Distribution patterns of east Australian humpback whales (Megaptera novaeangliae) in Hervey Bay, Queensland: a historical perspective
S. H. Stack A B , J. J. Currie A , J. A. McCordic A , A. F. Machernis A and G. L. Olson AA Pacific Whale Foundation, 300 Ma’alaea Road, Suite 211, Wailuku, Maui, HI 96793, USA.
B Corresponding author. Email: research@pacificwhale.org
Australian Mammalogy 42(1) 16-27 https://doi.org/10.1071/AM18029
Submitted: 24 April 2018 Accepted: 1 March 2019 Published: 24 April 2019
Journal Compilation © Australian Mammal Society 2020 Open Access CC BY-NC-ND
Abstract
Tourism activities are expanding in both terrestrial and marine environments, which can have detrimental effects on the target species. Balancing the amount of disturbance a population of animals receives against the educational value of tourism requires localised research and adaptive management. This study examined the distribution of humpback whales within Hervey Bay, Queensland, using data spanning 2004–16, just before the implementation of a commercial ‘swim-with-whales’ program. Spatial and temporal patterns of humpback whale calves were of particular interest given that they are more vulnerable to human-related disturbances than other group types. We found that humpback whales displayed a distinct spatial segregation in Hervey Bay based on pod composition. Most whales displayed a residency time of two to three days, with females having a somewhat shorter residency time than males. These findings suggest that humpback whales in Hervey Bay not only display temporal segregation dependent on maturation and reproductive status, but fine-scale spatial distribution based on pod composition. Understanding habitat preference and patterns of habitat use of humpback whales in Hervey Bay is critical for effective management of the newly sanctioned swim-with-whale tourism in Hervey Bay and the sustained recovery of humpback whales in this region.
Additional keywords: Balaenopteridae, habitat preference, population management
Introduction
As top predators of the marine environment, whales play a vital role in sustaining a healthy ecosystem by regulating the food flow of the ocean. Commercial whaling in the 20th century removed millions of large whales from the Southern Hemisphere (Clapham and Baker 2008), resulting in large consequences for the ecosystem below them, and the many species that live there. Both the east and west coast Australian humpback whale (Megaptera novaeangliae) populations were decimated by an estimated 95% from their pre-exploitation populations of ~27 000 individuals (DEH 2005). After the international moratorium on commercial whaling came into effect in 1985, the two Australian populations have experienced what is thought to be one of the highest rates of population increase in the world, at a rate of 10.9–11% (Noad et al. 2011a). In 1999, the east coast population was estimated to be 3160–4040 (DEH 2005) and in 2010, the absolute abundance estimate for the population was ~14 522 whales (Noad et al. 2011b). While the population has experienced tremendous recovery since the time of exploitation, threats such as vessel disturbance and collisions remain a concern with the potential for cumulative impacts on individuals.
Humpback whales in the eastern Australia Breeding Stock E-1 population migrate annually between subtropical breeding grounds along the north-east coast of Australia and high-latitude feeding areas in the Antarctic (e.g. Franklin et al. 2012; Constantine et al. 2014). During their southern migration from late-July to November, an estimated 30–50% (Bryden et al. 1989; Chaloupka et al. 1999) of humpback whales detour from their main migration route and travel into Hervey Bay (e.g. Paterson 1991; DEH 2005). Hervey Bay is a wide, horseshoe-shaped embayment bounded by the Queensland coast to the west and south and by Fraser Island to the east. Fraser Island provides protection from the prevailing winds, and water depth varies very little, with an average depth of ~18 m (Vang 2002), making Hervey Bay an ideal stopover site for whales to rest. Their average residency time in the bay is 1–3 days before continuing their migration towards Antarctica (Corkeron et al. 1994; Franklin 2012); however, extended stays of up to 22 days for females and 51 days for males have been recorded for some individuals (Franklin 2012). In particular, Platypus Bay, located along the north-western shores of Fraser Island, is where most whales aggregate during this stopover period (Kaufman et al. 1987; Forestell et al. 2003).
Humpback whales display distinct temporal segregation into and out of Hervey Bay based on age, sex, and reproductive status (Dawbin 1997; Franklin et al. 2011). Data indicate that Hervey Bay is utilised by immature males and females early in the season, followed by mature adults in the middle of the season, and lastly by mother–calf pairs (Franklin et al. 2011). Competition pods have also been observed in Hervey Bay, indicating that this region may provide mature males one last opportunity for breeding with females of various age classes before returning to Antarctica (Bryden et al. 1989; Corkeron 1995). For mothers with young calves, Hervey Bay is an ideal stopover site to rest, conserve energy, and provide food and protection for their calves before the calves’ first migration to their southern feeding ground (Franklin et al. 2011). While Hervey Bay is not a designated calving or breeding ground for humpback whales, it appears that it is utilised by multiple pod types for a variety of purposes (Corkeron 1995). This multifaceted use of the bay is logical considering the various age classes and reproductive stages of animals that utilise it during their migration.
As a result of Hervey Bay’s dynamic use by humpback whales and close proximity to sheltered waters from land, it has become a very reliable and highly sought-after whale-watching destination. The whale-watching industry experienced substantial growth in the late 1980s and early 1990s (Stoeckl et al. 2005; O’Connor et al. 2009), but factors, such as competition with areas that are more conveniently located near larger populations, have caused the industry in Hervey Bay to decline (Peake 2011). In an effort to jumpstart the regional economy, in 2014, the Queensland government initiated a trial ‘immersive whale watching’ program, which allows passengers to enter the water with the whales (Anon. 2014), the first of its kind for humpback whales in Australia. After an incident-free three-year trial, in 2017, the Australian Government permitted the ‘immersive whale watching’ program to become a permanent fixture in Hervey Bay, which was also supported by the Department of National Parks (Queensland Department of the Premier and Cabinet 2017).
The ‘swim-with’ industry has become an emerging form of tourism that is at the forefront of scientific evaluation to assess the impacts of interactions on marine mammal populations. Most swim-with activities occur with odontocete species, the responses of which are most frequently documented, but have also been known to occur with pinniped and mysticete species (Machernis et al. 2018). In a review specifically looking at swim-with-whales tourism, Rose et al. (2005) found that most programs are with humpback and minke whales in the Dominican Republic, Tonga, and the Great Barrier Reef. Swim-with interactions have also been documented with southern right whales (Lundquist 2007; Lundquist et al. 2008). In general, the literature suggests that animals’ behavioural responses to swim-with activities vary widely across species and locations and may be dependent on certain aspects of swimmers’ presence (Machernis et al. 2018). This consensus highlights the need to focus research efforts in areas where swim-with-whale tourism programs are emerging in order to evaluate its impact on regional whale populations.
A better understanding of how humpback whales utilise Hervey Bay is essential as the swim-with-whale tourism industry grows in this region. Mother-and-calf pairs are of particular importance given their use of this habitat to provide maternal care, and disturbance may result in energetic consequences that could cause population-level impacts. The current study examines the distribution of calf and non-calf pods of humpback whales in Hervey Bay, Australia, to determine whether there are differences in where these pod types occur. Data were collected from various whale-watching vessels over a period of 12 years, before the implementation of ‘swim-with-whale’ activities in this region, providing baseline information to humpback whale distribution patterns. This information will be crucial in helping to guide a precautionary management approach in this region that will reduce the level of disturbance to whales from an expanding ecotourism industry.
Methods
Study area
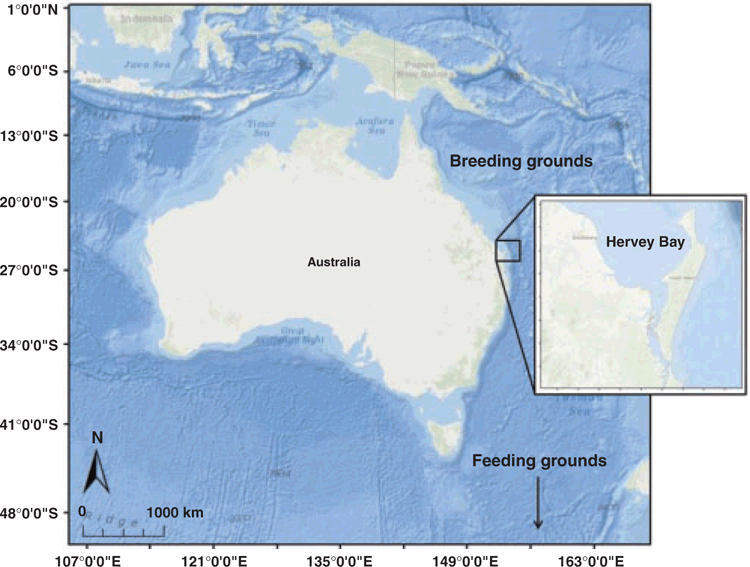
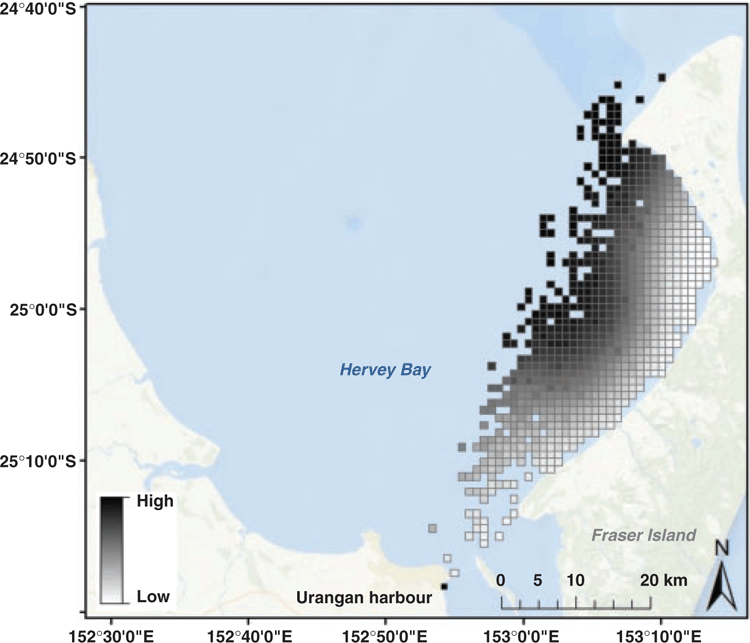
Hervey Bay is located at 25°00′S, 152°52′E on the east coast of Queensland, Australia (Fig. 1). It is a wide, shallow bay with an area of ~4000 km2, consisting of a sand and mud bottom, located ~175 nautical miles north of the Gold Coast. Most of the bay is 18 m deep, with depths increasing northward to more than 40 m, where the bay connects to the open ocean via an ~60-km-wide gap (Vang 2002). The bay is bounded by the Queensland coast to the west and south and by Fraser Island (126 km long) along a north-easterly axis. The bay is open to the South Pacific Ocean in the north, while the Great Sandy Strait enters the bay from the south (Corkeron 1995). At the northern tip of Fraser Island, the Great Sandy Spit separates the bay from the open ocean an additional 30 km north. This study was conducted within the Great Sandy Marine Park, an area covering ~6000 km2 of tidal land and waters, including Hervey Bay (Queensland Department of Environment and Science 2018). During the study period, a small fleet of 10–14 vessels conducted whale-watching operations annually within Hervey Bay. However, it should be noted that data on the number of annual whale-watching vessels were not available for the entire study period and the presented ranges may vary slightly.
Definitions
The following terms are defined here for clarification:
A pod was defined as either a lone (singleton) or a group of humpback whales within 100 m of each other, generally moving in the same direction, and coordinating their behaviour as well as speed of travel (Whitehead 1983; Corkeron et al. 1994). The term ‘pod’ used here does not imply stable groups.
A calf was defined as an individual whale visually estimated to be less than 50% of the length of the accompanying whale, less than one body length apart, and maintaining a constant and close relationship (e.g. Chittleborough 1965) with the adult whale, who is assumed to be the mother.
A subadult was defined as an individual whale estimated to be more than one year old and weaned from its mother, but not yet of a size consistent with achieving sexual maturity, i.e. 7–12 m long (Coughran and Gales 2010).
Calf pods were defined as pods containing one or more calves, while non-calf pods were defined as pods with no calves present. Each group could have any variation of additional whales present.
Data collection
Humpback whale sighting data were collected daily from various whale-watching vessels departing from Urangan Harbour between July and October over a 12-year period (2004–16, excluding 2013). Each whale-watching trip lasted 4 hours and followed a non-systematic search effort. Scanning for whales was completed by a researcher stationed on board and began once boats departed the harbour. Scanning was conducted with the naked eye and with binoculars looking for visual cues of humpback whale presence.
When a whale was sighted, the vessel approached the pod to a distance ≥100 m. Upon approach and subsequent observation, information on group size, composition, and behaviour were recorded. To ensure accurate group composition and size estimates, only sightings where group composition could be confirmed were included in subsequent analysis. Encounter location (latitude and longitude) was recorded using handheld GPS (Global Positioning System) when the vessel was ≤150 m from the focal pod. After the initial whale sighting, the whale-watching vessel navigated the area, randomly searching for more humpback whales. As such, the search effort was never consistently concentrated in a particular location or time. However, the nature of whale watching and the consistent track back to the harbour resulted in uneven search effort.
Postprocessing of fluke photographs followed Rankin et al. (2013), where quality and distinctiveness were graded by modified protocols for North Pacific humpback whales (Calambokidis et al. 2008). Each fluke was given a score of 1 through 5 (good to bad) for five criteria: (1) proportion of fluke visible, (2) fluke angle, (3) photographer/lateral angle, (4) focus, and (5) exposure/contrast. A cumulative score was calculated by summing the scores of the five criteria. Flukes were not considered for analysis if: (1) they had a cumulative score exceeding 14, or (2) they were scored a 4 or 5 for any single criterion, or (3) no picture was available of the fluke’s central notch.
Data preparation
To facilitate analysis, the study area was divided into 1 km × 1 km grid cells that covered 769 km2. For the entire 12-year period, each grid cell was summarised by monthly counts of: (1) total number of pods with calves present, and (2) total number of pods without calves present.
Each grid cell was characterised by the following variables: water depth, distance from shore, latitude, longitude. Water depth was expressed in metres and calculated as the mean depth within the grid cell taken from Geoscience Australia bathymetry dataset (resolution: 300 m) (Geoscience Australia 2013). Distance from shore was expressed as a positive value in kilometres and was determined using the near tool in ArcMap (ESRI 2017) to measure the distance from the centre of each grid cell to the nearest shoreline. Environmental covariates (water depth and distance from shore) were tested for pairwise correlations using the stats package in R (R-Core Team 2017). To account for non-normality in site covariates, the Spearman correlation coefficient (rs) was used to assess correlations. If site covariates were highly correlated (rs ≥ 0.7), the most biologically relevant variable was retained for subsequent analysis.
Residency time
For analysis of residency time, the sighting history for each individual whale was summarised per year from 1984–2016. Each record had dates of the first and last observations within each year that the individual was sighted, the sex of the whale, and, for females, lactation status (determined by the presence or absence of a calf). Whales that were sighted two or more times within a season were included in this analysis. Residency time in days was calculated by subtracting the last sighting date from the first sighting date for each individual recorded within each year.
Data analysis
Whale watches utilised a non-systematic search effort that did not incorporate equal coverage of the sampling area and employed a ‘search-and-find’ type of effort. Higby et al. (2012) completed a detailed analysis on the use of presence-only data collected from whale-watching vessels to determine humpback whale distribution. Despite some limitations, which are discussed below, Higby et al. (2012) found that this type of data had some advantages and its use in analysis of distribution was warranted.
Given the absence of vessel GPS tracks, subsequent analysis is based on presence-only data. This, in addition to addressing some issues of lack of vessel GPS tracks, also limits multiple zero counts in our dataset, reducing modelling bias and the need to account for zero-inflated models (Ridout and Demetrio 1992).
To ensure that no inferences are made on humpback whale distribution in areas where vessels did not travel, the final model predictions were made using only grid cells that had whale sightings throughout the 12-year study period. This significantly minimises the potential bias of predicting relative distribution in areas where the vessels did not travel. However, it is important to note that inferences on distribution are limited to the travelled region, which is appropriate for this study as sufficient coverage was achieved by pooling 12 years of data.
To ensure accuracy in data collection only trained researchers, independent of whale-watching vessel crew, were utilised to minimise: (1) the likelihood of misidentification of species, (2) inaccurate recording of sighting location, and (3) the misclassification of pod-composition (calf-pod and non-calf pod). To account for potential biases in detectability only trips where the Beaufort Sea State was ≤6 (Calambokidis and Barlow 2004; Andriolo et al. 2010) were included in subsequent analysis. As both the ‘calf’ and ‘non-calf’ groups contained adults, the differences in detectability between groups is thought to be minimal and any behavioural differences between pod type as it relates to detectability was not considered in this analysis.
The use of whale-watching vessels also presents the potential effort bias of seeking out pods encountered on previous trips. In part, stratification of the study area into grid cells helps address the issue of targeting high-use areas (Leaper et al. 1997; Macleod et al. 2004), with the 1-km2 grid cell division still allowing for investigation of fine-scale influences of environmental parameters on humpback whale distribution. However, it is important to note that the nature of whale watching in Hervey Bay limits the potential of relocating previously observed pods. It takes ~1 h to commute from the harbour to the primary whale-watching area and another hour to commute from the whale-watching area back to the harbour. These two hours, plus the additional hour between trips allows a whale at least three hours to move from its previous location before there is potential to resight that whale on a subsequent trip. Analysis of photo-ID data collected during this study from 2004–16 found that only 4.1% of whales that were photographically identified were seen twice on the same day over the study period.
Although we cannot completely discount the potential of resighting the same pod between trips, there would be limited impacts of this on modelling the relative distribution of humpback whales. The use of opportunistic data is thought to have little effect on assessing the spatial segregation of calf and non-calf pods, as these biases are demonstrated to have more significant effects on abundance estimates (Williams et al. 2006). These effort biases can be overcome in spatial analysis with sufficient coverage of environmental covariates within the survey area. Finally, the biases in data collection outlined for this study are experienced equally across calf and non-calf pods throughout the study period and are not thought to act independently on differing pod compositions. Therefore the implications of comparing relative distribution between calf and non-calf pods using presence-only data collected from whale-watching platforms is thought to be minimal.
To determine whether mother–calf pod distributions were influenced by environmental variables within the study area, a series of Generalised Additive Mixed Models (GAMM) were constructed, using the gamm4 package in R (Wood and Scheipl 2017). Depth, distance from shore, and location (latitude and longitude) were considered as potential explanatory variables for two response variables: (1) count of pods with calves, and (2) count of pods without calves. A Poisson error distribution with log-link function was utilised, with year as a random effect to account for temporal variation. The following model form was used:
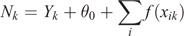
where f(xi) represents the smooth functions for each i explanatory variables, θ0 is an intercept term, and Nk is the expected count in a particular spatial grid cell k. The variable Yk represents the random variable for sample year.
Model selection and prediction
Model selection was based on the Akaike Information Criterion (AIC) to select the model with the lowest AIC value and highest explanatory power. Preliminary single-variable models were run to determine which variables to include in the full model. A reduced model was then constructed using all variables that showed significant effects in the full model. To ensure appropriate improvement in explanatory power, terms were retained only when the reduction in AIC value was greater than the number of terms added. The best reduced models, based on AIC, were compared with a null model containing only the random effect for year using a likelihood ratio test (Polansky and Robbins 2013). Only models that were significantly different (P ≤ 0.05) from the null model were selected as the final model. The final models were then used to predict counts of humpback whale pods with calves and pods without calves for each grid cell within the study area.
Results
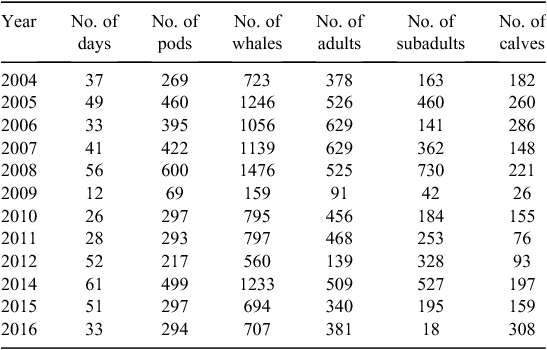
From 16 August 2004 to 17 October 2016, 479 days were spent on the water and 10 585 whales in 4112 pods were documented. It is important to note that these numbers do not reflect individual whales but rather a count of the humpback whales encountered on each day. Of these whales, 47.9% (n = 5071) were adults, 19.9% (n = 2111) were calves, and 32.2% (n = 3403) were subadults (Table 1). Adults accounted for the highest proportion of whales sighted, except in 2008, 2012, and 2014, when subadults made up the larger proportion (Table 1).
|
Residency in Hervey Bay
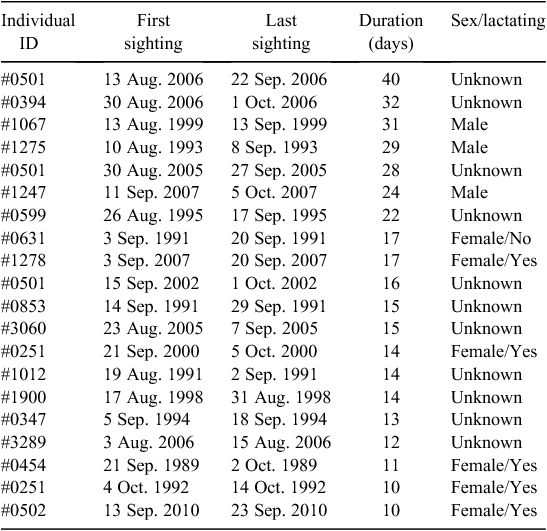
Throughout the study period, 748 whales were seen in Hervey Bay on two or more dates within the same season. Most of these individuals were observed for two (n = 276) or three (n = 112) days. Of the resighted whales, there were 20 cases of extended residency that spanned 10 or more days during this study period. The longest duration of stay was 40 days. The sightings, including the first and last date sighted, span of residency, and sex, if known, are summarised in Table 2.
|
Habitat modelling
Model analysis procedures found latitude, longitude and depth to be significant variables for modelling spatial distribution of both calf and non-calf pods in Hervey Bay (Tables S1 and S2, available as Supplementary Material to this paper). Including distance from shore and depth variables did not improve model fit, with top models for predicting both calf and non-calf pod counts including location (latitude and longitude) and month only (Tables S1 and S2).
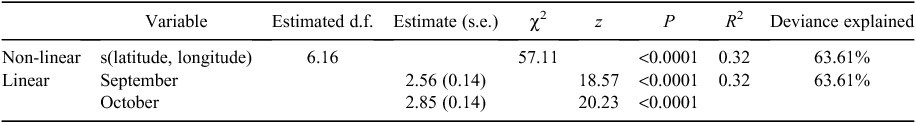
The best-fit model for count of calf sightings per grid cell included a smoothed interaction term between latitude and longitude and month (expressed as a factor), both found to be highly significant (Table 3). This model was significantly different from that of the null model containing only a random effect for year (Likelihood ratio test statistic = 1009.66, P < 0.0001) and explained 63% of the deviance (Table 3).
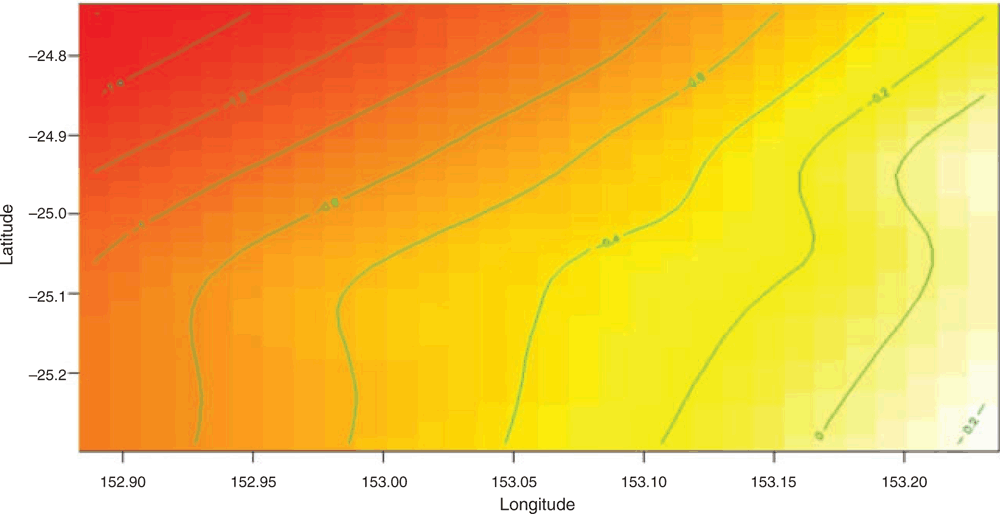
The model identified a non-linear trend in the relationship of location (latitude and longitude) to calf pod counts (Fig. 2). Calf pods were found in the greatest density in mid-longitude areas within Hervey Bay, between 153.08 and 153.20°E (Fig. 2).
The best-fit model for count of non-calf sightings per grid cell included a smoothed interaction term between latitude and longitude and month (expressed as a factor), with month found to be the most significant term (Table 4). This model was significantly different from that of the null model, containing only a random effect for year (Likelihood ratio test statistic = 220.28, P < 0.0001) and explained 32% of the deviance (Table 4).
The model identified a non-linear trend in the relationship between location (latitude and longitude) and non-calf pod counts (Fig. 3). When compared with calf pods, non-calf pods were located in a larger range of longitude within Hervey Bay, focussed around similar latitudes ranging from 25.2 to 24.8°S (Fig. 3).
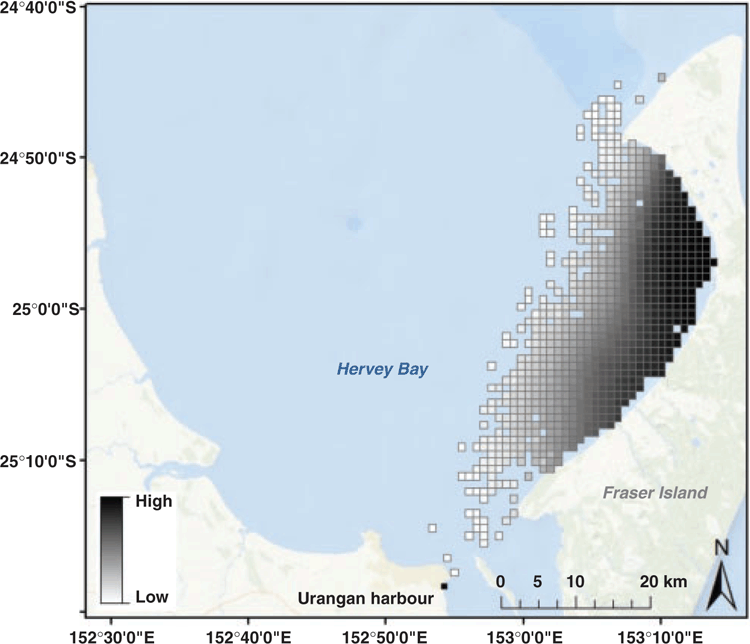
Model prediction showed that the highest counts of calf pods occurred in the waters near Fraser Island, with the highest predicted density occurring within the northern portion of the bay and alongside Fraser Island (Fig. 4). The model also showed that non-calf pod counts were low along the coastal waters of Fraser Island, with highest concentrations centred in the central and northern areas of the bay ~10–15 km off Fraser Island (Fig. 5). There was minimal overlap between areas of pods with calves and pods without calves, with the predicted distribution revealing a clear spatial segregation between these pod types.
Discussion
Distribution
Our results indicate that pods with and without a calf present were segregated and utilise different areas of Hervey Bay. Pods with a calf were predicted to occur in the northern portion of the Bay alongside Fraser Island, whereas pods with no calf were predicted to occur ~10–15 km off Fraser Island, in the central and northern areas of Hervey Bay. The distribution of each pod type was predicted by the same environmental variable (i.e. location), but each displayed a different relationship between the interactive terms. Calf pod distribution was predicted within both a narrow longitude and latitude range, whereas non-calf pod distribution was predicted within a wider latitude range, but more narrow longitude range. Water depth and distance to shore were not significant environmental predictors of distribution for either calf or non-calf humpback whale pods in our study. These results differ from those of other studies that have found that these two environmental variables influence the distribution of whale pods (e.g. Smultea 1994; Ersts and Rosenbaum 2003). Our differing results are likely explained by the geography and bathymetry of Hervey Bay. In terms of water depth as an environmental predictor, the average depth in Hervey Bay is 18 m (Vang 2002), with maximum depths of ~22 m and minimum depths of ~5 m along the coastline (Suzacq 2007). The bathymetry of the region gradually deepens into the centre of the bay by only a few metres, such that there are no stark differences in water depth that are likely to influence humpback whale distribution. In other geographic regions where there are steeper bathymetry gradients, calf pods are observed close to shore in shallow water, and non-calf pods are observed further offshore in deeper waters, possibly to facilitate breeding behaviour (Smultea 1994). Given the minimal changes in water depth within the study area, it is reasonable that depth is not a significant environmental predictor of whale distribution. However, given the predictive value of depth in other geographic regions for humpback whales, it was an important parameter to test for in Hervey Bay. Similarly, in terms of distance to shore, Hervey Bay is a semi-enclosed circle; there are no defined onshore or offshore areas. In our study, the predicted distribution of non-calf pods fell along a north-easterly axis, very close to shore in the north-eastern part of the bay close to Fraser Island, and further offshore (10–15 km) in the central part of the bay. In Hervey Bay, distance from shore is not correlated with deeper waters, such that the observed distribution may not be driven by either distance from shore or water depth, but rather a complex interaction between behavioural and biological requirements.
While segregation by water depth and distance to shore was not evident in our study, the spatial distribution of calf pods in Hervey Bay is consistent with previous findings indicating that the shallow, sheltered waters of Platypus Bay provide an important habitat for mother–calf pairs (Franklin et al. 2011). Similar habitat preferences have also been observed for mother–calf pairs on their breeding grounds in the Dominican Republic (Mattila et al. 1994), Madagascar (Ersts and Rosenbaum 2003), Brazil (Morete et al. 2007), Ecuador (e.g. Félix and Botero-Acosta 2011), Peru (Guidino et al. 2014), Central America (Rasmussen et al. 2007), and Hawaii (e.g. Smultea 1994; Craig et al. 2014). In general, mother–calf pairs tend to favour waters less than 50 m deep (e.g. Mattila et al. 1994; Oviedo and Solís 2008), and are commonly found in depths of less than 20 m (Ersts and Rosenbaum 2003; Félix and Botero-Acosta 2011); however, this is site-specific and dependent on the topography of the breeding ground. For example, in areas with steep shoreline gradients, such as Madagascar and Hawaii, mother–calf pairs are more common in shallower waters (Ersts and Rosenbaum 2003) with gradients observed at 100 m or less (Currie et al. 2018). In areas where shallow waters extend offshore, mother–calf distribution is more appropriately described as their distance from shore. In Brazil, females and their dependent calves displayed a preference for areas up to 10 km from shore (Félix and Botero-Acosta 2011). In Hervey Bay, our study supports these findings, with predicted calf pod distribution along the sheltered coast of Fraser Island. Although the trend for mother–calf pairs to prefer shallow, protective water on breeding grounds was also observed in Hervey Bay, it is important to note the unique geography of the bay and the use of the area as a migratory resting site, not a breeding ground. Since this study found that depth and distance to shore were not significant predictors of habitat preferences, the unique components of this specific region provide an opportunity to look at other factors that influence mother–calf distribution.
Several hypotheses have been suggested to explain habitat preference of mother–calf pods on their winter breeding grounds, including: (1) decreased predation pressure (Chittleborough 1953; Smultea 1994; Corkeron and Connor 1999), (2) reduced exposure to rough sea conditions (Whitehead and Moore 1982; Elwen and Best 2004; Félix and Botero-Acosta 2011), (3) conservation of energy (Whitehead and Moore 1982; Elwen and Best 2004), and/or (4) facilitation of social stratification, thereby reducing harassment and risk of injury to calves from sexually active males (e.g. Whitehead and Moore 1982; Smultea 1994; Craig et al. 2002, 2014; Ersts and Rosenbaum 2003). It is likely a combination of all four of these explanations that describe the observed habitat distribution of mother–calf pairs in Hervey Bay. However, since Hervey Bay is a migratory stopover, not a calving site, it is used by humpback whale females with older calves. Cartwright and Sullivan (2009) showed that older calves, like the ones seen in Hervey Bay, spend more time at rest or circling at the surface of the ocean. In Hervey Bay, energy conservation is likely a key driver in habitat selection, given that whales may be more vulnerable to increases in energy use during periods of resting (Braithwaite et al. 2015). Given the unique usage of this area as a migratory stopover, key drivers of habitat distribution may be more fine-scaled compared with larger breeding grounds.
In the broader sense, differential migratory timing appears to be one tactic that female humpback whales with a calf utilise to reduce energetic costs associated with male harassment (Craig et al. 2003). By arriving as one of the last groups to Hervey Bay, mother–calf pairs’ arrival coincides with the approximate departure time of mature males (Craig et al. 2003). Although mature males exhibit a preference for females without a calf, in order to maximise their reproductive success, they may also seek mating opportunities with maternal females (Craig et al. 2002). In Hervey Bay, mother–calf pairs presumably tuck closely along the west coast of Fraser Island to reduce their interaction with mature males and to minimise energy expenditure. This coastal protection affords mothers the opportunity to spend a large amount of time nursing their calves and reduce the increased energetic costs associated with being escorted by males (Craig et al. 2014). Conversely, our modelled prediction of non-calf pods supports the notion that females with the highest reproductive potential (i.e. non-lactating adult females) are found in a different location than lactating females, thus driving mature males towards the north-easterly axis of the bay to maximise reproductive potential (Craig et al. 2014). Lactating mothers are particularly vulnerable to disturbance while in a resting ground because if these whales have to use energy to avoid a source of disturbance, that action diverts energy away from lactation in mothers and therefore growth in calves (Braithwaite et al. 2015). Without the energetic deficits associated with postpartum malnutrition and lactation, non-calf pods are solely constrained by a lack of food and the need to subsist on stored energy reserves (Craig et al. 2003). Thus, they are not restricted to a specific area of the bay to help facilitate energetic conservation; rather, their fine-scale habitat distribution appears to be conspecific-driven by the location of the highest mating opportunities. While whales in Hervey Bay are migrating southward to their feeding grounds, they can also be involved in activities associated with mating, i.e. competition pods (Corkeron 1995).
Residency time
Braithwaite et al. (2015) carried out a modelling study examining the energetic requirements for female humpback whales migrating with a nursing calf. Their model showed that to minimise energy expenditure during migration, lactating females need to rest for 24–35 days and travel for 55–66 days (Braithwaite et al. 2015). These numbers align well with what is observed in Hervey Bay, with females having somewhat shorter residency times than males. Human activity has the potential to impact humpback whales in this resting area, causing them to be displaced from their resting ground without achieving adequate energetic benefits or by causing lactating females to engage in behaviours that allocate energy away from nursing their calves. It is unknown whether current levels of human activity in Hervey Bay present levels of disturbance significant enough to impact this population of humpback whales. Having baseline data on the residency times of male and female humpback whales in this resting ground will be valuable as we monitor this population’s status and recovery.
Limitations of this study
These results are bound by the limitations of data collected opportunistically aboard a whale-watching vessel. While data collected on a platform of opportunity can be very useful to evaluate species density and relative abundance to determine their spatial and temporal distribution (e.g. Henrys 2005; Kiszka et al. 2007; MacLeod et al. 2008; De Boer 2013), there are inherent biases in search effort. Departing from Urangan Harbour, whale watches are likely to limit their effort to where they first sight whales, which is typically in the lower portion of Hervey Bay, and therefore would not always continue to travel into the middle of the bay or towards the northern tip of Fraser Island. This means that the southern portion of Hervey Bay was travelled more heavily than the northern or central areas. Given that the analysis conducted was to determine whether mother–calf pod distribution is influenced by environmental variables within the study area, it was appropriate to summarise monthly counts for pods with and without calves per grid cell for the entire 12-year period to ensure good spatial and temporal coverage of the area and therefore minimise search effort biases.
Management implications and recommendations
The newly sanctioned ‘immersive whale watching’ program in Hervey Bay lacks a strong scientific assessment of the sustainability of such activities on humpback whales belonging to the Southern Hemisphere Breeding Stock E. This study provides a historical baseline against which comparison can be made in the future to determine whether whales are changing their distribution and/or residency in Hervey Bay. The timing of this study is crucial as it covers the period leading up to the introduction of a new form of tourism which has the potential to alter behavioural activity and energy use in this important resting ground. Further research is needed to assess the long-term repercussions of swimming with whales as a form of tourism, and we are continuing to monitor and research this topic as a follow-up to the current study.
Given the results of this study, mother–calf pairs may be the most vulnerable to human-related disturbance in the bay compared with other pod types, due to their preferential habitat distribution along Fraser Island’s protected western coast. While regulations prohibit allowing swimmers to enter the water with a calf present, these whales are still subject to vessel traffic. Fraser Island is also a popular tourist destination with many boats transiting along the western shoreline after departing from Urangan Harbour (Fig. S1). The parts of Platypus Bay that are preferred by mother–calf pairs overlap with areas of high vessel traffic from commercial whale-watching and recreational vessels. In other areas of the world, ‘swim-with’ activities have resulted in habitat displacement (e.g. Östman-Lind et al. 2004, Danil et al. 2005), altered activity budgets, and increased area avoidance (e.g. Samuels and Bejder 2004; Lundquist et al. 2008). Given the importance of the bay for humpback whales on their southern migration and the known impacts of ‘swim-with’ activities on cetaceans, understanding current patterns of habitat use is a prerequisite for effective management of newly introduced anthropogenic activities (e.g. Félix and Botero-Acosta 2011; Cartwright et al. 2012).
Platypus Bay is part of the Great Sandy Marine Park, which is divided into management zones. Within the Park there are existing regulations that apply to both vessels and swimmers when they are near a whale or dolphin, including approach distances and speed restrictions within the caution zones; however, there are no additional special management measures currently in place. There are regulations for swimming with whales to which commercial operators must adhere that are strict compared with those in other regions of the world – most notably banning swimmers from entering the water in the presence of a calf, requiring the use of a mermaid line, and banning swimmers from entering the water when whales are within 100 m of the vessel (Fraser Coast Industry Code of Practice 2018).
Conclusions
The aim of this study was to provide baseline information on humpback whale distribution in Hervey Bay in order to effectively evaluate how whales may alter their use of this resting ground in response to anthropogenic activities. Managing wildlife populations requires empirical data to determine which environmental and oceanographic features (e.g. water depth, distance to nearest shore) influence the distribution of a population within a specific area. This information is vital to ensure that human activities in the area, particularly the newly introduced swim-with-whale operations, are managed effectively. Continued research resulting in adaptive management actions will ensure the long-term viability of Hervey Bay as an important habitat for the eastern Australian humpback whale population.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank Pacific Whale Foundation’s members and supporters for providing the funding necessary to conduct this study. Our gratitude goes to Andrew Ellis and Scott Whitcombe for their logistical support in Hervey Bay and to Rachael Nidiffer, Jaimi Raveneau, Suzie Vallance, Emmanuelle Martinez, and numerous research interns for their assistance with data collection. Thank you to two anonymous reviewers for providing guidance that has greatly improved this manuscript.
References
Andriolo, A., Kinas, P. G., Engel, M. H., Martins, C. C. A., and Rufino, A. M. (2010). Humpback whales within the Brazilian breeding ground: distribution and population size estimate. Endangered Species Research 11, 233–243.| Humpback whales within the Brazilian breeding ground: distribution and population size estimate.Crossref | GoogleScholarGoogle Scholar |
Anon. (2014). Swimming with whales trial to start this winter. Fraser Coast Chronicle. Available at: https://www.frasercoastchronicle.com.au/news/swimming-whales-gets-tick-approval/2331323/?ref=hs [accessed 8 March 2018].
Braithwaite, J. E., Meeuwig, J. J., and Hipsey, M. R. (2015). Optimal migration energetics of humpback whales and the implications of disturbance. Conservation Physiology 3, cov001.
| Optimal migration energetics of humpback whales and the implications of disturbance.Crossref | GoogleScholarGoogle Scholar | 27293686PubMed |
Bryden, M. M., Corkeron, P. J., and Slade, R. W. (1989). Humpback whales (Megaptera novaeangliae) in Hervey Bay, Queensland 1988. Report to the Australian National Parks and Wildlife Service, Canberra.
Calambokidis, J., and Barlow, J. (2004). Abundance of blue and humpback whales in the eastern North Pacific estimated by capture–recapture and line‐transect methods. Marine Mammal Science 20, 63–85.
| Abundance of blue and humpback whales in the eastern North Pacific estimated by capture–recapture and line‐transect methods.Crossref | GoogleScholarGoogle Scholar |
Calambokidis, J., Falcone, E. A., Quinn, T. J., Burdin, A. M., Clapham, P. J., Ford, J. K. B., Gabriele, C. M., LeDuc, R., Mattila, D., Rojas-Bracho, L., Straley, J. M., Taylor, B. L., Urban R, J., Weller, D., Witteveen, B. H., Yamaguchi, M., Bendlin, A., Camacho, D., Flynn, K., Havron, A., Huggins, J. and Maloney, N. (2008). SPLASH: Structure of populations, levels of abundance and status of humpback whales in the North Pacific. Final report for Contract AB133F-03-RP-00078, US Department of Commerce Western Administrative Center, Seattle, Washington. Available at: https://faunalytics.org/wp-content/uploads/2015/05/SPLASH-contract-Report-May08.pdf [accessed 5 April 2019].
Cartwright, R., Gillespie, B., LaBonte, K., Mangold, T., Venema, A., Eden, K., and Sullivan, M. (2012). Between a rock and a hard place: habitat selection in female–calf humpback whale (Megaptera novaeangliae) pairs on the Hawaiian breeding grounds. PLoS One 7, e38004.
| Between a rock and a hard place: habitat selection in female–calf humpback whale (Megaptera novaeangliae) pairs on the Hawaiian breeding grounds.Crossref | GoogleScholarGoogle Scholar | 22666432PubMed |
Cartwright, R., and Sullivan, M. (2009). Behavioral ontogeny in humpback whale (Megaptera novaeangliae) calves during their residence in Hawaiian waters. Marine Mammal Science 25, 659--680.
Chaloupka, M., Osmond, M., and Kaufman, G. (1999). Estimating seasonal abundance trends and survival probabilities of humpback whales in Hervey Bay (east coast Australia). Marine Ecology Progress Series 184, 291–301.
| Estimating seasonal abundance trends and survival probabilities of humpback whales in Hervey Bay (east coast Australia).Crossref | GoogleScholarGoogle Scholar |
Chittleborough, R. G. (1953). Aerial observations on the humpback whale, Megaptera nodosa (Bonnaterre), with notes on other species. Australian Journal of Marine and Freshwater Research 4, 219–228.
| Aerial observations on the humpback whale, Megaptera nodosa (Bonnaterre), with notes on other species.Crossref | GoogleScholarGoogle Scholar |
Chittleborough, R. G. (1965). Dynamics of two populations of the humpback whale, Megaptera novaeangliae (Borowski). Australian Journal of Marine and Freshwater Research 16, 33–128.
| Dynamics of two populations of the humpback whale, Megaptera novaeangliae (Borowski).Crossref | GoogleScholarGoogle Scholar |
Clapham, P. J., and Baker, C. S. (2008). Whaling, modern. In ‘Encyclopedia of Marine Mammals’ (Eds W. F. Perrin, B. Wursig, and J. G. M. Thewissen.) pp. 1328–1332. (Academic Press: San Diego.)
Constantine, R., Steel, D., Allen, J., Anderson, M., Andrews, O., Baker, C. S., Beeman, P., Burns, D., Charrassin, J.-B., Childerhouse, S., Double, M., Ensor, P., Franklin, T., Franklin, W., Gales, N., Garrigue, C., Gibbs, N., Harrison, P., Hauser, N., Hutsel, A., Jenner, C., Jenner, M.-N., Kaufman, G., Macie, A., Mattila, D., Olavarria, C., Oosterman, A., Paton, D., Poole, M., Robbins, J., Schmitt, N., Stevick, P., Tagarino, A., Thompson, K., and Ward, J. (2014). Remote Antarctic feeding ground important for east Australian humpback whales. Marine Biology 161, 1087–1093.
| Remote Antarctic feeding ground important for east Australian humpback whales.Crossref | GoogleScholarGoogle Scholar |
Corkeron, P. J. (1995). Humpback whales (Megaptera novaeangliae) in Hervey Bay, Queensland: behaviour and responses to whale-watching vessels. Canadian Journal of Zoology 73, 1290–1299.
| Humpback whales (Megaptera novaeangliae) in Hervey Bay, Queensland: behaviour and responses to whale-watching vessels.Crossref | GoogleScholarGoogle Scholar |
Corkeron, P. J., and Connor, R. C. (1999). Why do baleen whales migrate? Marine Mammal Science 15, 1228–1245.
| Why do baleen whales migrate?Crossref | GoogleScholarGoogle Scholar |
Corkeron, P. J., Brown, M., Slade, R. W., and Bryden, M. M. (1994). Humpback whales, Megaptera novaeangliae (Cetacea: Balaenopteridae), in Hervey Bay, Queensland. Wildlife Research 21, 293–305.
| Humpback whales, Megaptera novaeangliae (Cetacea: Balaenopteridae), in Hervey Bay, Queensland.Crossref | GoogleScholarGoogle Scholar |
Coughran, D. and Gales, N. (2010). An unusual peak in recorded mortalities of humpback whales in Western Australia: normal stochastic variability or a regional indication of carrying capacity? Report to the International Whaling Commission, SC/62/SH24.
Craig, A. S., Herman, L. M., and Pack, A. A. (2002). Male mate choice and male–male competition coexist in the humpback whale (Megaptera novaeangliae). Canadian Journal of Zoology 80, 745–755.
| Male mate choice and male–male competition coexist in the humpback whale (Megaptera novaeangliae).Crossref | GoogleScholarGoogle Scholar |
Craig, A. S., Herman, L. M., Gabriele, C. M., and Pack, A. A. (2003). Migratory timing of humpback whales (Megaptera novaeangliae) in the central North Pacific varies with age, sex and reproductive status. Behaviour 140, 981–1001.
| Migratory timing of humpback whales (Megaptera novaeangliae) in the central North Pacific varies with age, sex and reproductive status.Crossref | GoogleScholarGoogle Scholar |
Craig, A. S., Herman, L. M., Pack, A. A., and Waterman, J. O. (2014). Habitat segregation by female humpback whales in Hawaiian waters: avoidance of males? Behaviour 151, 613–631.
| Habitat segregation by female humpback whales in Hawaiian waters: avoidance of males?Crossref | GoogleScholarGoogle Scholar |
Currie, J. C., Stack, S. H., McCordic, J. A., and Roberts, J. (2018). Utilizing occupancy models and platforms-of-opportunity to assess area use of mother–calf humpback whales. Open Journal of Marine Science 08, 276–292.
| Utilizing occupancy models and platforms-of-opportunity to assess area use of mother–calf humpback whales.Crossref | GoogleScholarGoogle Scholar |
Danil, K., Maldini, D., and Marten, K. (2005). Patterns of use of Maku’a Beach, O’ahu, Hawai’i, by spinner dolphins (Stenella longirostris) and potential effects of swimmers on their behavior. Aquatic Mammals 31, 403–412.
| Patterns of use of Maku’a Beach, O’ahu, Hawai’i, by spinner dolphins (Stenella longirostris) and potential effects of swimmers on their behavior.Crossref | GoogleScholarGoogle Scholar |
Dawbin, W. H. (1997). Temporal segregation of humpback whales during migration in Southern Hemisphere waters. Memoirs of the Queensland Museum 42, 105–138.
De Boer, M. N. (2013). Elusive marine mammals explored – charting under-recorded areas to study the abundance and distribution of cetaceans using multi-method approaches and platforms of opportunity. Ph.D. Thesis, Wageningen University, Wageningen, Netherlands.
Department of Environment and Heritage (2005). Humpback whale recovery plan 2005–2010. Department of Environment and Heritage, Canberra.
Elwen, S. H., and Best, P. B. (2004). Female southern right whales, Eubalaena australis: are there reproductive benefits associated with their distribution off South Africa? Marine Ecology Progress Series 269, 289–295.
| Female southern right whales, Eubalaena australis: are there reproductive benefits associated with their distribution off South Africa?Crossref | GoogleScholarGoogle Scholar |
Ersts, P. J., and Rosenbaum, C. (2003). Habitat preference reflects social organisation of humpback whales (Megaptera novaeangliae) on a wintering ground. Journal of Zoology 260, 337–345.
| Habitat preference reflects social organisation of humpback whales (Megaptera novaeangliae) on a wintering ground.Crossref | GoogleScholarGoogle Scholar |
ESRI (2017). ‘ArcGIS Desktop: Release 10.’ (Environmental Systems Research Institute: Redlands, CA.)
Félix, F., and Botero-Acosta, N. (2011). Distribution and behaviour of humpback whale mother calf pairs during the breeding season off Ecuador. Marine Ecology Progress Series 426, 277–287.
| Distribution and behaviour of humpback whale mother calf pairs during the breeding season off Ecuador.Crossref | GoogleScholarGoogle Scholar |
Forestell, P. H., Chaloupka, M., and Kaufman, G. D. (2003). Migratory characteristics of humpback whales (Megaptera novaeangliae) in Hervey Bay and the Whitsunday Islands, Queensland, Australia: 1993–1999. Final report to the Environment Protection Agency, Queensland Parks and Wildlife Service, Brisbane.
Franklin, T. (2012). The social and ecological significance of Hervey Bay, Queensland for eastern Australian humpback whales (Megaptera novaeangliae). Ph.D. thesis, Southern Cross University, Lismore, New South Wales.
Franklin, T., Franklin, W., Brooks, L., Harrison, P., Baverstock, P., and Clapham, P. (2011). Seasonal changes in pod characteristics of eastern Australian humpback whales (Megaptera novaeangliae), Hervey Bay 1992–2005. Marine Mammal Science 27, E134–E152.
| Seasonal changes in pod characteristics of eastern Australian humpback whales (Megaptera novaeangliae), Hervey Bay 1992–2005.Crossref | GoogleScholarGoogle Scholar |
Franklin, W., Franklin, T., Brooks, L., Gibbs, N., Childerhouse, S., Smith, F., Burns, D., Paton, D., Garrigue, C., Constantine, R., Poole, M. M., Hauser, N., Donoghue, M., Russell, K., Mattila, D. K., Robbins, J., Oosterman, A., Leaper, R., Harrison, P., Baker, S., and Clapham, P. (2012). Antarctic waters (Area V) near the Balleny Islands are a summer feeding area for some eastern Australian (E1 breeding group) humpback whales (Megaptera novaeangliae). Journal of Cetacean Research and Management 12, 321–327.
Fraser Coast Industry Code of Practice (2018). Immersive whale interactions in the Great Sandy Marine Park. Fraser Coast Tourism and Events, Special Publication, Queensland.
Geoscience Australia (2013). Bathymetry Raster Data Set. Geoscience Australia, Canberra.
Guidino, C., Llapapasca, M. A., Silva, S., Alcorta, B., and Pacheco, A. S. (2014). Patterns of spatial and temporal distribution of humpback whales at the southern limit of the southeast Pacific breeding area. PLoS One 9, e112627.
| Patterns of spatial and temporal distribution of humpback whales at the southern limit of the southeast Pacific breeding area.Crossref | GoogleScholarGoogle Scholar | 25391137PubMed |
Henrys, B. P. (2005). Spatial distance sampling modeling of cetaceans observed from platforms of opportunity. M.Sc. Dissertation, University of St Andrews, Scotland.
Higby, L. K., Stafford, R., and Bertulli, C. G. (2012). An evaluation of ad hoc presence-only data in explaining patterns of distribution: cetacean sightings from whale-watching vessels. International Journal of Zoology 2012, 1–5.
| An evaluation of ad hoc presence-only data in explaining patterns of distribution: cetacean sightings from whale-watching vessels.Crossref | GoogleScholarGoogle Scholar |
Kaufman, G. D., Smultea, M. A., and Forestell, P. H. (1987). Use of lateral body pigmentation patterns for photographic identification of east Australian (Area V) humpback whales. Cetus 7, 5–13.
Kiszka, J., Macleod, K., Van Canneyt, O., Walker, D., and Ridoux, V. (2007). Distribution, encounter rates, and habitat characteristics of toothed cetaceans in the Bay of Biscay and adjacent waters from platform-of-opportunity data. ICES Journal of Marine Science 64, 1033–1043.
| Distribution, encounter rates, and habitat characteristics of toothed cetaceans in the Bay of Biscay and adjacent waters from platform-of-opportunity data.Crossref | GoogleScholarGoogle Scholar |
Leaper, R., Fairbarns, R., Gordon, J., Hiby, A., Lovell, P., and Papastavrou, V. (1997). Analysis of data collected from a whalewatching operation to assess relative abundance and distribution of the minke whale (Balaenoptera acutorostrata) around the Isle of Mull, Scotland. Report of the International Whaling Commission 47, 505–511.
Lundquist, D. (2007). Behavior and movements of southern right whales: effects of boats and swimmers. Ph.D. Thesis, Texas A&M University, Texas, USA.
Lundquist, D., Sironi, M., Würsig, B., and Rowntree, V. (2008). Behavioural responses of southern right whales to simulated swim-with-whale tourism at Peninsula Valdes, Argentina. Journal of Cetacean Research and Management 60, 1–15.
Machernis, A. F., Powell, J. R., Engleby, L. K., and Spradlin, T. R. (2018). An updated literature review examining the impacts of tourism on marine mammals over the last fifteen years (2000–2015) to inform research and management programs. US Department of Commerce, NOAA. NOAA Technical Memorandum NMFS-SER-7.
Macleod, K., Fairbairns, R., Gill, A., Fairbairns, B., Gordon, J., Blair-Myers, C., and Parsons, E. C. (2004). Seasonal distribution of minke whales Balaenoptera acutorostrata in relation to physiography and prey off the Isle of Mull, Scotland. Marine Ecology Progress Series 277, 263–274.
| Seasonal distribution of minke whales Balaenoptera acutorostrata in relation to physiography and prey off the Isle of Mull, Scotland.Crossref | GoogleScholarGoogle Scholar |
MacLeod, C. D., Weir, C. R., Santos, M. B., and Dunn, T. E. (2008). Temperature-based summer habitat partitioning between white-beaked and common dolphins around the United Kingdom and Republic of Ireland. Journal of the Marine Biological Association of the United Kingdom 88, 1193–1198.
| Temperature-based summer habitat partitioning between white-beaked and common dolphins around the United Kingdom and Republic of Ireland.Crossref | GoogleScholarGoogle Scholar |
Mattila, D. K., Clapham, P. J., Vasquez, O., and Bowman, R. S. (1994). Occurrence, population composition, and habitat use of humpback whales in Samana Bay, Dominican Republic. Canadian Journal of Zoology 72, 1898–1907.
| Occurrence, population composition, and habitat use of humpback whales in Samana Bay, Dominican Republic.Crossref | GoogleScholarGoogle Scholar |
Morete, M. E., Bisi, T. L., and Rosso, S. (2007). Temporal pattern of humpback whale (Megaptera novaeangliae) group structure around Abrolhos Archipelago breeding region, Bahia, Brazil. Journal of the Marine Biological Association of the United Kingdom 87, 87–92.
| Temporal pattern of humpback whale (Megaptera novaeangliae) group structure around Abrolhos Archipelago breeding region, Bahia, Brazil.Crossref | GoogleScholarGoogle Scholar |
Noad, M. J., Dunlop, R. A., Paton, D., and Cato, D. H. (2011a). Absolute and relative abundance estimates of Australian east coast humpback whales (Megaptera novaeangliae). Journal of Cetacean Research Management. Sp Iss 3, 243–252.
Noad, M. J., Dunlop, R. A., Paton, D., and Kniest, H. (2011b). Abundance estimates of the east Australian humpback whale population: 2010 survey and update. IWC Report SC/63/SH22.
O’Connor, O. S., Campbell, R., Cortez, H., and Knowles, T. (2009). Whale watching worldwide: tourism numbers, expenditures and expanding economic benefits. A special report from the International Fund for Animal Welfare, IFAW and Economists at Large, Yarmouth, MA, USA.
Östman-Lind, J., Driscoll-Lind, A., and Rickards, S. (2004). Delphinid abundance, distribution and habitat use off the western coast of the island of Hawai’i. Southwest Fisheries Science Center Administrative Report LJ-04-02C, La Jolla, CA, USA.
Oviedo, L., and Solís, M. (2008). Underwater topography determines critical breeding habitat for humpback whales near Osa Peninsula, Costa Rica: implications for Marine Protected Areas. Revista de Biología Tropical 56, 591–602.
| 19256430PubMed |
Paterson, R. A. (1991). The migration of humpback whales (Megaptera novaeangliae) in east Australian waters. Memoirs of the Queensland Museum 30, 333–341.
Peake, S. (2011). An industry in decline? The evolution of whale-watching tourism in Hervey Bay, Australia. Tourism in Marine Environments 7, 121–132.
| An industry in decline? The evolution of whale-watching tourism in Hervey Bay, Australia.Crossref | GoogleScholarGoogle Scholar |
Polansky, L., and Robbins, M. M. (2013). Generalized additive mixed models for disentangling long‐term trends, local anomalies, and seasonality in fruit tree phenology. Ecology and Evolution 3, 3141–3151.
| Generalized additive mixed models for disentangling long‐term trends, local anomalies, and seasonality in fruit tree phenology.Crossref | GoogleScholarGoogle Scholar | 24102000PubMed |
Queensland Department of Environment and Science (2018). Great Sandy Marine Park. Available at: https://www.npsr.qld.gov.au/parks/great-sandy-marine/faq.html [accessed 21 August 2018].
Queensland Department of the Premier and Cabinet (2017). Swimming with whales in Hervey Bay gets green light after three year trial. Available at: http://statements.qld.gov.au/Statement/2017/9/10/swimming-with-whales-in-hervey-bay-gets-green-light-after-threeyear-trial [accessed 19 March 2018].
R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/
Rankin, R. W., Maldini, D. A., and Kaufman, G. R. (2013). Bayesian estimate of Australian humpback whale calving interval under sparse resighting rates: 1987 -2009. Journal of Cetacean Research and Management 13, 109–121.
Rasmussen, K., Palacios, D. M., Calambokidis, J., Saborio, M. T., Rosa, L. D., Secchi, E. R., Steiger, G. H., Allen, J. M., and Stone, G. (2007). Southern Hemisphere humpback whales wintering off Central America: insights from water temperature into the longest mammalian migration. Biology Letters 3, 302–305.
| Southern Hemisphere humpback whales wintering off Central America: insights from water temperature into the longest mammalian migration.Crossref | GoogleScholarGoogle Scholar | 17412669PubMed |
Ridout, M., and Demetrio, C. (1992). Generalized linear models for positive count data. Revista de Matemática e Estatística 10, 139–148.
Rose N, Weinrich M, Iníguez M, Finkle M (2005). Swim-with-whales tourism – an updated review of commercial operations. Report to the IWC Scientific Committee No SC/57/WW6.
Samuels, A., and Bejder, L. (2004). Chronic interaction between humans and free-ranging bottlenose dolphins near Panama City Beach, Florida. Journal of Cetacean Research and Management 6, 69–77.
Smultea, M. A. (1994). Segregation by humpback whale (Megaptera novaeangliae) cows with a calf in coastal habitat near the island of Hawaii. Canadian Journal of Zoology 72, 805–811.
| Segregation by humpback whale (Megaptera novaeangliae) cows with a calf in coastal habitat near the island of Hawaii.Crossref | GoogleScholarGoogle Scholar |
Stoeckl, N., Smith, A., Newsome, D., and Lee, D. (2005). Regional economic dependence on iconic wildlife tourism: case studies for Monkey Mia and Hervey Bay. Journal of Tourism Studies 16, 69–81.
Suzacq, L. D. L. P. (2007). Changes in abundance and distribution of humpback whales, Megaptera novaeangliae, in Hervey Bay Marine Park, Australia, based on aerial surveys conducted in 1992 and 2004. M.Sc. Thesis, University of South Florida, Florida, USA.
Vang, L. (2002). Distribution, abundance and biology of Group V humpback whales (Megaptera novaeangliae): a review. The State of Queensland Environmental Protection Agency, Conservation Management Report, Queensland.
Whitehead, H. (1983). Structure and stability of humpback whale groups off Newfoundland. Journal of Cetacean Research and Management 61, 1391–1397.
Whitehead, H., and Moore, M. J. (1982). Distribution and movements of West Indian humpback whales in winter. Canadian Journal of Zoology 60, 2203–2211.
| Distribution and movements of West Indian humpback whales in winter.Crossref | GoogleScholarGoogle Scholar |
Williams, R., Hedley, S. L., and Hammond, P. S. (2006). Modeling distribution and abundance of Antarctic baleen whales using ships of opportunity. Ecology and Society 11, art1.
| Modeling distribution and abundance of Antarctic baleen whales using ships of opportunity.Crossref | GoogleScholarGoogle Scholar |
Wood, S., and Scheipl, F. (2017). gamm4: Generalized Additive Mixed Models using ‘mgcv’ and ‘lme4’. R-package version 0.2–5. Available at: https://CRAN.R-project.org/package=gamm4