Investigation of free-living highland wild dogs near Grasberg Gold Mine, Papua Province, Indonesia
James K. McIntyre A # , Caitlin J. Curry B # , Lisa L. Wolf A , Hendra K. Maury
B # , Lisa L. Wolf A , Hendra K. Maury  D , Leonardo A. Numberi
D , Leonardo A. Numberi  D , Suriani Surbakti D and Brian W. Davis
D , Suriani Surbakti D and Brian W. Davis  C E *
C E *
A
B
C
D
E
# These authors contributed equally to this paper.
Handling Editor: Mark Eldridge
Abstract
Following reports of free-living wild dogs, Canis sp., resembling the descriptions and behaviours of New Guinea singing dogs in the Puncak Jaya area in 2015, a three-phase assessment was conducted in three expeditions occurring in 2016, 2018, and 2022. The goals of these studies were to determine the incidence, status, and natural history of wild dogs near the Grasberg Gold Mine in Papua Province, Indonesia. The assessments identified the existence of Highland wild dogs (HWD) through indirect and direct observations by using trail cameras and field observation. Additional quantitative information about their behaviour and morphology were collected through the capture of eight dogs. The captured dogs underwent comprehensive medical examinations, and biological samples were collected for genomic analyses to determine the genetic relationship between HWD and other canines. Additionally, four of the dogs were equipped with global positioning system (GPS) collars to study the ranging behaviour of HWD for the first time. Documenting the existence of HWD has further strengthened the establishment of Papua’s position as one of the world’s megadiverse regions.
Keywords: camera trap, collar, dingo, expedition, scat, singing dog, wild dog.
Introduction
The island of New Guinea is home to approximately 244 mammal species (Wilson and Reeder 2005). The largest extant carnivorous mammal is the New Guinea Highland wild dog (HWD), Canis sp., the wild progenitor of the captive New Guinea singing dog (NGSD) (Surbakti et al. 2020). These rarely observed canines are characterised by their unique tonal vocalisations that are resonate at a frequency not observed in any other living canids (Koler-Matznick et al. 2003). Little is known of their population size, distribution, home range, ecology, or interaction with humans. For over 65 years, the only direct documentation of HWD was anecdotal, based on sightings of individual dogs or reports of their characteristic vocalisations (McIntyre et al. 2019). The only empirical information about the existence of these dogs prior to these expeditions was the identification of canid scat and evidence from a partially eaten prey carcass (Helgen et al. 2011).
In the Puncak Jaya area of the Sudirman Range in the western central highlands of New Guinea, there is a population of free-ranging dogs with thick fur and relatively small bodies compared to other wild canids such as wolves and jackals, thought by inhabitants to be unique to the area (McIntyre et al. 2019). This area consists of barren rocks with limited vegetation apart from lichens and mosses. High mountain lakes and steep valleys dominate the landscape (PT Freeport Indonesia 2021). Only a few bird and mammal species have been observed to be adapted to this harsh environment (Helgen et al. 2011; Pujolar et al. 2022). At altitudes exceeding 4000 m above sea level (a.s.l.), this region contains the highest peak in Oceania and Australasia, Puncak Jaya (4884 m), as well as the large Grasberg copper and gold mine.
PT Freeport Indonesia (PTFI) is a mineral mining company that operates in the highland areas of Papua Province, Indonesia. They employ open pit and underground mining for one of the world’s largest copper and gold deposits (www.ptfi.co.id). To offset their impact on the surrounding environment, PTFI has reclaimed more than 1000 hectares around the Grasberg Mine in the Sudirman Range near Pucak Jaya (Fig. 1). Over time, this reclamation area has successfully become a biodiversity ecosystem for hundreds of species, including the HWD (PT Freeport Indonesia 2021).
Maps of (a) the distribution of trail cameras and visual sightings of New Guinea Highland wild dogs on the local topology, (b) elevation of Papua Province and (c) global location.
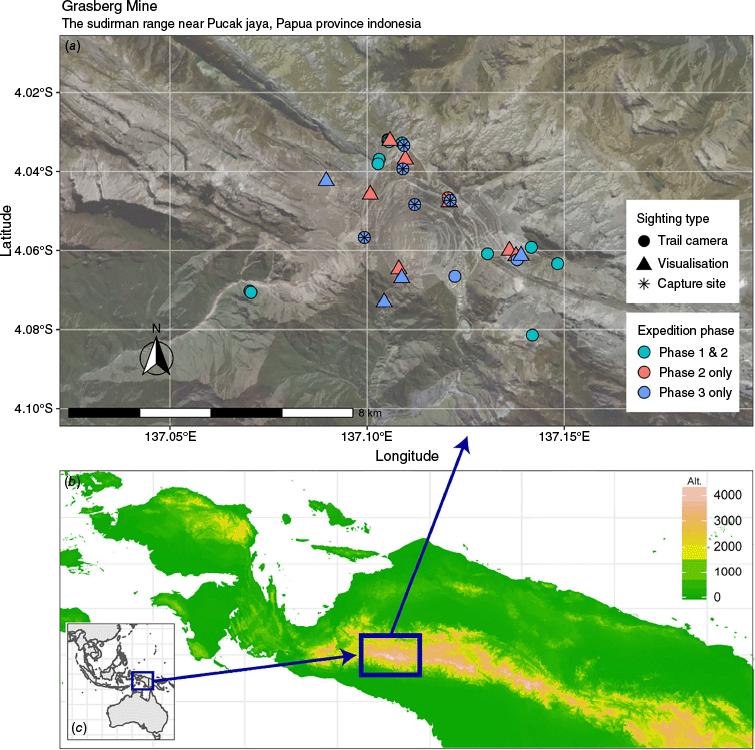
The HWD was recently documented to be monophyletic with dingo as a basal phylogenetic group to domestic canids by using genome-wide variation data derived from DNA collected in the 2018 expedition (Surbakti et al. 2020). The HWD exists in extremely challenging terrain to access, making in-person observation difficult. A systematic, interdisciplinary study supported by multiple available resources is needed to trace the existence and dynamics of HWD in Papua Province, Indonesia (West Papua). Documenting the existence of HWD further strengthens West Papua’s status as one of the world’s megadiverse regions (Mittermeier et al. 1997; Papua New Guinea 2021). A three-phase assessment aimed to acquire data and examine the population and habitat of HWD that inhabit the Puncak Jaya area was implemented in 2016, 2018, and 2022, in cooperation with the Grasberg Gold Mine. The purpose was to conduct a complete study of the elusive canid, including physical exams and measurements, health exams, collection of biological samples for genetic testing, and collecting data from global positioning system (GPS) tracking collars.
Materials and methods
Assessments were conducted in three phases scheduled to coincide with the anticipated breeding and whelping seasons, as well as an increase in the dogs’ activity level. Phase 1 took place over 10 days from 9 to 19 September 2016. Phase 2 lasted for 15 days, from 9 to 24 August 2018. Phase 3 spanned 20 days, from 6 to 26 October 2022. The field team consisted of individuals from the New Guinea Highland Wild Dog Foundation (NGHWDF) and University of Papua in 2016, NGHWDF and University of Cendrawasih in 2018, and NGHWDF, University of Cendrawasih, and University of Goettingen in 2022, each in cooperation with PTFI.
Study area
Research was conducted around the periphery of the Grasberg Gold Mine (4°3′10″S, 137°6′57″E) near Pucak Jaya, in Papua Province, Indonesia (Fig. 1). Puncak Jaya is the highest summit of Mount Jayawijaya in the Sudirman Range, western central highlands. This area comprises alpine meadows and rocky barrens, ranging from 3460 to 4400 metres above sea level (approximately 16,000 ft), with the highest regions located immediately below glaciers. As such, this research area is one of the most remote areas in West Papua. For each phase, the temperature fluctuated from 0°C at night to 11°C during the day. The rainy season lasts from June through to September. Mornings typically started clear, but temperatures quickly decreased with light to heavy rain during the remainder of the daylight hours and often continuing through the night. Snow flurries with ground accumulation were observed on occasion. Research during the dry season (April through to June) is preferred because these months have the least rainfall. Regrettably, these assessments were delayed to August through to October because of natural disasters, civil unrest, and the global pandemic in 2020.
Detection
Scents, lures, and baits, as described in McIntyre et al. (2019), were used as attractants for hair snares, trail cameras, and live traps. Food baits or cotton balls, wash cloths, or gauze pads saturated with scents, such as NGSD oestrous urine from captive animals in the USA, were placed on the ground or suspended with fishing line above the ground at varied heights. Hair snares were deployed in Phases 1 and 2. Hair snares consisted of 2-inch double-sided tape placed on rocks at shoulder height to HWD (Supplementary Fig. S3). Attractants were placed beneath the hair snares to elicit rubbing. Turf-covered ground hair snares with attractants in the centre were also set in the vicinity of live traps (Figs 2, S4). Moultrie M-990i Gen 2 trail cameras (Fig. S1) were set along well travelled game trails, overlooking attractant sites. Trail cameras were also set to view hair snares and live traps. Additionally, an EcoTec GC 300 predator caller with pre-programmed coyote calls was used when entering new areas, so as to arouse curiosity, elicit return calls, and entice the dogs to come into view. Field notes and photographs from trail cameras and during direct observation were used to identify individual dogs by their unique features.
Live capture and tracking
Live trapping was conducted during Phases 2 and 3 (Figs 2, S2). Cage traps were implemented in two stages, habituation (8 days) and capture (7 days). After installation and prior to setting the traps as live, attractants were placed inside and around the traps to draw the attention of the dogs and condition them to the traps. Boiled pieces of chicken were used as food baits inside the trap and oestrous urine collected from NGSD in the USA was used around the traps. The audio caller’s per-programmed call of the coyote (Canis latrans) howls was then played to evoke curiosity to further attract HWD to the trapping area. Last, a school bell was rung for HWD to associate the sound with chicken reward at the trap.
Captured HWD were sedated using a combination of 1.8 mL medetomidine (0.1 mg/kg) and 0.5 mL ketamine (2.5–3.0 mg/kg). Measurements of the dogs’ bodies were taken, and biological samples of blood, hair, tissue, saliva, and faeces were collected during veterinary examination. Blood was collected on FTA cards and in collection tubes, with EDTA or acid citrate as an anticoagulant (Surbakti et al. 2020). In addition to samples obtained during veterinary examination, faecal samples were also collected in the field along trails, and around staged live traps and placed in BD FecalSwab™ (Becton, Dickinson and Company) containers. Genomic work is ongoing to use these to document the microbiome and prey use of HWD.
Live-caught individuals were fitted with Lotek Pinnacle Pro L GPS collars for tracking, communicating via the Iridium satellite system, with mortality signal indication. Collars were programmed by the manufacturer to collect 12 GPS locations per day, static activity readings on all three axes every 6 s, with an approximately 18-month life span. To get a finer-scale resolution, one of the collars deployed in 2022 was configured to record readings every 10 min, taking a static activity reading on all three axes every second (Supplementary data, GPS Static Activity). The collars included the timed-release device (TRD) function that is designed to release after the life span expires. Special care was taken to ensure that no dogs were injured or harmed during capturing, handling, examining, collaring, or collection of samples. Dogs were reversed with atipamezole (0.25 mg/kg) and closely monitored while given time to recover in the covered cage before being released. It took approximately 2 h after reversal for dogs to be released.
Animal ethics
This work was performed with ethical approval of PT Freeport Indonesia, with the purpose and scope to examine habitat, biology and ethology of NGHWD; trap, immobilise, perform veterinary examinations; collect biological data; attach and deploy GPS monitoring collars; set trail cameras; set DNA traps; collect scats, scavenge or kill remains; collect anecdotal reports from locals; all under the auspices of a licensed veterinarian. Project ID: 123ABC456EFG.
Results
Detection
Twenty trail cameras were employed across the three phases (Fig. 1). Identification of individual HWD on the basis of unique physical characteristics was performed as described in McIntyre et al. (2019). Phase 1 identified 15 unique dogs (McIntyre et al. 2019). During Phase 2, 11 unique dogs were detected with an additional six incidental observations by members of the expedition not on trail cameras, including a deceased, pregnant female. Phase 3 identified 36 unique dogs observed within 46 occasions because of repeat sightings. The number of total observations of individual dogs with unique features was 68 across all three phases.
During Phase 1, scat samples (n = 24) at varying degrees of degradation were collected from remote areas between 3460 and 4150 m asl. Analyses for diet and genetic ancestry were performed, identifying primary food sources (Table S1) and the A29 mitochondrial haplotype, demonstrating a close relationship with other oceanic dogs (Surbakti et al. 2020). In Phase 2, an additional 10 fresh scat samples were collected. In Phase 3, 12 faecal samples were collected for future analyses, and 24 were analysed on the day of collection for parasites by using the flotation method and a compound microscope. Of the 24 samples, only two showed the presence of parasites. Further examination is planned to determine parasite load.
No dogs were observed, by using a trail camera, rubbing on hair snares during Phase 2, whereas 11 were observed during Phases 1 and 3. Hair was collected from ground level hair snares baited with attractants, but weather conditions made it unviable. Weather conditions were not conducive to this sampling method during the wet season and should be more successful during a drier season (April through to June). Because the HWD is the largest extant carnivorous mammal inhabiting this area and the apex predator, it was assumed that the majority of the scats were HWD scats. Genetic testing confirmed mitochondrial DNA to be of Canis familiaris origin (B. W. Davis, unpubl. data). Scats were found singularly and in groups, totalling 114 individual samples. When scats were found in groups, they were generally found within 1 m of each other, suggesting that these animals frequent the area for the purpose of defecation. Scats were never observed directly on a trail and were routinely deposited on the top of objects, ranging from isolated tufts of grass to water hoses discarded by mine employees.
Appearance
Dogs observed visually and with a trail camera ranged from cream-coloured to gradients of ginger/red to sable to black with small amounts of white (Fig. 3). Some darker dogs displayed tricolour patterning. There were varying degrees of white on the face blazes, muzzles, shoulder and neck collars, chest, undersides, forelegs, and tips of tails in an Irish spotting pattern.
Photos of highland wild dogs captures near Grasberg Gold Mine, demonstrating colour variation: (a) sable Irish, (b) black/tan, (c) ginger minimal white, (d) ginger Irish, (e) ginger socks, solid, and Irish puppies.

All observed female HWDs fell within the full-size range of captive NGSDs. However, three of the four males exceeded this range with respect to mass and weight (Table 1). Additionally, the upper carnassial length for six of the eight dogs was longer than recorded for captive NGSD (>1.6 cm (Koler-Matznick et al. 2003); Supplementary Table S1). These metrics are more similar to Australian dingoes (AUD) than NGSD (Koler-Matznick et al. 2003). The HWD ears were erect and triangular, situated on the top of their heads. Most HWDs had phenotypes consistent with that of captive NGSDs regarding shoulder height, length, and approximate weight (Table 1). All appeared healthy and fit with uniform well-furred coats. Only one dog appeared to be lean with ribs showing. This dog was a mother with three pups, likely devoting much of her energy to acquiring food and nursing the litter.
| Item | Adam | Blackie | Bali | Bernie | Bruce | George | Stella | White Socks | Captive NGSD range | |
|---|---|---|---|---|---|---|---|---|---|---|
| Phase | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | ||
| Coat colour | Ginger | Black | Ginger | Ginger | L Brown | Sable | Ginger | Ginger | ||
| Colour combo | Min.White | Black/Tan | Solid | Irish | Irish | Irish | Piebald | Socks | ||
| Sex | M | M | F | F | M | M | F | F | ||
| Age | Juvenile | Older | Adult | Young | Older | Older | Juvenile | Adult | ||
| Weight (kg) | 14.9 | 14.5 | 11.1 | 8 | 15.8 | 13.65 | 11.47 | 10.18 | 8.6–14.4 | |
| Height at withers (cm) | 41.5 | 44.5 | 37 | 40 | 52 | 48 | 45 | 51 | 31.8–45.7 | |
| Length – tail (cm) | 26 | 23.9 | 25.3 | 21 | 24 | 27 | 21 | 24 | 22.0–28.0 | |
| Length – body + tail (cm) | 84.5 | 88.9 | 79.8 | 92 | 107.4 | 113 | 97 | 95 | 71.0–91.0 | |
| Head length (cm) | 20.5 | 20.1 | 16.5 | 16 | 19 | 22 | 19 | 18 | 17.2–23.5 | |
| Ear height (cm) | 10.6 | 10.3 | 10.1 | 7 | 8 | 8 | 10 | 8 | 6.5–11.0 | |
| Condylobasal length (cm) | 10.4 | 10.4 | 8.4 | x | x | x | x | x | 14.1–17.4 | |
| Feet webbing | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1–3 |
Measurements for Bali, a dog discovered deceased, were made post-mortem. All other dogs were live captures. Age was estimated by appearance, greying, and dentition. NGSD ranges were taken from the captive population (Koler-Matznick et al. 2003).
Behaviour
Habituation to trapping systems in this area may not be necessary. According to local reports, near the mine, HWDs do not have predators, and are not hunted by humans, and dogs encountered by people in the wild are offered food owing to local custom. At the Grasberg study site, dogs readily approached and entered newly set traps, and would return to the traps occasionally, with one dog being trapped five times. Therefore, they are not as fearful of humans as are other species. This may not be true in more remote areas of New Guinea without human presence where a period of open-lock habituation to the traps could increase trapping success.
Scent rubbing behaviour was observed on or around assorted trail camera scents, baits, and lures, with 14 instances in Phase 2 and 15 in Phase 3. For example, these dogs rub the side of their cheek, jowls, and neck directly on the target scent, with their rump and tail high in the air. Dogs would also lie prone and directly run their entire side on the target scent. Additionally, a male dog was observed urinating and defecating on a target scent.
Dogs observed closer to the mine site were seen along roadways, atop rock tailing guard rails, and along cliff edges (Fig. 1). They exhibited relaxed behaviours until a vehicle slowed or stopped in their vicinity, then would stand, and appear wary. Any attempt to exit vehicles resulted in their retreat into the forest or rock barrens. HWDs observed along roadways associated with the mine appeared irregularly to forage for scraps left in discarded lunches provided for the mine workers. Food lures, such as chicken bones and cooked fish, were placed in areas around the mines where dogs had previously been observed. The dogs quickly consumed large amounts of food without chewing, before leaving for the security of remote rock barrens. The mines draw the dogs in from their remote habitats because of the ease of nutrition acquisition without considerable energy expenditure.
Firsthand observation and trail camera footage showed that HWD travelled in solitary, mixed sex pairs, in groups of three (mixed sex), and groups of four (mixed sex), moving both diurnally and nocturnally. In 2016, a female and three healthy puppies were photographed by trail camera by the Wanagon Lookout area. The same female was observed in 2018 by trail cameras in the same area, this time pregnant. A live trap had been set in the area. Once the pregnant female was discovered, the trap was removed from the area to avoid potential traumatisation. In 2019, it was reported that she was seen again with two pups in this same spot, indicating that this female chooses to whelp in the same spot. More pregnant females and bitches with pups need to be observed to definitively state an approximate breeding season.
In Phase 3, two groups were identified, the Surabaya group and the Kaimana Dump Lower (KDL) group. The Surabaya group consisted of six individuals. A senior mature female, ‘NeNe’ (ginger coloured with white socks), appeared be the ranking matriarch. The other seemingly leading member was an adult male, ‘George’ (white and sable with an Irish pattern). Five of the six were almost always seen in the same proximity. Another adult female, ‘Ruby’ (ginger with white socks), was a member of this group but was not always observed with the group. Two members of the group were likely to be yearling siblings, a male and female, ‘Mike’ and ‘Stella’ respectively. ‘Mike’ had an Irish pattern of white with black and brown rear legs, whereas ‘Stella’ had a white/cream body with ginger ears and ginger spot on her rump. The final member was a young adult female, ‘Scarlette’ (ginger with white socks). ‘Stella’ and ‘Mike’ were seen on several occasions running up to ‘NeNe’ and submissively crouching and licking her muzzle. ‘George’ and ‘NeNe’ were more aloof than the others and remained at the periphery of the group. ‘Stella’, ‘Mike’, and ‘Scarlette’ would readily approach food offerings from mine workers of deboned raw chicken. The five core members were always seen together, generally in their home area, but were also observed at other baiting stations by trail cameras 3 km and 5 km away from this area.
The KDL group consisted of eight individuals, including two adult males, two adult females, and four approximately 6-month-old puppies. This group also appeared to have a home area where they were most frequently observed. The social dynamics of this group was more difficult to discern. There was an adult male, ‘Bruce’ (ginger and white in colour), who appeared to have hierarchy over the other dogs. ‘Bruce’ would displace the other dogs at a food source. Another adult male, slighter in stature compared to ‘Bruce’, was ‘Brad Pitt’ (sable). An adult female, ‘Betty’ (solid ginger with white paws), was aloof and occasionally seen at the home area. Finally, ‘Brownie’, a sable female, had swollen teats and was probably actively nursing. A den site with a litter, however, was not found in or around their home area. It was assumed ‘Brownie’ was not the mother of the four puppies. The four ginger and white puppies were beyond nursing age and ‘Brownie’ was not observed associating with the puppies. However, ‘Brad Pitt’, the sable adult male, was observed in close proximity to the four puppies. Parentage of the puppies could not be determined by their behaviour.
Live capture and GPS tracking
Live captures were undertaken during Phases 2 and 3. During Phase 2, two live capture traps were deployed. Seven live capture nights resulted in two successful and two unsuccessful captures. Phase 3 saw 11 successful captures of 5 individuals. ‘White Socks’ was captured and released five times. Unsuccessful captures were a result of either trap malfunction or the dog escaping by pulling the rear panel inward.
Medical examinations were conducted, vital statistics taken, and biological samples collected on eight captured dogs, as well as a dog found deceased (Tables 1, S4). Pelage and morphology of captured dogs were consistent with what was previously described (McIntyre et al. 2019).
During Phase 2 examinations, GPS tracking collars were placed on the two captured individuals, ‘Adam’ (Fig. 3c) and ‘Blackie’ (Fig. 3b). GPS tracking collars were placed on two dogs captured in Phase 3, ‘George’ from the Surabaya group and ‘Bruce’ from the KDL group. The collars remotely track the dogs’ travel and activity to inform territory, distance travelled, activity periods, and feeding behaviour. In depth telemetry analysis was conducted and it determined that the dogs traversed steep, rocky and barren alpine mountain crevasses. The sampled animals had home ranges less than 128 km2, moved less than 56.8 km per day, and were found up to 4630 m above sea level (Allen et al. 2024).
GPS coordinates from Phase 2 placed collars were collected from 22 to 30 August 2018 for ‘Blackie’ and 19 August to 10 September 2018 for ‘Adam’. ‘Adam’ had 61 fixes and ‘Blackie’ had 209 fixes after data processing and outlier removal. Collars stopped transmitting data after 9 (24 August 2018 06:00:00 hours to 31 August 2018 06:22:00 hours) and 21 days (20 August 2018 08:00:00 hours to 11 September 08:00:00 hours) days respectively, although no mortality signals were received. Both collars exhibited variable sampling rates, with the most common sampling rate being at 20-min intervals, and the second-most common sampling rate being at 2-h intervals. Both individuals were observed after these dates, ‘Adam’ without a collar and ‘Blackie’ wearing his collar. There are multiple possibilities for the sporadic incoming data and early termination of transmission. Collar malfunction is the most likely reason, but we cannot rule out mis-programming or inability to make consistent contact with satellites.
GPS coordinates from Phase 3 placed collars were collected starting on 18 October 2022 for ‘Bruce’ and 19 October 2022 for ‘George’. The collar on ‘Bruce’ was set to transmit data for 1 year and was still transmitting at time of publication. ‘George’s’ collar transmitted for 21 days (from 19 October 2022 12:03:00 hours to 9 November 2022 20:52:00 hours). On the basis of this preliminary GPS data, ‘George’ was located 8 km away from the home area. Because the five core members were consistently seen together, it can be inferred that the rest of the group was also in a similar location.
Discussion
This study has provided unique insight into the dynamics of a free-living population of canids that has been recalcitrant to observation and study. These animals face novel challenges to life at high altitude, while contending with the incursion of humans into their home ranges, and have, until recently, been obscured from observation. The presence of HWD or related populations had not been empirically confirmed in their native habitat for more than half a century, with only anecdotal evidence supporting the existence of a wild-living dog in the Papua highlands (McIntyre et al. 2019).
On the basis of communications throughout the expeditions with rural tribal landowners, in some areas of New Guinea, wild dogs are viewed as trophies because of their rarity and difficulty to successfully hunt them. However, people in villages surrounding Grasberg Gold Mine properties revere these dogs and do not hunt them. In this way, privately owned properties create a safe haven for one of the rarest canids. In addition, there are no people or village dogs that compete with the HWD for their indigenous prey species around the mine. Although the mine has created a potential refuge for this population of HWDs, the proximity and increased likelihood for interaction with humans can be a threat to the population. There is potential for hybridisation and disease transmission from domestic village dogs in areas close to humans, as has been documented in AUD. Although hybridisation with domesticated individuals can compromise the adaptive fitness of wild populations (Sacks et al. 2011), there are no Papuan villages near the Grasberg Gold Mine properties, and Puncak Jaya is approximately 10 km from the nearest human settlement of Tembagapura. The lack of village dogs leads to lower likelihood of hybridisation with HWD, as is supported by our genetic findings (Surbakti et al. 2020). There have been instances of mine workers removing wild pups from den sites. Four anecdotal accounts of stolen pups all resulted in the death of pups. Also, the feeding of human food from box lunches by mine employees can alter their natural behaviour by habituating HWD to non-prey nutrition (Newsome et al. 2019; Behrendorff et al. 2023).
Biological samples have helped further clarify the relationships among HWD, NGSD, and AUD, and definitively determined the phylogeny of these canids (Surbakti et al. 2020). Some have argued that these canids are domestic dogs (Canis familiaris), others believe that they should be a separate classification (e.g. Canis hallstromi; Koler-Matznick et al. 2003); an important distinction because the outcome affects conservation status and protection, which will be debated in the context of the recent genetic data gathered during these sampling phases (Sandall et al. 2023). The sizes of some HWD individuals measured in this expedition exceed previously recorded size ranges and overlap with AUD. This increase in overall size for males could be due to numerous factors, including increased nutrition by human feeding, or local adaptation. Their build is more similar to the stocky conformation previously seen in NGSD, leading to questions about potential local adaptation that could be assessed with genetic data. There may also be a continuum of morphological characteristics that is absent when studying only the captive NGSD population. Additionally, we note that sexual dimorphism is more pronounced in this population than is observed in the captive NGSD population.
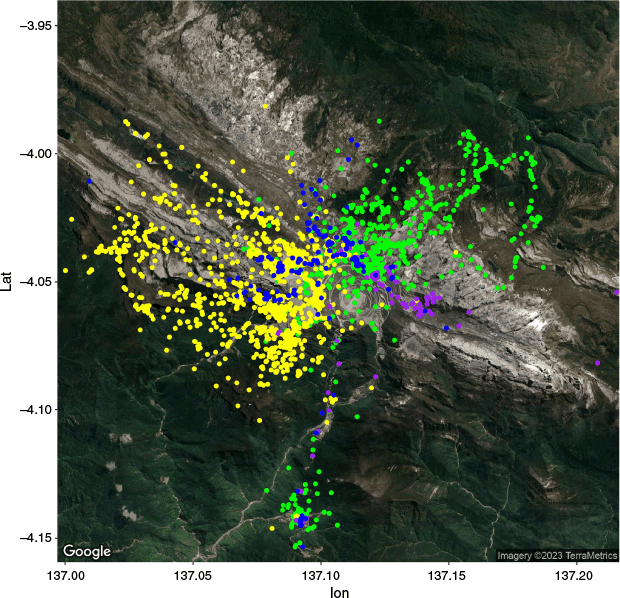
Previous estimates have shown that AUD living in unpopulated areas of Australia have larger (hundreds of km2) ranges than do AUD near mines (~10 km2) (Newsome et al. 2013). This could be associated with ease of access to nutrition near isolated human habitats. Preliminary data showed that the collared dogs are using manmade landscape features to navigate the landscape and possibly visit additional human settlements such as Tembagapura, likely in search of additional nutrients through scavenging or handouts (Fig. 4). These HWD covered a larger area during our collection period than that of mine-proximal AUD, but considerably smaller than that of wolves (Canis lupus), coyotes, and desert AUD (Poessel et al. 2016; Mancinelli et al. 2018). Data collected over a longer period is needed to determine whether these patterns are consistent over the 18-month life span of GPS collars. These data demonstrated, for the first time, the existence of distinctive ranges for HWD individuals (Allen et al. 2024). It also increased confidence that the studied HWD are part of a free-living population, and not simply free-ranging or feral dogs otherwise derived from human settlements. Importantly, the ranges of the dogs extend to extreme elevation changes. A vertical difference of 2500 m between the Grasberg mine and Tembagapura is significant and may represent one of the more challenging home ranges of canids when the exertion of altitude change is considered in the context of local adaptation.
GPS telemetry locations ending October 2023. Four dogs were successfully collared, two from each of 2018 and 2022 expedition. At the time of submission of this paper, both 2022 collars continued to broadcast locations. Expedition of 2022: ‘George’ (green) with 2603 points, ‘Bruce’ (yellow) with 2802 points. Expedition of 2018: ‘Blackie’ (purple) with 117 points, ‘Adam’ (blue) with 260 points. The 1 year of telemetry data from 2022 to 2023 showed that the ranges of ‘George’ and ‘Bruce’ overlap only at the Grasberg mine.
These assessments mark the beginning of an ongoing formal study. Although additional sites and populations of wild dogs need to be identified and examined, a permanent, multi-disciplinary research station should be established near Puncak Jaya to allow for ongoing, year-round study of this population, so as to build on the results of these assessments. It will also create opportunities to study the rich biodiversity of the remote area.
Data availability
Telemetry points and camera-trap photos are available by request from the corresponding author.
Declaration of funding
This project has been funded by PT freeport International, an affiliate of Freeport-McMoRan (FCX) and MIND ID Indonesia’s Mining Industry.
Acknowledgements
The research is dedicated to I. Lehr Brisbin Jr. We owe much gratitude for his inspiration. Our gratitude goes to PT Freeport Indonesia (PTFI), Indonesia, and the Grasberg mine for sponsoring this research and allowing us access. Thanks go to Paul Murphy, PTFI Senior advisor (retired), Andi Mukhsia, Vice President, PTFI Environmental Division, David Norris, PTFI Director of Environmental affairs, Gesang Setyadi, PTFI Environmental manager, Jim Dellinger, PTFI Environmental expert – resource development, Tito Puradyatmike, PTFI highland reclamation and monitoring general supervisor, and his crew (Chokie, Yanu, Eric, Richie, Arni, Forester, Isale, and Asep) for the special attention we received, by providing transportation, security, research assistance and invaluable information regarding the HWDs. Thanks go to Tumpal Sinaga, PTFI Supervisor of Biodiversity, Julia Mattner, PTFI botanist, Yohan Sunyoto, Manager of Environmental Highland, and Amiruddin, Superintendent HL QA/TA, PTFI, and Tom Hewitt of Adventure Alternatives. Thanks go to Johan Koibur and Deny Iyai of the University of Papua and Margaretha Pangau-Adam of the University of Cenderawasih. Special thanks go to research assistants Hendra Maury and Megan Selvig, and our veterinarian, Dr Berna Natalia Br Silaban. Many thanks go to Yahdi Jamur and Magdalena Friya, and their team at Antiga Films, for their professional film documentaries produced for our 2018 and 2022 expeditions.
References
Allen, B. L., Miller, C., Wolf, L., Maury, H. K., Numberi, L. A., Surbakti, S., Silaban, B. N. B, Kusuma, K. I., and McIntyre, J. K. (2024). Insights into the spatial ecology of the world’s most ancient dofg: high-altitude movements of New Guinea dingoes. Global Ecology and Conservation 56, e03264.
| Crossref | Google Scholar |
Behrendorff, L., King, R., and Allen, B. L. (2023). Efficacy of Management Efforts to Reduce Food-Related Dingo–Human Interactions and Conflict on K’gari (Fraser Island), Australia. Animals 13, 204.
| Crossref | Google Scholar |
Koler-Matznick, J., Brisbin, I. L., Feinstein, M., and Bulmer, S. (2003). An updated description of the New Guinea singing dog (Canis hallstromi, Troughton 1957). Journal of Zoology 261, 109-118.
| Crossref | Google Scholar |
Mancinelli, S., Boitani, L., and Ciucci, P. (2018). Determinants of home range size and space use patterns in a protected wolf (Canis lupus) population in the central apennines, Italy. Canadian Journal of Zoology 96, 828-838.
| Crossref | Google Scholar |
McIntyre, J. K., Wolf, L. L., Sacks, B. N., Koibur, J., and Brisbin, I. L. (2019). A population of free-living highland wild dogs in Indonesian Papua. Australian Mammalogy 42, 160-166.
| Crossref | Google Scholar |
Newsome, T. M., Ballard, G. A., Dickman, C. R., Fleming, P. J. S., and Van De Ven, R. (2013). Home Range, activity and sociality of a top predator, the dingo: a test of the resource dispersion hypothesis. Ecography 36, 914-925.
| Crossref | Google Scholar |
Newsome, T. M., Howden, C., and Wirsing, A. J. (2019). Restriction of anthropogenic foods alters a top predator’s diet and intraspecific interactions. Journal of Mammalogy 100, 1522-1532.
| Crossref | Google Scholar |
Papua New Guinea (2021). IUCN Green List. Available at https://iucngreenlist.org/country/papua-new-guinea/ [accessed 19 January 21].
Poessel, S. A., Breck, S. W., and Gese, E. M. (2016). Spatial ecology of coyotes in the Denver metropolitan area: Influence of the urban matrix. Journal of Mammal 97, 1414-1427.
| Crossref | Google Scholar |
PT Freeport Indonesia (2021). Biodiversity. Available at https://ptfi.co.id/en/biodiversity [accessed 19 January 2021].
Pujolar, J. M., Blom, M. P. K., Reeve, A. H., Kennedy, J. D., Marki, P. Z., Korneliussen, T. S., Freeman, B. G., Sam, K., Linck, E., Haryoko, T., Iova, B., Koane, B., Maiah, G., Paul, L., Irestedt, M., and Jønsson, K. A. (2022). The formation of avian montane diversity across barriers and along elevational gradients. Nature Communications 13, 268.
| Crossref | Google Scholar |
Sacks, B. N., Moore, M., Statham, M. J., and Wittmer, H. U. (2011). A restricted hybrid zone between native and introduced red fox (Vulpes vulpes) populations suggests reproductive barriers and competitive exclusion. Molecular Ecology 20, 326-341.
| Crossref | Google Scholar |
Sandall, E. L., Maureaud, A. A., Guralnick, R., McGeoch, M. A., Sica, Y. V., Rogan, M. S., Booher, D. B., Edwards, R., Franz, N., Ingenloff, K., Lucas, M., Marsh, C. J., McGowan, J., Pinkert, S., Ranipeta, A., Uetz, P., Wieczorek, J., and Jetz, W. (2023). A globally integrated structure of taxonomy to support biodiversity science and conservation. Trends in Ecology & Evolution 38, 1143-1153.
| Crossref | Google Scholar |
Surbakti, S., Parker, H. G., McIntyre, J. K., Maury, H. K., Cairns, K. M., Selvig, M., Pangau-Adam, M., Safonpo, A., Numberi, L., Runtuboi, D. Y. P., Davis, B. W., and Ostrander, E. A. (2020). New Guinea highland wild dogs are the original New Guinea singing dogs. Proceedings of the National Academy of Sciences of the United States of America 117, 24369-24376.
| Crossref | Google Scholar |



