Diagnosis, treatment and prevention of tuberculosis in children
Philip Britton A , Carlos M. Perez-Velez B C and Ben J. Marais A C DA The Children’s Hospital at Westmead
B Grupo Tuberculosis Valle-Colorado and Clínica León XIII, IPS Universidad de Antioquia, Colombia
C Sydney Emerging Infectious Diseases and Biosecurity Institute, The University of Sydney
D Corresponding author. Email: ben.marais@health.nsw.gov.au
NSW Public Health Bulletin 24(1) 15-21 https://doi.org/10.1071/NB12100
Published: 15 July 2013
Abstract
In Australia, tuberculosis notification rates have plateaued at a low level and disease is highly concentrated in immigrant communities where children may be affected. Many clinicians regard tuberculosis as an adult disease, hence it is rarely considered in the differential diagnosis of sick children. This paper provides a brief overview of the natural history of the disease in children to demonstrate the importance of taking a careful tuberculosis exposure history. It also provides guidance regarding the diagnosis, treatment and prevention of tuberculosis in children. The management of paediatric cases is not difficult if important differences with adult disease are carefully considered; these differences are discussed in detail.
Tuberculosis (TB) remains a major, but often unrecognised, cause of disease and death among women and children in TB endemic areas.1 Cases are highly concentrated in areas affected by poverty, social disruption, human immunodeficiency virus (HIV) infection and drug-resistant TB,2,3 with increased international travel and immigration posing major challenges to the control of TB. In Australia, TB incidence rates are among the lowest in the world at 5–6 per 100 000 population per year.4 However, rates are highly variable and up to 10 times higher in certain sub-groups of the population. More than 85% of cases occur in immigrant populations and represent imported infection, with the top five countries of origin being India, Viet Nam, the Philippines, China and Indonesia, where high rates of drug-resistant TB have been recorded.2,4 Evidence of local transmission is limited and restricted to particular disease clusters.5 New South Wales (NSW) reports the highest absolute number of TB cases within Australia.4,6 In 2008, children aged under 15 years constituted less than 5% of the disease burden (18/498),6 similar to other developed countries with minimal internal transmission and routine provision of post-exposure prophylaxis to young and vulnerable children.7,8 Despite low numbers of children with TB, Australian clinicians need to consider TB as part of the differential diagnosis, as cases are observed at regular intervals.9–13 This brief overview focuses on recent advances in diagnosis and on issues related to the clinical care of children with TB.
Natural history of disease
The pre-chemotherapy literature provides detailed natural history of disease descriptions which guide risk assessment and management.14,15 An observation is that most children (>90%) who progress to TB disease do so within the first 12 months after primary infection; this is referred to as the ‘window of risk’. Another observation is the pronounced bi-modal risk profile: very young children (aged less than 2 years) experience the greatest risk; a nadir occurs at around 5–10 years of age and then an increase is seen with the onset of puberty. This coincides with a radical shift in the disease spectrum. In young children, lymph node disease with or without airway compression predominates, due to exuberant lymph node responses and small pliable airways. Disseminated disease is also more common due to immature T-cell responses and poor disease containment. The sudden switch to adult-type TB that occurs around puberty, first in girls and then in boys, remains an enigma, but may shed light on key variables underlying individual vulnerability.16 It is important to remember that adolescent children with adult-type disease are highly infectious.17 Table 1 summarises some important differences between TB in adults and children.
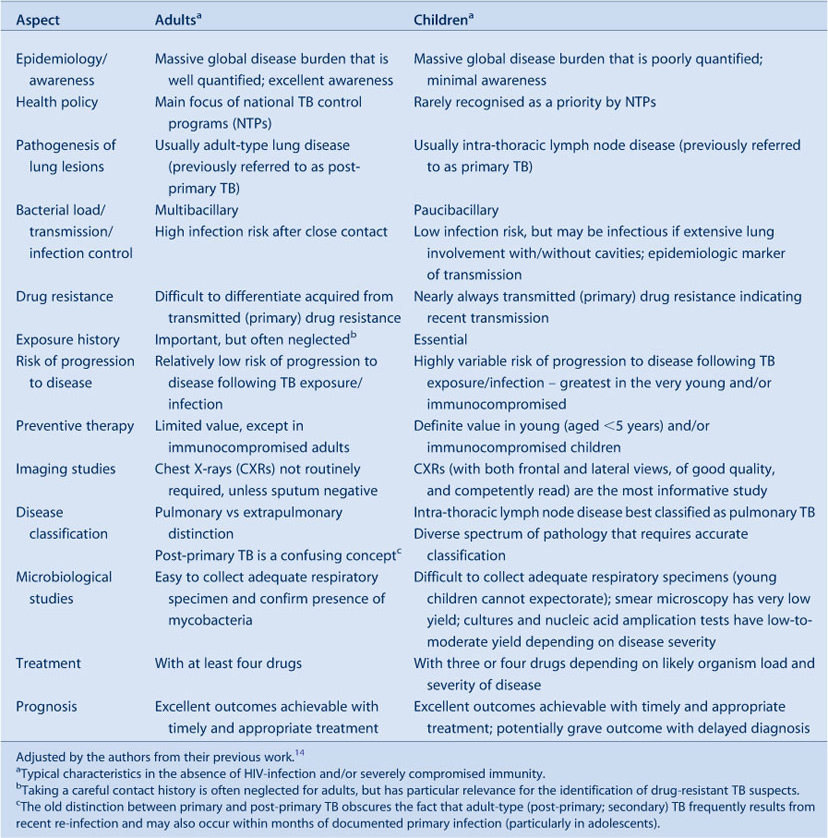
|
Diagnosis
Children are usually evaluated for TB as a result of immigrant screening, contact investigation or following presentation with symptoms or signs suggestive of TB disease. It is important to distinguish these different entry points since they influence the diagnostic work-up and interpretation of results (Figure 1). Mycobacterium tuberculosis infection detected during immigrant screening probably reflects remote past infection with reduced risk of disease progression, unless it is a young child or immunocompromised individual. M. tuberculosis infection detected during contact investigation is likely to be recent, implying a higher risk of disease progression, although this remains highly age-dependent. In this population, isolated radiographic findings in asymptomatic children are problematic, since transient elements of the Ghon/primary complex are frequently visualised and not necessarily indicative of active disease. Observational studies and current World Health Organization guidance suggest that symptom-based screening is adequate, at least in older children, and the complete absence of current symptoms is sufficient to rule out TB disease in this group.18,19 Table 2 provides an overview of investigations to establish a diagnosis of TB in children.
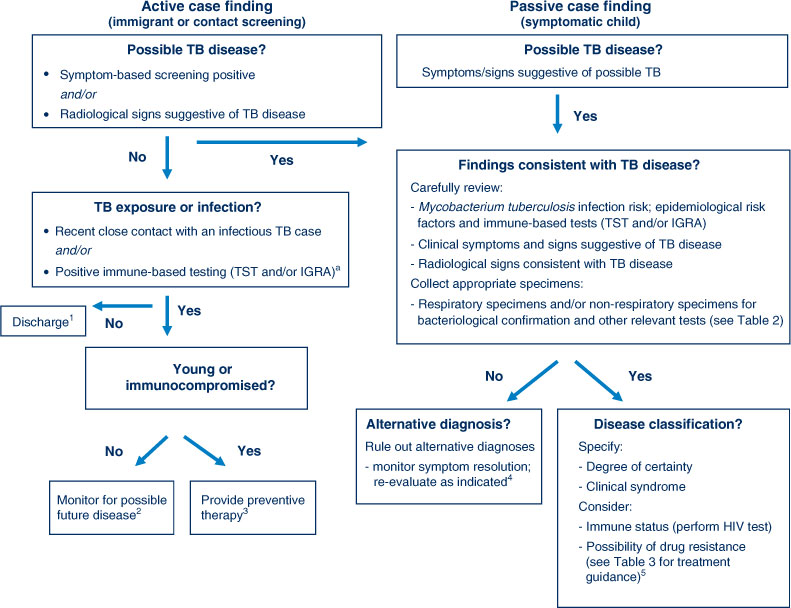
|

|
Clinical evaluation
Children rarely present with near pathognomonic signs of TB such as a TB gibbus; most clinical manifestations are non-specific. In fact, one of the remarkable features of intra-thoracic TB is the frequent absence of physical signs despite the presence of persistent non-remitting symptoms. Furthermore, despite minimal clinical findings, the clinician may be surprised by the radiographic extent of disease. The pathophysiological explanation for this discrepancy is not clear but may reflect the fact that TB often causes a vasculitis (as observed with TB meningitis) in addition to parenchymal involvement. This implies that both oxygen exchange and blood supply are reduced in affected parts of the lung, limiting the resultant ventilation:perfusion mismatch which may explain the frequent absence of acute respiratory distress despite extensive lung involvement.
A detailed history should explore the likelihood of recent (during the past 12 months) TB exposure and allow accurate symptom characterisation. This is important because poorly-defined symptoms have poor discriminatory power.20 Common constitutional symptoms include decreased appetite (recent crossing of weight centiles is most informative), fatigue or reduced playfulness, and fever. Despite TB being an infectious disease, fever is often absent, low-grade or intermittent. With lung involvement, children usually present with a persistent non-remitting cough that is unresponsive to standard first-line treatment. Airway compression may manifest as loud (large airway) wheezing that does not respond to bronchodilators. Clinical follow-up is a useful diagnostic tool in children with mild disease manifestations for whom the diagnosis cannot be made with certainty.20
Imaging studies
Chest radiography is generally the most informative investigation and should include both frontal and lateral views. Lateral views are important as they improve assessment of the mediastinum and hilar areas. Childhood intra-thoracic TB has a wide range of appearances associated with different disease entities, which justifies careful classification.21,22 Visible hilar adenopathy with or without airway compression is highly suggestive of TB disease. High-resolution chest computed tomography (CT) provides the most accurate visualisation of intra-thoracic structures,23 but due to the high cost and associated radiation exposure its use should be limited to complicated cases. CT and/or magnetic resonance imaging (MRI) is the best way to visualise extrapulmonary lesions, especially intra-cranial pathology. MRI is more sensitive for detecting brainstem lesions or early perfusion defects (infarcts) and also provides better evaluation of the spine and soft tissues.24
Laboratory studies
Immune-based tests are severely limited by their inability to differentiate M. tuberculosis infection from active disease, and neither the purified protein derivative tuberculin skin test (TST) or interferon-gamma release assays (IGRAs) (e.g. QuantiFERON-Gold In Tube®) offer a simple solution.25 IGRAs do not replace TSTs for the detection of M. tuberculosis infection in children and, like TSTs, cannot be used to exclude TB. In certain clinical situations IGRAs may be used in addition to TSTs to improve sensitivity and specificity in the detection of TB infection.25
Smear microscopy has poor sensitivity in young children, most of whom are paucibacillary and unable to expectorate; it has been largely superseded by culture and nucleic acid amplification tests (NAATs). In general, culture yields in children are lower than in adults, depending on the severity of disease as well as the quality, quantity and types of specimens collected. Two studies have evaluated the performance of the rapid NAAT-based Xpert® MTB/RIF assay in children, demonstrating similar performance characteristics to adult studies, with excellent specificity and detection of around 70% of culture-positive cases.26,27
Collecting adequate respiratory specimens in young children is problematic, but gastric aspirates, induced sputum (with or without laryngopharyngeal suction) and broncho-alveolar lavage (in select patients) offer feasible alternatives. A combination of specimens provides the best yield.28 Fine-needle aspiration biopsy has excellent utility in children with a peripheral lymph node mass.29 With tuberculous meningitis, slow clinical onset, cerebrospinal fluid pleocytosis (with total cell count <500) and elevated protein is highly suggestive.30 Despite the challenges discussed, bacteriological confirmation should always be attempted, although it should not delay treatment initiation in young and vulnerable children. TB can be diagnosed with relative certainty based on a combination of clinical, radiological, laboratory and histopathological (when feasible) findings consistent with TB disease, in association with epidemiological factors and/or immunological evidence of M. tuberculosis infection.
Treatment
If a diagnosis of TB disease is established, pragmatic disease classification guides management and facilitates case comparison. From a treatment perspective, likely bacillary load, anatomical location and the possibility of drug resistance are the most important variables to consider. If high bacillary loads are anticipated, the use of multiple drugs during the intensive phase of treatment reduces the risk of acquired drug resistance. Consideration should also be given to the possible involvement of ‘sanctuary sites’ such as the brain and cerebrospinal fluid (CSF), since drugs have highly variable CSF penetration.31 High and/or rising rates of drug-resistant TB, documented in many countries within the Asia-Pacific region, Eastern Europe and sub-Saharan Africa,2 make it necessary for clinicians to carefully scrutinise patients who resided in, or travelled through, these countries. The possibility of drug-resistant TB should be suspected following close contact with a drug-resistant source case; in residents of countries known to have a high prevalence of drug-resistant TB; or following contact with someone who died on TB treatment, is poorly adherent to therapy, or required more than one treatment course.
TB treatment aims to ensure long-term cure without serious adverse effects for the patient. From a public health perspective it is important to terminate transmission and prevent the emergence of drug resistance. Table 3 summarises the mode of action, main adverse effects and recommended dosages of first-line TB drugs, including dosage recommendations for children; sub-optimal drug levels result from using weight-adjusted adult doses.
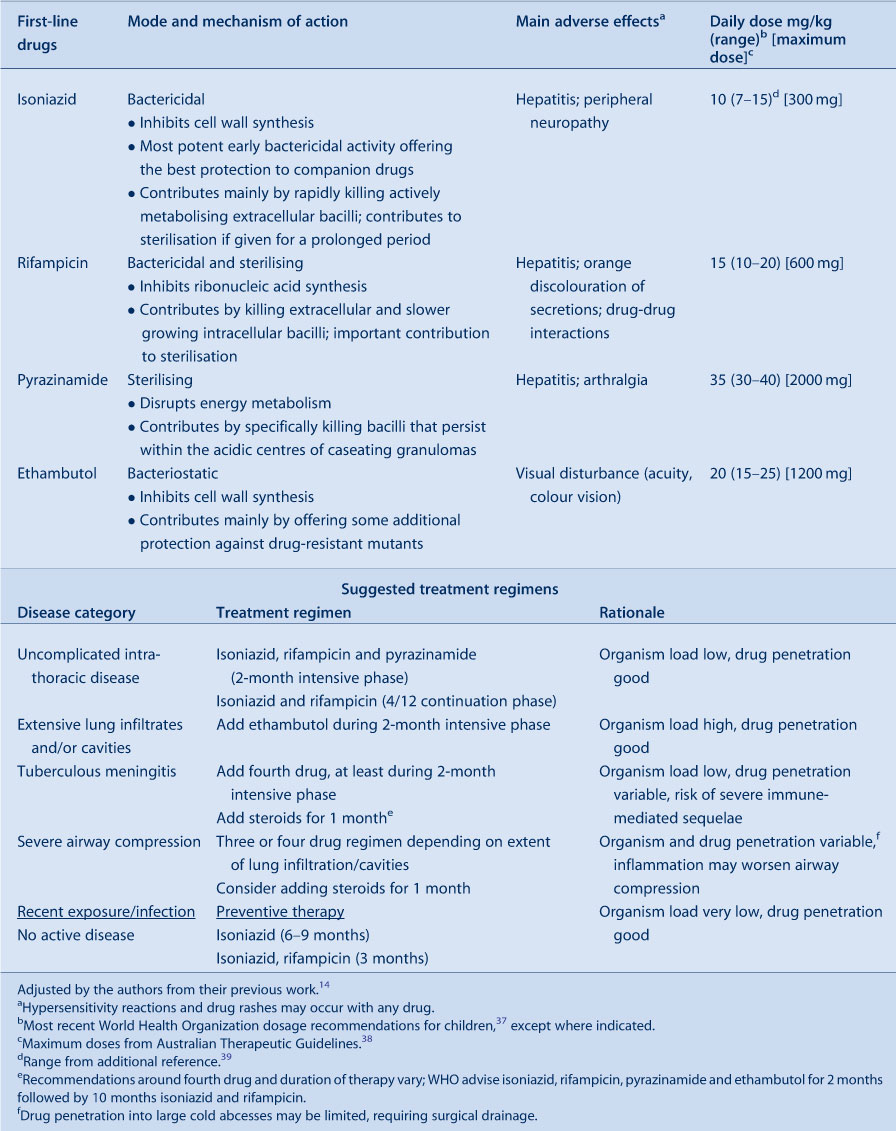
|
In the absence of drug resistance, the most likely cause of poor treatment response is non-adherence. If a child presents with a TB recurrence more than 6–12 months after treatment completion and clinical cure, it most likely represents re-infection. Standard first-line treatment would be appropriate (in the absence of risk factors for drug-resistant disease); there is no indication to use an escalated re-treatment regimen. With poor response to adherent therapy, careful re-evaluation of the original diagnosis and assessment for drug resistance is warranted. In NSW, all positive cultures undergo drug susceptibility testing, which provides additional motivation to achieve bacteriological confirmation. With drug-resistant TB, the basic principles of management are unchanged and excellent outcomes can be achieved.32 All children diagnosed with TB should be tested for HIV infection; management of co-infected children has been recently reviewed.33
Prevention
Prevention strategies include vaccination, pre- and post-exposure prophylaxis, treatment of ‘latent’ infection, and secondary prophylaxis (provided after completion of TB treatment). Bacille Calmette-Guérin (BCG) vaccination reduces the risk of disseminated (miliary) disease and tuberculous meningitis in very young children but protection is incomplete and it offers no consistent protection against adult-type TB.34 It is not included in routine vaccination schedules in Australia, however, it should be considered when vulnerable children (e.g. aged less than 2 years) are exposed to a high-risk environment, such as visiting a TB endemic country. Research to develop novel vaccines with improved efficacy and safety is ongoing.
Careful risk stratification identifies those at greatest need of preventive therapy following TB exposure. The target population for preventive therapy provision may vary in different settings depending on feasibility and available resources, but all young (aged less than 5 years) and/or immunocompromised children should receive preventive therapy following documented exposure/infection.19 With good adherence and in the absence of drug resistance, isoniazid monotherapy provides excellent protection following documented exposure/infection. However, parents are often reluctant to provide ‘treatment’ to an otherwise well child and ensuring good adherence is challenging. Treatment with isoniazid and rifampicin for 3 months has demonstrated equivalent efficacy and improved adherence compared to 9 months of treatment with isoniazid alone, with no increase in adverse events.35 Twelve doses of weekly rifapentine and isoniazid proved efficacious in a recent adult study,36 but this regimen cannot yet be recommended in children aged less than 12 years until more safety and efficacy data are available. In HIV-infected children on antiretroviral therapy, drug-drug interactions should be considered with all rifamycin-containing regimens.33
Conclusion
Children suffer a huge but under-recognised TB disease burden in endemic countries from which Australia continues to receive immigrants. Multiple challenges remain: to develop more effective vaccines, better diagnostics and shorter treatment regimens. However, it is worth emphasising that most children would be served well by the sensible application of existing tools.
References
[1] Marais BJ, Gupta A, Starke JR, El Sony A. Tuberculosis in women and children. Lancet 2010; 375 2057–9.| Tuberculosis in women and children.Crossref | GoogleScholarGoogle Scholar | 20488522PubMed |
[2] World Health Organization. Global tuberculosis report 2012. Geneva: World Health Organization; 2012.
[3] Raviglione M, Marais B, Floyd K, Lönnwroth K, Getahun H, Migliori GB, et al. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet 2012; 379 1902–13.
| Scaling up interventions to achieve global tuberculosis control: progress and new developments.Crossref | GoogleScholarGoogle Scholar | 22608339PubMed |
[4] Barry C, Waring J, Stapledon R, Konstantinos A, National Tuberculosis Advisory Committee, for the Communicable Diseases Network Australia Tuberculosis notifications in Australia, 2008 and 2009. Commun Dis Intell 2012; 36 82–94.
[5] Merritt TD, Sintchenko V, Jelfs P, Worthing M, Robinson B, Durrheim DN, et al. An outbreak of pulmonary tuberculosis in young Australians. Med J Aust 2007; 186 240–2.
| 17391086PubMed |
[6] Roberts-Witteveen AR, Christensen A, McAnulty JM. EpiReview: Tuberculosis in NSW, 2008. N S W Public Health Bull 2010; 21 174–82.
| EpiReview: Tuberculosis in NSW, 2008.Crossref | GoogleScholarGoogle Scholar | 20883656PubMed |
[7] Menzies HJ, Winston CA, Holtz TH, Cain KP, Mac Kenzie WR. Epidemiology of tuberculosis among US- and foreign-born children and adolescents in the United States, 1994-2007. Am J Public Health 2010; 100 1724–9.
| Epidemiology of tuberculosis among US- and foreign-born children and adolescents in the United States, 1994-2007.Crossref | GoogleScholarGoogle Scholar | 20634457PubMed |
[8] Sandgren A, Hollo V, Quinten C, Manissero D. Childhood tuberculosis in the European Union/European Economic Area, 2000 to 2009. Euro Surveill 2011; 16 19825
| 21457686PubMed |
[9] Tebruegge M, Ritz N, Connell T, Curtis N. A 2-year old girl with fever, cough, and tachypnoea. BMJ 2009; 338 b1210
| A 2-year old girl with fever, cough, and tachypnoea.Crossref | GoogleScholarGoogle Scholar | 19515707PubMed |
[10] Patradoon-Ho PS, Ambler RW. Universal post-arrival screening for child refugees in Australia: isn't it time? J Paediatr Child Health 2012; 48 99–102.
| Universal post-arrival screening for child refugees in Australia: isn't it time?Crossref | GoogleScholarGoogle Scholar | 22320270PubMed |
[11] Ritz N, Connell TG, Tebruegge M, Johnstone BR, Curtis N. Tuberculous dactylitis—an easily missed diagnosis. Eur J Clin Microbiol Infect Dis 2011; 30 1303–10.
| Tuberculous dactylitis—an easily missed diagnosis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbgtFSrsQ%3D%3D&md5=4790c50794213b5c5a849e1c0a554279CAS | 21491177PubMed |
[12] Thoo CH, Graf N, Hogan P. Erythema induratum in a Kenyan child. Australas J Dermatol 2008; 49 156–8.
| Erythema induratum in a Kenyan child.Crossref | GoogleScholarGoogle Scholar | 18638224PubMed |
[13] Blyth C, Waring J, Burgner D. Inconspicuous consumption: disseminated tuberculosis following untreated latent infection. J Paediatr Child Health 2004; 40 227–9.
| Inconspicuous consumption: disseminated tuberculosis following untreated latent infection.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c7itVaitg%3D%3D&md5=1adfe2e9057721d59e729b1a862239bfCAS | 15009555PubMed |
[14] Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med 2012; 367 348–61.
| Tuberculosis in children.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFegsrvI&md5=b44e0d8fe08d8cf5f893e2cbaa3e4ccfCAS | 22830465PubMed |
[15] Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of the pre-chemotherapy literature. Int J Tuberc Lung Dis 2004; 8 392–402.
| 1:STN:280:DC%2BD2c3ktlamsw%3D%3D&md5=b4817b583dcd61f20c892bd32bdba0d3CAS | 15141729PubMed |
[16] Donald PR, Marais BJ, Barry CE. Age and the epidemiology and pathogenesis of tuberculosis. Lancet 2010; 375 1852–4.
| Age and the epidemiology and pathogenesis of tuberculosis.Crossref | GoogleScholarGoogle Scholar | 20488519PubMed |
[17] Curtis AB, Ridzon R, Vogel R, McDonough S, Hargreaves J, Ferry J, et al. Extensive transmission of Mycobacterium tuberculosis from a child. N Engl J Med 1999; 341 1491–5.
| Extensive transmission of Mycobacterium tuberculosis from a child.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c%2FgslCqtQ%3D%3D&md5=ffe9e8b5a93639f9bacbcbd6e268718fCAS | 10559449PubMed |
[18] Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Enarson DA, Beyers N. Radiographic signs and symptoms in children treated for tuberculosis: possible implications for symptom-based screening in resource-limited settings. Pediatr Infect Dis J 2006; 25 237–40.
| Radiographic signs and symptoms in children treated for tuberculosis: possible implications for symptom-based screening in resource-limited settings.Crossref | GoogleScholarGoogle Scholar | 16511386PubMed |
[19] World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Geneva: World Health Organization; 2006.
[20] Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Lombard C, Enarson DA, et al. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics 2006; 118 e1350–9.
| A refined symptom-based approach to diagnose pulmonary tuberculosis in children.Crossref | GoogleScholarGoogle Scholar | 17079536PubMed |
[21] Marais BJ, Gie RP, Schaaf HS, Starke JR, Hesseling AC, Donald PR, et al. A proposed radiological classification of childhood intra-thoracic tuberculosis. Pediatr Radiol 2004; 34 886–94.
| A proposed radiological classification of childhood intra-thoracic tuberculosis.Crossref | GoogleScholarGoogle Scholar | 15300340PubMed |
[22] Gie RP. Diagnostic atlas of intrathoracic tuberculosis in children: a guide for low-income countries. Paris: International Union Against Tuberculosis and Lung Disease; 2003. Available at: http://www.theunion.org/index.php/en/resources/technical-publications/item/110-diagnostic-atlas-of-intrathoracic-tuberculosis-in-children (Cited 25 July 2012).
[23] Andronikou S, van Hoenacker FM, de Backer AI. Advances in imaging chest tuberculosis: blurring of differences between children and adults. Clin Chest Med 2009; 30 717–44.
| Advances in imaging chest tuberculosis: blurring of differences between children and adults.Crossref | GoogleScholarGoogle Scholar | 19925963PubMed |
[24] Pienaar M, Andronikou S, van Toorn R. MRI to demonstrate diagnostic features and complications of TBM not seen with CT. Childs Nerv Syst 2009; 25 941–7.
| MRI to demonstrate diagnostic features and complications of TBM not seen with CT.Crossref | GoogleScholarGoogle Scholar | 19107489PubMed |
[25] National Tuberculosis Advisory Committee Position statement on interferon-gamma release assays in the detection of latent tuberculosis infection. Commun Dis Intell 2012; 36 125–31.
[26] Nicol MP, Workman L, Isaacs W, Munro J, Black F, Eley B, et al. Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study. Lancet Infect Dis 2011; 11 819–24.
| Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study.Crossref | GoogleScholarGoogle Scholar | 21764384PubMed |
[27] Rachow A, Clowes P, Saathoff E, Mtafya B, Michael E, Ntinginya EN, et al. Increased and expedited case detection by Xpert MTB/RIF assay in childhood tuberculosis: a prospective cohort study. Clin Infect Dis 2012; 54 1388–96.
| Increased and expedited case detection by Xpert MTB/RIF assay in childhood tuberculosis: a prospective cohort study.Crossref | GoogleScholarGoogle Scholar | 22474220PubMed |
[28] Nicol MP, Zar HJ. New specimens and laboratory diagnostics for childhood pulmonary TB: progress and prospects. Paediatr Respir Rev 2011; 12 16–21.
| New specimens and laboratory diagnostics for childhood pulmonary TB: progress and prospects.Crossref | GoogleScholarGoogle Scholar | 21172670PubMed |
[29] Wright CA, Warren RW, Marais BJ. Fine needle aspiration biopsy: an undervalued diagnostic modality in paediatric mycobacterial disease. Int J Tuberc Lung Dis 2009; 13 1467–75.
| 1:STN:280:DC%2BD1MjmslOqtg%3D%3D&md5=720833adbc2575a931ab72943d222cedCAS | 19919763PubMed |
[30] Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, et al. Tuberculous meningitis: defining a uniform case definition for use in clinical research. Lancet Infect Dis 2010; 10 803–12.
| Tuberculous meningitis: defining a uniform case definition for use in clinical research.Crossref | GoogleScholarGoogle Scholar | 20822958PubMed |
[31] Donald PR. The chemotherapy of tuberculous meningitis in children and adults. Tuberculosis (Edinb) 2010; 90 375–92.
| The chemotherapy of tuberculous meningitis in children and adults.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtl2it7zL&md5=41a5ada479598206e0de5f6e16790d83CAS | 20810322PubMed |
[32] Seddon JA, Furin JJ, Gale M, Del Castillo Barrientos H, Hurtado RM, Amanullah F, et al. Caring for children with drug-resistant tuberculosis: practice-based recommendations. Am J Respir Crit Care Med 2012; 186 953–64.
| Caring for children with drug-resistant tuberculosis: practice-based recommendations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXpsVehsg%3D%3D&md5=924cb14d459b0673f075e715d2ece4eeCAS | 22983960PubMed |
[33] Marais BJ, Rabie H, Cotton MF. TB and HIV in children – advances in prevention and management. Paediatr Respir Rev 2011; 12 39–45.
| TB and HIV in children – advances in prevention and management.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M%2FlslWrtw%3D%3D&md5=76f55bf15807d0a16df9db512114db38CAS | 21172674PubMed |
[34] Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 2006; 367 1173–80.
| Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness.Crossref | GoogleScholarGoogle Scholar | 16616560PubMed |
[35] Spyridis NP, Spyridis PG, Gelesme A, Sypsa V, Valianatou M, Metsou F, et al. The effectiveness of a 9-month regimen of isoniazid alone versus 3- and 4-month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: results of an 11-year randomized study. Clin Infect Dis 2007; 45 715–22.
| The effectiveness of a 9-month regimen of isoniazid alone versus 3- and 4-month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: results of an 11-year randomized study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFahtrrP&md5=f7b29b89ee15e45866f9108042a54541CAS | 17712755PubMed |
[36] Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011; 365 2155–66.
| Three months of rifapentine and isoniazid for latent tuberculosis infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1WqtrnP&md5=d432909e6f761113cc57bf392df04820CAS | 22150035PubMed |
[37] Detjen AK, Macé C, Perrin C, Graham SM, Grzemska M. Adoption of revised dosage recommendations for childhood tuberculosis in countries with different childhood tuberculosis burdens. Public Health Action 2012; 2 126–32.
| Adoption of revised dosage recommendations for childhood tuberculosis in countries with different childhood tuberculosis burdens.Crossref | GoogleScholarGoogle Scholar |
[38] Antibiotic Expert Group. Therapeutic guidelines: antibiotic. Version 14. Melbourne: Therapeutic Guidelines Limited; 2010.
[39] Marais BJ, Graham SM, Maeurer M, Zumla A. Progress, challenges and concepts from childhood tuberculosis. Lancet (in press)

