatic18 whereas infection is nearly universal by 30 days in situ.16 For those patients who have an IUC in place for between 2 and 10 days, one in four will develop bacteriuria. Symptomatic infection develops in ~20% of patients with catheter-associated bacteriuria adding 1 to 2 hospital days to the length of stay (LOS).6 Bacteraemia develops in fewer than 4% of catheterised patients with bacteriuria, with reports of associated mortality rates ranging from 10 to 13%.12 Urinary catheterisation is also associated with patient discomfort and pain, restriction of activity and consequential discharge delays6 with the additional concern that CAUTIs ‘comprise one of the largest reservoirs of multidrug-resistant bacteria in healthcare settings’ (p. 41).1
Evidence-based guidelines
Evidence-based guidelines have been developed recently to assist in the reduction of CAUTI rates.5,18–20 Such guidelines include: restricting IUCs to where there are clear indications for their use and removing the catheter as soon as possible;19 usage reduction efforts should be directed towards those patient populations at highest risk of developing catheter complications;5,16,21 catheters should not be routinely used postoperatively;5 portable ultrasound bladder scanning can be effective in reducing unnecessary catheter insertions;5,19 and where appropriate, alternatives such as external catheters or intermittent catheterisation can be considered in selected population groups.5,18,21
Despite the availability of evidence-based guidelines, simple dissemination of this information is often not effective in changing clinical practice.6,7 For instance, data from the USA found that 30% of hospitals do not monitor urinary tract infection (UTI) rates, more than 50% do not monitor the number of patients with urinary catheters and more than 70% do not regularly monitor the duration and discontinuation of urinary catheters.6 Furthermore, fewer than 10% of hospitals in the USA have adopted stop order or reminder systems, despite the considerable evidence of their effectiveness in improving urinary catheter use.6,22 Such data highlights the complexity of sustainable translation of evidence and best practice in the prevention of CAUTI.22
Bundles
Sustainable intervention strategies addressing CAUTIs comprise several key elements. The first of these is the ‘care bundle’, defined as a collection of a small number of evidence-based practices or steps which are vital to achieving improvement in clinical outcomes.23 Saint et al.6 outline the ‘bladder bundle’ which utilises the simple mnemonic ‘ABCDE’. The bladder bundle focuses particularly on adherence to general infection control principles, on reducing catheter use by considering other alternatives and the regular evaluation of in situ catheters and their prompt removal.6
The adoption of a care bundle associated with a checklist is advocated as the optimal process to implement the guidelines.24 In particular, checklists assist in limiting the use of catheterisation, reduce the risk of acquiring a CAUTI should catheterisation be indicated and provide documentation to monitor and audit CAUTI rates.21 Furthermore, tailoring care bundles to local conditions fosters sustainability and fidelity of implementation.6
As part of a bundle, reminder systems have been instrumental in achieving reductions in unnecessary catheter use and CAUTI rates. For example, in one study physicians were prompted to remove inappropriate catheters by using a sticker placed on the patient’s medical record.25 In another study, staff utilised daily nurse reminders to physicians to remove urinary catheters 4 days after insertion. This reminder system resulted in the reduction of catheterisation duration in two out of five hospital departments and subsequently reduced CAUTI frequency rates.13 A systematic review and meta-analysis of interventions involving routine reminders and stop orders to prompt removal of catheters, resulted in a greater than 50% reduction in CAUTI episodes and a 37% decrease in catheter duration. The process highlights that stop orders and reminders, regardless of the technique used, can be simple, cost-effective tools to achieve reductions in CAUTI rates.26
Nurse-led implementation
Nursing staff have been identified as critical in ‘bundle’ interventions with nurse-led protocols for catheter removal under established guidelines being identified as an effective means to reduce IUC duration.27,28 It is recommended that nurses be nominated as project champions because they are often the clinician responsible for insertion, care and maintenance of IUCs.6,29 However, multidisciplinary team involvement is identified as a key feature in reducing CAUTI rates.6,30 Murphy et al.31 in a systematic review of interventions used to minimise IUC use in acute care, identified the need to change the culture of urinary catheter insertion through collaboration and communication between colleagues to address ‘ritualised’ (p 10.) insertion practices.
Much of the literature emphasises the importance of staff educational programs as part of the overall management strategy. Education of both new and long-term clinical staff should include indications for use of catheterisation as well as management and timely removal of catheters.5,19,32 The implementation of education strategies supplemented by surveillance and feedback systems have been demonstrated to lead to a reduction in CAUTI rates33 while audit and feedback of measurement data have been shown to reduce postoperative urinary catheter duration.5,9,28,33–35
Study aim
The aim of the CAUTI project was to develop and implement an innovative nurse-led ‘bundles’ model of care for the use and management of IUCs.
Ethics
This study was approved by the Hunter New England Human Research Ethics Committee. Ward and patient names were coded and data de-identified.
Method
Design
A pre and post intervention study was conducted and designed to proceed in three phases. Phases one and two are reported in this paper.
Phase one involved scoping the extent of the problem, exploration of the literature, collaboration with all stakeholders and development of evidence-based IUC insertion criteria, care bundles and guidelines for the nurse-led protocols.
Phase two involved further consultation with ward staff related to implementation strategies, nomination of ward champions to engage ward staff and assist in implementation of the nurse-led protocol, education of staff, development of education material such as DVDs and targeted resource materials such as stickers, posters and badges to increase awareness of the practice changes being implemented.
Phase three is an evaluation with the primary outcome targets being reduced IUC usage, days IUC in situ and incidence of CAUTI, improved staff knowledge and awareness of risks associated with IUC use, and bundle care compliance. This will be reported in a further paper.
Setting
Two acute care inpatient wards identified by the urology nurse consultant as anecdotally having the highest urinary catheter usage rates were chosen as the sites for the project. One ward was an adult surgical urology ward with 16 beds and the other an adult orthopaedic ward with 32 beds.
Data collection
Phase one
Infection control data was examined for a 3-month period between March and May 2013 to ascertain the current inpatient CAUTI rates for the two wards as a baseline to compare with post implementation. A clinical chart audit was carried out on all patients who had an IUC inserted in the two nominated wards over a 3-month period: March, April and May 2013. Audit data collected assisted in determining the need for improvement and identified trends related to IUC usage, days in situ, reason for insertion, date of removal and any presence of bacteria or CAUTI. Presence of CAUTI was determined using the Centers for Disease Control and Prevention definition.15
A pre implementation survey of nursing and medical staff working in both wards assessed their knowledge and skill levels related to: CAUTI risk and prevention, current guidelines and policy, and insertion management and removal of IUCs. Surveys were individually addressed to all nurses working on the two wards via the internal mailing system. Distribution to Medical Officers was opportunistic, on ward rounds or at clinical meetings. The results of this survey assisted to inform the development of the most appropriate educational resources and in providing a baseline to assess the effectiveness of implementation strategies and educational resources as well as provide trend data on rate of compliance with competency assessments.
Phase two: development and implementation
An evidence-based care bundle was developed for insertion and ongoing management of IUCs. A decision flowchart was developed for nurses to assess the need for the IUC and initiate IUC removal, and standardised guidelines for insertion and nurse-initiated removal criteria were developed. The development of these tools involved a comprehensive review of the literature and extensive collaboration with ward nursing staff, specialist nurses and medical staff in the areas of infection control, urology, continence management and orthopaedics.
Several nurses were engaged as ward champions for each ward to assist in communication and consultation processes and to oversee the bundle implementation process. Champions were also responsible for ongoing compliance monitoring to assess whether the model had been embedded in practice to ensure sustainability. Education sessions were held with all nursing staff across all shifts in both wards. These sessions provided the opportunity to consult with clinicians and get feedback and suggestions on the tools being developed and to advise on context-specific implementation strategies and educational resources required.
A variety of education tools were developed, such as a DVD outlining the bundles and the catheter insertion and management procedures. Information sessions were given to staff on the bundles and decision protocol, and champions again were responsible for supporting clinicians in the use of the bundles and protocol. Large colourful posters outlining the bundles and protocol were displayed in the wards, badges were worn by staff (champions) to improve awareness of the practice change and stickers were also used on patient charts and display boards to improve clinician awareness. Colour coding was consistent across all these tools.
A compliance audit sheet mapped to the care bundle and guidelines was developed and trialled before implementation, and optimised based on feedback from the ward champions. After implementation, compliance audits were carried out daily for 2 weeks to assist in the education of all clinicians, and to embed the new guidelines into clinical practice. The champions would be responsible for ongoing audits after this period.
Phase three
An evaluation will be undertaken 3 months post implementation in both wards. This will include a post implementation survey to assess knowledge and skill level improvement in staff, and a post implementation clinical chart audit over a period of 3 months to assess rate of IUC usage from January to March 2015. Compliance auditing will be conducted daily over a 2-week period. Infection control databases would identify any reduction in CAUTI rates within the two wards. There are then plans to implement the care bundle across the entire health district.
Data analysis
Quantitative clinical audit and educational survey data were descriptively analysed using SPSS Version 1836 to facilitate post implementation inferential comparison in the evaluation arm of the study (Phase three).
Results
Pre implementation clinical audit data
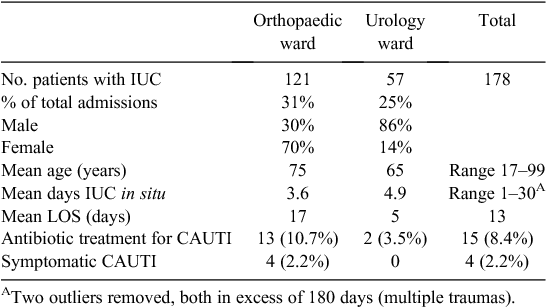
A total of 178 patients were identified as having an IUC in situ during their admission in the two study wards in the allocated pre intervention time period. Details of the audit are outlined in Table 1. Numbers audited in the orthopaedic ward were greater as they had more beds and a greater number of admissions. Up to 31% of admitted patients in the time period received an IUC. The mean age of patients was greater in the orthopaedic ward. The majority of catheterised patients were female in the orthopaedic ward and male in the urology ward, reflecting the different type of patient demographic in each ward. The predominant admission diagnosis in the orthopaedic ward was hip or femur fracture (n = 101). In the urology ward the most common admission diagnosis was benign prostatic hyperplasia requiring surgery (25%, n = 14), followed by renal and bladder cancer (18%, n = 10).
|
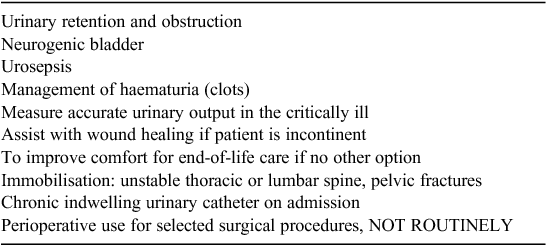
In the orthopaedic ward where it is routine practice to insert an IUC into all patients with a fractured hip or femur, the reason documented for all of these insertions was ‘preoperative’ or ‘intraoperative’. The most common documented reasons for IUC insertion were: pre or intraoperatively (59%, n = 105), monitor urine output (11%, n = 20) and irrigation (10%, n = 18). There were two patients where no reason was documented at all and hip fracture was given as the reason in one instance only. Another six were documented as in situ on admission and these patients had been transferred from other facilities. In the orthopaedic ward there were only eight patients who had IUC insertion that were considered appropriate based on the indications for IUC insertion developed as part of this project and outlined in Table 2. The majority of patients had an IUC inserted in either the emergency department (ED) (47%, n = 84) or operating theatre (OT) (38%, n = 68) and mostly by registered nurses (RN) (59%, n = 104). Only 10 IUC’s were inserted in the wards and 15 were inserted in other facilities. In the urology ward reasons for IUC insertion were well documented in all but one chart audit and were all appropriate when compared with indications for IUC insertion guidelines developed by this project team.
A documented request to insert a catheter was made on 131 occasions (74%); however, the remaining 47 patient charts audited (26%) had no documented request. Requests for catheterisation were made by medical officers but there was one occasion of a nurse-initiated insertion. In general, catheters were inserted for described conditions preoperatively, to monitor urinary output, to irrigate the bladder, intraoperatively, or due to trauma, urinary tract infection, or haematuria. The mean length of stay (LOS) was 13 days and mean days the IUC was left indwelling was 3.9 days overall, ranging from 1 to 30 days and was higher in the urology ward. However days in situ was unable to be calculated from 20 patient charts as either an insertion or removal date and time was not recorded anywhere in the patient notes.
|
Catheters were recorded as removed for a variety of reasons. Catheters were removed because they either became dislodged (n = 2), leaked (n = 2) and one was recorded as removed after 10 days due to contamination. On many occasions there was no documented evidence of a request for removal, or no reason provided. At least 50% of the time there was a documented request for removal of the catheter (unspecified ‘yes’ n = 53) or by a registrar (n = 39); however, on 63 occasions it was cited as unknown who had made a request for removal. Adding the ‘yes’ responses (unspecified) and the unknowns together, a request for removal can be said to have occurred obscurely on at least 116 (70%) occasions.
CAUTI rate
In all, 81% (n = 144) of charts recorded a CSU or MSU’s as being taken, 25% (n = 36) having an identified bacterial growth and 61% (n = 22) of these patients were treated with oral (n = 17) or intravenous (n = 5) antibiotics. There were 15 (8.4%) patients treated for CAUTI from the whole group (Table 1): 12 of these patients had a primary diagnosis of hip or femur fracture and 10 were female. However, only 4 of the 15 cases (2.2%) were identified as symptomatic CAUTI based on the definition15 and all were fracture patients from the orthopaedic ward. However, there were two other patients with laboratory-confirmed CAUTI not included in this number because they did not meet the criteria outlined15 as no other symptoms were documented in their history notes. The mean duration the IUC was in situ in the CAUTI group was 6.5 days, with a median of 4.5 days and a range of 3–15 days. However two patients were not included in this calculation as there was no documentation of insertion or removal dates. There was no removal date recorded for the two patients in the urology ward treated for CAUTI.
The educational survey
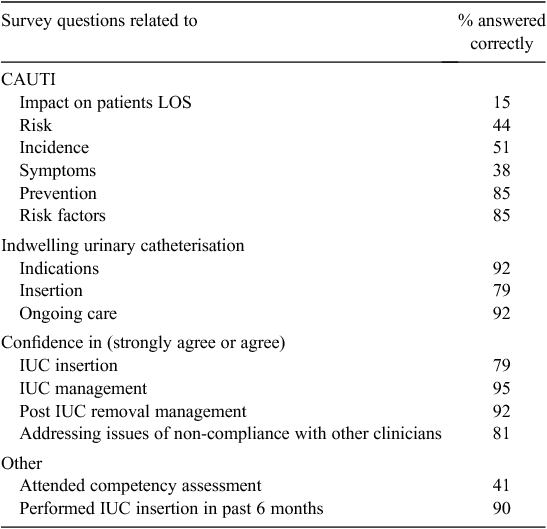
There were 90 surveys distributed to all nurses across both wards via internal mail and 20 to medical staff opportunistically at clinical meetings and ward rounds. Response rate for nurses was disappointing at 32% (n = 29) and 50% (n = 10) for medical officers. The majority (77%) of respondents were employed in these wards fulltime. Questions and results are detailed in Table 3. There was a considerable gap in knowledge identified in respondents related to the risk and incidence of CAUTI. When asked about the patient risk and prevention of CAUTI, respondents were correct only between 15 and 50% of the time; however, they had a greater knowledge of IUC management and CAUTI prevention strategies with 85 to 92% accuracy.
|
Only 41% (n = 16) of respondents had completed the compulsory competency assessment but over 87% (n = 34) of respondents had catheterised a patient in the past 6 months. There were 18 respondents (46%) who had catheterised a male or female patient in the last 6 months and who had not passed competency assessment. Fourteen of these respondents had not had any other education on urinary catheterisation and five of these were medical officers. However, almost 80% (n = 31) agreed that they felt confident when inserting a urinary catheter. The majority of respondents reported being confident in the care of patients post catheter insertion (95%) and post catheter removal (92%), but 18% of respondents did not feel confident in addressing issues of non-compliance of care with other staff members. Several staff commented that they would like further education and the development of a decision-making tool to help staff in deciding if a patient requires an IUC.
Development of the intervention bundle and guidelines
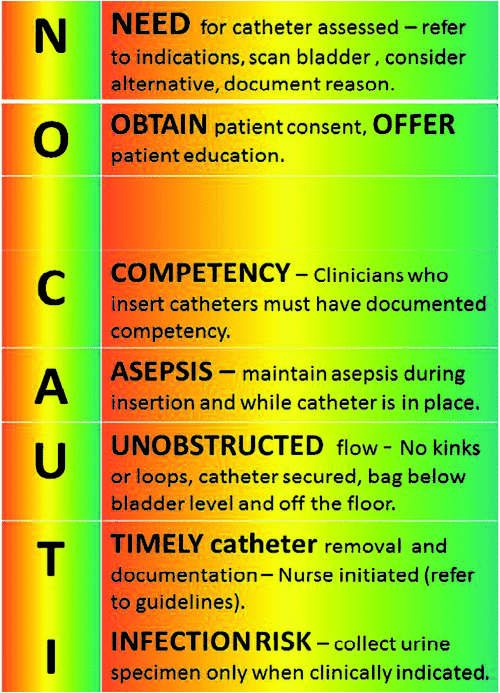
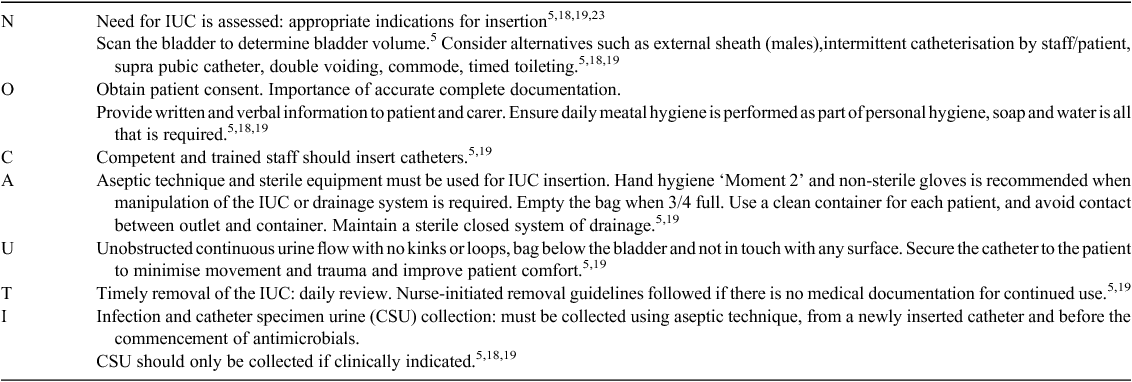
The development of the bundle and guidelines was informed by an extensive literature review and collaborative consultation with key stakeholders including the: Director of Infection Prevention Services, Director of Urology Services, orthopaedic surgeons, Director of Emergency Department, clinical nurse consultants in infection control and urology, and nursing staff in the two participating wards. The bundle (Fig. 1) steers away from the traditional ABC mnemonics,6 using NO CAUTI as the acronym to reinforce the message that CAUTI is a risk with all urinary catheterisations and that prevention is our key aim. The bundle places emphasis on assessing the need for catheter insertion and timely removal, documentation, patient education and consent, clinical insertion competency, asepsis and care maintenance. Table 4 outlines the evidence base that supports the content of the bundle.
|
|
A compliance audit tool was developed based on the bundle format and trialled in wards that provided valuable feedback to further optimise the tool. Guidelines were also developed in the form of a decision flowchart to assist nursing staff in initiating IUC removal (Fig. 2), and criteria for IUC insertion were reviewed and updated after consultation with the above stakeholder group.

|
A new catheterisation pack was developed which included all equipment in the one pack, such as documentation stickers and securing devices. This was done in consultation with all units who insert urinary catheters such as operating theatre, obstetrics, ED, general wards and community clinicians so that one standard pack would aim to fulfil the needs across all services and sectors. Clinicians agreed to trial the generic pack and then give feedback.
An awareness campaign was implemented where education sessions were held in the wards and the ED, champions were nominated and engaged as part of the implementation team, badges were worn by the project team and ward staff to increase awareness, and posters were displayed in ward areas with insertion and removal criteria and the care bundle. Consistent bright colours were used to colour code all tools associated with the project (Fig. 1). The project team developed an educational DVD available to all staff on the organisation’s intranet outlining the insertion techniques in detail for male and female catheterisations and ongoing care while the IUC remained in situ.
Discussion
Internationally the literature reports that a quarter of all inpatients have an indwelling catheter inserted during their hospital stay4,5 and on many occasions the insertion is based on ritualistic practices, with no clinical indication for insertion.31
The data derived from the first two phases of this study has identified considerable inconsistencies in IUC insertion and management practices. It is important to note here that there was no routine monitoring of IUC usage across the facility or health district and at the time of the study it was difficult to identify UTI rates. This influenced the scope of this study which was limited to two wards. There was an identified routine practice of IUC insertion in female hip and femur fracture patients. This routine practice does not align with the guidelines developed as part of this study related to indications for IUC insertion. However extensive consultation has occurred with orthopaedic surgeons and the ED consultants as part of the process which should reduce routine IUC insertions without indication. These IUCs are generally inserted in the ED or OT and the reason recorded for insertion in all instances except one is either ‘preoperative’ or ‘intraoperative’. In the study period 75 female patients with hip or femur fractures were included in the orthopaedic ward cohort and all received IUC routinely either in ED or in OT, if they did not already have one in place when transferred from elsewhere. This practice no doubt accounts for the high IUC rate of 31% in patients admitted to the orthopaedic ward compared with the urology ward (25%) and 15% to 25% cited in international literature.4,5 Ten of these 75 female patients were treated for CAUTI, which is 13% of all female patients admitted to the orthopaedic ward with a hip or femur fracture. The findings from this study reflect a natural gender bias related to reported CAUTI rates due to the high number of females with hip and femur fractures and the larger numbers included from the orthopaedic ward that make up this cohort.
A unique feature of this study is the differentiation between symptomatic and asymptomatic CAUTI which has been somewhat neglected in many previous studies.4,5,14 According to the CAUTI definition used for this study,15 only four patients were identified as having symptomatic CAUTI despite 15 being treated for CAUTI. However, two other patients did have laboratory-confirmed CAUTI but had no documentation of the symptoms outlined as part of the criteria outlined,15 so were omitted from the final numbers. There were challenges when applying this definition uniformly using retrospective documentation alone as well as in this elderly cohort who were dealing with many other complications and comorbidities.
Again it is important to note that the presence of bacteria in the urine (bacteriuria) of otherwise healthy patients with an IUC is generally asymptomatic and this typically resolves with the removal of the catheter. Nonetheless, antimicrobial treatment is commonly used in patients with ASB16 even though it has not been shown to be beneficial.17 This has highlighted an important issue which needs more attention and is something that the team will focus on in their future education.1,16,17
Collaborative consultation with the orthopaedic, urology and ED and relevant specialists in the development of the insertion guidelines and care bundles has led to the creation of concise decision tools to guide insertion practices with the aim of reducing unnecessary catheterisations and standardising insertion practices.
Findings related to location of insertion, where the majority of IUCs were inserted in the ED changed the focus of our implementation strategy to include implementation in the ED department, as well as the wards. This will inform future implementation strategies district-wide in 2015.
Certainly, the lack of adequate patient documentation has been highlighted as one area needing major work and strategies to address this have been developed as part of the nurse-led protocol. A sticker highlighting all required documentation was developed and made available in the sterile catheter insertion pack. This sticker is utilised as part of the procedure and placed in the patient’s history notes. The bundle concept has been implemented by many organisations, however, the bundle alone is not sufficient to achieve sustained changes in clinical practice.
Evidence indicates that interventions should also incorporate staff educational programs, compliance monitoring and feedback systems.6,9,29,35 Our findings highlighted the poor compliance, with compulsory assessments at only 41% across the two wards. To address this issue, part of the intervention strategy was the development of a DVD made available on the health intranet for easy access. The nominated champions were charged with assessment responsibility at ward level and the assessment was added to MO orientation programs. Further, the project team have engaged and educated all clinical educators across the site with ‘train the trainer’ capacity and competency assessment responsibilities. They are now directly responsible for monitoring compliance.
Education of both new and long-term clinical staff should include indications for use of catheterisation as well as management and timely removal of catheters.5,19,32 The implementation of education strategies supplemented by surveillance systems has been demonstrated to lead to a reduction in CAUTI rates33 while audit and feedback of measurement data has been shown to reduce postoperative urinary catheter duration.34 This project considered all of these elements, as well as a decision tool to guide staff in assessing the need for IUC use on inpatients, a removal decision flowchart (Fig. 2) as well as supportive educational resources including a DVD accessible across the health district via the organisation’s intranet. The care bundles were developed in collaboration with medical and nursing staff across speciality areas, to ensure general agreement and enable standardisation of the protocol as routine practice across facilities and specialties.
Improving awareness was a major consideration in the implementation plan and initiatives such as colour-coded stickers, posters and badges have all been utilised to facilitate clinicians asking questions about IUC use and the risks and potential adverse outcomes for patients. The project team have made an effort to provide education about CAUTI risk and disseminate the protocol, guidelines and other initiatives developed during the course of this study. The tools in this project were developed using a strong evidence base and the most up-to-date best practice initiatives. There has been considerable interest expressed across both rural and metropolitan areas within the health district, as well as at the state level, to roll out the implementation more widely. A full evaluation will occur 3 months post implementation with a view to complete implementation at a district level in 2015.
Conclusion
Urinary catheter-associated infections have a significant impact on patient morbidity and increase costs in the acute healthcare context. The development of a systematic and standardised approach to IUC care for inpatients using bundle care interventions will potentially reduce IUC use, provide a clear pathway for nurse-initiated IUC removal and reduce the incidence of CAUTI. Ongoing compliance monitoring is essential to ensure that these practices are fully embedded and sustained.
Conflicts of interest
On behalf of all authors there is no conflict of interest to declare.
Funding
The project was funded by an internal innovation scholarship provided by the Innovation Support, Hunter New England Local Health District.
Acknowledgement
The authors thank Dr John Ferguson for his support, guidance and sponsorship thoughout the course of the project.
References
[1] Association for Professionals in Infection Control and Epidemiology. Guide to the elimination of catheter-associated urinary tract infections (CAUTIs): developing and applying facility-based prevention interventions in acute and long-term care settings. Washington: APIC; 2008.[2] Nasr A. State of the globe: catheterisations continue to cultivate urinary infections. J Global Infect Dis. 2010; 2 81–2.
| State of the globe: catheterisations continue to cultivate urinary infections.Crossref | GoogleScholarGoogle Scholar |
[3] Gokula RRM, Hickner JA, Smith MA. Inappropriate use of urinary catheters in elderly patients at a midwestern community teaching hospital. Am J Infect Control 2004; 32 196–9.
| Inappropriate use of urinary catheters in elderly patients at a midwestern community teaching hospital.Crossref | GoogleScholarGoogle Scholar |
[4] Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control 2000; 28 68–75.
| Clinical and economic consequences of nosocomial catheter-related bacteriuria.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c7ktlCitQ%3D%3D&md5=f89414e0e7ce06f6f770c435a528a294CAS | 10679141PubMed |
[5] Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol 2010; 31 319–26.
| Guideline for prevention of catheter-associated urinary tract infections 2009.Crossref | GoogleScholarGoogle Scholar | 20156062PubMed |
[6] Saint S, Olmsted RN, Fakih MG, Kowalski CP, Watson SR, Sales AE, Krein S. Translating healthcare-associated urinary tract infection prevention research into practice via the bladder bundle. Jt Comm J Qual Patient Saf 2009; 35 449–55.
| 19769204PubMed |
[7] Oman KS, Makic MBF, Fink R, Schraeder N, Hulett T, Keech T, Wald H. Nurse-directed interventions to reduce catheter-associated urinary tract infections. Am J Infect Control 2012; 40 548–53.
| Nurse-directed interventions to reduce catheter-associated urinary tract infections.Crossref | GoogleScholarGoogle Scholar | 22047997PubMed |
[8] Salgado CD, Karchmer TB, Farr BM. Prevention of catheter-associated urinary tract infections. 4th edn. Wenzel RP, ed. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 297–311.
[9] Aboelela SW, Stone PW, Larson EL. Effectiveness of bundled behavioural interventions to control healthcare-associated infections: a systematic review of the literature. J Hosp Infect 2007; 66 101–8.
| Effectiveness of bundled behavioural interventions to control healthcare-associated infections: a systematic review of the literature.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2szjtl2iug%3D%3D&md5=bd28f8e49343be062aac2540b3293be5CAS | 17320242PubMed |
[10] Wald HL, Kramer AM. Nonpayment for harms resulting from medical care: catheter-associated urinary tract infections. JAMA 2007; 298 2782–4.
| Nonpayment for harms resulting from medical care: catheter-associated urinary tract infections.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVGmurzL&md5=eab5f71adea5f430a7583f7aeb54659bCAS | 18165672PubMed |
[11] Centers for Medicare and Medicaid Services. Medicaid program: payment adjustment for provider-preventable conditions including health care-acquired conditions. Fed Regist 2011; 76 32815–38.
[12] Klevens RM, Edwards JR, Richards CL, Horan TC, Gaynes RP, Pollock DA. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 2007; 122 160–6.
| 17357358PubMed |
[13] Crouzet J, Bertrand X, Venier AG, Badoz M, Husson C, Talon D. Control of the duration of urinary catheterization: impact on catheter-associated urinary tract infection. J Hosp Infect 2007; 67 253–7.
| Control of the duration of urinary catheterization: impact on catheter-associated urinary tract infection.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2snlvVKqug%3D%3D&md5=ce282f162be6fd72a2377e65f678eb54CAS | 17949851PubMed |
[14] Trautner B. Management of catheter-associated urinary tract infection (CAUTI). Curr Opin Infect Dis 2010; 23 76–82.
| Management of catheter-associated urinary tract infection (CAUTI).Crossref | GoogleScholarGoogle Scholar | 19926986PubMed |
[15] Centers for Disease Control and Prevention. Device-associated Module CAUTI. Urinary Tract Infection (Catheter-Associated Urinary Tract Infection [CAUTI] and Non-Catheter-Associated Urinary Tract Infection [UTI]) and Other Urinary System Infection [USI]) Events USA; 2015. Available from: http://www.cdc.gov/nhsn/pdfs/pscManual/7pscCAUTIcurrent.pdf [verified March 2015]
[16] Maki DG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis 2001; 7 342–7.
| Engineering out the risk for infection with urinary catheters.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MzpsFSlsA%3D%3D&md5=1a5885c731ee499798b05fbe29e22789CAS | 11294737PubMed |
[17] Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious diseases society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40 643–54.
| Infectious diseases society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults.Crossref | GoogleScholarGoogle Scholar | 15714408PubMed |
[18] Tenke P, Kovacs B, Bjerklund Johansen TE, Matsumoto T, Tambyah PA, Naber KG. European and Asian guidelines on management and prevention of catheter-associated urinary tract infections. Int J Antimicrob Agents 2008; 31 S68–78.
| European and Asian guidelines on management and prevention of catheter-associated urinary tract infections.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXptlSitQ%3D%3D&md5=1d5e31cb72b411ca1435bee7d74e3e28CAS | 18006279PubMed |
[19] Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambayh PA, Tenke P, Nicolle LE. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010; 50 625–63.
| Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America.Crossref | GoogleScholarGoogle Scholar | 20175247PubMed |
[20] Lo E, Nicolle L, Classen D, Arias KM, Podgorny K, Anderson DJ, et al Strategies to prevent catheter-associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol 2008; 29 S41–50.
| Strategies to prevent catheter-associated urinary tract infections in acute care hospitals.Crossref | GoogleScholarGoogle Scholar | 18840088PubMed |
[21] Mitchell B, Ware C, McGregor A, Brown S, Wells A, Stuart RL, Wilson F, Mason M. ASID (HICSIG)/AICA Position Statement: Preventing catheter-associated urinary tract infections in patients. Healthc Infect 2011; 16 45–52.
| ASID (HICSIG)/AICA Position Statement: Preventing catheter-associated urinary tract infections in patients.Crossref | GoogleScholarGoogle Scholar |
[22] Mourad M, Auerbach A. Improving use of the ‘other’ catheter: comment on ‘reducing inappropriate urinary catheter use’. Arch Intern Med 2012; 172 260–1.
| Improving use of the ‘other’ catheter: comment on ‘reducing inappropriate urinary catheter use’.Crossref | GoogleScholarGoogle Scholar | 22231612PubMed |
[23] Institute for Healthcare Improvement. Bundle up for safety. Cambridge; 2011. Available from: http://www.ihi.org/resources/Pages/ImprovementStories/BundleUpforSafety.aspx [verified March 2015]
[24] Health Protection Scotland. Targeted literature review: what are the key infection prevention and control recommendations to inform a urinary catheter maintenance care quality improvement tool? Scotland: NHS; April 2012.
[25] Bruminhent J, Keegan M, Lakhani A, Roberts IM, Passalacqua J. Effectiveness of a simple intervention for prevention of catheter-associated urinary tract infections in a community teaching hospital. Am J Infect Control 2010; 38 689–93.
| Effectiveness of a simple intervention for prevention of catheter-associated urinary tract infections in a community teaching hospital.Crossref | GoogleScholarGoogle Scholar | 21034979PubMed |
[26] Meddings J, Rogers MA, Macy M, Saint S. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis 2010; 51 550–60.
| Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients.Crossref | GoogleScholarGoogle Scholar | 20673003PubMed |
[27] Newman DK. CAUTIon: carefully manage indwelling urinary catheters. Nurs Manage 2009; 40 20–2.
| CAUTIon: carefully manage indwelling urinary catheters.Crossref | GoogleScholarGoogle Scholar |
[28] Parry MF, Grant B, Sestovic M. Successful reduction in catheter-associated urinary tract infections: focus on nurse-directed catheter removal. Am J Infect Control 2013; 41 1178–81.
| Successful reduction in catheter-associated urinary tract infections: focus on nurse-directed catheter removal.Crossref | GoogleScholarGoogle Scholar | 23768439PubMed |
[29] Fakih MG, Rey JE, Pena ME, Szpunar S, Saravolatz LD. Sustained reductions in urinary catheter use over 5 years: bedside nurses view themselves responsible for evaluation of catheter necessity. Am J Infect Control 2013; 41 236–9.
| Sustained reductions in urinary catheter use over 5 years: bedside nurses view themselves responsible for evaluation of catheter necessity.Crossref | GoogleScholarGoogle Scholar | 22980514PubMed |
[30] DeNyse A, Olmsted R. Interdisciplinary team approach decreased CAUTI rates in a rehabilitation setting. Infect Dis Newsl 2011; 24 8
[31] Murphy C, Fader F, Prieto J. Interventions to minimise the initial use of indwelling urinary catheters in acute care: a systematic review. Int J Nurs Stud 2014; 51 4–13.
| Interventions to minimise the initial use of indwelling urinary catheters in acute care: a systematic review.Crossref | GoogleScholarGoogle Scholar | 23332716PubMed |
[32] Health Protection Surveillance Centre. Guidelines for the prevention of catheter-associated urinary tract infection. Dublin, Ireland; 2011. Available from: http://www.hpsc.ie/A-Z/MicrobiologyAntimicrobialResistance/InfectionControlandHAI/UrinaryCatheters/Publications/File,12913,en.pdf [verified March 2015]
[33] Marigliano A, Barbadoro P, Pennacchietti L, D’Errico MM, Prospero E, CAUTI Working Collaborative Group Active training and surveillance: 2 good friends to reduce urinary catheterization rate. Am J Infect Control 2012; 40 692–5.
| Active training and surveillance: 2 good friends to reduce urinary catheterization rate.Crossref | GoogleScholarGoogle Scholar | 22632823PubMed |
[34] Wald HL, Kramer AM. Feasibility of audit and feedback to reduce postoperative urinary catheter duration. J Hosp Med 2011; 6 183–9.
| Feasibility of audit and feedback to reduce postoperative urinary catheter duration.Crossref | GoogleScholarGoogle Scholar | 21480488PubMed |
[35] Fakih MG, Watson SR, Greene MT, Kennedy EH, Olmsted RN, Krein SL, Saint S.. Reducing inappropriate urinary catheter use: A statewide effort. Arch Intern Med 2012; 172 255–60.
| Reducing inappropriate urinary catheter use: A statewide effort.Crossref | GoogleScholarGoogle Scholar | 22231611PubMed |
[36] Statistical Package for the Social Sciences. SPSS Statistics for Windows, Version 18.0. Chicago: SPSS Inc; 2009.

