Health today versus health tomorrow: does Australia really care less about its future health than other countries do?
Nancy Devlin A C and Paul Scuffham BA Centre for Health Policy, Melbourne School of Population and Global Health, The University of Melbourne, 207 Bouverie Street, Melbourne, Vic. 3010, Australia.
B Centre for Applied Health Economics, Menzies Health Institute Queensland, Griffith University, G40_8.83, Gold Coast Campus, Southport, Qld 4222, Australia. Email: p.scuffham@griffith.edu.au
C Corresponding author. Email: nancy.devlin@unimelb.edu.au
Australian Health Review 44(3) 337-339 https://doi.org/10.1071/AH20057
Submitted: 6 April 2020 Accepted: 19 May 2020 Published: 1 June 2020
Abstract
Economic evaluation provides important evidence on value for money in health care and is routinely used in health technology assessment processes. The relevant costs and benefits of health care that are considered may arise now and/or in the future, and the relative importance placed on costs and benefits in the future is reflected in the discount rate applied to them. In this paper we note that Australia appears to apply one of the highest discount rates in the world to the assessment of future healthcare benefits. At a time when healthcare systems worldwide are calling for a rebalance of effort towards prevention, Australia’s discount rate risks pulling resource allocation in precisely the opposite direction, locking in institutional short-sightedness to funding decisions.
Introduction
The use of cost-effectiveness analysis to assess value for money in health care has gained widespread acceptance and use. It is most commonly used to assess new healthcare technologies via health technology assessment (HTA) processes. HTA considers the context for the use of a new technology, including the intended population, the clinical evidence on its safety, efficacy and effectiveness relative to the technology most likely to be displaced (i.e. comparator), the evidence on cost-effectiveness and the financial implications.1 HTA is intended to support healthcare decision making through use of systematic, transparent, scientific methods. For example, evidence on cost-effectiveness is routinely considered by Australia’s Pharmaceutical Benefits Advisory Committee (PBAC), which was one of the world’s first HTA organisations and the first, in 1993, to mandate pharmaceutical companies to include economic evaluation evidence in their submissions for reimbursement.2 The use of economic evaluation continues to grow apace as the world’s most highly populated countries (i.e. China, India, Brazil, Mexico) take bold steps towards universal health care, and put HTA processes into place as a means of managing demand and cost. Even the US, which famously once banned cost-effectiveness analysis of health care by an act of federal law, is now enthusiastically taking up the use of cost-effectiveness analysis via the Institute for Clinical and Economic Review.
Indeed, such is the predominance of cost-effectiveness evidence in healthcare decision making globally that health economics could be argued to have achieved more widespread use and positive impact than almost any other field of applied economics.
In essence, this approach to economic evaluation assesses the costs and benefits of an intervention relative to the costs and benefits of an existing treatment, and the incremental changes are expressed as an incremental cost-effectiveness ratio (ICER).
That sounds straightforward, but there are many aspects of economic evaluation methods that rely on value judgements. Importantly, ‘value for money’ can never be assessed in an entirely objective value-judgement-free way: what ‘value’ means depends on the perspective from which it is assessed. These methodological issues can appear to be quite arcane, but they are important because they can have a substantial bearing on the evidence presented and decisions about who gets access to treatments.
An example of this is the way that future costs and benefits are factored into the analysis. Things that happen in the future are ‘discounted’ (i.e. they carry less weight) than costs and benefits in the short term. This includes, for example, the years of life gained in the future by preventing a premature death now. Nearly all examples of cost-effectiveness analysis discount future costs and benefits in this way, and the higher the discount rate used, the less importance is attached to costs and benefits that occur in the future. Where all else is equal, a higher discount rate tips the balance towards approving technologies that yield benefits in the short term.
The standard rationale for discounting includes the existence of pure time preference (a preference for benefits today rather than in the future), uncertainty over future outcomes (a ‘bird in the hand…’) and the expectation of positive economic growth, meaning a dollar today is ‘worth more’ than a dollar in the future when we are richer.
What discount rate should be used, and what exactly it represents, is something health economists continue to debate, which does not make this issue any easier for policy makers to engage with. There are two competing schools of thought on what the discount rate should be based on.3 The first proposes that the discount rate should reflect the opportunity cost of capital used in the project (i.e. the benefits to society that the funds would have returned if used elsewhere in the economy), and the second proposes that the discount should reflect ‘pure time preference’, namely society’s willingness to give up consumption now in order to have greater consumption in the future.
Further, there is debate about whether costs and benefits should be discounted at the same or at different rates.4 For example, as countries grow richer, they spend an increasing percentage of gross domestic product (GDP) on health care; this is consistent with the idea that the marginal value of health is increasing and that future benefits should be discounted at a lower rate than future costs.5 That is, as we get wealthier, we place a greater value on health relative to what it costs to generate that health.
In the 1970s and 1980s, a discount rate of 5% was often used in published studies and became the default discount rate. This 5% discount rate was introduced in the PBAC guidelines in 1995,2 with the basis for the choice of that discount rate being unknown but referenced by those 1995 guidelines to an associated ‘Background Document’ that is now unavailable. Nevertheless, this 5% discount rate, applied to both costs and benefits, has remained for the past 25 years.
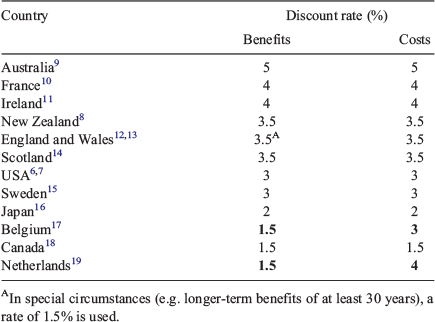
Most countries currently have lower discount rates. For example the discount rates currently recommended for use in the US and New Zealand are 3%6,7 and 3.5%8 respectively. The discount rates currently being used in HTA in a range of countries are given in Table 1, which lists countries in rank order from the highest to lowest discount rates applied to future health in HTA.
|
Notably, Australia is at the top of this list. Of course, this is not a comprehensive list of all HTA systems, but we are not aware of any country that uses a higher discount rate in its HTA process. Is there any evidence to suggest that there are underlying factors, either in terms of preferences or the financial markets, that justify Australia being so different from these other countries in this respect? We doubt it. This is one league table that Australia does not necessarily want to be at the top of.
Because the discount rate can be important to conclusions about cost-effectiveness, HTA bodies generally ask for evidence on how sensitive results are to the use of both higher and lower discount rates. Interestingly, the PBAC recommendations for sensitivity analysis are 3.5% and 0%, both of which are lower than the PBAC’s base-case discount rate of 5%. The Medical Services Advisory Committee (MSAC), which appraise therapeutic and diagnostic services and devices, also use a base-case 5% discount rate for both costs and outcomes, and recommend sensitivity analyses that include 0% for outcomes alone and then 0% for both costs and outcomes jointly. Using a 5% discount rate as the base-case means the base case reflects the worst-case scenario in terms of the value of future benefits: they are minimised compared with those in the sensitivity analyses.
What are the implications of using relatively high discount rates? Healthcare interventions that reduce risks of ill-health (i.e. preventative health care), such as lipid-lowering drugs, antihypertensives, breast and bowel cancer screening programs and immunisations, will appear less cost-effective and may not be recommended in Australia. Compared with other countries, risk-reducing interventions will appear less cost-effective even if the clinical benefits and costs of delivery are roughly the same. Similarly, other interventions with long time horizons for accruing benefits, such as gene-editing technologies, which offer the promise of lifetime ‘cures’ for diseases, will also appear less cost-effective in Australia compared with other countries simply because of the choice of discount rate.
It is likely that differences in which healthcare interventions are approved for government subsidy are most apparent between Australia compared with the Netherlands and Belgium, where lower discounts rates are used for benefits compared with costs. In England, the National Institute for Health and Care Excellence (NICE) applies a lower discount rate of 1.5% to benefits that extend over long time frames (at least 30 years into the future),13 although the selective use of lower discount rates for benefits creates other issues.20 The application of discount rates for costs and benefits is clearly being handled in very different ways across the world and, in many cases, the rationale for the choice of discount rate is poorly justified.21 However, health is valued more highly as we get wealthier5 and, arguably, it is appropriate to recognise this in how we assess value for money in health care. Further, as real, risk-free interest rates fall close to zero in financial markets, there will be growing pressure to reduce discount rates in many countries.
At a time when healthcare systems are continually calling for a rebalance of effort towards prevention and with public health issues more important than ever, Australia’s discount rate is resulting in the systematic undervaluation of long-term health benefits. This makes some healthcare interventions appear not cost-effective and less likely to be recommended for government funding. This pulls healthcare resource allocation in precisely the opposite direction to supporting prevention and locks in institutional short-sightedness to funding decisions. It is time for the PBAC and MSAC to reconsider how they weigh up health today versus health tomorrow in HTA.
Competing interests
Paul Scuffham has contracts with the Australian Government’s Department of Health to undertake HTA for the MSAC and PBAC. Nancy Devlin has no competing interests to declare.
Acknowledgements
The authors did not receive any funding to write this article. All opinions and views expressed in this article are the sole responsibility of the authors.
References
[1] Department of Health, Australian Government. Guidelines for preparing a submission to the Pharmaceutical Benefits Advisory Committee, Version 5.0, September 2016. 2016. Available at: https://pbac.pbs.gov.au/content/information/files/pbac-guidelines-version-5.pdf [verified 16 May 2020].[2] Department of Health, Australian Government. Guidelines for the pharmaceutical industry on preparation of submissions to the Pharmaceutical Benefits Advisory Committee: including major submissions involving economic analyses. 1995. Available at: https://pbac.pbs.gov.au/information/printable-version-of-guidelines.html [verified 16 May 2020].
[3] Drummond M, Sculpher M, Claxton K, Stoddart G, Torrance G. Methods for the economic evaluation of health care programmes. 4th edn. Oxford: Oxford University Press; 2015.
[4] Claxton K, Paulden M, Gravelle H, Brouwer W, Culyer AJ. Discounting and decision making in the economic evaluation of health care technologies. Health Econ 2011; 20 2–15.
| Discounting and decision making in the economic evaluation of health care technologies.Crossref | GoogleScholarGoogle Scholar | 21154521PubMed |
[5] Gravelle H, Smith D. Discounting the health effects in cost benefit and cost effectiveness analysis. Health Econ 2001; 10 587–99.
| Discounting the health effects in cost benefit and cost effectiveness analysis.Crossref | GoogleScholarGoogle Scholar | 11747043PubMed |
[6] Neumann P, Sanders G, Russell L, Siegal J, Ganiats T. Cost effectiveness in health and medicine. New York: Oxford University Press; 2017.
[7] Institute for Clinical and Economics Review (ICER). ICER’s reference case for economic evaluations: principles and rationale. 2018. Available at: http://icer-review.org/wp-content/uploads/2018/07/ICER_Reference_Case_July-2018.pdf [verified 17 May 2020].
[8] Pharmaceutical Management Agency (PHARMAC). 8.2 Recommended discount rate. In: Prescription for pharmacoeconomic analysis. 2017. Available at: https://https://www.pharmac.govt.nz/medicines/how-medicines-are-funded/economic-analysis/pfpa/8-discounting/#recommended [verified 25 May 2020].
[9] Pharmaceutical Benefits Advisory Committee. 3A.9 Uncertainty analysis: model inputs and assumptions. 2016. Available at: https://pbac.pbs.gov.au/section-3a/3a-9-uncertainty-analysis-model-inputs-and-assumptions.html [verified 20 February 2020].
[10] Haute Autorité de Santé. Choices in methods for economic evaluation. Paris: Haute Autorité de Santé; 2012.
[11] Health Information and Quality Authority. Guidelines for the economic evaluation of health technologies in Ireland. 2019. Available at: https://www.hiqa.ie/sites/default/files/2019-07/HTA-Economic-Guidelines-2019.pdf [verified 17 May 2020].
[12] National Institute for Health and Care Excellence. 5.6 Discounting. In: Guide to the methods of technology appraisal 2013. 2013. Available at: https://www.nice.org.uk/process/pmg9/chapter/the-reference-case#discounting [verified 20 February 2020].
[13] National Institute for Health and Care Excellence. Discounting of health benefits in special circumstances. 2011. Available at: https://www.nice.org.uk/guidance/ta235/resources/osteosarcoma-mifamurtide-discounting-of-health-benefits-in-special-circumstances2 [verified 25 May 2020].
[14] Scottish Medicines Consortium. Guidance to manufacturers for completion of new product assessment form (version 2.1). Glasgow: Scottish Medicines Consortium; 2007.
[15] Dental and Pharmaceutical Benefits Board (TLV). Change in the Dental and Pharmaceutical Benefits Board General Guidance on Economic Evaluations. Stockholm: Dental and Pharmaceutical Benefits Board; 2017. Available at: https://www.tlv.se/download/18.467926b615d084471ac3230c/1510316374332/TLVAR_2017_1.pdf [verified 25 May 2020]. [In Swedish].
[16] Shiroiwa T, Fukuda T, Ikeda A, Takura T, Moriwaki K. Development of an official guideline for the economic evaluation of drugs/medical devices in Japan. Value Health 2017; 20 372–8.
| Development of an official guideline for the economic evaluation of drugs/medical devices in Japan.Crossref | GoogleScholarGoogle Scholar | 28292481PubMed |
[17] Belgian Health Care Knowledge Centre. Belgian guidelines for economic evaluations and budget impact analysis. 2nd edn. Brussels: Belgian Health Care Knowledge Centre; 2012.
[18] CADTH. CADTH methods and guidelines. Guidelines for the Economic Evaluation of Health Technologies: Canada. 4th edn. 2017. Available at: www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf [verified 20 February 2020].
[19] Netherlands National Health Care Institute. Guideline for economic evaluations in healthcare. Diemen: National Health Care Institute; 2016. Available at: https://english.zorginstituutnederland.nl/publications/reports/2016/06/16/guideline-for-economic-evaluations-in-healthcare [verified 25 May 2020].
[20] O’Mahony JF, Paulden M. NICE’s selective application of differential discounting: ambiguous, inconsistent, and unjustified. Value Health 2014; 17 493–6.
| NICE’s selective application of differential discounting: ambiguous, inconsistent, and unjustified.Crossref | GoogleScholarGoogle Scholar | 25128041PubMed |
[21] Attema AE, Brouwer WBF, Claxton K. Discounting in economic evaluation. Pharmacoeconomics 2018; 36 745–58.
| Discounting in economic evaluation.Crossref | GoogleScholarGoogle Scholar | 29779120PubMed |


