Immediate action required to prevent another Australian avian extinction: the King Island Scrubtit
Matthew H. Webb A D , Mark Holdsworth B , Dejan Stojanovic A , Aleks Terauds A , Phil Bell C and Robert Heinsohn AA Fenner School of Environment and Society, Australian National University, Canberra, ACT 0200, Australia.
B Forest Hill Wildlife Consultants, 360 Forest Hill Road, Sanford, Tas. 7020, Australia.
C Department of Zoology, University of Tasmania, Sandy Bay, Tas. 7005, Australia.
D Corresponding author. Email: Matthew.Webb@anu.edu.au
Emu 116(3) 223-229 https://doi.org/10.1071/MU15099
Submitted: 25 September 2015 Accepted: 1 December 2015 Published: 7 March 2016
Journal Compilation © BirdLife Australia 2016
Abstract
For small and rapidly declining populations acting fast to prevent extinction is crucial. However, many endangered species receive little attention or management action. Action paralysis can prevail for several reasons, particularly for data deficient species when conservation resources are scarce. Here we draw attention to one of the world’s rarest birds, the King Island Scrubtit (Acanthornis magnus greenianus), a subspecies of a monotypic genus. Recognised as critically endangered for more than two decades, conservation action is virtually non-existent despite a rapid population decline. To establish current baseline information using a repeatable cost-effective monitoring methodology we surveyed 154 sites at eight locations as well as additional sites within the agricultural matrix. We detected the King Island Scrubtit at 28 sites in three locations (Nook Swamp, Colliers Swamp and Pegarah State Forest). At these locations, we estimated overall occupancy to be 0.35 (s.e. 0.05) and detectability to be 0.68 (s.e. 0.05) during a single site visit. We estimate the current area of occupancy of the bird to be <1 km2 and declining. This study documents previously unrecognised threats (acid sulfate soils, macropod browsing and wind-throw) and provides a path forward to population recovery. Our results highlight the need for urgent action to prevent Australia’s next avian extinction.
Introduction
The world is experiencing a global extinction crisis, and anthropogenic change is recognised as the key driver (Barnosky et al. 2011). The International Union for Conservation of Nature (IUCN) Red List has been developed to evaluate the threat status of species and is widely considered to be one of the best starting points for conservation of species (Rodrigues et al. 2006; Mace et al. 2008). The criteria for listing require a basic level of information about where species occur, population size and trajectory, and life-history attributes (IUCN Standards and Petitions Subcommittee 2014). However, for many species these data are not available (Butchart and Bird 2010; Morais et al. 2013). For these data deficient species, identifying and addressing critical gaps in knowledge is required before conservation actions – if necessary – can be initiated (Good et al. 2006). In reality, however, conservation resources are scarce and the management for data deficient species often remains in stasis.
For rapidly declining threatened species or populations, acting fast to implement conservation measures to avert extinction is crucial (Martin et al. 2012), particularly for very small populations (Meek et al. 2015). Thus, there is a strong imperative to overcome inaction. Several processes can lead to paralysis in decision-making for conservation actions (Fisher 2011; Griffiths and Dos Santos 2012; Meek et al. 2015) particularly when conservation resources are scarce (Bottrill et al. 2009). Recently, there has also been a major increase in studies that address conservation decision-making when information, time and resources are limited (Ng et al. 2014). In any case, new biological data can be essential for diagnosing why a species is declining, and in determining the best management options (Canessa et al. 2015).
The King Island Scrubtit (Acanthornis magnus greenianus) is a subspecies of a monotypic genus listed as critically endangered under the Australian Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act). Despite being recognised as critically endangered for more than two decades (Garnett et al. 2011), and the preparation of a recovery plan (Threatened Species Section 2012), very little quantitative information on the subspecies is available (Higgins and Peter 2002; Donaghey 2003, 2011) and almost no on-ground conservation action has been initiated (Threatened Species Section 2012). Further, there has been no systematic evaluation of threats and potential allocation of conservation resources to the species.
To address the poor state of knowledge and lack of action for the King Island Scrubtit, our objectives were to: (1) develop a population monitoring protocol; (2) determine baseline distribution, occupancy and detectability; (3) re-evaluate the conservation status of the subspecies; and (4) reassess threatening processes. We use this information to propose a path towards population recovery and habitat management, and evaluate whether the conservation status of the King Island Scrubtit has improved since listing. We discuss our approach in the context of species languishing on threatened species lists and with the problems posed by a lack of information and very limited management resources.
Materials and methods
Study system
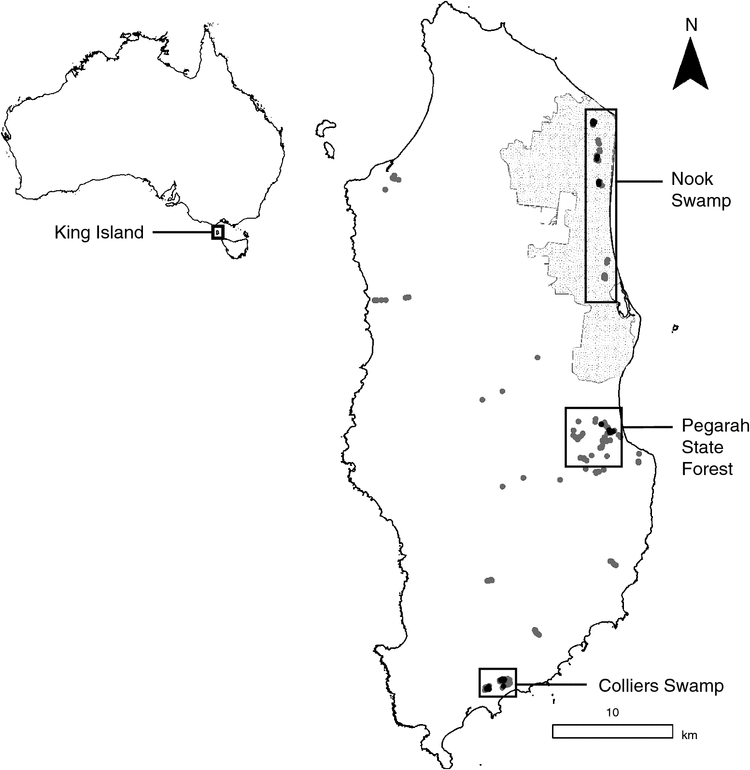
King Island is 1100 km2 in area and located in the Bass Strait, between mainland Australia and Tasmania (Fig. 1) with a temperate, maritime climate. King Island has been severely deforested and extant native vegetation cover has been reduced to 33.5% of the island area, with most of the island now covered in non-forest vegetation (Barnes et al. 2002). Anthropogenic changes to King Island have resulted in the extinction of eight vertebrate species, and five other resident vertebrates are of conservation concern (Threatened Species Section 2012). The King Island Scrubtit was historically widespread in Swamp paperbark (Melaleuca ericifolia) forests and damp, dense, ferny gullies in wet sclerophyll forests (Higgins and Peter 2002). They are now confined to relict patches of Swamp paperbark forest and are highly vulnerable to loss of this habitat (Garnett et al. 2011). However, this habitat continues to be degraded and lost. For example, in 2007, a wildfire in the Nook Swamps (Fig. 1; Fig. S1 in Supplementary material, available online only) burned 90% of Swamp paperbark forest at this location, previously considered the stronghold of the species (Resource Management and Conservation Division 2007).
The King Island Scrubtit National Recovery Plan is incorporated into the King Island Biodiversity Management Plan (Threatened Species Section 2012). The subspecies has been recognised as critically endangered since 1990 (Garnett and Crowley 2000) and was subsequently listed under the Environment Protection and Biodiversity Act 1999 in 2002. Despite the conservation status of the Scrubtit, few management actions have been undertaken and there is currently no recovery team for the species. Historically, King Island Scrubtits occurred at only five localities with an estimated population size of 200 breeding adults (Garnett and Crowley 2000). Over the subsequent two decades, King Island Scrubtits have apparently continued to decline, so that <50 individuals are now estimated to persist at only two locations, with the most recent estimate of area of occupancy being 5 km2 (Garnett et al. 2011).
Field survey
We implemented an occupancy survey for King Island Scrubtits across its range. We established 154 sites across the island (Fig. 1), excluding unfavourable habitats such as pasture, grassland, heathland and saltmarshes, and undertook three sampling sessions (December 2011, April 2012 and May 2015). Sites were selected within potential habitat (i.e. Melaleuca and wet Eucalyptus forest) and places where King Island Scrubtits have historically been recorded (Donaghey 2011; Garnett et al. 2011). Eight primary locations were sampled as well as additional sites within the agricultural matrix. At historical locations we attempted to sample as much potentially suitable habitat as practicable. Navigating dense Melaleuca swamp on King Island can be challenging and the exact location of survey points was sometimes constrained by dense, impenetrable stands of stinging nettles (Urtica incisa). In 2011, the sampling protocol involved 5-minute passive observations followed by 5 minutes of intermittent call-playback for ~20 seconds (which typically elicits a strong response in the Tasmanian nominate species; M. H. Webb, pers. obs.). In 2012 and 2015, surveys were reduced to 5 minutes using intermittent call-playback only because few detections were gained through passive observation. To minimise double counting, sites were ~100 m apart. During each sampling session, individual sites were visited up to three times. Over the three sampling sessions sites were surveyed between two and six times.
Analytical approach
To address objectives one (population monitoring) and two (distribution, occupancy, detectability), we followed an occupancy modelling approach (MacKenzie et al. 2002). For simplicity, we primarily used the program PRESENCE (Hines 2012) modelling occupancy (Ψ) and detectability (p) for: (1) pooled data from all surveys for each occupied region, and (2) pooled data from all three survey periods for all sites.
We generated two predictor variables for each site: (1) presence–absence of old growth Swamp paperbark, which we defined as >15 m in height, and (2) presence–absence of dense understorey or structural complexity (e.g. coarse woody debris) or both combined. We also tested an interaction term between these two variables. To test our results in PRESENCE, we also fitted analogous zero-inflated binomial models using the EM algorithm (Webb et al. 2014) and mixed-effects models (where detectability was assumed to be constant) using the lme4 package in the software R (Bates et al. 2013; R Development Core Team 2015).
Area of Occupancy (AOO) is a measure of the area of suitable habitat occupied by a species within its overall extent of occurrence (Criterion B; IUCN Standards and Petitions Subcommittee 2014). Using the extent or change in AOO as a proxy for change in population size has a sound empirical basis for sedentary species (He 2012). To address objective three (evaluation of conservation status), we estimated AOO in two ways. First, we mapped core habitat within occupied locations in ArcMap 10.2.1 (ESRI, Redlands, CA) based on our observations of habitat availability and suitability during field surveys. Second, we placed a 100-m buffer around all sites where King Island Scrubtits were detected during surveys. Overlapping buffers were dissolved to produce an estimate of AOO. We considered a 100-m radius (~3.1 ha) to be a generous assumption of territory size during breeding, based on territory sizes of related species (Higgins and Peter 2002).
To address objective four (identify threats) we inspected each survey site in this study and recorded all processes visible to the casual observer that may threaten the King Island Scrubtit or its habitat, and reviewed relevant management documents related to King Island. We then compiled these observations and assigned a severity rank based on the observed frequency with which the threat was encountered, and the scale of the impact caused by the process.
Results
King Island Scrubtits were recorded at only three locations: (1) Nook Swamp, Lavinia Nature Reserve, (2) Colliers Swamp, and (3) Fraser River in the Pegarah State Forest (where birds had been considered locally extinct; Garnett et al. 2011). Scrubtits were detected at 28 of 83 sites within these locations (Fig. 1). The mean number of King Island Scrubtits counted at a site, given detection, was 1.5 (range 1–3). No King Island Scrubtits were recorded at any of the other 67 sites scattered across the island.
In the Nook Swamp, King Island Scrubtits were detected in three isolated patches of swamp forest that did not burn during the 2007 fire (e.g. Fig. S1 in Supplementary material). Each patch was separated by >2 km. In Pegarah State Forest, King Island Scrubtits were only detected in riparian forest along the Fraser River. In Colliers Swamp, King Island Scrubtits were detected in both the north-east and west of the swamp.
Occupancy modelling
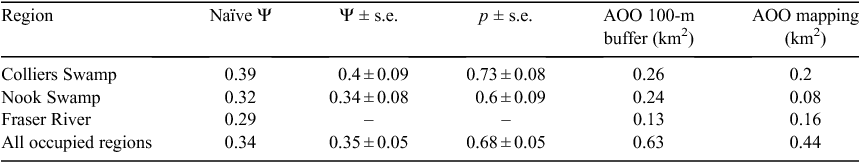
In the zero-inflated binomial models implemented in PRESENCE, constant occupancy models were the best, based on Akaike’s Information Criterion (AIC) scores (adjusted for small sample size – QAICc; Burnham and Anderson 2002). Estimates from these models are shown in Table 1. Too few King Island Scrubtits were detected at Fraser River to model Ψ or p. In the simpler, binomial mixed-models (where detection was also assumed to be constant), the best models (as indicated by AIC) included both the old-growth and dense-understorey variables (but not the interaction term). However, the explanatory power of these variables in the model was extremely low (Wald test P > 0.05). Further support for the importance of old-growth Swamp paperbark and dense understorey in King Island Scrubtit occupancy was also provided by the best EM algorithm zero-inflated binomial (ZIB) model, which included both terms as significant (Wald test, old-growth Swamp paperbark P = 0.0002, and dense understorey P = 0.001) but only when detection was assumed to be constant. Habitat at all 28 sites where King Island Scrubtits were detected was characterised by mature Swamp paperbark >15 m in height with a dense understorey or structural complexity created by coarse, woody debris (Fig. S2 in Supplementary material). While the EM algorithm models provide some interesting insights into the factors driving King Island Scrubtit occupancy, we only report on the estimates from PRESENCE models here as they provide standard errors and did not have the same convergence issues as the EM algorithm models.
Area of occupancy and vegetation mapping
Our two methods for estimating AOO produced similar results except for Nook Swamp, where on-ground mapping provided a considerably lower estimate (Table 1). Our rudimentary mapping of suitable habitat produced an AOO of <0.5 km2, and buffering all sites where King Island Scrubtits were detected resulted in an AOO of 0.63 km2.
When compared with publically available mapping of vegetation (TASVEG 3.0; DPIPWE 2013) we detected widespread errors in vegetation classification at occupied sites. Although all of our detections were in forest dominated by Swamp paperbark or, less frequently, Blackwood (Acacia melanoxylon) forest with subdominant Swamp paperbark, only 54% of those sites were correctly classified in existing vegetation mapping (Table 2).

|
Conservation status and threats
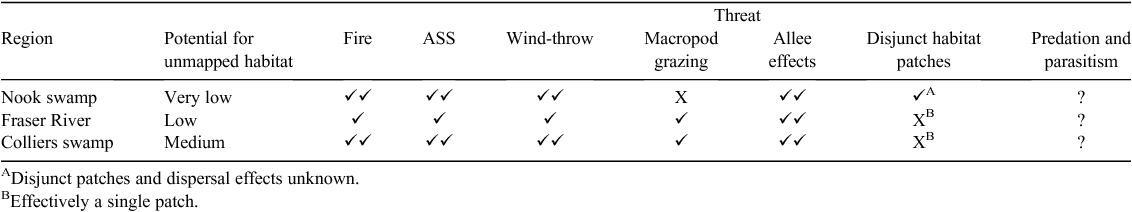
Several threats to the King Island Scrubtit have been identified (Garnett et al. 2011; Threatened Species Section 2012), the most important of which is the impact of fire on remaining habitat. Fire continues to decimate native vegetation across the island and it is clear that the remaining Scrubtit habitat is extremely vulnerable to this threat. Although not well understood, the effect of other threats, such as predation by feral cats (Felis catus), parasitic ticks and nest parasitism by cuckoos is likely to have an increased level of impact on such a tiny and dispersed population. During this study we also identified acid sulfate soils (ASS), wind-throw and herbivore browsing as additional processes threatening the King Island Scrubtit (Table 3).
|
Potential or actual ASS are widespread throughout King Island (King Island Natural Resource Management Group 2010). When exposed to air, the sulphides in ASS form sulphuric acid and anthropogenic hydrological change is a known cause of ASS-induced vegetation death (Sammut et al. 1996; Cook et al. 2000). Hydrological patterns on King Island have been substantially altered as a result of burning peat deposits (Corbett 2010) and widespread agricultural drainage (M. H. Webb, pers. obs.) and these factors exacerbate the impacts of ASS by exposing soil to air. A review of available information on the distribution of ASS on King Island revealed all current sites (and their drainage basins) where King Island Scrubtits occur, and some historical sites support potential or actual ASS (e.g. Gurung 2006; DPIPWE 2009). Further, it was noted that for the Ramsar-listed Lavinia Nature Reserve, ‘disturbance of potential or actual acid sulphate soils may impact the critical components and services of the site’ (DPIPWE 2014). We contend ASS is likely to be a current and future contributing factor to the loss and degradation of habitat of King Island Scrubtits and may be an underlying factor that contributed to historical loss of habitat. Likewise, the effects of ASS may explain undiagnosed dieback of mature Swamp paperbark in the Nook Swamp that escaped the 2007 fire observed in this study.
The second previously undocumented cause of habitat loss we observed was wind-throw (i.e. tree collapse as a result of wind) of mature Swamp paperbark, which we observed at two of the three locations where King Island Scrubtits were recorded (Table 3). For example, in Colliers Swamp one single event destroyed 0.5–1 ha of habitat (Fig. S3 in Supplementary material). The few remaining patches of habitat in the Nook Swamp are being similarly affected owing to increased exposure to wind following the 2007 fire.
The third previously undocumented threatening process we observed was heavy browsing by macropods, which are known to be super-abundant on King Island. Norton and Johannson (2010) estimate the population of Bennett’s (Red-necked) Wallaby (Macropus rufogriseus) to be >500000 individuals. Browsing pressure observed during this study was so severe in parts of Colliers Swamp, Pegarah State Forest and several other sites that understorey structure has been greatly simplified and King Island Scrubtit were not recorded at these sites (Fig. S4 in Supplementary material).
Discussion
Our results show that the King Island Scrubtit is on the verge of extinction. Small passerines can be rescued from extinction by management intervention (e.g. Black Robin (Petrocia traversi); Butler and Merton 1992) but delays will reduce the chances of success. Conservation actions for the King Island Scrubtit need to be implemented urgently owing to the high chance of stochastic events and issues associated with a lack of genetic diversity (i.e. inbreeding) also known as the Allee effect (Courchamp et al. 2008). Our study is the first to quantify Ψ and p of this critically endangered bird using a standardised sampling protocol. We rediscovered an extant subpopulation of King Island Scrubtits where they had been presumed locally extinct since the 1970s (Pegarah State Forest), expanded the area known to be occupied by another subpopulation (Colliers Swamp), and confirmed local extinction at three historical sites.
In addition to the 28 sites at which King Island Scrubtits were detected, 24 other sites supported suitable habitat (old-growth Swamp paperbark with structural complexity in the understorey) and all were in the three occupied regions. In the Nook and Colliers Swamps, all detections of King Island Scrubtits were in swamp forest where Swamp paperbark was the dominant canopy species. Where we detected Scrubtits in riparian vegetation along the Fraser River, the dominant canopy vegetation varied from Swamp paperbark to Blackwood with Swamp paperbark occurring as a subdominant (Fig. S5 in Supplementary material). Although our results clearly highlight the importance of Swamp paperbark, we suspect Scrubtits once occupied forests on King Island where Swamp paperbark was absent but this forest has been destroyed or severely degraded. Furthermore, because available vegetation mapping (Table 2) performs poorly at identifying the correct vegetation community there is a need for detailed on-ground habitat mapping for the King Island Scrubtit.
Our study demonstrates that King Island Scrubtits are in a substantially worse state than previously assumed, and the total AOO for this subspecies is <1 km2. This is much smaller than the threshold IUCN criterion for a critically endangered species (i.e. <10 km2; IUCN 2012) and represents a reduction in range compared with other recently reported estimates of AOO (i.e. 5 km2; Garnett et al. 2011). Taken together, our data confirm the criteria for listing as critically endangered under the EPBC Act (Garnett et al. 2011; IUCN Standards and Petitions Subcommittee; Szabo et al. 2012).
We suggest that an occupancy modelling framework is better suited to monitoring the population of King Island Scrubtits than direct counts or estimates of abundance. Given that King Island Scrubtits are chronically understudied and their conservation is under-resourced, using estimates of Ψ and AOO as a proxy for population size (e.g. MacKenzie et al. 2006) is a cost-effective monitoring approach because it can be undertaken quickly, with minimal resourcing. Our survey protocol resulted in birds being highly detectable (P = 0.68), thus providing a high degree of confidence for absences at sites with fewer visits. However, at some sites, King Island Scrubtits did not respond with a call in response to call-playback and were only visually detected, highlighting the need for experienced observers, familiar with the behaviour of the species. Our study provides a baseline for future monitoring, and our repeatable, low-cost methodology improves the likelihood that monitoring may be undertaken even when resources are limited.
Cumulative anthropogenic loss of habitat has dramatically diminished availability of habitat for King Island Scrubtits, so that the species is effectively corralled into small relicts of suitable habitat. Garnett et al. (2011) report that the population of King Island Scrubtits was estimated to have declined from 200 mature individuals to ~50 in a decade. Given our extremely small estimate of AOO and considering the size of territories of related passerines (Higgins and Peter 2002), our results indicate that a population estimate of 50 mature individuals may in fact be optimistic. Furthermore, there are no demographic data for the subspecies. This extremely small estimate of AOO and population size makes this subspecies highly vulnerable to Allee effects and stochastic events (for example fire; see below), which are known to affect populations of other small, island-dwelling animals (Angulo et al. 2007; Brashares et al. 2010).
Our study also highlights the ongoing loss, fragmentation and degradation of King Island Scrubtit habitat. Fire has converted a large patch of high-quality habitat (Nook Swamp) into scattered, small relict patches of swamp forest embedded in a matrix of unsuitable degraded habitat. Furthermore, the loss of peat soils along the western edge of the swamp caused by the 2007 fire has changed local hydrological patterns and lowered the watertable of this Ramsar-listed site (Corbett 2010) and ASS almost certainly now pose an immediate threat to large areas of regenerating swamp forest. Our observations of Swamp paperbark dieback at various locations indicates that ASS may already be affecting remaining King Island Scrubtit habitat. To prevent resources being naively misdirected to inappropriate locations future habitat restoration or establishment will need to consider the potential interactions between ASS and changed hydrological patterns resulting from fire, agricultural drainage and rainfall patterns. Similarly, it may be important to develop site-specific techniques to ameliorate the effects of ASS at currently occupied locations.
We also observed wind-throw and macropod browsing as additional and widespread threats to the species. Wind-throw is a known problem in forests where the canopy is opened after disturbance (Gibbons et al. 2008), and in the small habitat fragments where King Island Scrubtits persist, we observed extensive damage to habitat from this process (e.g. Fig S3 in Supplementary material). Likewise, super-abundant marsupial herbivores can have strong effects on the structure of native vegetation (Howland et al. 2014) and this is recognised as a major problem on King Island (Norton and Johannson 2010). Suppression of the understorey by browsing herbivores may diminish habitat quality for Scrubtits. Given the small area of occupied habitat available, these processes may pose a serious risk to local persistence of King Island Scrubtit subpopulations.
This study exemplifies the value of targeted research to inform the current status of a species and identify potential new threats. Furthermore, our study involved minimal investment of personnel and time (effectively 4 weeks of surveys over three survey periods) but yielded critical new information. Although our study highlights the perilous state of the King Island Scrubtit population, it also shows how valuable new information can help target limited resources and, as discussed below, that several critical conservation actions may involve little investment to be successful.
From paralysis to action
To move from conservation paralysis to action requires an understanding of the reasons why inaction prevails. Fear of failure, aversion to controversy, or lack of resources and information can often cause action paralysis (Doremus and Pagel 2001; Martin et al. 2012; Meek et al. 2015). Recognising potential roadblocks to conservation and discounting others may assist the decision process for the King Island Scrubtit (Meek et al. 2015). For King Island Scrubtits, we argue that there is ample information to make informed decisions to act, but the decision-making process would greatly benefit from increased information sharing and collaboration. For example, although detailed mapping of ASS is available, it is not mentioned in any management document relevant to the King Island Scrubtit and has not been recognised as a source of habitat loss (e.g. Corbett 2010; Resource Management and Conservation Division 2007; Garnett et al. 2011; Threatened Species Section 2012). Similarly, data collation, directed habitat mapping, management of fire and browsing herbivores, and assessments of water quality in collaboration with relevant experts may help to move from paralysis to action. We advocate urgent action to: (1) manage potential Allee effects by translocating individuals between subpopulations; (2) map the extent of suitable habitat across King Island; (3) identify suitable sites for habitat augmentation; (4) activate experimental exclusion of herbivores to improve habitat; (5) develop an emergency response plan in case of stochastic events; (6) review the existing recovery actions in light of our new data; and (7) implement already identified recovery actions.
The King Island Scrubtit is on the brink of extinction but lessons learnt from recent and near extinctions (e.g. Martin et al. 2012) should be heeded if this subspecies is to survive. Recovering the relict population of King Island Scrubtits poses a considerable but not insurmountable conservation challenge. We argue that decisive action and long-term commitment from conservation agencies and the King Island community and the formation of a National Action Group or Recovery Team to guide recovery actions is critical to the survival of the subspecies. We call for immediate action to avoid another Australian avian extinction.
Acknowledgements
We thank the King Island and Cradle Coast Natural Resource Management Groups and several members of the King Island community. Adrian Webb and Justine Shaw provided valuable comments on the manuscript.
References
Angulo, E., Roemer, G. W., Berec, L., Gascoigne, J., and Courchamp, F. (2007). Double Allee effects and extinction in the Island Fox. Conservation Biology 21, 1082–1091.| Double Allee effects and extinction in the Island Fox.Crossref | GoogleScholarGoogle Scholar | 17650257PubMed |
Barnes, R. W., Duncan, F., and Todd, C. S. (2002). ‘The Native Vegetation of King Island, Bass Strait.’ Nature Conservation Report 02/6. (Nature Conservation Branch, Department of Primary Industries, Water and Environment: Hobart.)
Barnosky, A. D., Matzke, N., Tomiya, S., Wogan, G. O. U., Swartz, B., Quental, T. B., Marshall, C., McGuire, J. L., Lindsey, E. L., Maguire, K. C., Mersey, B., and Ferrer, E. A. (2011). Has the Earth’s sixth mass extinction already arrived? Nature 471, 51–57.
| Has the Earth’s sixth mass extinction already arrived?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXis1Cktbo%3D&md5=3e12cbb41948d1348cd4569134e56ad6CAS | 21368823PubMed |
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2013). lme4: linear mixed-effects models using Eigen and S4. R package version 1.0–4. Available at http://CRAN.R-project.org/package=lme4 [Verified 10 August 2015].
Bottrill, M. C., Joseph, L. N., Carwardine, J., Bode, M., Cook, C., Game, E. T., Grantham, H., Kark, S., Linke, S., McDonald-Madden, E., Pressey, R. L., Walker, S., Wilson, K. A., and Possingham, H. P. (2009). Finite conservation funds mean triage is unavoidable. Trends in Ecology & Evolution 24, 183–184.
| Finite conservation funds mean triage is unavoidable.Crossref | GoogleScholarGoogle Scholar |
Brashares, J. S., Werner, J. R., and Sinclair, A. R. E. (2010). Social ‘meltdown’ in the demise of an island endemic: Allee effects and the Vancouver Island Marmot. Journal of Animal Ecology 79, 965–973.
| Social ‘meltdown’ in the demise of an island endemic: Allee effects and the Vancouver Island Marmot.Crossref | GoogleScholarGoogle Scholar | 20546064PubMed |
Burnham, K. P., and Anderson, D. R. (2002). ‘Model Selection and Multi-Model Inference.’ 2nd edn. (Springer Verlag: New York.)
Butchart, S. H. M., and Bird, J. P. (2010). Data deficient birds on the IUCN Red List: what don’t we know and why does it matter? Biological Conservation 143, 239–247.
| Data deficient birds on the IUCN Red List: what don’t we know and why does it matter?Crossref | GoogleScholarGoogle Scholar |
Butler, D., and Merton, D. (1992). ‘The Black Robin: Saving the World’s Most Endangered Bird.’ (Oxford University Press: Auckland.)
Canessa, S., Guillera-Arroita, G., Lahoz-Monfort, J. J., Southwell, D. M., Armstrong, D. P., Chadès, I., Lacy, R. C., and Converse, S. J. (2015). When do we need more data? A primer on calculating the value of information for applied ecologists. Methods in Ecology and Evolution , .
| When do we need more data? A primer on calculating the value of information for applied ecologists.Crossref | GoogleScholarGoogle Scholar |
Cook, F. J., Hicks, W., Gardner, E. A., Carlin, G. D., and Froggatt, D. W. (2000). Export of acidity in drainage water from acid sulphate soils. Marine Pollution Bulletin 41, 319–326.
| Export of acidity in drainage water from acid sulphate soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhs1agsQ%3D%3D&md5=8251b90ef3ac6202d30f5c1a7bc24f09CAS |
Corbett, K. D. (2010). Lavinia State Reserve, King Island. Post- fire geomorphology and vegetation assessment. Report 1. Assessment of peat deposits, fire damage and drainage features. Cradle Coast Natural Resource Management Group, Tas.
Courchamp, F., Berec, J., and Gascoigne, J. (2008). ‘Allee Effects in Ecology and Conservation.’ (Oxford University Press: Oxford, NY.)
Department of Primary Industries, Parks, Water and Environment (DPIPWE) (2009). Tasmanian Acid Sulfate Soil Management Guidelines. Available at http://www.dpipwe.tas.gov.au/Documents/ASS-Guidelines-FINAL.pdf [Verified 9 February 2016].
Department of Primary Industries, Parks, Water and Environment (DPIPWE) (2014). Information Sheet on Ramsar Wetlands (RIS) – 2009–2014 version. Available at http://www.environment.gov.au/water/topics/wetlands/database/pubs/5-ris.pdf [Verified 18 January 2016].
Department of Primary Industries, Parks, Water and Environment (DPIPWE) (2013). Tasmanian Vegetation Monitoring and Mapping Program (including TASVEG). Resource Management and Conservation Division. Available at http://dpipwe.tas.gov.au/conservation/flora-of-tasmania/monitoring-and-mapping-tasmanias-vegetation-(tasveg) [Verified 18 January 2016].
Donaghey, R. (Ed.) (2003). ‘The Fauna of King Island. A Guide to Identification and Conservation Management.’ (King Island Natural Resource Management Group: Currie: King Island.)
Donaghey, R. (2011). Survival, distribution and recovery of the King Island Scrubtit in Lavinia State Reserve following the 2007 wildfire. Cradle Coast Natural Resource Management Group, Tas. Available at http://www.kingislandnaturalresources.org/publications/KI_srubtitReport-Final2010.pdf [Verified 15 July 2015].
Doremus, H., and Pagel, J. E. (2001). Why listing may be forever: perspectives on delisting under the U.S. Endangered Species Act. Conservation Biology 15, 1258–1268.
| Why listing may be forever: perspectives on delisting under the U.S. Endangered Species Act.Crossref | GoogleScholarGoogle Scholar |
Fisher, D. O. (2011). Cost, effort and outcome of mammal rediscovery: neglect of small species. Biological Conservation 144, 1712–1718.
| Cost, effort and outcome of mammal rediscovery: neglect of small species.Crossref | GoogleScholarGoogle Scholar |
Garnett, S. T., and Crowley, G. M. (2000). ‘Action Plan for Australian Birds 2000.’ (Environment Australia: Canberra.)
Garnett, S. T., Szabo, J. K., and Dutson, G. (2011). ‘Action Plan for Australian Birds 2010.’ (CSIRO Publishing: Melbourne.)
Gibbons, P., Cunningham, R. B., and Lindenmayer, D. B. (2008). What factors influence the collapse of trees retained on logged sites?: A case-control study. Forest Ecology and Management 255, 62–67.
| What factors influence the collapse of trees retained on logged sites?: A case-control study.Crossref | GoogleScholarGoogle Scholar |
Good, T. C., Zjhra, M. L., and Kremen, C. (2006). Addressing data deficiency in classifying extinction risk: a case study of a radiation of Bignoniaceae from Madagascar. Conservation Biology 20, 1099–1110.
| Addressing data deficiency in classifying extinction risk: a case study of a radiation of Bignoniaceae from Madagascar.Crossref | GoogleScholarGoogle Scholar | 16922226PubMed |
Griffiths, R. A., and Dos Santos, M. (2012). Trends in conservation biology: progress or procrastination in a new millennium? Biological Conservation 153, 153–158.
| Trends in conservation biology: progress or procrastination in a new millennium?Crossref | GoogleScholarGoogle Scholar |
Gurung, S. (2006). Tasmanian acid drainage reconnaissance. Report 2. Distribution of acid sulphate soils in Tasmania. Tasmanian Geological Survey Record 2001/06. Mineral Resources Tasmania, Hobart, Tas. Available at http://www.mrt.tas.gov.au/portal/documents/10184/38472/UR2001_06_REPORT.pdf/847335e8-6be8-4436-996a-1ae29b6a237a [Verified 15 July 2015].
He, F. (2012). Area-based assessment of extinction risk. Ecology 93, 974–980.
| Area-based assessment of extinction risk.Crossref | GoogleScholarGoogle Scholar |
Higgins, P. J., and Peter, J. M. (Eds) (2002). ‘Handbook of Australian, New Zealand and Antarctic Birds. Vol. 6: Pardalotes to Shrike-thrushes.’ (Oxford University Press: Melbourne.)
Hines, J. E. (2012). PRESENCE 6.9 – Estimates patch occupancy rates and related parameters. United States Geological Survey – Patuxent Wildlife Research Centre. Available at http://www.mbr-pwrc.usgs.gov/software/presence.html [Verified 1 September 2014].
Howland, B., Stojanovic, D., Gordon, I. J., Manning, A. D., Fletcher, D., and Lindenmayer, D. B. (2014). Eaten out of house and home: impacts of grazing on ground-dwelling reptiles in Australian grasslands and grassy woodlands. PLoS One 9, e105966.
| Eaten out of house and home: impacts of grazing on ground-dwelling reptiles in Australian grasslands and grassy woodlands.Crossref | GoogleScholarGoogle Scholar | 25501680PubMed |
IUCN Standards and Petitions Subcommittee. (2014). Guidelines for using the IUCN Red List categories and criteria, version 11.0. Available at http://www.iucnredlist.org/documents/RedListGuidelines.pdf [Verified 10 June 2015].
King Island Natural Resource Management Group (2010). King Island natural resource management strategy 2010 to 2020. King Island Natural Resource Management Group Inc., Currie, King Island, Tas.
Mace, G. M., Collar, N. J., Gaston, K. J., Hilton-Taylor, C., Akcakaya, H. R., Leader-Williams, N., Milner-Gulland, E. J., and Stuart, S. N. (2008). Quantification of extinction risk: IUCN’s system for classifying threatened species. Conservation Biology 22, 1424–1442.
| Quantification of extinction risk: IUCN’s system for classifying threatened species.Crossref | GoogleScholarGoogle Scholar | 18847444PubMed |
MacKenzie, D., Nichols, J., Lachman, G., Droege, S., Royle, J., and Langtimm, C. (2002). Estimating site occupancy rates when detection probabilities are less than one. Ecology 83, 2248–2255.
| Estimating site occupancy rates when detection probabilities are less than one.Crossref | GoogleScholarGoogle Scholar |
MacKenzie, D., Nichols, J., Royle, J., Pollock, K., Bailey, L., and Hines, J. (2006). ‘Occupancy Estimation and Modelling: Inferring Patterns and Dynamics of Species Occurrence.’ (Elsevier: San Diego, CA.)
Martin, T. G., Nally, S., Burbidge, A. A., Arnall, S., Garnett, S. T., Hayward, M. W., Lumsden, L. F., Menkhorst, P., McDonald-Madden, E., and Possingham, H. P. (2012). Acting fast helps avoid extinction. Conservation Letters 5, 274–280.
| Acting fast helps avoid extinction.Crossref | GoogleScholarGoogle Scholar |
Meek, M. H., Wells, C., Tomalty, K. M., Ashander, J., Cole, E. M., Gille, D. A., Putman, B. J., Rose, J. P., Savoca, M. S., Yamane, L., Hull, J. M., Rogers, D. L., Rosenblum, E. B., Shogren, J. F., Swaisgood, R. R., and May, B. (2015). Fear of failure in conservation: the problem and potential solutions to aid conservation of extremely small populations. Biological Conservation 184, 209–217.
| Fear of failure in conservation: the problem and potential solutions to aid conservation of extremely small populations.Crossref | GoogleScholarGoogle Scholar |
Morais, A. R., Siqueira, M. N., Lemes, P., Maciel, N. M., De Marco, P., and Brito, D. (2013). Unraveling the conservation status of Data Deficient species. Biological Conservation 166, 98–102.
| Unraveling the conservation status of Data Deficient species.Crossref | GoogleScholarGoogle Scholar |
Ng, C. F., McCarthy, M. A., Martin, T. G., and Possingham, H. P. (2014). Determining when to change course in management actions. Conservation Biology 28, 1617–1625.
| Determining when to change course in management actions.Crossref | GoogleScholarGoogle Scholar | 25155429PubMed |
Norton, T., and Johannson, N. (2010). Implications of browsing by native wildlife on improved pastures and native vegetation communities on King Island, Tasmania. Tasmanian Institute of Agricultural Research, University of Tasmania, Hobart, Tas.
R Development Core Team (2015). ‘R: A Language and Environment for Statistical Computing.’ (R Foundation for Statistical Computing: Vienna, Austria.) Available at http://www.R-project.org/ [Verified 1 August 2015].
Resource Management and Conservation Division (2007). King Island 2007 fires: impact on natural values. Unpublished report to the Tasmanian Parks and Wildlife Service. Biodiversity Conservation Branch, Resource Management and Conservation Division, Department of Primary Industries and Water, Hobart.
Rodrigues, A. S. L., Pilgrim, J. D., Lamoreux, J. F., Hoffmann, M., and Brooks, T. M. (2006). The value of the IUCN Red List for conservation. Trends in Ecology & Evolution 21, 71–76.
| The value of the IUCN Red List for conservation.Crossref | GoogleScholarGoogle Scholar |
Sammut, J., White, I., and Melville, M. (1996). Acidification of an estuarine tributary in eastern Australia due to drainage of acid sulfate soils. Marine and Freshwater Research 47, 669–684.
| Acidification of an estuarine tributary in eastern Australia due to drainage of acid sulfate soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XmvF2jtLk%3D&md5=46924e06c3ab54f1ffae906d825959b2CAS |
Szabo, J. K., Butchart, S. H. M., Possingham, H. P., and Garnett, S. T. (2012). Adapting global biodiversity indicators to the national scale: a Red List Index for Australian birds. Biological Conservation 148, 61–68.
| Adapting global biodiversity indicators to the national scale: a Red List Index for Australian birds.Crossref | GoogleScholarGoogle Scholar |
Threatened Species Section (2012). King Island biodiversity management plan. Department of Primary Industries, Parks, Water and Environment, Hobart.
Webb, M. H., Wotherspoon, S., Stojanovic, D., Heinsohn, R., Cunningham, R., Bell, P., and Terauds, A. (2014). Location matters: using spatially explicit occupancy models to predict the distribution of the highly mobile, endangered Swift Parrot. Biological Conservation 176, 99–108.
| Location matters: using spatially explicit occupancy models to predict the distribution of the highly mobile, endangered Swift Parrot.Crossref | GoogleScholarGoogle Scholar |