Factors affecting hepatitis C treatment intentions among Aboriginal people in Western Australia: a mixed-methods study
Amineh Rashidi A D , Peter Higgs
A D , Peter Higgs  B and Susan Carruthers C
B and Susan Carruthers C
A School of Nursing and Midwifery, Building 21, Room 409, Edith Cowan University, Joondalup Campus, WA 6207, Australia.
B Department of Public Health, La Trobe University, Plenty Road, Bundoora, Vic. 3083, Australia. Email: P.Higgs@latrobe.edu.au
C Peer Based Harm Reduction WA, Bunbury Site, Suite 21 & 22, 7 Aberdeen Street, Perth, WA 6230, Australia. Email: swcdw@harmreductionwa.org
D Corresponding author. Email: a.rashidi@ecu.edu.au
Australian Health Review 44(5) 755-762 https://doi.org/10.1071/AH19194
Submitted: 8 September 2019 Accepted: 20 March 2020 Published: 28 August 2020
Journal Compilation © AHHA 2020 Open Access CC BY-NC-ND
Abstract
Objective The aim of this study was to identify the hepatitis C treatment intentions of Aboriginal people living with hepatitis C virus (HCV) in Western Australia.
Methods This study used a mixed-methods design. In the cross-sectional survey, 123 Aboriginal people who inject drugs and self-report as living with hepatitis C completed a purpose-designed questionnaire. In the qualitative phase, 10 participants were interviewed about the factors influencing their future intentions to undertake hepatitis C treatment.
Results Analysis of the survey data revealed significant associations between an intention to undertake hepatitis C treatment and support, community attachment, stable housing and stigma. In addition, there was a high overall level of expressed intention to undertake HCV treatment, with 54% of participants responding positively. Analysis of the qualitative data supported quantitative findings, revealing concerns about stigma, lack of social support and unstable housing as factors affecting the intention to undertake hepatitis C treatment.
Conclusion This mixed methods study with Aboriginal people living with self-reported HCV indicates interventions focused on reducing stigma and unstable housing could positively affect hepatitis C treatment intentions. These findings have implications for developing holistic programs to promote and support people on hepatitis C treatment.
What is known about the topic? Substantial knowledge gaps need to be resolved if HCV elimination among Aboriginal Australians is to be achieved. Current research has prioritised non-Aboriginal communities.
What does this paper add? This study found that stigma and unstable housing require attention if Aboriginal Australians are to obtain the full benefits of direct acting antiviral (DAA) hepatitis C treatment.
What are the implications for practitioners? Reducing stigma (in the primary healthcare setting) and providing access to stable housing are vital components of supportive, non-judgemental and culturally appropriate care for Aboriginal people. This study highlights the importance of education for nurses and other primary care providers to increase engagement in the hepatitis cascade of care. To achieve this, scaling-up of HCV treatment engagement, trained Aboriginal community healthcare workers and HCV treatment advocates must mobilise and support Aboriginal people to avoid the negative effects of stigma, build positive and enabling relationships and reinforce positive attitudes towards DAA hepatitis C treatment.
Additional keywords: injecting drug use.
Introduction
Globally, people who inject drugs are the predominant population affected by hepatitis C virus (HCV).1 Recent Australian data show a 15% increase in the notification rate of hepatitis C diagnoses among the Aboriginal and Torres Strait Islander population (from 138 per 100 000 population in 2012 to 173 per 100 000 population in 2016). Over a similar period, the rate of hepatitis C diagnoses in the non-Aboriginal population decreased by 12% (from 43.6 per 100 000 population in 2013 to 38.4 per 100 000 population in 2017).2 There are several well-documented health and socioeconomic issues that increase the health inequalities of Aboriginal people compared with their non-Aboriginal counterparts.3 These issues could be associated with insufficient clinical care and access to health promotion interventions coupled with a lack of disease prevention services.4 In addition, poverty, homelessness and mental health issues may be associated with a higher prevalence of HCV among Aboriginal people compared with non-Aboriginal people.5 Cultural respect is a key element for ensuring Aboriginal people receive effective primary care, and this includes an understanding of the social determinants of health, as well as how individuals communicate their health needs, and developing services that are culturally responsive so as to achieve the most optimal care outcomes.6
Australia has been at the forefront of HCV elimination globally. In February 2016, the Australian government placed direct-acting antivirals (DAAs) on the Pharmaceutical Benefits Scheme (PBS) for anyone living with HCV.7 Increasing access to these highly tolerable and effective medications has seen a considerable increase in the rate of treatment uptake since their introduction.7 Surveillance data from the Kirby Institute highlights the rapid increases in interferon-free treatment uptake between 2015 and December 2018 (from 3430 people to over 70 000 people).8 According to the Australian Needle Syringe Program Survey, 3.7% of Aboriginal people reported undergoing HCV treatment in 2015.9 However, there are no current published data on the progress towards HCV elimination among Aboriginal people in the context of new DAA treatment.8 Thus, factors that affect the intentions of Aboriginal people to undertake HCV treatment need to be examined.8
Sentinel surveillance data with people attending needle and syringe programs shows that HCV prevalence rates are higher for Aboriginal people who inject drugs,10 with Aboriginal and Torres Strait Islander respondents being almost twice as likely to report receptive needle and syringe sharing as non-Aboriginal respondents.11 Recent data indicate that the longer a person has been living with HCV, the greater the severity of liver disease and the higher the likelihood of developing liver cancer and early death.12 Consequently, there is a need to address the high prevalence of hepatitis C in Aboriginal people. The focus of the present study was to identify factors affecting treatment intention among Aboriginal people living with HCV, with the aim of informing relevant interventions and targeted strategies to enable increased treatment uptake for Aboriginal people living with HCV.
Methods
A cross-sectional survey and semistructured interviews were conducted between January and June 2015 in Western Australia (WA). Participants who self-identified as Aboriginal and were living with hepatitis C were recruited from the peer-based services, namely the Western Australian Substance Users Association (WASUA) in Perth and Bunbury, HepatitisWA, the WA Aids Council (WAAC) Needle Syringe Exchange Program (NSEP) fixed site and WAAC NSEP mobile van services, which operate in eight locations in the Perth metropolitan area.
All participants in the present study self-identified as Aboriginal, were current injecting drug users (defined as at least monthly injecting over past 6 months), self-reported as having received an HCV diagnosis from a healthcare professional more than 6 months prior to the interview (confirmatory tests were not performed), were HCV treatment naïve and were currently living in WA. Written informed consent was obtained from all participants.
The first phase of data collection for this project involved semistructured face-to-face interviews with eligible participants (n = 10). Participants were recruited by using posters, which were placed in selected settings where injecting drug users could see them during routine visits. Potential participants contacted one of the authors (AR) by telephone to ask further questions, after which appointments for interviews were arranged with eligible participants. The duration of the interviews ranged between 30 and 45 min and the interviews were conducted (by RA) onsite in the premises of the organisations involved.
The second phase of the study consisted of a survey. Survey participants (n = 123) were not required to have completed Phase 1 of the study. Recruitment for the survey was undertaken through the distribution of study posters and information sheets at the venues listed above, and potential participants contacted the author (AR) directly by telephone to determine eligibility and to arrange a time to complete the questionnaire. Participants completed the questionnaire in a private room at the recruitment site or in the WAAC mobile van.
Ethics approval for the study was received from Curtin University (Reference no. 12/198) and the Fremantle Hospital Human Research Ethics Committee (HR 77/2012). All participants were reimbursed for their time and effort with a supermarket voucher to the value of an A$35 (qualitative arm) and A$20 (survey).
Study instrument
The survey instrument was adapted from previous Australian studies and included questions regarding sociodemographics and drug history,13 length of time since diagnosis,14 feeling a part of the HCV-positive community,15 perceived HCV discrimination and perceived stigma around disclosure,16 social support,17 lifestyle changes18 and any intention to start treatment in the next 5 years.19
Feeling a part of HCV-positive community
To establish participants’ feelings about community attachment, they were asked 12 questions, including sources of help seeking for the management of HCV, emotional support from community-based organisations, the need for HCV services and information about HCV treatment.
Perceived HCV discrimination and stigma
The scale used to establish perceptions of HCV discrimination and stigma16 consisted of 18 items; nine items measured perceived HCV discrimination and nine items examined perceived stigma around disclosing HCV status.
Lifestyle since HCV diagnosis
Participants were asked whether any changes in their lifestyle had occurred since their HCV diagnosis. The scale used consisted of five items: changes in diet, reduction or abstinence from alcohol, increased exercise, HCV check-ups and any use of complementary medicines.18
Social support
The scale used to assess social support17 contained 12 items, which measured the level of support from family and friend(s), as well as from healthcare providers if the participant had chosen to undertake HCV treatment.
Treatment intention
Participants were asked whether they intended to undertake treatment on four levels: in the next 12 months; in the next 1–2 years; in the next 2–5 years; or not for at least another 5 years.19
Responses from the Likert scale were transformed into dichotomous variables, namely ‘Disagree’ (grouping together the first three responses, Strongly Disagree, Disagree, and Neutral) and ‘Agree’ (the last two responses, Agree and Strongly Agree); the response ‘Neutral’ was included in the first group. This scoring system was adopted from previous Australian studies5,20,21 that measured HCV treatment intention and suggested that ‘Neutral’ did not necessarily mean ‘Agree’. ‘Disagree’ and ‘Agree’ were recategorised into ‘No’ and ‘Yes’ respectively. Median age was used as a cut-off point to convert age into a binary variable.22 Heroin or amphetamine were the only drug choices available in the survey; the duration of injecting drug use was dichotomised into ‘8–10 years’ or ‘more than 11 years’ and the frequency of injection reported as either ‘once a day’ or ‘more than once a day’.
Data analysis
Interview data were transcribed verbatim in Microsoft (Redmond, WA, USA) Word and then uploaded into NVivo 10 (QSR International, Melbourne, Vic., Australia) to identify and organise participants’ interview narratives. One of the authors (AR) performed initial and axial coding to organise and review the data, and then placed them into codes in order to find connections between data. Coded data were read repeatedly for emerging themes and analysed by one author (AR). A second author (PH) reviewed the data and refined the coding with further discussion until consensus was reached.
Data from the questionnaires were coded and entered into SPSS version 22 (IBM Corp., Armonk, NY, USA). Descriptive analysis was used to describe the characteristics of the sample. Logistic regression was used to determine the predictors of HCV treatment intention. In unadjusted analyses, variables at the P < 0.10 level were considered for the multivariate model. In the multivariate analysis, significant differences were assessed at the P < 0.05 level. In all two-sided statistical tests, P < 0.05 was considered significant.
Results
Qualitative findings
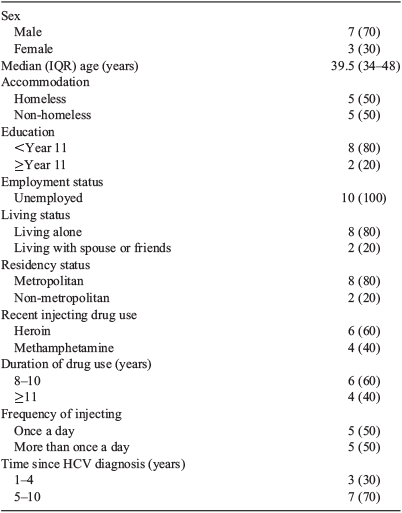
More than two-thirds (n = 7) of participants were men. Participants ranged in age from 34 to 48 years with a median age of 39.5 years. Half the participants reported stable accommodation, with most living alone. All participants were unemployed and reported their main source of income as government benefits (Table 1). Three key themes were evident from the interviews, namely stigma and shame, support and unstable housing. These are outlined below and supported by narratives from participants.
|
Stigma and shame
Most participants (n = 8) reported not publicly disclosing their HCV status and linked their low intention to enter HCV treatment to fear of exclusion from the community, their family and the injecting drug use community. One participant described his apprehension about HCV treatment as follows:
I’m too scared to go on HCV treatment, as I did not tell anyone that I’m HCV positive, especially my parents and especially my friends. I don’t want to reveal my HCV by going on HCV treatment. It would be so embarrassing. I didn’t want to be left out them and not feel attached to them. I believe it is a very common reason that has stopped people going on HCV treatment. (Male, 38 years)
Participants believed that stigma remains central to the experiences of being an injecting drug user living with HCV, so enduring treatment with this stigma would expose them to emotional distress:
Stigma is attached to HCV for a long time and going on HCV treatment with this stigma attached to it is very difficult and it hurts you emotionally and it could have an impact on your mental health. (Male, 34 years)
Support
Few participants reported having family support and, without it, they felt a lack of confidence to undertake HCV treatment. Participants affirmed that a lack of support led them to not seek HCV treatment due to loss of self-esteem, hope and self-efficacy. For example, having a personal reminder to take HCV medication on time during the HCV treatment course in case they missed doses was seen as an important part of having treatment:
I have family here, but they are not helpful. They are drug users too…I have to manage everything on my own if I go on HCV treatment, and keep thinking about who is gonna [sic] remind me to take my medication on time if I go on HCV treatment. I strongly believe that taking medication on schedule and on time is really important to get the best result out of treatment. I know myself that I am not good at these things. Someone has to be with you to remind you and keeping you on the track until finishing treatment. So, how can I go without having support from my family? When you don’t have support, it means you don’t have confidence and hope to be fully committed to treatment. (Male, 43 years)
Some participants believed that the lack of a supportive family stopped them from undergoing HCV treatment. They believed that living with their family would enable them to receive better care and support and, importantly, to follow the treatment regime and commit to treatment completion:
I live on my own. I don’t have relationship with them, because of my HCV, they try to stay away from me. So I can’t rely on them at all. Living with family gives you luxury to feel relaxed, comfortable, and importantly you don’t need to be worry about forgetting or missing medication. When you can’t communicate with your family, which means there is no help and empathy, it makes you unwilling to take HCV treatment. (Female, 39 years)
Unstable housing
Participants who were homeless (n = 5) at interview described this vulnerability as increasing the likelihood of them sharing injecting equipment, exchanging sex for drugs and having unprotected sex. They expressed frustration with their lack of healthy food, inadequate sleep, difficulty storing HCV medications and a commitment to start the treatment journey. They reported not having the resources to undertake HCV treatment and that it did not make sense for them to do so while they lived in such unstable environments. They perceived the ongoing struggle of being homeless as a demotivating factor with regard to HCV treatment:
You know, I’m homeless over the last two years. How can I go on HCV treatment while I am homeless, how can I keep my medication? How can I be on time for my medicine? How can I follow doctor instruction for taking my medicine? As a homeless person, I do everything to earn money for my expenses…so I can’t do it. When I am desperate for the drugs and I don’t have a clean needle, I use other users’ needles. I don’t eat, sleep or shower properly. At the moment, without a house, I just want to live and spend my life. (Female, 40 years)
Homeless participants noted that they could not register themselves with healthcare providers in order to commence HCV treatment without a stable address and contact number. Timely taking of medication was raised again and again as important by participants:
I have been living on the streets for the past three years. As you know, I need stable accommodation with a stable address and contact number to be able to enrol in HCV treatment. So if I want to take HCV treatment, I need to go through a registration process. How can I take my medicine on a certain time, how can I be on track day by day until finishing the treatment? At this stage, it is not possible. Having a stable address is one of the key parts to go on HCV treatment. (Male, 40 years)
Quantitative survey results
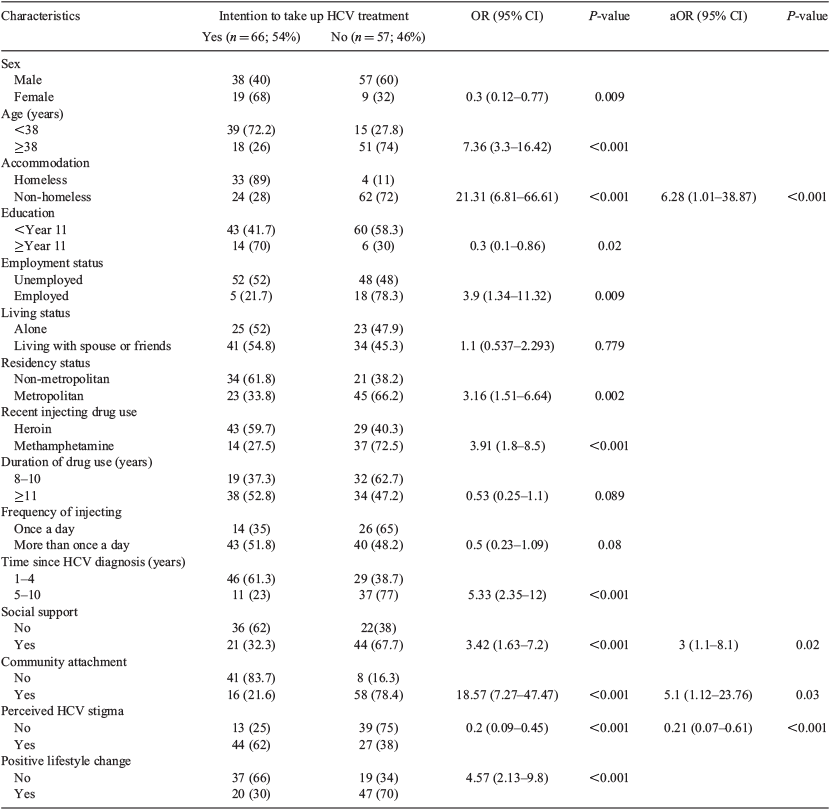
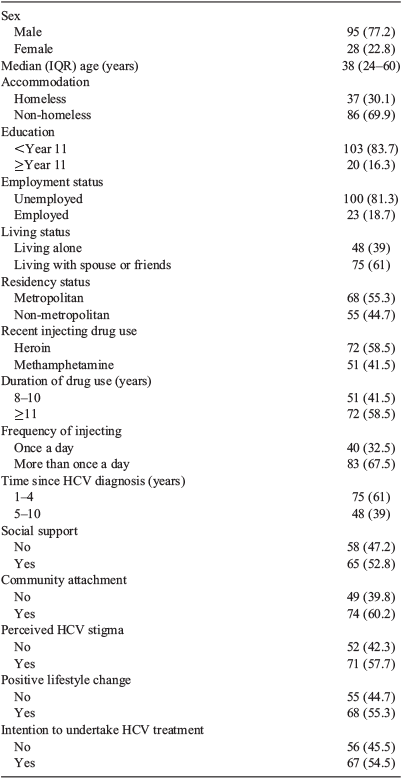
Over three-quarters of respondents were male (77.2%) and the median age of participants was 38 years (Table 2). Almost one-third (30%) described themselves as homeless and almost half (44.7%) were from a non-metropolitan area. More than 80% of participants were unemployed and received government benefits. Participants identified either heroin or amphetamine as their preferred drug, with almost 60% of participants reporting mostly injecting heroin over the past 6 months. All participants had been injecting for longer than 8 years, with almost 60% injecting for 11 years or longer. Half the study participants reported being diagnosed with HCV for between 1 and 4 years before the interview, with a little over half (54%) indicating an intention to undertake future HCV treatment.
|
Factors associated with intention to undertake HCV treatment
In the bivariate analysis, most of the variables were significantly associated with an intention to undertake HCV treatment. Only the ‘living status’ variable was not significantly associated with an intention to undertake HCV treatment. The factors that remained significantly associated with intention to take up HCV treatment after multivariate analysis included accommodation status, support, perceived stigma and community attachment. Participants who reported an intention to seek HCV treatment were more likely to have social support (adjusted odds ratio (aOR) 3; 95% confidence interval (CI) 1.1–8.1) and to have stable accommodation (aOR 6; 95% CI 1.01–38.87). In addition, those participants who intended to take up treatment were more likely to interact with their Aboriginal community (aOR 5.1; 95% CI 1.12–23.76). However, those reporting no intention to undergo treatment were more likely to perceive HCV stigma (aOR 0.21; 95% CI 0.07–0.61; Table 3).
Discussion
Limited attention has been paid to the experiences of Australian Aboriginal people living with HCV, particularly in WA. A clinical and epidemiological study indicates that this population is overrepresented in the HCV epidemic but historically under-represented in HCV treatment.23 The quantitative data in the present study indicate that approximately half the participants (54%) expressed an intention to undertake treatment in the future. Brener et al. used a similar instrument to measure HCV treatment intention in their study of 203 Aboriginal people living in New South Wales with HCV and found that 66% planned to go treatment in the future.24 The high levels of HCV treatment intention found in the study of Brener et al.24 is perhaps not surprising given the imminent arrival of DAA treatment when that study was conducted. The higher efficacy of DAA treatment and the fewer reported side-effects of DAAs than previous interferon-based HCV treatments25 have made treatment a more viable option.
The stigma associated with drug injecting and/or HCV has been identified previously as a key reason for reduced intention to engage with HCV treatment.24,26 Some participants in the qualitative phase of the present study reported that they felt healthcare services treated them differently from people with other chronic diseases. They reported that these services did not meet their needs or their expectations, and they assumed that the substandard care they received was because of their status as someone who was injecting drugs. Other studies have also reported stigmatised individuals are less likely to have a healthcare consultation and are less likely to take up treatment.27,28 Even with the availability of DAA treatment, stigma needs to be addressed among communities affected by HCV so the full benefits can be obtained from DAA treatment.8
The present study is the first with Aboriginal people in WA to find a relationship between community attachment and greater intent to enter HCV treatment, reflecting the importance of community attachment in undertaking HCV treatment. As indicated by the data, those participants with a stronger sense of attachment to an Aboriginal community had a higher intention to take up HCV treatment. This suggests that a strong sense of belonging to the community may act as a buffer against the stigma of living with chronic illness by building positive relationships within the community and fostering a positive outlook on health.29 That is, community attachment increases the intention to undertake treatment and may be seen as protecting participants against the negative effects of stigma, as well as encouraging and supporting treatment engagement. This support may provide opportunities that enable participants to obtain a deeper understanding of what is involved in treatment and treatment outcomes. Hearing positive stories or seeing successful treatment outcomes of DAA treatment from peers has been found to increase the intention of First Nation Peoples to undertake HCV treatment in other settings.30 Peer support can also play a significant role in motivating and encouraging injecting drug users with HCV to accomplish treatment.31–33 This may be true for Aboriginal people with a strong network within their community, where peer support can improve engagement and opportunities to undertake HCV treatment.26
Lack of support was another main reason for treatment refusal specified by the narratives of participants in the qualitative phase of this study. The absence of social support has been identified as a key factor for treatment refusal in similar studies with Aboriginal and non-Aboriginal people.24 However, the quantitative data indicated that 68% believed they would have the necessary social support if they undertook HCV treatment. A possible reason for this discrepancy is that more people in the qualitative arm of the study lived alone and reported family breakdown, and so lacked strong and intimate relationships with their families. In addition, the family members of some of the participants in the qualitative phase were reported to be heavy drug users or to have other chronic illnesses, further reducing the support available.
Unstable housing was identified as an important reason for reporting no intention to seek HCV treatment. Homeless participants in the present study reported that they focused on the day-to-day problems of living on the street, including lack of regular sleep, physical exhaustion and daily anxiety. Some participants reported they injected unsafely and that they consumed large amounts of alcohol. It was difficult for them to follow medical advice about following the strict schedule of daily medication. This is confirmed by the quantitative analysis, which found that stable accommodation was a significant predictor of a high intention to undertake HCV treatment in both the univariate and multivariate analyses. This is in line with other studies that reported unstable housing to be associated with low intention to take up HCV treatment.34
As noted earlier, many clinicians advocate that for DAAs to be effective, strict adherence is important; hence, unstable housing may remain as a factor deterring homeless people from starting their treatment. Recent evidence indicates that daily or weekly dosing of DAAs can support and enhance adherence and optimise treatment outcomes.35 Strategies such as extending DAA treatment beyond the planned duration is one practical way of reducing the effect of missing, forgetting or skipping doses.36 Other similar strategies that provide support to build confidence and enable people to attempt treatment are also worthy of attention. Concerns remain that people experiencing homelessness lack awareness of DAA treatment and are less likely to initiate treatment.37 Therefore, further efforts are required to provide in-depth information of DAAs to this population. One focus may include people who have successfully completed DAA treatment and looking to them as advocates through peer support, including telephone calls and/or text messaging.
This study is limited by the absence of serological testing to confirm self-reported HCV status, although previous Australian studies demonstrate good validity and reliability with self-reported data.38 In addition, there is the potential for social desirability bias, because participants may have reported an intention to undertake HCV treatment to avoid stigmatisation shame when talking to the interviewer. Additional limitations include the cross-sectional study design, which could only estimate the intention to undertake treatment at one point in time, whereas participants’ intention to undertake treatment may change over time, especially as DAA treatments become more widely available.
Despite the increased availability of DAA treatment for people living with HCV, data on the uptake of HCV treatment among Aboriginal people remain limited. As noted above, the implementation of effective strategies to extend DAA treatment to Aboriginal people living with HCV must include culturally appropriate and targeted interventions focused on maximising treatment uptake among Aboriginal people. Further efforts are required to promote locally focused health approaches that improve the daily living conditions and make structural changes that increase access to HCV treatment programs. Doing this, together with trained Aboriginal community healthcare workers and HCV treatment advocates, could help avoid stigmatisation while at the same time supporting those more vulnerable, including homeless, individuals. Such structural changes will encourage engagement in HCV treatment, optimise treatment outcomes and support broader HCV elimination goals.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
This research did not receive any specific funding.
References
[1] Grebely J, Larney S, Peacock A, Colledge S, Leung J, Hickman M, Vickerman P, Blach S, Cunningham EB, Dumchev K, Lynskey M. Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction 2019; 114 150–66.| 30035835PubMed |
[2] Kirby Institute. Bloodborne viral and sexually transmitted infections in Aboriginal and Torres Strait Islander people: annual surveillance report. Sydney: Kirby Institute; 2018.
[3] Mitrou F, Cooke M, Lawrence D, Povah D, Mobilia E, Guimond E, Zubrick S. Gaps in Indigenous disadvantage not closing: a census cohort study of social determinants of health in Australia, Canada, and New Zealand from 1981–2006. BMC Public Health 2014; 14 201
| Gaps in Indigenous disadvantage not closing: a census cohort study of social determinants of health in Australia, Canada, and New Zealand from 1981–2006.Crossref | GoogleScholarGoogle Scholar | 24568143PubMed |
[4] Gracey M, King M. Indigenous health part 1: determinants and disease patterns. Lancet 2009; 374 65–75.
| Indigenous health part 1: determinants and disease patterns.Crossref | GoogleScholarGoogle Scholar | 19577695PubMed |
[5] Brener L, Wilson H, Jackson LC, Johnson P, Saunders V, Treloar C. The role of Aboriginal community attachment in promoting lifestyle changes after hepatitis C diagnosis. Health Psychol Open 2015; 2 1–10.
| The role of Aboriginal community attachment in promoting lifestyle changes after hepatitis C diagnosis.Crossref | GoogleScholarGoogle Scholar |
[6] Freeman T, Edwards T, Baum F, Lawless A, Jolley G, Javanparast S, Francis T. Cultural respect strategies in Australian Aboriginal primary health care services: beyond education and training of practitioners. Aust N Z J Public Health 2014; 38 355–61.
| Cultural respect strategies in Australian Aboriginal primary health care services: beyond education and training of practitioners.Crossref | GoogleScholarGoogle Scholar | 25091076PubMed |
[7] Kirby Institute. Monitoring hepatitis C treatment uptake in Australia (Issue 9). Sydney: UNSW Sydney, Kirby Institute; 2018.
[8] Burnet Institute and Kirby Institute. Australia’s progress towards hepatitis C elimination: annual report 2019. Melbourne: Burnet Institute; 2019.
[9] Kirby Institute. Bloodborne viral and sexually transmitted infections in Aboriginal and Torres Strait Islander people: surveillance and evaluation report. Sydney: Kirby Institute; 2016.
[10] Doyle M, Maher L, Graham S, Wand H, Iversen J. Hepatitis C virus prevalence and associated risk factors among Indigenous Australians who inject drugs. Aust N Z J Public Health 2018; 42 52–6.
| Hepatitis C virus prevalence and associated risk factors among Indigenous Australians who inject drugs.Crossref | GoogleScholarGoogle Scholar | 29168317PubMed |
[11] Kirby Institute. Australian Needle and Syringe Program Survey national data report 2012–2016: prevalence of HIV, hepatitis C and injecting and sexual behaviour among NSP attendees. Sydney: UNSW Sydney, Kirby Institute; 2017.
[12] Alavi M, Law MG, Valerio H, Grebely J, Amin J, Hajarizadeh B, Selvey C, George J, Dore G. Declining hepatitis C virus-related liver disease burden in the direct-acting antiviral therapy era in New South Wales, Australia. J Hepatol 2019; 71 281–8.
| Declining hepatitis C virus-related liver disease burden in the direct-acting antiviral therapy era in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar | 31078544PubMed |
[13] Loxley W, Carruthers S, Bevan J. In the same vein: first report of the Australian study of HIV and injecting drug use. Perth: National Centre for Research into the Prevention of Drug Abuse, Curtin University of Technology; 1995.
[14] McNally S, Sievert W, Pitts MK. Now, later or never? Challenges associated with hepatitis C treatment. Aust N Z J Public Health 2006; 30 422–7.
| Now, later or never? Challenges associated with hepatitis C treatment.Crossref | GoogleScholarGoogle Scholar | 17073222PubMed |
[15] Rawstorne P, Prestage G, Grierson J, Song A, Grulich A, Kippax S. Trends and predictors of HIV-positive community attachment among PLWHA. AIDS Care 2005; 17 589–600.
| Trends and predictors of HIV-positive community attachment among PLWHA.Crossref | GoogleScholarGoogle Scholar | 16036245PubMed |
[16] Treloar C, Brener L, Wilson H, Jackson LC, Saunders V, Johnson P, Gray R, Newland J. Evaluation of NSW Health Aboriginal Hepatitis C Program. Sydney: Centre for Social Research in Health Arts and Social Sciences, UNSW Australia; 2014.
[17] Zimet GD, Dahlem NW, Zimet SG, Farley GK. The multidimensional scale of perceived social support. J Pers Assess 1988; 52 30–41.
| The multidimensional scale of perceived social support.Crossref | GoogleScholarGoogle Scholar |
[18] Horwitz R, Brener L, Treloar C. Evaluation of an integrated care service facility for people living with hepatitis C in New Zealand. Int J Integr Care 2012; 12 e229
| 23593067PubMed |
[19] Treloar C, Hull P, Dore G, Grebely J. Knowledge and barriers associated with assessment and treatment for hepatitis C virus infection among people who inject drugs. Drug Alcohol Rev 2012; 31 918–24.
| Knowledge and barriers associated with assessment and treatment for hepatitis C virus infection among people who inject drugs.Crossref | GoogleScholarGoogle Scholar | 22612899PubMed |
[20] Alavi M, Krahe M, Fortier E, Dunlop AJ, Balcomb AC, Day CA, Treloar C, Bath N, Haber PS, Dore GJ, Grebely J, ETHOS Study Group Effect of treatment willingness on specialist assessment and treatment uptake for hepatitis C virus infection among people who use drugs: the ETHOS study. J Viral Hepat 2015; 22 914–25.
| Effect of treatment willingness on specialist assessment and treatment uptake for hepatitis C virus infection among people who use drugs: the ETHOS study.Crossref | GoogleScholarGoogle Scholar | 25996567PubMed |
[21] Treloar C, Hull P, Dore G, Grebely J. Knowledge and barriers associated with assessment and treatment for hepatitis C virus infection among people who inject drugs. Drug Alcohol Rev 2012; 31 918–24.
| Knowledge and barriers associated with assessment and treatment for hepatitis C virus infection among people who inject drugs.Crossref | GoogleScholarGoogle Scholar | 22612899PubMed |
[22] Tolmie A, Muijs D, McAteer E. Quantitative methods in educational and social research using SPSS. Milton Keynes: McGraw-Hill Education; 2011.
[23] Cooper CL, Bailey RJ, Bain VG, Anderson F, Yoshida EM, Krajden M, Marotta P, Canadian Pegasys Study Group Outcomes of peginterferon alpha-2a and ribavirin hepatitis C therapy in Aboriginal Canadians. Can J Gastroenterol 2008; 22 677–80.
| Outcomes of peginterferon alpha-2a and ribavirin hepatitis C therapy in Aboriginal Canadians.Crossref | GoogleScholarGoogle Scholar | 18701944PubMed |
[24] Brener L, Wilson H, Jackson LC, Johnson P, Saunders V, Treloar C. Experiences of diagnosis, care and treatment among Aboriginal people living with hepatitis C. Aust N Z J Public Health 2016; 40 S59–64.
| Experiences of diagnosis, care and treatment among Aboriginal people living with hepatitis C.Crossref | GoogleScholarGoogle Scholar | 26123616PubMed |
[25] Madden A, Hopwood M, Neale J, Treloar C. Beyond interferon side effects: what residual barriers exist to DAA hepatitis C treatment for people who inject drugs? PloS One 2018; 13 e0207226
| 30500863PubMed |
[26] Treloar C, Jackson C, Gray R, Newland J, Wilson H, Saunders V, Johnson P, Brener L. Care and treatment of hepatitis C among Aboriginal people in New South Wales, Australia: implications for the implementation of new treatments. Ethn Health 2016; 21 39–57.
| Care and treatment of hepatitis C among Aboriginal people in New South Wales, Australia: implications for the implementation of new treatments.Crossref | GoogleScholarGoogle Scholar | 25665723PubMed |
[27] von Hippel C, Brener L, Horwitz R. Implicit and explicit internalized stigma: relationship with risky behaviors, psychosocial functioning and healthcare access among people who inject drugs. Addict Behav 2018; 76 305–11.
| Implicit and explicit internalized stigma: relationship with risky behaviors, psychosocial functioning and healthcare access among people who inject drugs.Crossref | GoogleScholarGoogle Scholar | 28889059PubMed |
[28] Hatzenbuehler ML. Structural stigma and health inequalities: research evidence and implications for psychological science. Am Psychol 2016; 71 742–51.
| Structural stigma and health inequalities: research evidence and implications for psychological science.Crossref | GoogleScholarGoogle Scholar | 27977256PubMed |
[29] Sellers RM, Shelton JN. The role of racial identity in perceived racial discrimination. J Pers Soc Psychol 2003; 84 1079–92.
| The role of racial identity in perceived racial discrimination.Crossref | GoogleScholarGoogle Scholar | 12757150PubMed |
[30] Pearce ME, Jongbloed K, Demerais L, MacDonald H, Christian WM, Sharma R, Pick N, Yoshida EM, Spittal PM, Klein MB. ‘Another thing to live for’: supporting HCV treatment and cure among Indigenous people impacted by substance use in Canadian cities. Int J Drug Policy 2019; 74 52–61.
| ‘Another thing to live for’: supporting HCV treatment and cure among Indigenous people impacted by substance use in Canadian cities.Crossref | GoogleScholarGoogle Scholar | 31525640PubMed |
[31] Swan D, Long J, Carr O, Flanagan J, Irish H, Keating S, Keaveney M, Lambert J, McCormick PA, McKiernan S, Moloney J. Barriers to and facilitators of hepatitis C testing, management, and treatment among current and former injecting drug users: a qualitative exploration. AIDS Patient Care STDS 2010; 24 753–62.
| Barriers to and facilitators of hepatitis C testing, management, and treatment among current and former injecting drug users: a qualitative exploration.Crossref | GoogleScholarGoogle Scholar | 21138381PubMed |
[32] Grebely J, Knightb E, Genoway K, Viljoen M, Khara M, Elliottc D, Gallagher L, Storms M, Raffa JD, DeVlaming S, Duncan F. Optimizing assessment and treatment for hepatitis C virus infection in illicit drug users: a novel model incorporating multidisciplinary care and peer support. Eur J Gastroenterol Hepatol 2010; 22 270–7.
| Optimizing assessment and treatment for hepatitis C virus infection in illicit drug users: a novel model incorporating multidisciplinary care and peer support.Crossref | GoogleScholarGoogle Scholar | 20425880PubMed |
[33] Grebely J, Tyndall MW. Management of HCV and HIV infections among people who inject drugs. Curr Opin HIV AIDS 2011; 6 501–7.
| Management of HCV and HIV infections among people who inject drugs.Crossref | GoogleScholarGoogle Scholar | 22001894PubMed |
[34] Harris M, Rhodes T. Hepatitis C treatment access and uptake for people who inject drugs: a review mapping the role of social factors. Harm Reduct J 2013; 10 7
| 23651646PubMed |
[35] Read P, Gilliver R, Kearley J, Lothian R, Cunningham EB, Chronister KJ, Dore GJ. Treatment adherence and support for people who inject drugs taking direct-acting antiviral therapy for hepatitis C infection. J Viral Hepat 2019; 26 1301–10.
| Treatment adherence and support for people who inject drugs taking direct-acting antiviral therapy for hepatitis C infection.Crossref | GoogleScholarGoogle Scholar | 31299127PubMed |
[36] Cunningham EB, Hajarizadeh B, Amin J, Litwin AH, Gane E, Cooper C, Lacombe K, Hellard M, Read P, Powis J, Dalgard O. Adherence to once-daily and twice-daily direct-acting antiviral therapy for hepatitis C infection among people with recent injection drug use or current opioid agonist therapy. Clin Infect Dis 2019; ciz1089
| 31677262PubMed |
[37] Beiser ME, Smith K, Ingemi M, Mulligan E, Baggett TP. Hepatitis C treatment outcomes among homeless-experienced individuals at a community health centre in Boston. Int J Drug Policy 2019; 72 129–37.
| Hepatitis C treatment outcomes among homeless-experienced individuals at a community health centre in Boston.Crossref | GoogleScholarGoogle Scholar | 30962036PubMed |
[38] Nielsen S, Gassowski M, Wenz B, Bannert N, Bock C-T, Kücherer C, Ross RS, Bremer V, Marcus U, Zimmermann R, DRUCK Study Group Concordance between self-reported and measured HIV and hepatitis C virus infection status among people who inject drugs in Germany. Hepatol Med Policy 2016; 1 8
| 30288312PubMed |