Use and cost of Medicare Benefits Schedule and Pharmaceutical Benefits Scheme services following inpatient rehabilitation for acquired disability in Australia
Samantha J. Borg A * , David N. Borg
A * , David N. Borg  A , Michele M. Foster A , Ryan Bell A B , Jessica Bowley A and Timothy Geraghty A B
A , Michele M. Foster A , Ryan Bell A B , Jessica Bowley A and Timothy Geraghty A B
A The Hopkins Centre, Menzies Health Institute Queensland, Griffith University, Brisbane, Qld, Australia.
B Division of Rehabilitation, Princess Alexandra Hospital, Brisbane, Qld, Australia.
Australian Health Review 47(2) 165-174 https://doi.org/10.1071/AH22118
Submitted: 14 May 2022 Accepted: 26 November 2022 Published: 22 December 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of AHHA. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Objectives This study explored publicly funded health system and patient expenditure in the post-acute phase following discharge from inpatient acquired brain injury (ABI) or spinal cord injury (SCI) rehabilitation. The secondary aim was to explore sociodemographic and injury characteristics associated with high costs.
Methods This was a prospective cohort study. 153 patients (ABI: n = 85; SCI: n = 68) who consented to the use of their Medicare data were recruited between March 2017 and March 2018, at the point of discharge from ABI or SCI specialist rehabilitation units. The main outcome measure involved linkage of the Medicare Benefits Schedule (MBS) and Pharmaceutical Benefits Scheme (PBS) data for the 12 months following discharge from rehabilitation. Bayesian penalised regression was used to determine characteristics associated with high costs.
Results The median number of MBS items used in the 12 months after discharge was 33 (IQR: 21–52). General practitioners and allied health services were accessed by 100% and 41% of the cohort, respectively. The median MBS system cost (in Australian dollars) was $2006 (IQR: $162–$3090). Almost half (46%) of the participants had no MBS patient expenditure. The median PBS system cost was $541 (IQR: $62–$1574). For people with ABI, having a traumatic injury or one comorbidity was associated with lower PBS system costs by on average $119 and $134, respectively. We also found that hospitalisation in ABI was associated with higher PBS system costs, by on average $669.
Conclusion There was evidence of high and variable MBS and PBS costs, raising concerns about financial hardship. Future research should focus on identifying any unmet service and prescription needs in the post-acute rehabilitation phase for these populations.
Keywords: acquired brain injury, healthcare costs, Medicare Benefits Schedule, primary care, Pharmaceutical Benefits Scheme, rehabilitation, service use, spinal cord injury.
Medicare was established to provide subsidised medical and hospital services to minimise disadvantage for Australian citizens. This is particularly important for acquired disability populations for whom complex care needs would otherwise be hindered by catastrophic financial pressures in lieu of subsidy systems. Acquired disability is often associated with routine, lifelong health and rehabilitation service use, the absence of which can contribute to premature decline in functioning, placing significant pressure on the Australian healthcare system.1 As such, subsidy systems are critical to equitable and affordable access for people who sustain an acquired brain injury (ABI) or spinal cord injury (SCI), and avoidance of a compromised recovery and further decline in health status.1–3 In Australia this includes the Medicare Benefits Schedule (MBS): a national health insurance system that provides no-cost public hospital access and out-of-hospital medical services, at no or ‘minimal’ cost to the service user for eligible items;4 and the Pharmaceutical Benefits Scheme (PBS): a national government medication subsidy program for eligible prescriptions.4,5
Despite being clinically distinct, ABI and SCI are both populations with complex rehabilitation and care needs that are characterised by routine health system utilisation, with greater hospital and primary care use than the general population, which in turn account for considerably higher costs.6–8 Engagement with post-acute primary health and rehabilitation services is a critical transition pathway for many ABI and SCI patients.9–11 This ongoing health service mix is heterogenous, meaning that the combination and service costs after inpatient rehabilitation varies.12 Although some in these clinical cohorts are eligible for the different lifetime care schemes in Australia, most also rely on Medicare and federal subsidies, either fully or in part, to fund primary and specialist care, medication prescriptions and allied health services expenditure.13
Although the complex needs and high health costs for these clinical cohorts are well-documented, there is a paucity of data detailing the specific service use and costs in the early, post-acute phase in Australia. As such, this exploratory study had two aims: (1) to describe MBS and PBS item use and the associated costs in the 12 months after discharge from inpatient ABI and SCI units; and (2) to explore whether sociodemographic and injury characteristics were associated with higher MBS and/or PBS costs.
Methods
Study design
Using a prospective cohort design, participants were followed for 12 months after discharge from specialist ABI or SCI rehabilitation units.14 Data on each participant were extracted from electronic medical records, and via Medicare-linked data.15 Ethics approval for the project was granted from the Metro South Human Research Ethics Committee (HREC/16/QPAH/684; SSA/16/QPAH/685) and the Griffith University Human Research Ethics Committee (2016/915). The study followed the principles outlined in the Declaration of Helsinki, except for registration in a public database.
Setting and participants
Participants were recruited at the point of discharge, from the specialist ABI or SCI rehabilitation units, at the Princess Alexandra Hospital (PAH), Queensland, Australia. The PAH is a major tertiary facility in the Metro South Health and Hospital Service, serving an estimated 1 million people and providing state-wide ABI and SCI rehabilitation services.16
A convenience sample of participants was recruited between March 2017 and March 2018. Participants were recruited as part of a larger study investigating access and wellbeing after acquired disability.14 Eligibility criteria included: (i) a new diagnosis of SCI or ABI; (ii) age ≥18 years; and (iii) capacity to provide informed consent, or consent via a substitute decision maker on behalf of the patient. All participants or their decision makers provided written informed consent.
Data measures
Sociodemographic, injury-related, hospital length of stay (acute hospital plus specialist rehabilitation stay), discharge variables and comorbidities were extracted from electronic medical records. This included: age, gender, marital status, education, living situation, injury type and severity, funding support and discharge destination. Traumatic brain injury severity was classified as mild, moderate or severe according to published guidelines using the Glasgow Coma Scale,17 and injury level for SCI as paraplegia or tetraplegia. SCI completeness was based on the American Spinal Injury Association Impairment Scale (AIS), in which a grading of A relates to a complete injury and levels B–D represent varying levels of an incomplete SCI.18 MBS and PBS data for the 12 month period after rehabilitation discharge were accessed via data linkage.
The MBS items were grouped into 10 categories: general practitioner (GP), specialist, medical practitioner, nursing, allied health, psychology, optometric, diagnostic procedures and investigations, pathology services, and therapeutic procedures. These categories are largely based on the broad MBS item categories (Supplementary Table S1).15 The PBS data include the medication item code and descriptions and dispensing date for medicines eligible under the National Health Act 1953, for which a claim was processed.5 MBS and PBS costs are reported as the benefit paid, that is, the amount of a service or prescription covered by the schedule or scheme (henceforth, system costs), and patient out-of-pocket costs, consisting of the balance paid by patients for the service or prescription above the Medicare reimbursement (henceforth, patient costs). MBS items that do not incur patient out-of-pocket costs are considered bulk-billed and are accounted for under system costs.
Medicare and the Pharmaceutical Safety Net protect against high out-of-pocket costs.4 Once an individual (or family group, if applicable) reaches a certain out-of-pocket amount during a calendar year, all subsequent expenditure will have a higher amount subsidised, ultimately reducing all subsequent out-of-pocket costs.19 Safety nets are automatically calculated and taken into consideration in the MBS costings data.
Data analysis
All analyses were undertaken using R.20 First, MBS and PBS use and costs were summarised for the cohort and by injury type as count (percent) or median (IQR). Second, Bayesian penalised regression was used to conduct an exploratory analysis of factors associated with the MBS and PBS system and patient costs. Bayesian penalised regression is a variable selection method that guards against overfitting, with the estimates of unimportant coefficients in the model shrunk towards zero.21 This is achieved by specifying the shrinkage prior distributions (i.e. horseshoe (d.f. = 1)) for all coefficients in the model.21 MBS patient costs were modelled using a zero-inflated negative binomial response distribution. All other outcomes were logged and modelled with a Gaussian response distribution.
The predictor variables of age (standardised; mean = 0, s.d. = 1), gender (levels: male, female), hospital length of stay (standardised; mean = 0, s.d. = 1), living situation (levels: living alone, living with others), funding support (levels: no, yes), injury aetiology (levels: traumatic, non-traumatic), comorbidities (levels: 0, 1, >1) and hospitalisations (levels: no, yes) were considered for inclusion in each model. When fitting the SCI models, we also considered injury completeness (levels: incomplete, complete). Injury severity (levels: mild, moderate, severe) was considered when fitting the ABI models. Because the sample sizes of ABI and SCI were relatively small, exploratory plots were used to identify potentially important predictors of each outcome variable before model fitting. The ratio of the number of expected non-zero coefficients to the number of expected zero coefficients was set to 1/5. The models were fitted using Stan with the brms interface.22
In interpreting the regression coefficients, we focused on effects for which the chance of the effect being greater or less than zero (depending on the direction of the effect) was at least 90%. This standard is suited to the exploratory nature of the analysis, and to acknowledge the relatively low threshold we use the terminology ‘evidence’ when referring to the results. Markov chain Monte Carlo procedures (8 chains, each with 2000 iterations, and a 50% burn-in) were used to generate posterior estimates of expected values. Posterior estimates of interest were: (i) the probability that a regression coefficient was greater than zero (Pr β > 0) or less than zero (Pr β < 0) and (ii) the mean and 90% credible interval (CrI) of regression coefficients.
Results
Sample characteristics
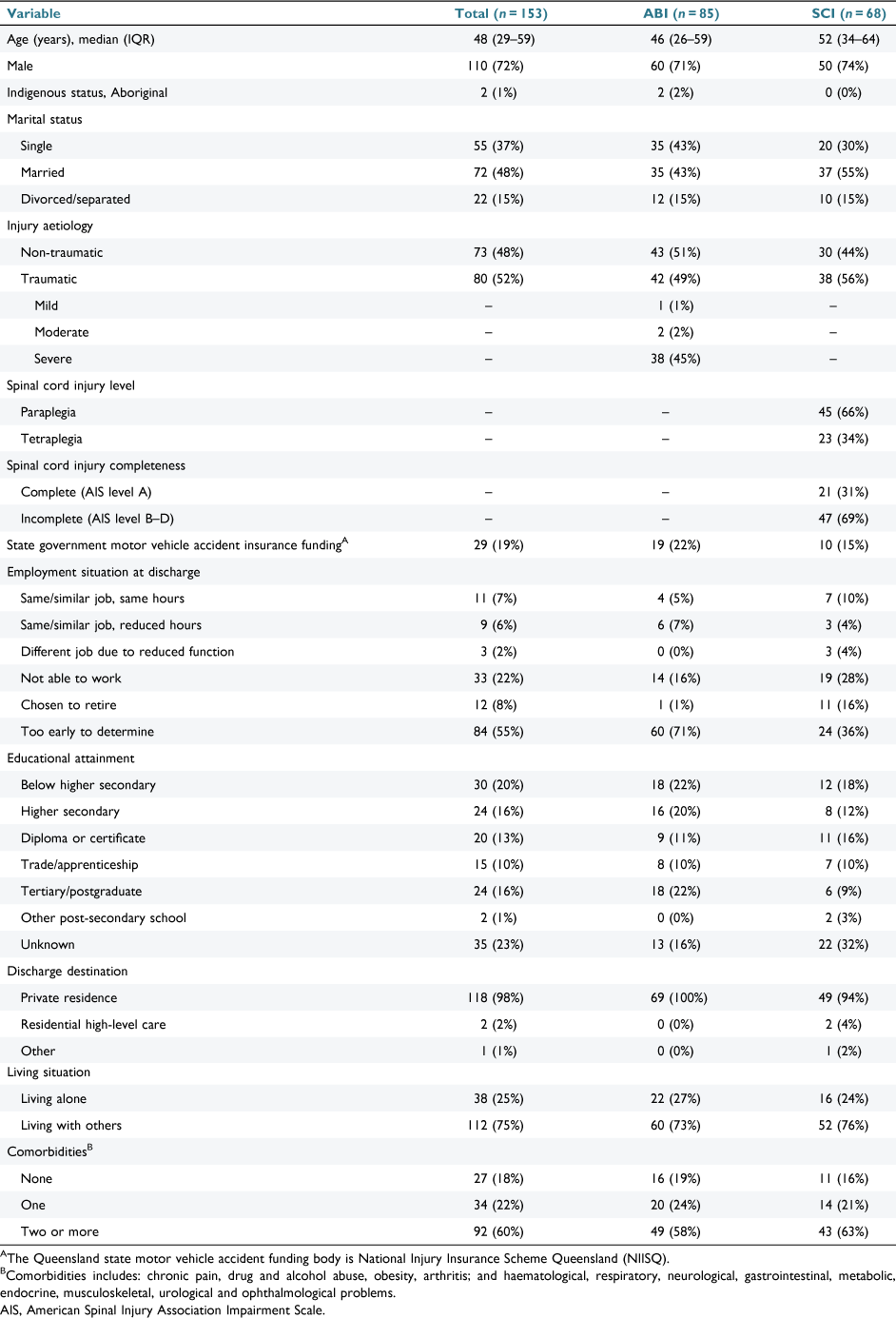
In total, MBS and PBS data were obtained for 153 participants (ABI: n = 85; SCI: n = 68). Six participants (4%, all ABI) appeared in the MBS but not the PBS dataset. Participants were mostly male (72%), had a median age of 48 years, and there was an even representation across traumatic and non-traumatic aetiologies (Table 1).
|
MBS items
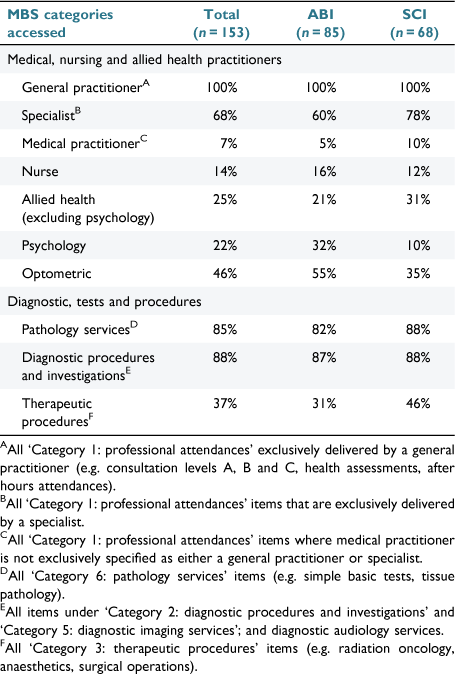
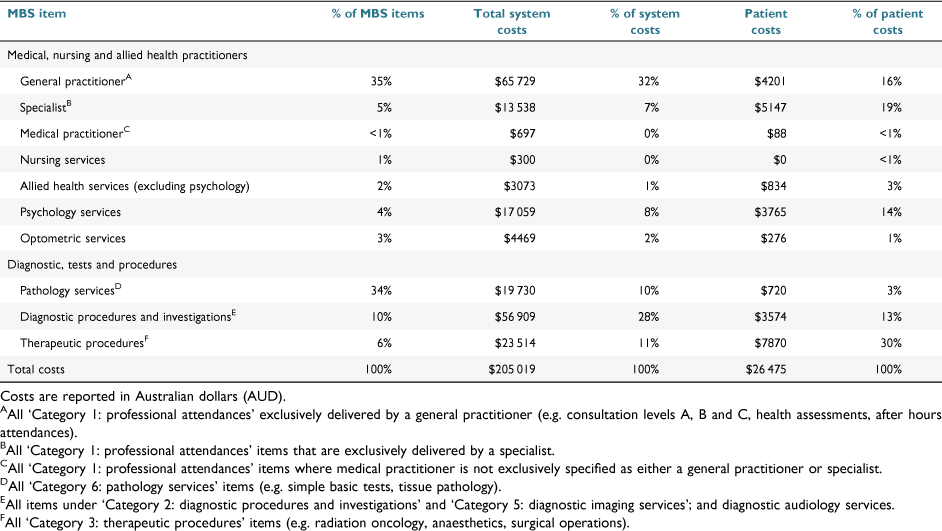
A total of 6513 (ABI: n = 3203; SCI: n = 3310) MBS items were accessed in the 12 months following discharge. The median number of items per participant was 33 (IQR: 21–52; range: 4–189). Pathology services (36%), GP appointments (34%) and diagnostic procedures (10%) accounted for 80% of all MBS items used. The proportion of participants accessing MBS services is given in Table 2. GPs and allied health services were accessed by 100% and 41% of the full cohort (i.e. ABI and SCI), respectively.
|
MBS costs
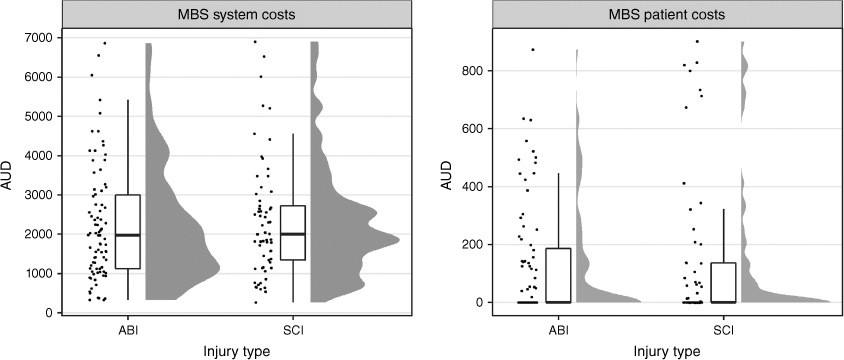
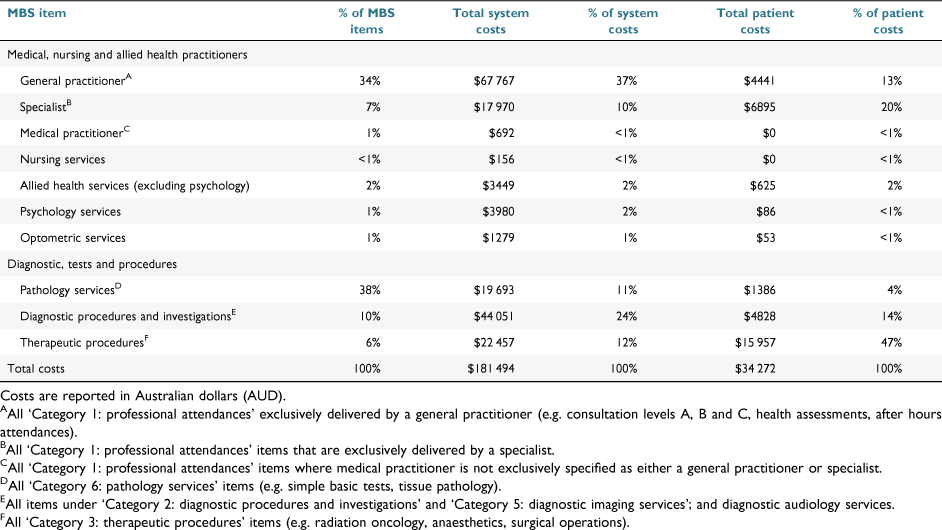
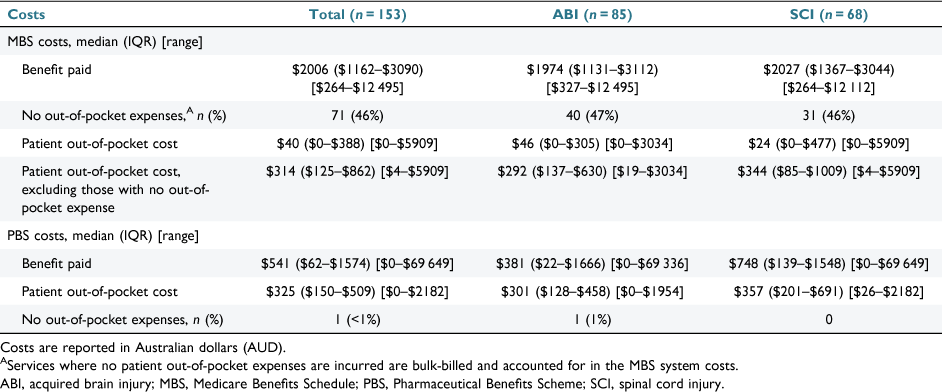
The total MBS system costs (in Australian dollars) for the full cohort were $386 513, for which ABI accounted for 53% ($205 019/$386 513) and SCI accounted for 47% ($181 494/$386 513). MBS system and patient costs are given in Table 3 for ABI and Table 4 for SCI. The median system cost was $2006 (IQR: $1162–$3090; range: $264–$12 495), which was similar across injury type, while MBS median patient costs were lower for participants with SCI (Table 5, Fig. 1). Of the full cohort, 46% incurred no out-of-pocket costs, having been bulk-billed for all MBS items used during the 12 month observation period (Table 5). MBS patient out-of-pocket costs were $60 747 for the full cohort. For patients who did incur MBS out-of-pocket expenses, the median patient expenditure for the 12 month observation period was $314 (IQR: $125–$862). MBS patient and system costs are shown in Fig. 1.
|
|
|
Factors associated with MBS costs
We did not find any evidence that any of the sociodemographic or injury-related variables explored had an effect on MBS system or patient costs, for both ABI and SCI.
PBS items
Prescription data were available for 147 participants and involved 6016 dispensed prescriptions (ABI: n = 2662; SCI: n = 3354). The median dispensed prescriptions per ABI and SCI participants was 26 (IQR: 13–57; range: 1–120) and 40 (IQR: 20–63; range: 3–249), respectively. Oxycodone and pregabalin were among the top five dispensed medications for both the ABI and SCI groups (Supplementary Table S2).
PBS costs
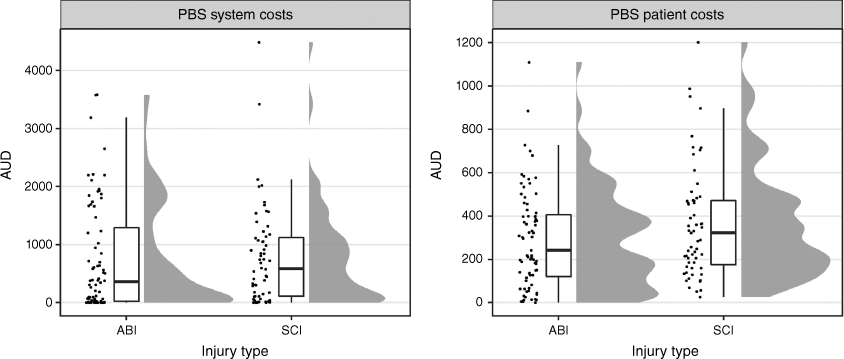
The total PBS system costs for all 147 participants (ABI: n = 79; SCI: n = 68) were $412 829, of which ABI accounted for 52% ($215 798/$412 829) and SCI accounted for the remaining 48% ($197 031/$412 829). The median system costs for the full cohort were $541 (IQR: $62–$1574; range: $0–$69 649) and were higher for those with SCI (Table 5). PBS patient and system costs are shown in Fig. 2. PBS patient costs for all participants were $66 599 (ABI: $28 954; SCI: $37 645). The median PBS patient cost was $325 (IQR: $150–$509; range: $0–$2182) for the full cohort (Table 5).
Factors associated with PBS costs
Traumatic brain injuries were associated with lower PBS system costs compared with non-traumatic brain injuries (Pr β < 0 = 0.929), by on average $119 (90% CrI = –$2, $375). There was also evidence of lower PBS system costs for those with one comorbidity compared with no comorbidities (Pr β < 0 = 0.903), by on average $134 (90% CrI = –$5, $414). There was evidence of higher PBS system costs for people with ABI who were hospitalised in the first 12 months after discharge (Pr β > 0 = 0.933), by on average $669 (90% CrI = −$3, $2438).
For people with ABI, there was evidence of a very small increase in PBS patient costs with age (Pr β > 0 = 0.911), with a 16.8 year change in age associated with a $29 (90% CrI = –$2, $96) higher cost. For ABI, having two or more comorbidities was associated with higher PBS patient costs compared with those with no comorbidities (Pr β > 0 = 0.995), by on average $190 (90% CrI = $65, $336), and compared with those with only one comorbidity (Pr β > 0 = 0.990), by on average $164 (90% CrI = $44, $304).
It was unclear whether people with SCI who had two or more comorbidities had higher PBS system costs (Pr β > 0 = 0.894) compared with those with only one comorbidity (β = $1041; 90% CrI =−$27, $3560). We did not find evidence of effects of any predictor variable on PBS patient costs for people with SCI.
Discussion
Medicare use and costs, and PBS costs in the first year after hospital discharge were highly variable in people with ABI and SCI. Our exploratory analysis shows that for those with ABI, there was evidence that having a traumatic injury or only one comorbidity was associated with lower PBS system costs, whereas hospitalisation was associated with higher PBS system costs. A very small increase in PBS patient cost was associated with a large increase in age (~17 years), with higher PBS patient costs associated with having multiple comorbidities. We did not find any evidence that any of the studied sociodemographic or injury-related variables were associated with MBS system or patient costs. The presence of comorbidities in the ABI cohort was more costly to the individual. There was no evidence of comorbidities being more costly for people with SCI, despite comparable comorbidities to those with ABI. Although not generalisable, our findings indicate high MBS and PBS use and large variability in system and patient costs in the first 12 months following discharge, which is likely to be a reflection of the heterogeneity across and within these population groups.
Median patient out-of-pocket costs in this study are below that seen in other clinical populations (Table 5), for example, in the 12 months following diagnosis of a major cancer.23 However, this needs to be considered in certain contexts. First, although numerous studies have undertaken linkage processes investigating Medicare use and/or costs, there is substantial heterogeneity among the studies, making comparisons difficult. Previous studies vary in relation to population (e.g. injury-specific or general population), observation period (e.g. up to 10 year follow-up24) and the Medicare items studied (e.g. mental health25 or telehealth26 items). Second, the costs identified in our study account for only a portion of the overall lifetime costs for these two cohorts, and further, were not representative of the full breadth of costs for our data collection period. For example, inpatient hospital costs, for which people with traumatic SCI and ABI typically incur a higher number of hospitalisations and inpatient hospital days in the 12 months following injury,27 are funded at the State government level.
From a practical standpoint, this study provides an important initial benchmark of Medicare use and costs in two groups of people with severe and often permanent, acquired disabilities with complex health and rehabilitation needs. These initial findings can contribute to the identification of longer-term service use patterns and their relation to wellbeing and inequities/hardships, which could deter help-seeking behaviours. Additionally, they could also be used to understand the impacts of service interruptions, including those resulting from the coronavirus (COVID-19) pandemic or increasing natural disasters. Further studies should consider similar benchmarking in these rehabilitation populations, using larger sample sizes, from multiple Australian states. Such studies may also need to consider any impact of outside insurance schemes and system-level funding on Medicare use, including the National Disability Insurance Scheme (NDIS). The NDIS, which was progressively rolled out in specific regions across Queensland from 2017 to January 2019,28 was not accounted for in the current study due to incomplete coverage during the time of recruitment (March 2017–March 2018), extending into the 12 month observation period (until March 2019). Although the NDIS does not cover medical services, future studies will need to take into consideration that allied health are one of the largest groups of registered providers and supports for people receiving NDIS funding.29
The median MBS system cost was similar across injury type, at ~$2000 (Table 5). In contrast, PBS system costs were higher for SCI (Table 5), which may be explained by differences in polypharmacy between SCI and ABI. Our exploratory modelling showed that traumatic brain injury had lower PBS system costs. This could be due to traumatic injuries being more compensable, for which insurance schemes would offset prescription and service costs. In the current context, this includes the National Injury Insurance Scheme Queensland (NIISQ), which is a no-fault based scheme that provides lifetime treatment and care for anyone who sustains a serious personal injury in a motor vehicle accident on or after 1 July 2016.30 Nearly one in five participants in the current study were covered by NIISQ (22% for those with ABI and 15% in SCI), and although NIISQ coverage was not found to be important based on our exploratory modelling, this does not preclude NIISQ from being an important consideration for costings in larger sample sizes, or in studies with longer observation periods.
We found that, in general, Medicare services were accessed by people with ABI and SCI to a similar extent as other Australians with varying long-term health conditions (e.g. diabetes, kidney disease).31 However, our cohort accessed some services more, such as medical specialists (68% versus 53%), which may reflect the immediate post-acute needs of our cohort.31 Of interest, some services that accounted for only a small proportion of MBS items, incurred significant patient or system cost. For example, therapeutic procedures represented 6% of MBS items for those with SCI yet accounted for 47% of patient out-of-pocket costs (Table 4). Medical specialist appointments were similar, accounting for between 5% and 7% of MBS items for those with ABI or SCI and contributing between 19% and 20% of patient costs (Tables 3, 4).
Considering the entire sample, the median MBS patient cost ($314 for those who incurred out-of-pocket costs) and PBS patient cost ($325) over the 12 months appear modest. However, there is large variability within these costs. Despite nearly half the cohort incurring no MBS patient expenditure, the upper range of patient costs was $5909; and $2183 for pharmaceutical costs (Table 5). This variability is concerning given that people with ABI and SCI typically face financial hardship and have lower incomes compared with the general population. Cross-sectional research for people with SCI in Australia in 2018 identified that half of the cohort relied on disability or aged pensions, and more than 40% earned less than the weekly minimum income,32 which during 2018 was equivalent to $719 per week (or $19 per hour).33 Similar trends are seen in Australian ABI populations, for whom there is inequality, including lower levels of engagement in paid work and earnings when compared with Australians without disability.34 Lower incomes can lead to cost-related non-adherence for medical and pharmaceutical needs. In a Canadian SCI cohort, cost-related non-adherence for prescription medications was reported at 37%, with opioids and antidepressants among the most frequently avoided.35 It is not unreasonable to assume that Australian SCI rates are similar.31,36 In 2020–21, cost-related medication avoidance rates for individuals with varying long-term health conditions was 1 in 20, while medical specialist avoidance was evident among 1 in 17.31 There is evidence that cost-related medical avoidance disproportionality affects those with greater levels of disability or illness, and socioeconomic disadvantage.31 This raises concerns about the potential for avoidance of services or prescriptions due to high out-of-pocket costs, increasing the risk of unmet needs in this critical post-acute period. Given that financial hardships can negatively affect anxiety, quality of life and, ultimately, recovery,13,37 cost-related prescription and medical service avoidance is a major concern for Australians with ABI and SCI. As such, its interaction with unmet need and health outcomes warrants future investigation.
We caution against generalisation of the findings due to the relatively small sample size and the recruitment of participants from a single tertiary hospital. The data reported specifically relate to eligible MBS and PBS items and do not account for services accessed outside of Medicare, or commonly used over-the-counter medications. Although less than half (41%) of the full cohort used allied health services, this is likely to be underrepresented because the data did not account for privately funded services, including those covered by the National Injury Insurance Scheme, or accessed through ambulatory services. Although this study was concerned with MBS and PBS use and costs, we noted that oxycodone (a Schedule 8 restricted opioid analgesic) and pregabalin (used in the management of neuropathic pain38) were among the most frequent PBS prescriptions. This raises concerns regarding persistent pain as an ongoing complication and the appropriate use of these medications, and warrants further investigation. We emphasise that the results from our exploratory analysis should be interpreted with caution, given that the characteristics of participants in the current study may not be comparable to other ABI or SCI groups. The small sample may have precluded the identification of relatively smaller but practically important effects. Future studies with targeted hypotheses are needed to confirm our findings.
Conclusion
This study demonstrates the potential for financial hardship during a critical period of transition back to the community for people with ABI and SCI. Many individuals had high patient costs, which raises concerns about financial hardship, particularly because of the long-term burden of ABI and SCI and the limited capacity of these populations to absorb such costs. It remains a responsibility of both the system and service providers to continue to work together to minimise patient costs for people with ABI and SCI, as well as other vulnerable population groups in Australia.
Supplementary material
Supplementary material is available online.
Data availability
The R code used to generate the analyses are available upon request from the corresponding author. A limited, non-identifiable dataset may also be made available upon request.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
References
[1] Williams E, Martini A, Jackson H, et al. Time between acquired brain injury and admission to community-based rehabilitation: differences in cognitive and functional gains. Brain Inj 2020; 34 713–722.| Time between acquired brain injury and admission to community-based rehabilitation: differences in cognitive and functional gains.Crossref | GoogleScholarGoogle Scholar |
[2] Demir Y, Köroğlu Ö, Tekin E, et al. Factors affecting functional outcome in patients with traumatic brain injury sequelae: our single-center experiences on brain injury rehabilitation. Turk J Phys Med Rehabil 2019; 65 67–73.
| Factors affecting functional outcome in patients with traumatic brain injury sequelae: our single-center experiences on brain injury rehabilitation.Crossref | GoogleScholarGoogle Scholar |
[3] Scivoletto G, Morganti B, Molinari M. Early versus delayed inpatient spinal cord injury rehabilitation: an Italian study. Arch Phys Med Rehabil 2005; 86 512–516.
| Early versus delayed inpatient spinal cord injury rehabilitation: an Italian study.Crossref | GoogleScholarGoogle Scholar |
[4] Foster M, Fleming J. The health care system in Australia. In: Taylor S, Foster M, Fleming J, editors. Health care practice in Australia. Melbourne: Oxford University Press; 2008. pp. 46–73.
[5] Australian Institute of Health and Welfare. Pharmaceutical Benefits Scheme (PBS) data collection. Australian Government. 2022. Available at https://www.aihw.gov.au/about-our-data/our-data-collections/pharmaceutical-benefits-scheme [accessed 14 April 2022].
[6] Dismuke CE, Walker RJ, Egede LE. Utilization and Cost of Health Services in Individuals With Traumatic Brain Injury. Glob J Health Sci 2015; 7 156–169.
| Utilization and Cost of Health Services in Individuals With Traumatic Brain Injury.Crossref | GoogleScholarGoogle Scholar |
[7] Dryden DM, Saunders LD, Jacobs P, et al. Direct health care costs after traumatic spinal cord injury. J Trauma 2005; 59 441–447.
| Direct health care costs after traumatic spinal cord injury.Crossref | GoogleScholarGoogle Scholar |
[8] Ronca E, Scheel-Sailer A, Koch HG, et al. Health care utilization in persons with spinal cord injury: part 2-determinants, geographic variation and comparison with the general population. Spinal Cord 2017; 55 828–833.
| Health care utilization in persons with spinal cord injury: part 2-determinants, geographic variation and comparison with the general population.Crossref | GoogleScholarGoogle Scholar |
[9] Turner BJ, Fleming J, Ownsworth T, Cornwell P. Perceived service and support needs during transition from hospital to home following acquired brain injury. Disabil Rehabil 2011; 33 818–829.
| Perceived service and support needs during transition from hospital to home following acquired brain injury.Crossref | GoogleScholarGoogle Scholar |
[10] Conneeley AL. Transitions and Brain Injury: A Qualitative Study Exploring the Journey of People with Traumatic Brain Injury. Brain Impair 2012; 13 72–84.
| Transitions and Brain Injury: A Qualitative Study Exploring the Journey of People with Traumatic Brain Injury.Crossref | GoogleScholarGoogle Scholar |
[11] Foster M, Legg M, Hummell E, et al. Right people, right time? A qualitative study of service access experiences of adults with acquired brain injury following discharge from inpatient rehabilitation. Brain Impair 2020; 22 92–107.
| Right people, right time? A qualitative study of service access experiences of adults with acquired brain injury following discharge from inpatient rehabilitation.Crossref | GoogleScholarGoogle Scholar |
[12] Chen A, Bushmeneva K, Zagorski B, et al. Direct cost associated with acquired brain injury in Ontario. BMC Neurol 2012; 12 76
| Direct cost associated with acquired brain injury in Ontario.Crossref | GoogleScholarGoogle Scholar |
[13] Legg M, Foster M, Jones R, et al. The impact of obstacles to health and rehabilitation services on functioning and disability: a prospective survey on the 12-months after discharge from specialist rehabilitation for acquired brain injury. Disabil Rehabil 2021; 44 5919–5929.
| The impact of obstacles to health and rehabilitation services on functioning and disability: a prospective survey on the 12-months after discharge from specialist rehabilitation for acquired brain injury.Crossref | GoogleScholarGoogle Scholar |
[14] Legg M, Foster M, Parekh S, et al. Trajectories of Rehabilitation across Complex Environments (TRaCE): design and baseline characteristics for a prospective cohort study on spinal cord injury and acquired brain injury. BMC Health Serv Res 2019; 19 700
| Trajectories of Rehabilitation across Complex Environments (TRaCE): design and baseline characteristics for a prospective cohort study on spinal cord injury and acquired brain injury.Crossref | GoogleScholarGoogle Scholar |
[15] Australian Government. MBS Online: Medicare Benefits Schedule. Department of Health. 2021. Available at http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Downloads-211101 [accessed 14 April 2022].
[16] Metro South Health. About us. Queensland Government. 2022. Available at https://metrosouth.health.qld.gov.au/about-us [accessed 14 April 2022].
[17] Ponsford J, Janzen S, McIntyre A, et al. INCOG recommendations for management of cognition following traumatic brain injury, part I: posttraumatic amnesia/delirium. J Head Trauma Rehabil 2014; 29 307–320.
| INCOG recommendations for management of cognition following traumatic brain injury, part I: posttraumatic amnesia/delirium.Crossref | GoogleScholarGoogle Scholar |
[18] American Spinal Injury Association. International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI). 2022. Available at https://asia-spinalinjury.org/wp-content/uploads/2019/04/ASIA-ISCOS-IntlWorksheet_2019.pdf [accessed 21 September 2022].
[19] Australian Government. Medicare Safety Nets. 2022. Available at https://www.servicesaustralia.gov.au/medicare-safety-nets [accessed 21 September 2022].
[20] R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2022. Available at https://www.R-project.org/
[21] van Erp S, Oberski DL, Mulder J. Shrinkage priors for Bayesian penalized regression. J Math Psychol 2019; 89 31–50.
| Shrinkage priors for Bayesian penalized regression.Crossref | GoogleScholarGoogle Scholar |
[22] Bürkner PC. brms: An R Package for Bayesian Multilevel Models Using Stan. J Stat Softw 2017; 80 1–28.
| brms: An R Package for Bayesian Multilevel Models Using Stan.Crossref | GoogleScholarGoogle Scholar |
[23] Rodriguez-Acevedo AJ, Chan RJ, Olsen CM, et al. Out-of-pocket medical expenses compared across five years for patients with one of five common cancers in Australia. BMC Cancer 2021; 21 1055
| Out-of-pocket medical expenses compared across five years for patients with one of five common cancers in Australia.Crossref | GoogleScholarGoogle Scholar |
[24] Laursen B, Helweg-Larsen K. Health service use in adults 20–64 years with traumatic brain injury, spinal cord injury or pelvic fracture. A cohort study with 9-year follow-up. BMJ Open 2012; 2 e001521
| Health service use in adults 20–64 years with traumatic brain injury, spinal cord injury or pelvic fracture. A cohort study with 9-year follow-up.Crossref | GoogleScholarGoogle Scholar |
[25] Byles JE, Dolja-Gore X, Loxton DJ, et al. Women’s uptake of Medicare Benefits Schedule mental health items for general practitioners, psychologists and other allied mental health professionals. Med J Aust 2011; 194 175–179.
| Women’s uptake of Medicare Benefits Schedule mental health items for general practitioners, psychologists and other allied mental health professionals.Crossref | GoogleScholarGoogle Scholar |
[26] Snoswell CL, Arnautovska U, Haydon HM, et al. Increase in telemental health services on the Medicare Benefits Schedule after the start of the coronavirus pandemic: data from 2019 to 2021. Aust Health Rev 2022; 46 544–549.
| Increase in telemental health services on the Medicare Benefits Schedule after the start of the coronavirus pandemic: data from 2019 to 2021.Crossref | GoogleScholarGoogle Scholar |
[27] Mitchell RJ, Cameron CM, McClure R. Patterns of health care use of injured adults: a population-based matched cohort study. Injury 2017; 48 1393–1399.
| Patterns of health care use of injured adults: a population-based matched cohort study.Crossref | GoogleScholarGoogle Scholar |
[28] Foster M, Hummell E, Fisher K, et al. Organisations adapting to dual aspirations of individualisation and collaboration in the National Disability Insurance Scheme (NDIS) market. Aust J Publ Admin 2022; 81 127–144.
| Organisations adapting to dual aspirations of individualisation and collaboration in the National Disability Insurance Scheme (NDIS) market.Crossref | GoogleScholarGoogle Scholar |
[29] National Disability Insurance Scheme. Allied health providers. 2022. Available at https://www.ndis.gov.au/providers/working-provider/allied-health-providers#role [accessed 21 September 2022].
[30] National Injury Insurance Scheme Queensland. About the scheme. 2022. Available at https://niis.qld.gov.au/about-the-scheme/ [accessed 21 September 2022].
[31] Australian Bureau of Statistics. Patient Experiences in Australia: Summary of Findings. 2022. Available at https://www.abs.gov.au/statistics/health/health-services/patient-experiences-australia-summary-findings/latest-release [accessed 14 April 2022].
[32] Borg SJ, Geraghty T, Arora M, et al. Employment outcomes following spinal cord injury: a population-based cross-sectional study in Australia. Spinal Cord 2021; 59 1120–1131.
| Employment outcomes following spinal cord injury: a population-based cross-sectional study in Australia.Crossref | GoogleScholarGoogle Scholar |
[33] Fair Work Commission. National Minimum Wage Order 2018. 2018. Available at https://www.fwc.gov.au/documents/awardsandorders/html/pr606629.htm [accessed 21 September 2022].
[34] Kavanagh AM, Krnjacki L, Aitken Z, et al. Intersections between disability, type of impairment, gender and socio-economic disadvantage in a nationally representative sample of 33,101 working-aged Australians. Disabil Health J 2015; 8 191–199.
| Intersections between disability, type of impairment, gender and socio-economic disadvantage in a nationally representative sample of 33,101 working-aged Australians.Crossref | GoogleScholarGoogle Scholar |
[35] Gupta S, McColl MA, Smith K, et al. Prescription medication cost, insurance coverage, and cost-related nonadherence among people with spinal cord injury in Canada. Spinal Cord 2020; 58 587–595.
| Prescription medication cost, insurance coverage, and cost-related nonadherence among people with spinal cord injury in Canada.Crossref | GoogleScholarGoogle Scholar |
[36] Duckett S, Stobart A, Lin L. Not so universal: How to reduce out-of-pocket healthcare payments. 2022. Available at https://grattan.edu.au/report/not-so-universal-how-to-reduce-out-of-pocket-healthcare-payments/ [accessed 14 April 2022].
[37] Borg DN, Foster MM, Legg M, et al. The Effect of Health Service Use, Unmet Need, and Service Obstacles on Quality of Life and Psychological Well-Being in the First Year After Discharge From Spinal Cord Injury Rehabilitation. Arch Phys Med Rehabil 2020; 101 1162–1169.
| The Effect of Health Service Use, Unmet Need, and Service Obstacles on Quality of Life and Psychological Well-Being in the First Year After Discharge From Spinal Cord Injury Rehabilitation.Crossref | GoogleScholarGoogle Scholar |
[38] Widerström-Noga E. Neuropathic Pain and Spinal Cord Injury: Phenotypes and Pharmacological Management. Drugs 2017; 77 967–984.
| Neuropathic Pain and Spinal Cord Injury: Phenotypes and Pharmacological Management.Crossref | GoogleScholarGoogle Scholar |