Pneumocystis canis pneumonia in dogs
Elizabeth Ralph A , George Reppas B , Catriona Halliday C , Mark Krockenberger D and Richard Malik E FA Animal Referral Hospital, 250 Parramatta Road, Homebush West, NSW 2140, Australia
B Vetnostics, 60 Waterloo Road, North Ryde, NSW 2113, Australia
C Centre for Infectious Diseases and Microbiology Laboratory Services, ICPMR, Westmead Hospital, Westmead, NSW 2145, Australia
D Faculty of Veterinary Science, The University of Sydney, NSW 2006, Australia
E Centre for Veterinary Education, The University of Sydney, NSW 2006, Australia
F Corresponding author. Email: Richard.Malik@sydney.edu.au
Microbiology Australia 36(2) 79-82 https://doi.org/10.1071/MA15026
Published: 17 March 2015
Pneumocystis canis is a potential cause of life-threatening interstitial fungal pneumonia in dogs. It is seen almost exclusively in two canine breeds, miniature Dachshunds and Cavalier King Charles Spaniels (CKCS)1. Historically, Australian veterinarians had a key role in the documentation of this entity and its conspicuous breed associations2–4. Affected Dachshunds and CKCS are likely to have an inherited immunodeficiency that predisposes them to infection with this commensal organism of the respiratory tract and pharynx1,2,5,6. A high index of suspicion is required to make a timely diagnosis and save affected patients, as these dogs cope poorly with anaesthesia and other measures to procure the specimens required to make a definitive diagnosis. Possible co-infection with Bordetella bronchiseptica must be considered when determining antimicrobial strategies. Affected dogs occasionally have a previous or concurrent history of generalised demodicosis5,7,8. With early intervention, affected dogs can be saved, although some require life-long therapy to prevent recurrence. The future challenge is to develop fast molecular techniques to diagnose P. canis† pneumonia (PCP)7,9 and to determine the underlying immune defect in over-represented breeds through the rapidly advancing field of canine genomics10.
Pneumocystis spp. are ubiquitous commensals of the respiratory tract of many mammalian species, including dogs1. This group of organisms has the potential to cause life-threatening pneumonia in a wide range of mammals, including rats, pigs, horses and goats1. P. jirovecii has the same pathogenic potential in people1. Airborne droplet transmission from subclinically affected normal dogs, often transmitted from bitch to pup soon after birth, is suspected of being the means for transmission1. PCP results from the effects of masses of organisms within the alveolar spaces, combined with the associated inflammatory response of the host1–6.
Historically, most canine cases had been reported in young (less than one-year-old) miniature Dachshunds1–3,6,11. The first documentation of this entity was provided in a paper from the University of Sydney (UoS) by Farrow and colleagues2, although a brilliant immunoparasitologist at McMaster Laboratory (Robert J. Love) provided key immunologic insights.‡ The disease in Dachshunds was characterised further by South African veterinarians, with Lobetti and colleagues adapting human treatment strategies to successfully manage their canine cases1,11. The best radiological description of PCP was provided by Kirberger12, although this has been complemented by recent advances in imaging such as digital radiology and computed tomography (CT). Although PCP continues to be seen in Dachshunds in South Africa and the USA, the great majority of cases encountered in Australia over the past 20 years have been in adult CKCS, the first detailed report being provided by Paul Canfield and colleagues from UoS in 19934. This breed preponderance was then detected in the UK5,13 and subsequently Europe, the USA and Japan7. ‘Cavaliers’ tend to get the disease as young adult dogs4,5,13. There may be antecedent or concurrent footprints of immune deficiency, such as generalised demodicosis5,8.
In people, the disease is best known as a complication of HIV/AIDS, although it is also seen in transplant recipients and other patients receiving immunosuppressive drug regimens. A very well characterised case cluster in Australia that closed a transplant ward at Westmead Hospital was reported by Sharon Chen, Wieland Meyer and their ANZMIG colleagues14. In Arabian horses and related breeds, foals with autosomal recessive severe combined immunodeficiency syndrome (analogous to the like-named SCID condition of men and mice) typically died of PCP in the neonatal period15, and this was common in the Camden district in the 1970s. A test was developed for this genetic disease of horses, and nowadays the condition is hardly encountered, a cogent example of genetic counselling and preventative veterinary medicine16.
In dogs, the nature of the immune defect in Dachshunds and Cavaliers has not been determined at the molecular level. Poor lymphocyte stimulation (despite normal lymphocyte numbers in peripheral blood) has been documented in miniature Dachshunds1,2. IgA, IgM and IgG concentrations are subnormal in affected members of this breed6. Subsequent assessment of CKCS with pneumocystosis also reveals immunoglobulin deficiencies and decreased lymphocyte function5,7. These immunodeficiencies persist after resolution of the infection with effective antimicrobial therapy. Studies of the involvement of adaptive cell-mediated immunity and innate immunity (including the potential involvement of Toll-like receptors) would be sensible but have not yet been performed.
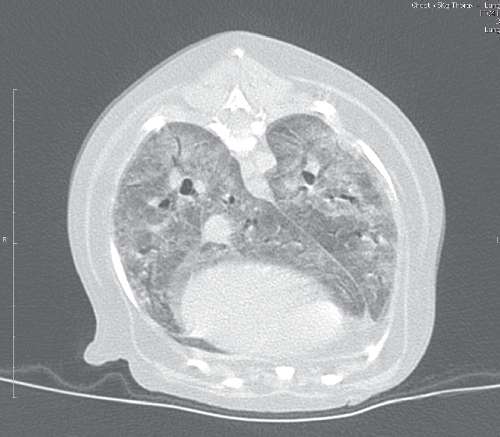
Most dogs with PCP present with respiratory signs, including dyspnoea, tachypnoea, increased breath sounds on thoracic auscultation or cyanotic mucous membranes. The presence of a cough is variable, and this sign is frequently absent. Duration of signs prior to presentation for veterinary attention can be as long as four weeks1–4. Haematologic and serum biochemical abnormalities are non-specific1. Thoracic radiographs reveal diffuse, bilaterally symmetrically increased radiodensity of the pulmonary parenchyma, classically described as a miliary-interstitial to alveolar pattern (Figure 1)1,12,17. There is often radiological evidence of right-sided heart enlargement and pulmonary hypertension, and this can be confirmed echocardiographically4. Solitary opacities, cavitary lesions or a non-symmetric radiographic changes are less commonly observed1,12. Severe long-standing cases may develop emphysema1. The CT findings in canine PCP are dramatic (Figure 2), providing a better appreciation of the lung pathology and disclosing regional lymphadenomegaly.
|
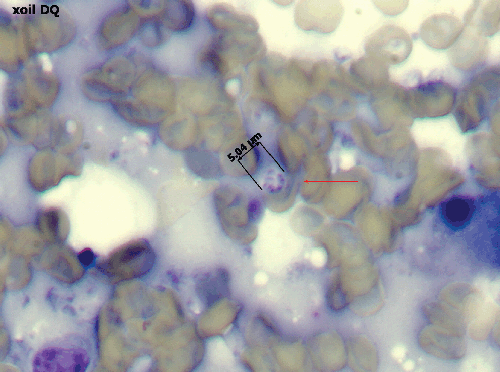
Diagnosis can be challenging in a veterinary context, with identification of P. canis ‘cysts’ in bronchoalveolar lavage fluid (BAL) or transthoracic fine needle aspirates of lung generally being required. Intact cysts are distinctive, being 5–10 µm in diameter and containing 4–8 dark-staining intracystic bodies (2–3 µm in diameter; Figure 3). PCR testing of BAL fluid or lung aspirates is uncommon in veterinary laboratories, but should probably be attempted more often7,9, as the requirement to perform open lung biopsies to secure a diagnosis is unnecessarily invasive given the characteristic nature of the imaging findings and the strong breed associations. If BAL fluid specimens are obtained, they should be subjected to routine bacterial culture as well as microscopy and PCR, as Bordetella bronchiseptica can be an important co-morbidity in canine PCP pneumonia. If a BAL is not done and treatment is based on a presumptive diagnosis, then doxycycline should be given as well as trimethoprim sulfonamide to cover this possibility. Research is currently focused on development of qPCR assays for canine P. carinii strains, as assays developed for P. jirovecii may not detect P. canis associated with infections in dogs1. Panfungal PCR with sequence analysis provides an interim molecular diagnostic strategy, as does genus-specific Pneumocystis PCR assays7–9.
|
Treatment includes appropriate antimicrobial therapy, using intravenous trimethoprim sulfonamide combinations, and less commonly nebulised pentamidine, along with appropriate supportive care (e.g. supplementary oxygen, nebulisation, chest physiotherapy). Long-term ventilation of affected dogs is challenging and beyond the financial limits of most owners. There is no zoonotic potential for humans in close contact with a Pneumocystis infected dogs, as the organisms in humans and dogs are distinct and no evidence of cross-species transmission has been documented.
As thoughtful and responsible veterinary physicians, we should be banking DNA from all dogs with confirmed and presumptive PCP. The most likely way to eliminate this condition is by harnessing the power of canine genomics to detect any underlying genetic defect, utilising either a genome-wide association study or whole genome sequencing of ‘Trios’ of affected and closely related dogs10. It is much better to try and prevent this severe mycosis by screening the breeding population using a molecular genetic test, rather than to have to treat a severely affected individual. This is where vets have an advantage over medics, we can use eugenics!
References
[1] Lobetti, R. (2014) Pneumocystosis. In Canine and Feline Infectious Disease (Sykes, J.E., ed.) Saunders, Philadelphia, pp. 689–692.[2] Farrow, B.R. et al. (1972) Pneumocystis pneumonia in the dog. J. Comp. Pathol. 82, 447–453.
| Pneumocystis pneumonia in the dog.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE3s%2FosVSgtw%3D%3D&md5=1fa4c6d91e173602d7ecbeb6c69aefa6CAS | 4265168PubMed |
[3] Copland, J.W. (1974) Canine pneumonia caused by Pneumocystis carinii. Aust. Vet. J. 50, 515–518.
| Canine pneumonia caused by Pneumocystis carinii.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE2M%2FptVSmug%3D%3D&md5=f0460079789f52c1b055f256f3629e1dCAS | 4548647PubMed |
[4] Canfield, P.J. et al. (1993) Pneumocystis pneumonia in a dog. Aust. Vet. Pract. 23, 150–154.
[5] Watson, P.J. et al. (2006) Immunoglobulin deficiency in Cavalier King Charles Spaniels with Pneumocystis pneumonia. J. Vet. Intern. Med. 20, 523–527.
| Immunoglobulin deficiency in Cavalier King Charles Spaniels with Pneumocystis pneumonia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28zgs1Kntg%3D%3D&md5=c3466683b3a2ca3c8d0943e0461e90eaCAS | 16734084PubMed |
[6] Lobetti, R. (2000) Common variable immunodeficiency in miniature dachshunds affected with Pneumocystis carinii pneumonia. J. Vet. Diagn. Invest. 12, 39–45.
| Common variable immunodeficiency in miniature dachshunds affected with Pneumocystis carinii pneumonia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c7lt1KgsQ%3D%3D&md5=21173ad699021bd4c6cbd718251c1effCAS | 10690774PubMed |
[7] Hagiwara, Y. et al. (2001) Pneumocystis carinii pneumonia in a Cavalier King Charles Spaniel. J. Vet. Med. Sci. 63, 349–351.
| Pneumocystis carinii pneumonia in a Cavalier King Charles Spaniel.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MvpsVantA%3D%3D&md5=1c68dca09fac1227c570616beaa4fbe1CAS | 11307943PubMed |
[8] Furuta, T. et al. (1994) Spontaneous Pneumocystis carinii infection in the dog with naturally acquired generalised demodicosis. Vet. Rec. 134, 423–424.
| Spontaneous Pneumocystis carinii infection in the dog with naturally acquired generalised demodicosis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2czhtVOlsA%3D%3D&md5=c049225c304b496d7ecbd089d64bb37cCAS | 8036776PubMed |
[9] English, K. et al. (2001) DNA analysis of Pneumocystis infecting a Cavalier King Charles spaniel. J. Eukaryot. Micro. 48, 106S.
| DNA analysis of Pneumocystis infecting a Cavalier King Charles spaniel.Crossref | GoogleScholarGoogle Scholar |
[10] Karlsson, E.K. and Lindblad-Toh, K. (2008) Leader of the pack: gene mapping in dogs and other model organisms. Nat. Rev. Genet. 9, 713–725.
| Leader of the pack: gene mapping in dogs and other model organisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVSitrnJ&md5=47fbf4d2698d323fc56d48d0a68fb0a8CAS | 18714291PubMed |
[11] Lobetti, R.G. et al. (1996) Pneumocystis carinii in the miniature dachshund: case report and literature review. J. Small Anim. Pract. 37, 280–285.
| Pneumocystis carinii in the miniature dachshund: case report and literature review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK28zpvVanuw%3D%3D&md5=3939fda4a6203615bd6f109964d2dd4cCAS | 8965482PubMed |
[12] Kirberger, R.M. and Lobetti, R.G. (1998) Radiographic aspects of Pneumocystis carinii pneumonia in the miniature Dachshund. Vet. Radiol. Ultrasound 39, 313–317.
| Radiographic aspects of Pneumocystis carinii pneumonia in the miniature Dachshund.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1czntl2jtQ%3D%3D&md5=446ec0b0e514b9766ea9e2ee49d2b2dcCAS | 9710133PubMed |
[13] Ramsey, I.K. et al. (1997) Pneumocystis carinii pneumonia in two Cavalier King Charles spaniels. Vet. Rec. 140, 372–373.
| Pneumocystis carinii pneumonia in two Cavalier King Charles spaniels.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s3nvFaitA%3D%3D&md5=fa88248140634d76b1b639e50804388fCAS | 9133724PubMed |
[14] Phipps, L.M. et al. (2011) Nosocomial Pneumocystis jirovecii pneumonia: lessons from a cluster in kidney transplant recipients. Transplantation 92, 1327–1334.
| Nosocomial Pneumocystis jirovecii pneumonia: lessons from a cluster in kidney transplant recipients.Crossref | GoogleScholarGoogle Scholar | 22129760PubMed |
[15] Perryman, L.E. (2004) Molecular pathology of severe combined immunodeficiency in mice, horses, and dogs. Vet. Pathol. 41, 95–100.
| Molecular pathology of severe combined immunodeficiency in mice, horses, and dogs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXivFylsLw%3D&md5=9cdf8b9ff9698f743500a336c878aaa7CAS | 15017021PubMed |
[16] Ainsworth, D.M. et al. (1993) Recognition of Pneumocystis carinii in foals with respiratory distress. Equine Vet. J. 25, 103–108.
| Recognition of Pneumocystis carinii in foals with respiratory distress.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s3itlKntg%3D%3D&md5=8e3ce55f772f5a72abb6febd0a02355bCAS | 8467767PubMed |
[17] Cabañes, F.J. et al. (2000) Pneumocystis carinii pneumonia in a Yorkshire terrier dog. Med. Mycol. 38, 451–453.
| Pneumocystis carinii pneumonia in a Yorkshire terrier dog.Crossref | GoogleScholarGoogle Scholar | 11204883PubMed |
†The literature refers to Pneumocystis carinii infection of dogs, although recent taxonomic developments suggest this name should be probably restricted to organisms that infect rats. One conference Abstract suggests the name Pneumocystis canis9, although Pneumocystis special form canis may be a safer term in the interim. For the purposes of this article we have used the term P. canis for simplicity.
‡The first report of PCP pneumonia in dogs was actually from Germany in 1955. The patient was a nine-week-old sheepdog pup with Pneumocystis forms in its lungs, hilar lymph nodes and myocardium. Sedlmeier, H. and Dahme, E. (1955) Pneumocystis carinii-Infektion biem Hund. Zentralbl. Allg. Path. 93, 150–155.
Biographies
Elizabeth Ralph is a veterinarian and has recently finished her residency in small animal internal medicine and is well on the way to her goal of becoming a small animal internal medicine specialist. Elizabeth has also worked with the Department of Primary Industries during the Equine Influenza outbreak in 2007 and with her local government as a member of the Companion Animal Advisory Committee. Her professional interests include small animal internal medicine, especially immune-mediated disease.
George Reppas is a specialist veterinary pathologist at Vetnostics (the veterinary division of Laverty Pathology NSW) and is involved in all aspects of veterinary laboratory medicine. He has been instrumental in establishing a successful veterinary cytology department within Vetnostics over the past decade. His interests include the application of advanced diagnostic techniques such as immunocytochemistry and flow cytometry in veterinary cytology and haematology as well as establishing protocols for PCR testing of some infectious agents on cytology specimens.
Catriona Halliday is the Senior Scientist in charge of the Clinical Mycology Reference Laboratory at Westmead Hospital where she has been working for over 14 years. Her research interests have focused on the development and implementation of culture independent tests to aid in the rapid diagnosis of invasive fungal infections, in particular invasive aspergillosis.
Dr Mark Krockenberger graduated from the University of Sydney in 1993, and has worked in dairy and small animal practice in Australia and United Kingdom. After completing a PhD on cryptococcosis in koalas in 2001, Mark accepted a Lecturer in Veterinary pathology at the University of Sydney. After rising through the ranks, he is now an Associate Professor and head of the Diagnostic Laboratory. His research includes the host–pathogen–environment interactions of cryptococcosis, diseases of koalas and diseases of Australian wildlife. He is especially interested in the pathogenesis of fungal disease, including in laboratory models.
Richard Malik is a consultant in small animal medicine that has a special interest in infectious diseases of companion animals. He is particularly interested in viral diseases of cats, fungal diseases especially those caused by Cryptococcus species, mycobacteria, saprophytic pathogens such as Burholderia, Prototheca and Pythium and most recently parasitic diseases. Richard works for the Centre for Veterinary Education where he facilitates feline distance education programs and develops life-long learning strategies for vets in practice.



