Attributes and behaviours of crude oils that naturally inhibit hydrate plug formation
Zachary Aman A , William G.T. Syddall B , Paul Pickering B , Michael Johns A and Eric F. May AA Fluid Science and Resources Division, The University of Western Australia.
B Woodside Energy.
The APPEA Journal 55(2) 416-416 https://doi.org/10.1071/AJ14051
Published: 2015
Abstract
The severe operating pressures and distances of deepwater tiebacks increase the risk of hydrate blockage during transient operations such as shut-in and restart. In many cases, complete hydrate avoidance through chemical management may be cost prohibitive, particularly late in a field’s life.
For a unique subclass of crude oils, however that have not been observed to form a hydrate blockage during restart, active hydrate prevention may be unnecessary.
In the past 20 years, limited information has been reported about the chemical or physical mechanisms that enable this particular non-plugging behaviour. This extended abstract demonstrates a systematic method of characterising this oil, including:
physical property analysis that includes and builds upon ASTM standards;
water-in-oil emulsion behaviour; and,
the effect of oil on hydrate blockage formation mechanics.
This last set of experiments uses a sapphire autoclave to allow direct observation of hydrate aggregation and deposition, combined with resistance-to-flow measurements. The effect of shut-ins and restarts on the oil’s plugging tendency is also studied in these experiments. The method was tested with several Australian crude oils, some of which exhibited non-plugging behaviour.
In general, these particular crude oils do not form stable water-in-oil emulsions but do form stable non-agglomerating hydrate-in-oil dispersions. The oils suppress hydrate formation rates and their resistance-to-flow does not increase significantly when the amount of hydrate present would normally form a plug.
Introduction
Gas hydrates are ice-like inclusion compounds where a molecular lattice of water surrounds light hydrocarbon species such as methane or ethane (Sloan and Koh, 2007).
Hydrates are typically stable at high pressure and low temperature conditions, which are readily achieved in subsea oil and gas pipelines. After a variable induction time inside the hydrate stability zone, solid nucleation will commonly proceed at the water-hydrocarbon interface (Walsh et al, 2009) where the guest species is readily available (Barnes et al, 2014).
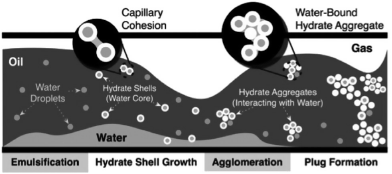
In severe cases, hydrate formation may completely plug the flowline, resulting in costly remediation efforts (Creek et al, 2011). Turner et al (2009), in collaboration with J. Abrahamson, proposed a four-step mechanism to describe hydrate plug formation in oil-continuous systems (Fig. 1):
emulsification of liquid water in the continuous oil phase, which was discussed by Boxall et al (2012);
hydrate shell growth around the water droplet (Taylor et al, 2007);
particle aggregation through capillary liquid bridges (Aman et al, 2011) and deposition on the pipeline wall (Grasso et al, 2014); and,
aggregate jamming, which increases viscous forces and, for a fixed pressure drop, reduces or eliminates flow (Lafond et al, 2013).
|
Previous studies by Sjoblom et al (2010) reported new experimental studies on hydrate non-plugging oils, where moderate hydrate volume fractions would not result in pipeline blockage. Qualitative observations from the field labelled these oils as non-plugging, and Sjoblom et al (2010) proposed that the functional explanation for their behaviour was related to the adsorption of natural organic surfactants on the hydrate particle surface; however, natural surfactants that reduce hydrate aggregation potential may not be required to prevent hydrate plug formation in cases where either the continuous phase viscosity or shear rate are appreciably high.
In this study, a comprehensive laboratory-based characterisation of hydrate plug formation has been deployed to qualify non-plugging behaviour in an Australian crude oil.
The characterisation of non-plugging oils provides crucial information to support a pivotal business decision in deepwater oil and gas investments where the traditional engineering approach is to prevent the system from ever cooling inside the hydrate stability zone. Insulating or burying the pipeline and depressurising the line on shut-in may prevent hydrate formation for tiebacks in moderate water depths; however, adding insulation may significantly increase the cost of the flowline.
For deepwater tiebacks where an insulate-and-blowdown strategy is not viable, the injection of thermodynamic hydrate inhibitors is required; these anti-freeze chemicals may cost around US$80 per barrel of water produced. Sloan et al (2011) estimated that the capital expenditure associated with complete hydrate prevention can be around US$500 million for a typical deepwater development. With some exceptions, hydrate non-plugging oils may reduce or even eliminate this required investment and significantly improve the production profitability.
Laboratory characterisation methods
Laboratory experiments were performed at the University of Western Australia under an adaptation of the test procedures described by Sjoblom et al (2010). These are the results for one crude oil. The dead oil density was measured to be 0.91 +/- 0.02 g/ml by volume displacement. Infinite shear viscosity was measured on a TA Instruments HR-3 hybrid rheometer using a two degree cone-and-plate geometry that was calibrated directly against standard fluids; the dead oil viscosity was 165 cP at 20°C and 639 cP at 5°C. Inherent water content in the crude oil was measured at 0.05 vol% by the ASTM 4007 standard technique, in which no solids content was detected.
The crude oil behaviour was evaluated through three types of measurement: interfacial activity, emulsion stability and hydrate dispersion stability. An abbreviated version of each procedure is shown in this extended abstract and additional details are provided in the associated references.
In all measurements, the aqueous phase was a solution of 3.5 wt% NaCl in deionised water. To avoid contamination between oil species, each piece of laboratory equipment was cleaned with 60-second sequential rinses of toluene, ethanol and acetone; the equipment was then dried in air.
Interfacial activity was evaluated for both water-oil and hydrate-oil systems respectively, using interfacial tensiometry and micromechanical force (MMF) measurements. Water-oil interfacial tension (IFT) was captured with a ThetaLite TL101 apparatus from Attension Instruments; this apparatus employs a pendant drop technique with automated droplet recognition as described by Aman et al (2013).
The water-oil IFT was measured for both the pure crude oil and for diluted mixtures of 1–10 wt% crude oil in mineral oil (Sigma-Aldrich). While the former measurement provides a basis for evaluating the hydrophilicity and effectiveness of the natural surfactants, the latter measurement provides qualitative insight into the amount of surfactants in the oil phase.
Crude oil activity at the hydrate-oil interface was evaluated by measuring the cohesive force between two cyclopentane hydrate particles before and after exposure to crude oil. A detailed explanation of this procedure is provided by Dieker et al (2009), where cyclopentane hydrate particles are attached to calibrated carbon fibre cantilevers connected to motorised micromanipulators and examined with an Olympus IX-73 inverted microscope.
As 1–5 wt% crude oil is injected in the continuous cyclopentane phase, hydrate cohesive forces decrease from the baseline value. Aman et al (2011) quantified the water-oil contribution to this cohesive force, allowing for estimation of the hydrate-oil interfacial energy when exposed to a crude oil. If the crude oil contains hydrate-specific active species, as discussed by Erstad et al (2009), the hydrate interparticle cohesive force will decrease disproportionately to the water-oil interfacial tension.
Emulsion stability was evaluated using three experimental techniques—bottle stability tests, NMR-based droplet size distributions and visual microscopy. Each emulsion was prepared according to the technique of Sjoblom et al (2010), where the crude oil was mixed in a high-speed (17,800 RPM) homogeniser for three minutes; during mixing, the aqueous phase was added dropwise to the oil phase.
Emulsions were created at 30%, 50%, 70% and 90% water-cut and were ripened for 24 hours before use to ensure metastability was achieved. In bottle stability tests, 200 ml samples were photographed periodically for 300 hours; the volume of the clarified water phase was tracked by analysing calibrated images with ImageJ (Rasband). Emulsion breakage was characterised through an increase in the clarified water content across this observation window. Droplet size distributions were captured at 20°C by placing samples of each emulsion in an ACT-Aachen 1 Telsa Halbach Array permanent magnet, operating at a 1H resonance frequency of 43 MHz.
The NMR measurement was configured with a pulsed field gradient (PFG) of 4 ms duration at an observation time of 250 ms; further information about the inversion of this data to obtain a droplet size distribution is described by Johns (2009).
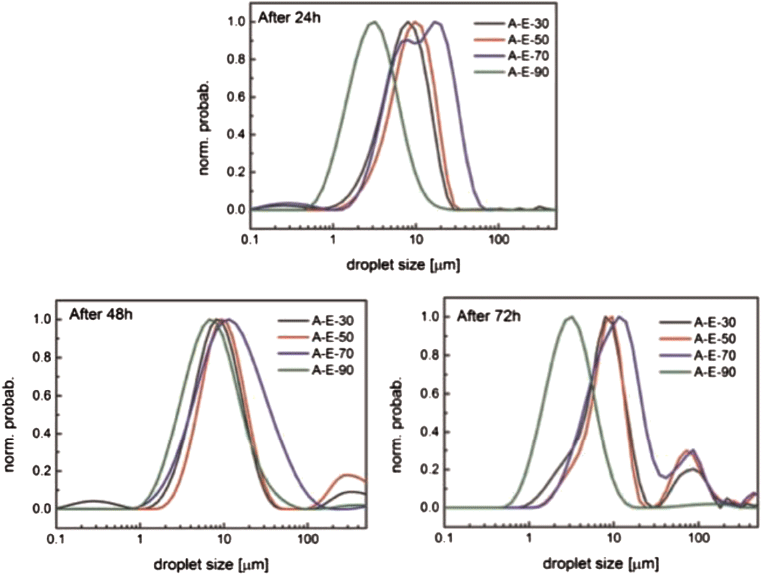
For each of the four water-cuts studied (30–90%), NMR droplet size distributions were captured after 24-, 48- and 72 hours of ripening; emulsion breakage was characterised through an increase in the mean droplet diameter and distribution range.
Finally, visual microscopy was performed on each emulsion at 20°C, using an Olympus IX-73 inverted light microscope with a 20x objective lens; the microscope was equipped with integrated recording equipment and an LED light source.
Hydrate dispersion stability was evaluated using a DB Robinson-type sapphire autoclave apparatus, presented by Aman et al (2014a). In each experiment, the 1” autoclave was filled with a pre-determined quantity of pure crude oil and the aqueous phase to achieve a total volume of 18 ml; this is the quantity required to submerge the impeller blades without creating an unmixed volume.
The autoclave was submerged in a glycol bath with temperature control, pressurised with approximately 75 bar of ultra-high purity methane, sealed and cooled from 20°C to 1°C at a rate of 1°C/hr. During the experiment, cell pressure and temperature were measured at 30-second intervals with resolutions of 0.1 bar and 0.2 C; the torque required to maintain constant mixing velocity (900–1,000 RPM) was also recorded. In stirred cell geometries, the torque evolution during hydrate formation is an analogous resistance-to-flow measurement to frictional pressure drop in a flow loop. A severe hydrate plug was characterised by an increase of at least 10.5 times in the torque value during the hydrate formation period (Skiba et al, 2011).
Results and discussion
The interfacial tension between pure crude oil and the brine solution was 29.0 mN/m (Fig. 2). Compared to the non-plugging crude oils reported by Sjoblom et al (2010), this comparatively high interfacial tension value suggests the crude oil studied does not contain strong natural surfactants.
The equilibrium interfacial tension for the paraffin oil and brine was approximately 55 mN/m; the addition of crude oil decreased this equilibrium interfacial tension by about 50%. The effect of crude oil on hydrate interparticle cohesive force was captured with the MMF apparatus discussed above.
The baseline force between hydrate particles in liquid cyclopentane phase is 4.3 +/- 0.4 mN/m without any crude oil present (Aman et al, 2012).
The cohesive force decreased by a maximum of about 50% at this higher oil fraction of 5 wt%, which is consistent with the level of surfactant activity observed in the interfacial tension measurements.
Both interfacial activity studies confirm that the crude oil under investigation does not contain a rich surfactant profile when compared to the inferential non-plugging oil criteria by Sjoblom et al (2010).
The stability of water-in-oil emulsions was first measured using an inverted light microscope after the emulsions were ripened for 48 hours. The results showed a distribution of water droplet diameters from 1–50 microns. The droplet size distribution (DSD) was monitored through low-field NMR measurements (Fig. 2), with mean droplet diameters in the range of 2–20 microns after up to 72 hours of ripening.
For the initial measurement at 24 hours, the DSDs illustrate that emulsions with higher water-cut tend to have smaller droplets. There are two reasons for this observation. First, the samples were taken from the oil phase, which precludes accounting for the large droplets that coalesce after mixing. Second, the use of a constant total kinetic energy transfer (through constant mixing rate and time) will generate smaller droplets with higher density or lower viscosity (Boxall et al, 2012).
Both reasons apply when replacing oil with water in an isochoric system. The DSDs show that, after 72 hours, the 30–70% water-cut emulsions begin breaking, resulting in the appearance of droplets with diameters above 100 microns.
|
Bottle stability tests showed that only the 30% water-cut emulsion remained fully emulsified in 30 minutes of mixing, with three-quarters of the water remaining emulsified after 300 hours.
For the 50–70% water-cut systems, which are often those at a high risk of hydrate plug formation, only 25% of the water remained emulsified initially, with 5–15% of the water phase remaining emulsified after 300 hours. The 90% water-cut system broke immediately after mixing with a small fraction (< 5%) of the water remaining in the oil phase; this result illustrates why large water droplets were not observed in the DSD results. Together, the data supports the hypothesis that this crude oil is not a strong emulsifier, with emulsions above 30% water-cut always resulting in a free water phase.
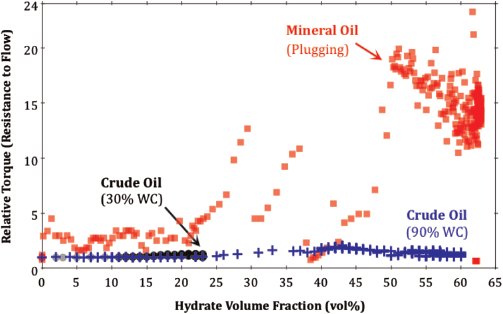
Conventionally, the inability to hold an emulsion would suggest that this crude oil is not a suitable candidate for non-plugging behaviour. To test this hypothesis clearly, the torque required to maintain constant mixing velocity was measured during methane hydrate formation in the crude oil at 30% and 90% water-cut.
The results show that the torque observed in the presence of hydrates relative to that observed prior to hydrate formation did not increase beyond 1.5, even when up to 60 vol% hydrate was present in the oil phase (Fig. 3). This result is compared to an equivalent experiment with mineral oil where the relative torque increased by a factor of 20 once 50 vol% hydrate was present in the system.
|
Fig. 3 illustrates that the crude oil under investigation is clearly non-plugging as resistance-to-flow does not increase appreciably when a large fraction of hydrate is present in the system. This result is unexpected, as the interfacial activity and emulsion stability of this crude oil are inconsistent with the criteria provided by Sjoblom et al (2010).
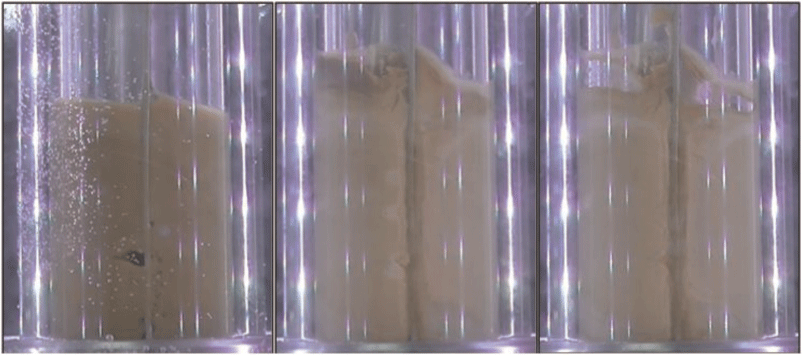
To reconcile this observation, video images captured during hydrate formation in the autoclave were analysed and compared with the corresponding torque measurements. Example images are provided in Fig. 4, and reveal a novel observation—neither a hydrate film nor discrete hydrate particles are present on the wall throughout the experiment. That is, the viscous crude oil (600 cP at 5°C) exhibits sufficient stress at the wall so as to prevent the build-up of hydrate at the wall. This observation provides one explanation for the torque data reported in Fig. 3—severe torque increases are only observed when the mixing blades interact with crystalline deposits on the wall.
In this context, the mild increases in resistance-to-flow are likely the result of solid particles suspended in the crude oil; the growth of these aggregates is contained likely because they are fractured by the same shear stresses exerted by the fluid on the wall.
|
Summary
This study presents the formulation and results of laboratory tests to characterise the severity of hydrate plugs formed in a crude oil. Based on the protocols developed in previous investigations, experimental procedures were devised to evaluate the crude oil’s interfacial activity, emulsion stability and hydrate dispersion stability.
The crude oil under investigation had a high dead-oil density and viscosity (0.91 g/ml and 165 cP at 20°C, respectively).
Water-oil and hydrate-oil interfacial activity tests confirmed independently that the crude oil was not rich in natural surfactants, with an equilibrium water-oil interfacial tension of 29.0 mN/m.
In agreement with these studies, the oil was unable to successfully stabilise a water-oil emulsion above 30% water-cut without continuous mixing. Based on the limited previous studies of non-plugging oils, these criteria would suggest the present crude oil would tend to form hydrate plugs; however, when placed in a high-pressure sapphire autoclave, this crude oil was able to sustain up to 60 vol% hydrate without a significant increase in resistance-to-flow (motor torque), which characterises it as a non-plugging oil.
The reasons for this unique behaviour were resolved by analysing experimental images of hydrate formation in the crude oil, which showed a lack of hydrate film growth or particle deposition on the autoclave wall. The authors hypothesise that the high crude oil viscosity and, consequently, shear stress under flow are sufficient to prevent hydrate particle deposition on the wall or aggregation in the continuous phase. This is the first reported observation of non-plugging behaviour in a non-emulsifying crude oil.
References
Aman, Z.M., Akhfash, M., Johns, M., and May, E. (2014a). Methane hydrate bed formation in a visual autoclave: cold restart and Reynolds Number dependence. Journal of Chemical and Engineering Data 60, 409–17.Aman, Z.M., Brown, E.P., Sloan, E.D., Sum, A.K., and Koh, C.A. (2011). Interfacial mechanisms governing cyclopentane clathrate hydrate adhesion/cohesion. Physical Chemistry Chemical Physics 13, 19,796–806.
Aman, Z.M., Joshi, S.E., Sloan, E.D., Sum, A.K., and Koh, C.A. (2012). Micromechanical cohesion force measurements to determine cyclopentane hydrate interfacial properties. Journal of Colloid and Interface Science 376, 283–8.
Aman, Z.M., Olcott, K., Pfeiffer, K., Sloan, E.D., Sum, A.K., and Koh, C.A. (2013). Surfactant adsorption and interfacial tension investigations on cyclopentane hydrate. Langmuir 29, 2,676–82.
Aman, Z.M., Pfeiffer, K., Vogt, S.J., Johns, M.L., and May, E.F. (2014b). Corrosion inhibitor interaction at hydrate–oil interfaces from differential scanning calorimetry measurements. Colloids and Surfaces A: Physicochemical and Engineering Aspects 448, 81–7.
Barnes, B.C., Knott, B.C., Beckham, G.T., WU, D.T., and Sum, A.K. (2014). Reaction coordinate of incipient methane clathrate hydrate nucleation. Journal of Physical Chemistry B 118, 13,236–43.
Boxall, J.A., KOH, C.A., Sloan, E.D., Sum, A.K., and Wu, D.T. (2012). Droplet size scaling of water-in-oil emulsions under turbulent flow. Langmuir 28, 104–10.
Creek, J.L., Subramanian, S., and Estanga, D., 2011—New Method for Managing Hydrates in Deepwater Tiebacks. Society of Petroleum Engineers Offshore Technology Conference. Houston, Texas, 2–5 May.
Dieker, L.E., Aman, Z.M., George, N.C., Sum, A.K., Sloan, E.D., and Koh, C.A. (2009). Micromechanical adhesion force measurements between hydrate particles in hydrocarbon oils and their modifications. Energy and Fuels 23, 5,966–71.
Erstad, K., Hoiland, S., Fotland, P., and Barth, T. (2009). Influence of petroleum acids on gas hydrate wettability. Energy and Fuels 23, 2,213–9.
Grasso, G.A., Lafond, P.G., Aman, Z.M., Zerpa, L.E., Sloan, E.D., Koh, C.A., and Sum, A.K., 2014—Hydrate Formation Flowloop Experiments. 8th International Conference on Gas Hydrates, Beijing, China. July 28–August 1.
Johns, M.L. (2009). NMR studies of emulsion. Current Opinion in Colloid and Interface Science 14, 178–83.
Lafond, P.G., Gilmer, M.W., Koh, C.A., Sloan, E.D., Wu, D.T., and Sum, A.K. (2013). Orifice jamming of fluid-driven granular flow. Physical Review E 87, 1–8.
Rasband, W., 2013—ImageJ, National Institute of Health. Accessed 1 February 2013. <http://imagej.nih.gov/ij/>.
Sjoblom, J., ’Afvrevoll, B., Jentoft, G., Lesaint, C., Palermo, T., Sinquin, A., Gateau, L., Barre, L., Subramanian, S., Boxall, J., Davies, S., Dieker, L., Greaves, D., Lachance, J., Rensing, P., Miller, K., Sloan, E.D., and Koh, C.A. (2010). Investigation of the hydrate plugging and non-plugging properties of oils. Journal of Dispersion Science and Technology 31, 1,100–19.
Skiba, S., Aman, Z.M., Joshi, S., Sum, A.K., Sloan, E.D., and Koh, C.A., 2011—Measurements to Characterise the Plugability of Crude Oils. 7th International Conference on Gas Hydrates. Edinburgh, United Kingdom. 17–21 July.
Sloan, E.D., and Koh, C.A., 2007—Clathrate Hydrates of Natural Gases. Boca Raton, Florida: CRC Press, Taylor & Francis Group.
Sloan, E.D., Koh, C.A., and Sum, A.K., 2011—Natural Gas Hydrates in Flow Assurance. Houston: Gulf Professional Publishing, Elsevier Inc.
Taylor, C.J., Miller, K.T., Koh, C.A., and Sloan, E.D. (2007). Macroscopic investigation of hydrate film growth at the hydrocarbon/water interface. Chemical Engineering Science 62, 23,6524–33.
Turner, D., Miller, K., and Sloan, E. (2009). Methane hydrate formation and an inward growing shell model in water-in-oil dispersions. Chemical Engineering Science 64, 3,996–4004.
Walsh, M.R., Koh, C.A., Sloan, E.D., Sum, A.K., and Wu, D.T. (2009). Microsecond simulations of spontaneous methane hydrate nucleation and growth. Science 326, 1,095–8.

Zachary M. Aman is an associate professor of mechanical and chemical engineering at the University of Western Australia. His research focuses on the management and prevention of gas hydrate and asphaitene blockages in subsea oil and gas pipelines, as well as the transport of oil through the water column during deepwater blowout. He holds a BSc and a PhD in chemical engineering from the Colorado School of Mines, with a research focus on the interfacial phenomena of cyclopentane hydrate at the school’s Center for Hydrate Research. Member: SPE, Australian Computer Society, the American Institute of Chemical Engineers and the Institution of Chemical Engineers. |

William G. T. Syddall is a senior flow assurance engineer at Woodside Energy Ltd. He is working as a flow assurance lead for a heavy oil development at Woodside Energy Ltd. He has had an interest in the rheology of dispersions for more than 14 years which started in the design and operation of two offshore heavy oil fields in Northern China. He holds a BSc in applied chemistry and an MEng in chemical engineering from Curtin University, Perth. |

Paul F. Pickering is a principal flow assurance engineer at Woodside Energy Ltd, where he works in the design of oil and gas production systems. He has worked with multiphase flow and modelling of fluid flow systems, most notably with the foundation of FEESA Ltd and the invention of the Maximus Integrated Production Modelling software. He holds an MEng and a PhD in chemical engineering from Imperial College, London, with a research focus on transient instabilities in multiphase flows. |

Michael L. Johns is the professor of chemical and process engineering at the University of Western Australia and is the chemical engineering discipline head. His research focuses primarily on the development and application of mobile magnetic resonance (MR) techniques with a particular focus on systems relevant to the oil and gas industry. He has previously developed and commercialised an MR method to characterise emulsions and the fouling of desalination module units. He has published more than 120 peer-reviewed publications. |

Eric F. May is the Chevron Chair in gas process engineering at the University of Western Australia (UWA). He has been conducting experimental research in fluid science for more than a decade and was awarded the Malcolm McIntosh Prize for Physical Scientist of the Year as part of the 2012 Prime Minister’s Prizes for Science. He teaches undergraduate and industry courses in thermodynamics and gas processing and has helped create a new chemical engineering degree at UWA. His research group works closely with industry and is conducting projects in LNG production, flow assurance, CO2 sequestration and fluid property prediction. |


