Where in the beef-cattle supply chain might DNA tests generate value?
Alison L. Van Eenennaam A C and Daniel J. Drake BA Department of Animal Science, University of California, 1 Shields Avenue, Davis, CA 95616, USA.
B University of California Cooperative Extension, 1655 South Main Street, Yreka, CA 96097, USA.
C Corresponding author. Email: alvaneenennaam@ucdavis.edu
Animal Production Science 52(3) 185-196 https://doi.org/10.1071/AN11060
Submitted: 26 April 2011 Accepted: 27 November 2011 Published: 2 February 2012
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Abstract
DNA information has the potential to generate value for each sector of the beef-cattle industry. The value distribution among sectors (breeding, commercial, feedlot, processing) will differ depending on marketing. The more descendants an animal produces, the more valuable each unit of genetic improvement becomes. Therefore, the value of using DNA testing to increase the accuracy of selection and accelerate the rate of genetic gain is highest in the breeding sector, particularly for replacement stud animals. There is a lesser value associated with increasing the accuracy of yearling commercial bulls. The cost to DNA test commercial sires will likely be incurred by breeders before sale, and must be recouped through higher bull sale prices or increased market share. Commercial farmers could also use DNA tests to improve the accuracy of replacement female selection. This assumes the development of DNA tests that perform well for the low-heritability traits that directly affect maternal performance (e.g. days to calving) in commercial cattle populations. DNA tests may provide the sole source of information for traits that are not routinely measured on commercial farms. In that case, DNA test information will provide new selection criteria to allow for genetic improvement in those traits. As DNA test offerings mature to have improved accuracy for traits of great value to the feedlot (e.g. feed conversion, disease resistance) and processing (e.g. meat quality) sectors, the added value derived from DNA-enabled selection for these traits will need to be efficiently transferred up the beef production chain to incentivise continued investment. The widespread adoption of DNA testing to enhance the accuracy of selection will likely require an approach to share the value realised by downstream sectors of the beef-cattle industry with those upstream sectors incurring DNA collection and testing expenses.
Additional keywords: marker-assisted management, marker-assisted selection.
Introduction
The beef industry is characterised by several different production tiers or sectors that vary in their investment opportunities for the application of DNA technologies. These tiers can broadly be divided into the seed-stock (or bull selling) sector, the commercial (or bull buying) sector, feedlots and processors. DNA information may be used for a variety of purposes, including parentage assignment, testing for recessive traits and genetic defects, marker-assisted selection, and marker-assisted management. The usefulness and value of DNA information will differ among the sectors, and producers need to understand their own particular production and marketing circumstances when considering adopting DNA-based technologies for the purpose of increased profitability. Industry structure and market signals are also important factors in determining the value derived from investment in DNA testing. The beef industries in Australia (AU) and the United States (US) differ markedly in structure, and this has implications for industry adoption of DNA testing. The purpose of the present paper is to consider how DNA information might be used by the different sectors of the beef industry, and to compare and contrast the value proposition associated with its use in the AU and US beef industries.
A comparison of the US and AU beef-cattle industries
The US beef-cattle industry is 3–4 times the size of the AU industry, but there are almost 20 times more US beef-cattle operations (NASS 2011). In AU, operations with fewer than 100 head of cattle represent 30% (12 017) of farms, but account for just 3% of the beef-cattle herd (ABARE 2010). In the US, 90% (692 050) of farms have fewer than 100 head, and this accounts for almost half (46%) of the US beef herd (Tables 1, 2).The average US herd has 41 cows whereas the average AU herd has 321 cows. In the AU industry, 80% of beef cows are located on farms with over 400 head, and more than a quarter (3.5 million cows) are located on the 441 properties carrying in excess of 5400 mother cows (Fig. 1).
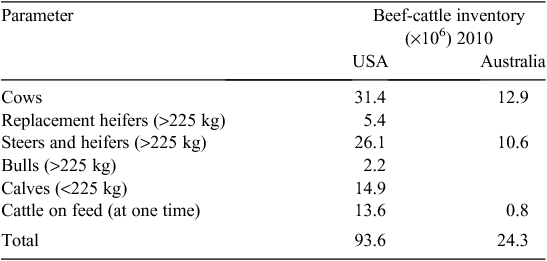
|
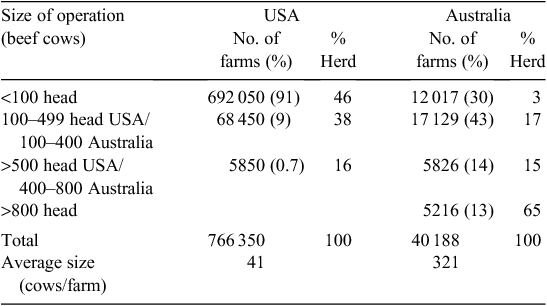
|
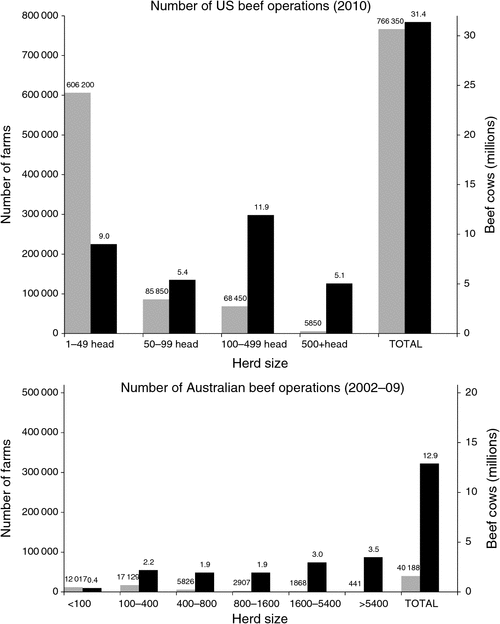
|
Almost all US cattle are finished in feedlots. Producers have a choice of selling cattle as either weanlings or grass-fed yearlings, or retaining ownership through the feedlot phase to harvest. Because of the high proportion of small-scale producers, most sell their cattle at auction for commodity prices before feedlot entry. For the less than 1% of producers with more than 500 head, ~1/3 of the calf crop is sold at weaning, 1/3 as grass-fed yearlings, and the remaining 1/3 are retained through harvest (T. Field, National Beef Cattlemen’s Association, pers. comm.). The large number of small US beef producers is in marked contrast to the concentration in the feedlot industry where the 260 feedlots that have a one-time capacity of more than 16 000 head feed ~60% of the nation’s cattle (Table 3). At any one point in time, there are ~13.6 million US cattle on feed, and 26 million head were fed in 2009. In contrast, AU fed only 2.3 million head in 2009, with ~1/2 of these animals being long-fed (~300 days) for the high-value export market.
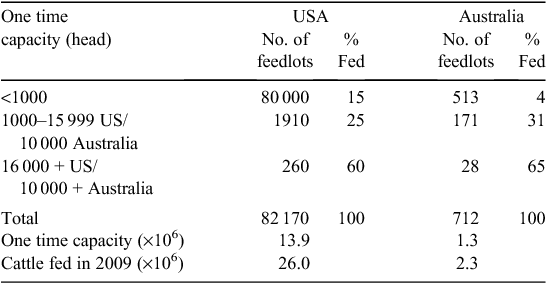
|
In AU, animals produced for slaughter are sold into the following four markets: directly for slaughter, for live export, to feedlots, or to breeders and/or for store purposes. When beef-cattle producers in both northern and southern Australia were ranked by farm financial performance, the top 25% of producers were found to sell a greater proportion of their cattle ‘over the hook’ and fewer via auction than were the lower-performing producers (ABARE 2010). Over the hook is an Australian term for sales direct to the processing plant, with price determined on the abattoir grid (usually on carcass weight and fat depth specifications), and is somewhat analogous to the US term of retained ownership where producers feed cattle and receive a price from the processor. Sale price varies in accordance with how well animals meet specifications, and the buyer can more accurately determine whether the product meets specifications such as weight and fat cover. If product falls outside these specifications, the price is discounted accordingly. This approach provides direct feedback to producers and may provide incentive for producers to modify their breeding and production systems to meet buyers’ requirements.
From the perspective of genetic evaluation, there are also major differences between the two countries. In AU, the genetic evaluation system known as BREEDPLAN is used to evaluate all breeds within AU (Graser et al. 2005). The BREEDPLAN analytical software was developed by the Animal Genetics and Breeding Unit (AGBU), a joint unit of the University of New England and New South Wales Department of Primary Industries, with research and development funding primarily provided by Meat and Livestock Australia. BREEDPLAN software is licenced to the Agricultural Business Research Institute (ABRI), which provides genetic evaluation services to a large number of clients. The software uses pedigree and performance data recorded predominantly by seed-stock breeders. These data are commonly channelled through several Breed Societies to the databases of the National Beef Recording Scheme at ABRI. An associated software program, BreedObject, calculates economic values for a given set of farm production and marketing parameters and combines the BREEDPLAN estimated breeding values (EBVs) into a total production AU$Index EBV for whole-industry economic merit (Barwick and Henzell 2005). AGBU has developed a national genotype database that stands ready to house DNA-based (genotypes and molecular breeding values) and phenotypic data.
In contrast, national genetic evaluations in the US are performed by several different groups primarily led by breed associations, although the system is currently in a state of flux. Historically, four land-grant universities, namely Colorado State University, Cornell University, University of Georgia and Iowa State University, were involved in beef-cattle genetic evaluation (Garrick and Golden 2009), although some breeds (e.g. Brahman, Hereford) chose to run their evaluations through BREEDPLAN. However, with decreases in federal and state support for these institutions, there was a move several years ago to shift routine evaluation activities away from the land-grant system. Breed associations stepped up to the task of producing genetic evaluations. In doing so, they did not adopt the unified approach envisioned by the National Beef Cattle Evaluation Consortium (NBCEC) where a single national body would form to undertake genetic evaluation activities. Rather evaluation entities emerged in a somewhat disjointed fashion. A subsidiary of the American Angus Association, AngusGenetics Inc., runs evaluations for Angus, the North American Limousin Foundation and the American Gelbvieh Association. The American Simmental Association undertakes a joint evaluation with Red Angus. And a third group, Genetic Performance Solutions, does evaluations for the American Brangus, Braunvieh, Akaushi and Texas Longhorn breeds. This situation of multiple independent entities is unlikely to be sustainable in the long run due to the limited number of people that are being trained with the appropriate quantitative genetics expertise to perform the genetic evaluations. The need to incorporate DNA information with pedigree and performance data to enable DNA-assisted genetic evaluations is also likely to be complicated by the lack of a single data repository for all breeds. Finally, there are no well defined selection objectives for national beef-cattle improvement in the US (Garrick and Golden 2009).
Seed-stock sector
The seed-stock sector can be partitioned into a few nucleus herds that lead genetic change, and many multiplier herds that produce and market sons from outstanding industry bulls. It is hard to get accurate estimates of the numbers that belong in each group. In a study of American Red Angus, it was found that ~3% of the total herds in the breed association were supplying animals represented as grandparents in the pedigree (Marquez et al. 2010). The low reproductive rate of beef cattle means that there is a relatively large number of elite breeding females in the nucleus sector as compared with pigs and poultry (Amer et al. 2007). Even so, it has been estimated that less than 5% of cows belong to the bull-breeding sector (Garrick and Golden 2009).
Genetic markers provide an approach for parentage identification. DNA testing for pedigree verification is mandatory for some breeds, and random testing is mandated by others. The obvious value to the breed association is to correct pedigree recording errors. Pedigree errors reduce the rate of genetic gain to below that which is possible and predicted. The ability to use DNA to assign parentage also offers the opportunity for breeders to use multi-sire pastures which offers several benefits. Having multiple sires present in with a group of cows results in higher fertility, precludes sire failure and reduces the calving interval. It also minimises the number of pastures needed, thereby allowing for better pasture management. Additionally, it reduces the labour cost and need to disturb animals at birth, thereby improving both maternal–offspring bonding and worker safety. Parentage tests currently range in price from US$13 to US$25, and vary in type from microsatellite-based tests to SNP tests of ~100 loci.
There are many DNA-based tests for simply inherited traits including coat colour, horned-status and recessive genetic defects. These tests range in price from US$20–100 per sample. There are large numbers of genetic abnormalities in cattle, occurring in a variety of breeds. These defects have had a significant impact on specific cattle populations. Naturally occurring recessive genetic defects are common in all species, including humans. The average human carries ~2000 deleterious recessive alleles, of which one to two are thought to be lethal (Sunyaev et al. 2001). Such numbers are likely true for cattle. Recessive conditions become evident only when certain lines of cattle are used very heavily, such that both cows and bulls have common ancestors in their pedigree, thereby allowing a rare genetic defect to become homozygous in their offspring.
Genetic defects are often propagated as a result of specific trait selection. In dogs, it has been noted that each of the 50 most popular breeds has one aspect of breed type that predisposes the breed to a genetic disorder (Asher et al. 2009). For example, bull dogs are prone to airway obstruction syndrome, and King Charles spaniels are affected by a reduced-size malformation of the skull related to selection for skull conformations that are steep caudally. Perhaps the most famous example of a genetic defect in 20th century beef breeding was ‘snorter’ dwarfism which became an issue in Angus and Hereford cattle during the 1940s and 1950s. A detailed history of snorter dwarfism and the efforts to eliminate it from the Hereford breed is described in a book entitled ‘The Battle of the Bull Runts’ (McCann 1974). This genetic defect was uncovered as a result of strong selection pressure for animals with small stature. Ultimately, the cause of this mutation was traced back to a bull named St Louis Lad, born in 1899. A 1956 survey of Hereford breeders in the USA identified 50 000 dwarf-producing animals in 47 states (Whitlock et al. 2008). Through detailed pedigree analysis and test crosses, the American Hereford Association, in concert with breeders and scientists, virtually eliminated the problem from the breed. Because carrier status was difficult to prove and required expensive progeny testing, some entire breeding lines were eliminated. This situation can be contrasted to the speed with which genetic testing has allowed 21st-century breeders to quickly and accurately determine the carrier status of their animals.
The Angus breed has recently had to manage three simply inherited, single locus-recessive genetic conditions. These include two lethal conditions, namely arthrogryposis multiplex (AM, ‘curly calf syndrome’) and neuropathic hydrocephalus (NH). The first is caused by a chromosomal deletion that occurred in Rito 9J9 of B156 7T26, (American Angus registration no. 9682589, born 29 October 1979). The second occurred as a result of a single DNA base-pair mutation in his grandson, the widely used GAR Precision 1680 (American Angus registration no. 11520398, born 6 September 1990). The widespread use of this superior carcass-trait bull spurred on by an increased selection emphasis on carcass traits increased the probability of this bull showing up on both sides of many Angus pedigrees, thereby uncovering the presence of any recessive lethal mutations. The third condition is a non-lethal autosomal genetic defect called congenital contractural arachnodactyly (CA, ‘fawn calf syndrome’) that is caused by a deletion of ~54 kbp. The inheritance pattern of these conditions can be calculated by applying straightforward Mendelian inheritance rules, i.e. 25% affected calves expected from a mating of carriers.
It is difficult to get exact frequencies of these recessive alleles in the US Angus population, but some educated estimates can be made. Of the 96 247 Angus animals that had been tested for AM in the US as of November 2009, 20% (5168 bulls and 14 361females) were found to be carriers, and 80% (23 638 bulls and 53 080 females) were free. When considering the 466 225 Angus animals with a registration number higher than16 000 000 (i.e. born after January 2007 and likely to be test candidates) as of November 2009; 96 247 were tested, and 19 773 were carriers of AM. If it is assumed that the frequency in the untested animals was the same as that in the tested animals, then the upper limit of the AM deletion allele would be a frequency of 10.2%. Presumably, the tested animals were a subset of potential carriers, and so if we assume instead that all of the untested animals are unrelated to GAR Precision 1680 (i.e. free of AM), then the frequency of the AM deletion allele in the US Angus would be 2.1%. This sets the upper and lower limits of the AM deletion allele frequency, although this frequency will decrease over time as a result of genetic testing and breed-association registration policies. Nearly 10% of 934 AI sires representing a broad cross-section of registered Angus genetics were found to be carriers of NH (Beever 2009), giving a mutant NH allele a frequency of 5%. Given the number of calves reported with this condition, this frequency appears to be higher than expected. This may be due to fetal loss associated with this condition. Finally, 39 CA carriers were identified in a population of 1256 AI sires. This corresponds to a heterozygote frequency of ~3.1% and an allele frequency of ~1.5% (Beever 2010).
Angus Australia, in collaboration with ABRI, uses GeneProb (Kinghorn 1997), a software program to determine the probability of each animal in a large dataset being a homozygote and heterozygote, to track five genetic conditions, with weekly analysis involving almost 1.3 million animals. Estimates in the AU population are therefore quite precise (Teseling and Parnell 2011). In 2010, the heterozygote frequency of calves born into the AU Angus herd was estimated to be 2.4% AM, 4.3% NH and 4.4% CA, decreased from a high of 5.7% AM in 2005, 6.6% NH in 2005 and 5.1% CA in 2004, in part as a result of the availability of DNA tests (C. Teseling, pers. comm.).
Genetic tests for AM, NH and CA became available 1 January 2009, 15 June 2009 and 4 October 2010, respectively. As of May 2011, 148 677 AM, 110 215 NH and 35 162 CA tests, respectively, had been performed by American Angus Association members since testing for these defects began (B. Schumann, pers. comm.). Assuming these tests cost AU$25 each, this amounts to over AU$7.35 million in testing costs in the US Angus population alone (Table 4). In AU, 17 344 AM, 14 598 NH and 3049 CA tests had been performed as of March 2011 for these three conditions, respectively. This amounts to testing costs of approximately AU$875 000 ($34 991 × $25) in AU testing costs. The use of GeneProb significantly reduced the number of animals that needed to be tested in AU (Teseling and Parnell 2011). Although these testing costs remain substantial, they are dwarfed by what it would have cost to eliminate all of the descendants of GAR Precision 1680 and Rito 9J9 of B156 7T26 from the Angus breed that tested free (e.g. 24 489 AU animals) in both countries. The speed with which these genetic tests were developed is testament to the power of having access to the bovine genome-sequence information, and is perhaps the greatest success story of genomics never told. The proactive response of the breed association in making genotypes available also helped address the problem rapidly and transparently. It is important to realise that although genetic defects can be catastrophic for individual breeders, they do not have to be catastrophic for the industry. The sooner a defect is recognised and the genetic cause identified, the sooner it can be eradicated from the population.
|
DNA information also offers the opportunity to increase the rate of genetic gain by increasing the accuracy of EBVs, especially for traits where records are available only after selection (e.g. carcass traits). The annual rate of genetic change in the seed-stock sector is dictated by four interacting components. These are (1) the intensity of selection, (2) the generation interval (or average age of parents when offspring are born), (3) the amount of genetic variation and (4) the accuracy of selection. Annual advances from selection will be maximised when a few of only the very best candidates are selected and used widely at an early age. In practice, the accuracy of selection of young animals is limited for many of the economically relevant traits, either because the traits have low heritability (e.g. reproductive traits), and/or they can be measured only late in life (e.g. longevity and carcass attributes) and/or under challenging conditions (e.g. disease or nutritional stress). The value of DNA information to improve accuracy at the time of making selection decisions is therefore an important factor in determining whether DNA information will lead to increased economic returns (Garrick and Van Eenennaam 2008).
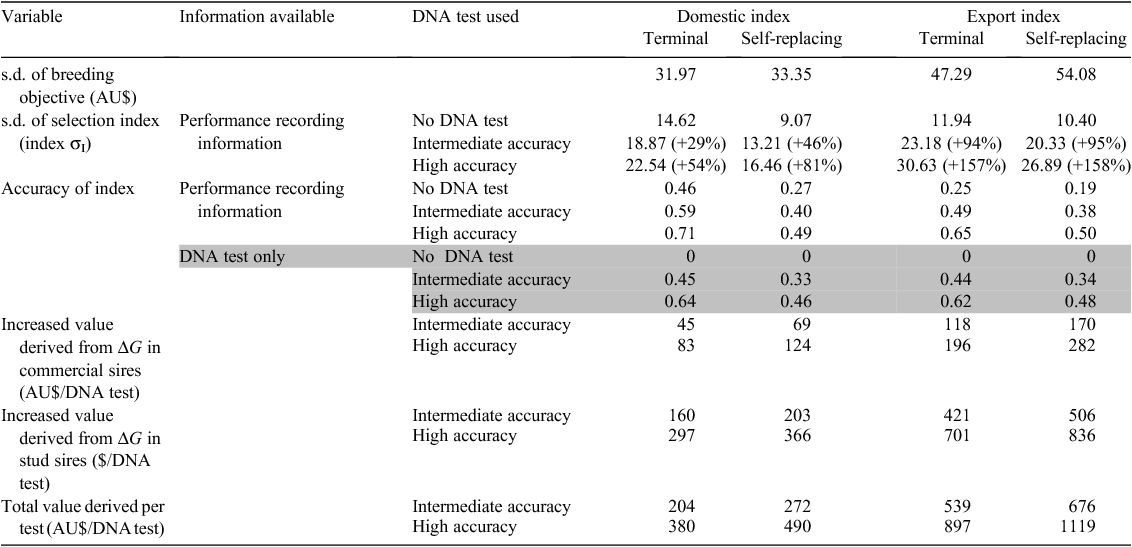
Van Eenennaam et al. (2011) estimated the value derived from using DNA information to increase the accuracy of beef sire selection in a closed seed-stock herd. Breeding objectives for commercial production systems targeting domestic and export markets were examined using multiple-trait selection indices developed for the AU cattle industry. The response to conventional selection based on phenotypic performance records was compared with that obtained following the inclusion of information from DNA tests of varying power. In one case, the DNA test explained a percentage of the additive genetic variance equal to the heritability of all traits in the breeding objective and selection criteria (high accuracy), and in the other case, to one-half of this amount (intermediate accuracy). DNA testing increased the selection response between 29% and 158%. The value of this improvement above that obtained using traditional performance recording ranged from AU$89 to AU$565 per commercial bull, and from AU$5332 to AU$27 910 per stud bull. Assuming that the entire bull-calf crop was tested to achieve these gains and that the top 3% were selected as replacement stud sires and the sale of the remaining top half as commercial bulls, the value generated ranged between AU$204 and AU$1119 per test (Table 4). Genetic gain in traits that resulted in direct revenue to the processing sector accounted for 23–85% of the returns generated by the selection of superior commercial sires, depending on the target market (export v. domestic), selection index (self-replacing v. terminal), and initial index accuracy in the absence of DNA information. These results suggest the development of high-accuracy DNA tests for beef-cattle selection could be beneficial from an industry-wide perspective. However, the return on testing to the seed-stock operator will strongly depend on efficient transfer of revenue derived from genetic improvement in processor traits up the production chain to the seed-stock sector incurring the costs of genotyping.
The study of Van Eenennaam et al. (2011) modelled a scenario where all of the young bulls being sold from the seed-stock to the commercial cow-calf sector were DNA tested, and the inferior half were not used for breeding on the basis of results. This emphasises the sometimes overlooked fact that although DNA tests may improve the accuracy of EBVs, EBVs will not always move in the desired direction. The resultant re-ranking of bulls will move some bulls up, and an equal number down! This has proven to be a source of frustration for some producers who anticipated that purchasing a DNA test would improve both accuracy and the bull’s ranking. In the scenario modelled, every bull marketed for breeding would have to be priced to cover the cost of a superior bull’s DNA test results as well as that of a bull rejected for sale. A major determinant of seed-stock profitability is the proportion of young bulls that can be sold for breeding, and eliminating half of possible sale bulls from contention may be unrealistic. Some seed-stock breeders may be interested in using DNA information only to improve the accuracy of replacement stud-bull selection for their own herd, and not to additionally select the better half of the commercial bulls for sale as was modelled in the Van Eenennaam et al. (2011) study. If a breeder instead chose to sell the entire physically sound bull-calf crop, the value associated with selecting the better commercial sire candidates would be eliminated because selection intensity would drop to zero. However, selling additional commercial bulls would increase the value of replacement stud bulls due to the higher number of descendants this larger group of commercial sale bulls would produce.
The promise of using DNA information to improve the accuracy of national genetic evaluations on young animals is starting to be realised, at least for Angus cattle. To incorporate DNA test information into genetic evaluations, it is necessary to estimate the accuracy of DNA tests in a validation or calibration population outside the discovery set of animals. This process provides the parameters required for their incorporation into genetic evaluations. In the US, this is being carried out by genomics companies in collaboration with some breed associations (MacNeil et al. 2010; Northcutt 2011), whereas in AU, this is being carried out in collaboration with AGBU (Johnston et al. 2011). Genomic-enhanced EBVs are produced using DNA and traditional (performance records, pedigree) information sources. In the US, there is an agreement between Angus Genetics Inc. and both Igenity (Duluth, GA) and Pfizer Animal Genetics (Kalamazoo, MI) to calculate genomic-enhanced expected progeny differences for multiple carcass traits using their tests in conjunction with American Angus Association breed association data. The results of genomic predictions from both Igenity profile for Angus and Pfizer HD 50K for Angus are being incorporated weekly into genetic evaluations for growth, residual average daily gain, calving ease, docility, yearling scrotal circumference and height, mature weight and carcass trait breeding values calculated from phenotypic measurements and pedigree for Angus cattle in the US (Northcutt 2011). In AU, Angus Australia and Pfizer Animal Genetics signed an agreement that enables the full integration of Pfizer Animal Genetics’ molecular value predictions from the HD 50K for Angus into Angus BREEDPLAN (Table 5).

|
Data so far suggest that prediction equations developed for one breed are unlikely to be highly accurate in another. The NBCEC has encouraged discussion about how to develop DNA tests to improve the accuracies of EPDs for breeds other than Angus. Even within Angus, tests trained in North American Angus were associated with less genetic variation when used in the AU and New Zealand Angus population, and required regional recalibration for that population and production system (Table 5). One of the requirements in DNA test development is access to a large training population of genotyped animals from the target breed or its crosses. Some breed associations (e.g. Hereford) are starting to develop such populations, with an eye towards developing breed-specific DNA tests. A similar approach is being implemented in AU, where breed associations are establishing reference ‘beef information nucleus’ populations to provide training and validation populations for breed-specific DNA-test development.
The purebred seed-stock sector is not a large proportion of the national cattle population. Assuming it is 5% of the national herd, this equates to ~1 840 000 US and 645 000 AU cows and heifers. If all bull calves from these cows were tested, assuming a 90% calving rate, that would amount to ~828 800 and 290 000 tests per year, in the US and AU, respectively. Nucleus herds are an even smaller subset of this group. If we assume nucleus herds make up 3% of the seed-stock sector, then this brings down the number of cows making up the nucleus to 55 200 and 19 350 in the US and AU, respectively. The small size of this market is unlikely to generate sufficient returns to attract continued private-sector investment, suggesting there may be a need to secure public-sector and industry funds to develop genomic technologies for improving the accuracy of selection in the seed-stock sector.
Commercial sector
In the commercial cow-calf sector, the principal determinants of income are the number of sale animals and the value per sale animal (Garrick and Golden 2009). Genetic improvement in the commercial sector is largely realised through the purchase of breeding males from multiplier seed-stock herds. As discussed previously, commercial producers may derive value from using DNA information to improve the accuracy of identifying above-average herd sires. However, producers would want this information at the time of purchase and so testing costs would be incurred by the seed-stock producer, and recouped by an increased price at the time of bull purchase. Improving the accuracies associated with these breeding males would have an additional risk-reduction benefit of potential importance to commercial producers, especially for traits such as calving ease where there may be a high cost associated with low-accuracy genetic predictions.
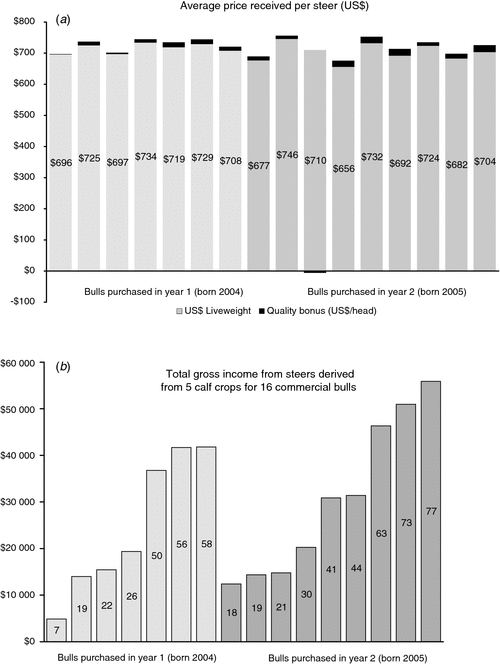
A bull has two qualities of value to commercial producers. One is the ability to impregnate cows, and the other is the ability to pass genes for superior performance on to his offspring. In the absence of the former, the importance of the latter is moot. In large commercial ranches in northern California, we have found that sire prolificacy varies dramatically among apparently healthy bulls that have undergone a breeding soundness examination (Van Eenennaam et al. 2007b). Recently, we compiled data on all of the steer progeny derived from two cohorts of 16 bulls purchased in successive years that all served as herd sires for 5 breeding seasons in multi-sire breeding groups on a commercial ranch (Fig. 2). Offspring were marketed at an average of 314 days of age to the feedlot, and although the producer did not retain ownership he participated in a program that required selection for specific carcass attributes and rewarded carcass quality with a premium paid back to the producer. The average gross return including the quality premium derived from the steer progeny of each sire was US$721 (Fig. 2a), but the total gross revenue derived from all male offspring of each bull ranged from US$4881 to US$55 889 (Fig. 2b) due to differences in sire prolificacy. This huge discrepancy in calf numbers shows how certain bulls in a multi-sire team may disproportionately influence herd genetics and affect profit.
|
The BreedObject software program was used to develop a custom AU$Index for this US commercial production system. Reproduction (cow weaning rate) was the target trait of by far the most importance (42%) in this self-replacing herd (W. Upton, pers. comm.). Melton (1995) suggested that US cow-calf producers who market calves at weaning should have a relative economic emphasis of 47% on reproduction, 24% on production and 30% on carcass traits, whereas those that retain ownership should increase their emphasis to 40% on carcass and 29% on production traits, and decrease their reproduction emphasis to 31%. In this case, where the producer-derived value associated with improving carcass quality was small compared with the total carcass liveweight value, the relative emphasis on reproduction in the $Index remained high. It should be noted that the producer in this case reported additional benefits of participating in the partnership program. These included a genuine interest in producing a quality product for the consumer, a preferred supplier status, and a predictable sale price. This final circumstance is not the case for many smaller US producers who are subject to the vagaries of the auction yard on sale day.
In more general terms, even though nearly all US calves go through the feedlot and are sold on a carcass-quality basis, most commercial producers sell at weaning. Their financial returns are tied very closely to the number of calves, a function of reproduction, and less to growth and even less to carcass traits. In contrast, many AU cattle are sold at a much older age directly for slaughter, and an increasing number are being sold ‘over the hook’ where sale price may vary in accordance with how well animals meet specifications. Additionally, direct consignment sales to the feedlot also reward suppliers selecting on the long-fed high-quality Japanese export market index, which puts an emphasis on marbling. This means there is a financial incentive for AU producers to modify their production system and genetic improvement program to meet carcass requirements. If DNA testing can improve the pre-selection accuracy of EBVs for the suite of reproductive, growth and carcass traits that are generating value to the AU beef industry, their use may be associated with a greater return to producers in AU than in US cattle production systems due to market failure in the latter.
Parentage assignment also allows for the development of on-farm genetic evaluations (Dodds et al. 2005). This offers the opportunity for large commercial farms to produce their own young sires by developing a bull-breeding herd and testing their bulls in multi-sire settings, and using DNA to resolve the offspring paternity at a later date (Pollak 2005). Candidate herd bulls could then be selected on the basis of their progeny-test performance, or possibly on the basis of marker scores if DNA tests are shown to be accurate in commercial or crossbred cattle populations. This approach can considerably reduce the cost of progeny testing. This technique is widely used in the New Zealand sheep industry, but has seen only limited use in the beef industry.
Development of herd sires is an opportunity best suited to operations large enough to spread the costs associated with developing and evaluating young sires. The 441 AU properties carrying in excess of 5400 mother cows would seem to be likely candidates. Garrick (2008) argued that given the relative size of commercial versus the seed-stock sector, there are likely to be more elite animals in the commercial sector. The problem is that there are typically no performance data or EBV information collected on commercial cattle, and so it is not possible to identify superior animals. DNA testing may provide an avenue for commercial animals to become selection candidates. In the Van Eenennaam et al. (2011) study, it was shown that the hypothetical ‘intermediate accuracy’ DNA test provided the same index accuracy as collecting BREEDPLAN performance records on an animal (Table 4). Replacing performance recording with DNA testing in this way would only be feasible if tests were dependably accurate in the populations being tested, and a source of phenotyped individuals remained available for re-estimation of marker effects and the proportion of genetic variation associated with the tests.
DNA testing could also be used to select replacement commercial females, many of which have no EBV information. The beef industry would benefit greatly from improvement in traits directly affecting maternal performance (Roughsedge et al. 2005). The value of testing heifers will depend on the information available at the time of selection, the power of the test and the selection intensity. The latter is dependent on the calving and replacement rates. The break-even cost of testing all of the potential replacement heifers in a commercial herd with a replacement rate of 20% and 45 potential replacement heifers born per 100 cows per year when using the intermediate-accuracy DNA test (index accuracy ~0.33) would be ~AU$13 and ~AU$24 for domestic and export maternal indices, respectively, using the assumptions outlined in Van Eenennaam et al. (2011), and that the commercial producer was not performance recording (i.e. had no other data on which to base heifer-replacement decisions). In practice, selection for replacement heifers is frequently driven by age because heifers that are born later in the calving season are too immature to be cycling in time for the first potential breeding season. This criterion tends to put indirect selection on fertility traits of the dam (e.g. days to calving). Commercial producers would typically select on at least a visual estimate of a heifer’s 400-day weight. This individual record decreases the break-even value of the information provided by the intermediate-accuracy test to ~AU$8 for the domestic maternal index. These estimates again reflect the whole-industry value of genetic improvement in both production and processor traits.
Replacement commercial female selection involves a much larger proportion of the national herd than does seed-stock testing. However, the value derived per test is less because commercial cows produce fewer descendants. Unless DNA tests are developed that have high accuracy for maternal traits, they should be used in conjunction with available phenotypic data. And here is the quandary when developing tests for replacement female selection. Traits that are of the most economic value to self-replacing herds are low-heritability reproductive traits, including age at first calving, reproductive success and replacement rate (Roughsedge et al. 2005). Research results suggest that large numbers of records will be required to obtain accurate DNA tests for low-heritability traits (Goddard 2009; Hayes et al. 2009). Further, such tests are the most difficult to validate because there is a paucity of cattle populations with sufficient phenotypic data to estimate the accuracy of new genetic tests for those traits. However, because commercial producers often have little information on which to base their replacement heifer selection decisions, DNA testing provides an attractive approach to obtain previously absent criteria on reproductive traits prior to selection, although such tests will need to be inexpensive to be commercially viable.
Feedlot sector
Marker-assisted management (MAM) is the process of using the results of DNA-marker testing to predict the future phenotype of the animal being tested and sort individual cattle into management groups that are most likely to achieve specific end points. This process is akin to using a visible characteristic; only DNA information is not readily observable and thus requires additional expense to obtain. In the feedlot sector, costs and revenues are determined by prices and quantities. Revenues are generated by the number of sale animals, their weight and carcass premium or discounts, whereas costs are determined by carcass-growth efficiency, mortality and disease incidence. There exists the potential to use DNA information to manage feedlot cattle. Predicting the difference between the performance of the best and worst animal in the feedlot is of little importance, the economically relevant question is ‘How can I use the DNA test information to profitably sort cattle into management groups?’ Any pre-sorting of lines to reduce the variation in the pen at the end of the feeding period has the potential to be cost-effective.
Consider an example where an AU$10/head DNA test is used to divide a set of 100 cattle into four pens. To break even, the management measures implemented on the basis of the DNA-test results would need to either save AU$10 in costs or result in an extra $10 in income per head. Management measures might include more precisely managing time (e.g. feeding sorted pens a different number of days), and more precisely, applying technology (e.g. using alternative implant or β-agonist strategies) and/or targeting pens to different value-added markets. The costs and benefits associated with these management options will depend on feedlot-specific factors and market conditions, including the difference between the price received for the choice and select quality grade (choice : select spread), other carcass premiums and discounts, cost of feed, daily fixed pen costs, carcass value and saleable carcass weight. The ability of the feedlot manager to fully dilute their overhead costs requires that pens be near capacity. It is problematic for large beef feedlots to partially harvest pens of cattle. Furthermore, half-empty pens can attract only half the yardage fees of full pens. It makes economic sense for feedlots to keep pens full, and to harvest the entire pen when its revenue over costs is maximised.
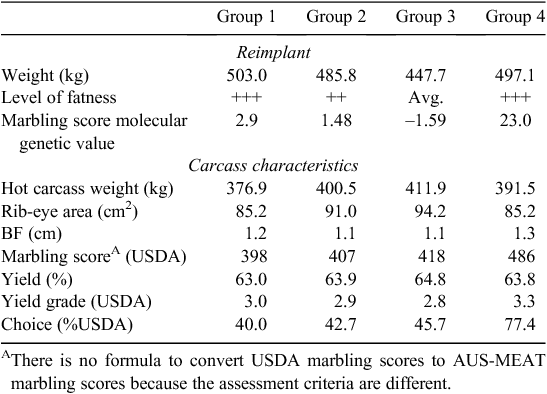
Cattle need to be genotyped for MAM sorting and it takes some time to generate the DNA-test results. In the absence of an approach to share genotypes between sectors, it is generally not possible to use markers to sort cattle on arrival. A strategy that some feedlots have used is to take a DNA sample at receiving and then sort cattle or manage them differently at hormone reimplantation. In 2009, Bill Kolath from Cargill Meat Solutions (Wichita, KS) gave a presentation at the Beef Improvement Federation that outlined how that company used DNA-test information provided to them by MetaMorphix Inc. (MMI, Davis, CA). On arrival at the feedlot, a nasal swab was taken and sent for DNA analysis. MAM decisions we made at the time of hormone reimplantation using the genetic information and body composition of each animal. These included how long to feed the animal, and which, if any, growth-promoting technologies (e.g. hormone implants) to use. Animals were sorted into one of four groups, the goal being to allow the animal the ability to reach its genetic potential while being managed within a group setting. The reimplant and carcass characteristics of each of the four groups are shown in Table 6. Cargill Cattle Feeders was reportedly DNA-testing all 700 000 animals entering their feedlots each year before late October 2009. However, at that time it was announced that Cargill would no longer invest in DNA testing. According to a report in the Washington Business Journal (Sinha 2009), the decision was made because it was found that DNA testing was ‘too expensive to justify in a recession’. This was in part due to volatility in the choice : select spread. MetaMorphix Inc. subsequently announced bankruptcy in early 2011 (Clabaugh 2011).
|
Some studies have reported the usefulness of using a single SNP in the leptin gene for sorting feedlot cattle (Engler et al. 2009). The ‘exon 2’ SNP was one of the first DNA markers discovered with an effect on traits of importance to beef-cattle production (Buchanan et al. 2002). This marker was commercialised as a tool for marker-assisted selection in the early 2000s. Subsequent reports in the literature have been conflicting. Some have found a significant association between this marker and the level of fatness (Kononoff et al. 2005), whereas other studies (Barendse et al. 2005; Casas et al. 2005) and an independent assessment or ‘validation’ by the NBCEC (Van Eenennaam et al. 2007a) found that there was no evidence of a significant association between the inheritance of the ‘T’ allele and increased marbling score in the validation populations examined.
This leptin SNP test is currently being marketed for feedlot marker-assisted management by Quantum Genetics (Saskatoon, SK, Canada) and is being used selectively by Cactus Feeders, the second largest feeding company in the US. They reported a significant interaction between leptin genotype and Zilpaterol Hydrochloride (ZH), a β-adrenergic agonist used as a growth-enhancing agent to increase weight gain in feedlot cattle (Engler et al. 2009). In a randomised complete block design study using 4179 steers in eight blocks, Engler et al. (2009) reported leptin genotype × ZH interactions for marbling score and percentage of carcasses stamped USDA Choice. They found that when steers were not fed ZH, the ‘TT’ steers had significantly greater marbling scores and a greater percentage of carcasses stamped USDA Choice or better than did ‘CC’ steers (63.6% v. 47.9%; P < 0.01). However, when steers were fed ZH, it effectively negated this leptin marker effect, and no differences (P > 0.30) in marbling scores or percentages of carcasses stamped USDA Choice or better were detected between the genotypes (42.9% v. 46.5% USDA Choice or better for ‘TT’ and ‘CC’, respectively; P > 0.30). Cactus Feeders is now using the leptin marker on a limited basis to manage cattle in their feedlot where previously all animals received ZH. In a randomised complete block design study using 2696 steers in 10 blocks at Cactus Feeders, it was found that managing cattle by using this marker in conjunction with phenotypic measurements returned a gross margin of US$13 per head before the costs of genotyping and sorting were considered. This margin improvement was generated through a combination of increased carcass price, and increased saleable-carcass weight (saleable meat %) minus the cost of additional feed fed to sorted cattle (M. Engler, pers. comm.).
Academic economic simulations examining the use of the leptin marker are conflicting in that some conclude that the ‘TT’ cattle are more profitable, whereas others conclude that ‘CC’ cattle are most profitable (DeVuyst et al. 2007; Lusk 2007; Lambert 2008). This is partially the result of the varying market conditions assumed in the different studies. However, a common finding among these studies was that there was some value associated with using the leptin marker to sort cattle into different management groups, but that this value did not outweigh the prevailing cost of leptin testing in the US (US$40–50; DeVuyst et al. 2007). There appears to be limited value associated with using DNA tests to sort cattle on quality grade alone, especially given the variability of the choice : select spread. Recent industry events would tend to support this conclusion. Tests that focus on only single-trait management in a feedlot setting are not likely to capture enough value for the feedlot operator to pay for the test. It is likely that in the future tests will be developed for important feedlot profit drivers (e.g. disease resistance, feed efficiency), which have an impact on multiple outcomes. These developments, along with the continued decline in genotyping costs, may lead to cost-effective approaches to feedlot MAM. The cost and difficulty of obtaining sufficient phenotypes on these hard-to-measure traits should not be underestimated. However, if a large feedlot incentivised DNA collection and genotyping of animals before entry into the feedlot through breeder and producer partnerships and then routinely collected feedlot phenotypes, large databases would soon accumulate. It may be that the combined value derived from using DNA-test information for multiple purposes, in combination with the rapidly declining cost of genotyping, will ultimately push the economics of DNA-based technologies over the tipping point towards more widespread industry adoption (Van Eenennaam 2011).
Conclusions and recommendations
There is opportunity to derive value from DNA information in each of the different sectors that make up the beef industry. At the current time, the costs of genotyping tend to exceed the value that is returned to any single sector. Using genetic tests in combination with performance records to increase the accuracy of EBVs in the seed-stock sector has the potential to generate large returns. Improvements in the accuracy of predicting the genetic merit of stud animals at a young age will affect many descendants, thereby amplifying the value of each unit of genetic improvement. However, increased vertical integration or more efficient price signalling throughout the beef production chain will be required to enable the seed-stock sector to capture the value of this genetic gain. Reproductive traits are a major profit driver of self-replacing herds and DNA tests have the potential to provide previously absent selection criteria for commercial replacement heifer selection. The development of DNA tests for low-heritability reproductive traits will be contingent on the availability of large training populations. Such tests will need to be inexpensive because commercial animals produce fewer descendants from which to recoup testing costs. Feedlots could also use DNA information for marker-assisted management, although again the slim margins associated with feeding cattle will impose a low ceiling on testing costs. Ideally, cattle would be genotyped once early in life and genotypes shared among production sectors to derive the maximum value from the fixed DNA-collection and -extraction costs. Many seed-stock breeders are already collecting DNA for pedigree verification and genetic defect testing, and there would seem to be an opportunity to spread the costs of this investment across the different sectors of the beef industry to extract the maximum value from that information. Groups that can organise themselves to take advantage of the rapidly declining cost of genotyping and capture the cumulative supply-chain value derived from using DNA information for multiple purposes (traceability, parentage, genetic defects, selection, marker-assisted management, product differentiation) will be ideally positioned to fully realise the nascent potential of genomic information.
Acknowledgements
The author thanks Bryce Schumann, Chief Executive Officer, American Angus Association; David Buchanan, Professor, North Dakota State University; Mike Engler, President and Chief Executive Officer, Cactus Feeders; Tom Field, Director of Producer Education,National Cattlemen’s Beef Association; Jennie Pryce, Senior Scientist, Victorian Department of Primary Industries; Carel Teseling, Breed Development and Information Manager, Angus Australia; and Wayne Upton, Extension Specialist, Animal Genetics and Breeding Unit, University of New England, for helpful discussions and/or providing data for the preparation of this manuscript. A. L. Van Eenennaam acknowledges support from the National Research Initiative Competitive Grant No. 2009-55205-05057 from the USDA National Institute of Food and Agriculture.
References
ABARE (2010) Australian beef: financial performance of beef cattle producing farms, 2007–08 to 2009–10. ABARE Project 3364, Canberra.ALFA (2011) Australian Lot Feeders Association/Meat and Livestock Australia Feedlot Survey October – December 2010. Available at http://www.feedlots.com.au/images/stories/SURVEY/mrdec10.pdf [Verified 25 August 2011]
Amer PR, Nieuwhof GJ, Pollott GE, Roughsedge T, Conington J, Simm G (2007) Industry benefits from recent genetic progress in sheep and beef populations. Animal 1, 1414–1426.
Asher L, Diesel G, Summers JF, McGreevy PD, Collins LM (2009) Inherited defects in pedigree dogs. Part 1: disorders related to breed standards. Veterinary Journal (London, England) 182, 402–411.
| Inherited defects in pedigree dogs. Part 1: disorders related to breed standards.Crossref | GoogleScholarGoogle Scholar |
Barendse W, Bunch RJ, Harrison BE (2005) The leptin C73T missense mutation is not associated with marbling and fatness traits in a large gene mapping experiment in Australian cattle. Animal Genetics 36, 86–88.
| The leptin C73T missense mutation is not associated with marbling and fatness traits in a large gene mapping experiment in Australian cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXit1Ggsbs%3D&md5=0feb1a44bd347fdae8723d1d90b1b171CAS |
Barwick SA, Henzell AL (2005) Development successes and issues for the future in deriving and applying selection indexes for beef breeding. Australian Journal of Experimental Agriculture 45, 923–933.
| Development successes and issues for the future in deriving and applying selection indexes for beef breeding.Crossref | GoogleScholarGoogle Scholar |
Beever JE (2009) An update on neuropathic hydrocephalus in cattle. Available at http://www.angus.org/NH_Summary.pdf [Verified 25 August 2011]
Beever JE (2010) Update on contractural Arachnodactyly. [Available at http://www.angus.org/pub/CA/CA_Summary.pdf [Verified 25 August 2011]
Buchanan FC, Fitzsimmons CJ, Van Kessel AG, Thue TD, Winkelman-Sim DC, Schmutz SM (2002) Association of a missense mutation in the bovine leptin gene with carcass fat content and leptin mRNA levels. Genetics, Selection, Evolution. 34, 105–116.
| Association of a missense mutation in the bovine leptin gene with carcass fat content and leptin mRNA levels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjslKgsb0%3D&md5=20a26aa80de4a6867bddef2cf3c3816bCAS |
Casas E, White SN, Riley DG, Smith TPL, Brenneman RA, Olson TA, Johnson DD, Coleman SW, Bennett GL, Chase CC (2005) Assessment of single nucleotide polymorphisms in genes residing on chromosomes 14 and 29 for association with carcass composition traits in Bos indicus cattle. Journal of Animal Science 83, 13–19.
Clabaugh J (2011) MetaMorphix seeks buyer. Washington Business Journal. Available at http://www.bizjournals.com/washington/news/2011/02/04/metamorphix-seeks-buyer.html [Verified 25 August 2011]
DeVuyst EA, Bullinger JR, Bauer ML, Berg PT, Larson DM (2007) An economic analysis of genetic information: leptin genotyping in fed cattle. Journal of Agricultural and Resource Economics 32, 291–305.
Dodds KG, Tate ML, Sise JA (2005) Genetic evaluation using parentage information from genetic markers. Journal of Animal Science 83, 2271–2279.
Engler M, Defoor P, Marquess L (2009) Impact of a leptin SNP and zilpaterol hydrochloride on growth and carcass characteristics of finishing steers. In ‘Beef Improvement Federation 41st annual research symposium’. Available at http://www.bifconference.com/bif2009/ab_c2_5_engler.html [Verified 25 August 2011]
Garrick DJ (2008) How whole genome selection may affect National Cattle Evaluation. In ‘Beef Improvement Federation 40th annual research symposium’. Available at http://www.bifconference.com/bif2008/ppt/DorianGarrick_GP.pdf [Verified 25August 2011]
Garrick DJ, Golden BL (2009) Producing and using genetic evaluations in the United States beef industry of today. Journal of Animal Science 87, E11–E18.
| Producing and using genetic evaluations in the United States beef industry of today.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1M3mtVWjsA%3D%3D&md5=e6051f91f66e937748e23c04ba157600CAS |
Garrick DJ, Van Eenennaam AL (2008) ‘No bull’ discussion on genetic markers. In ‘2008 Florida beef cattle short course’. pp. 33–38. (University of Florida Cooperative Extension). Available at http://animal.ifas.ufl.edu/extension/beef/shortcourse/2008/Garrick.pdf [Verified 25 August 2011]
Goddard M (2009) Genomic selection: prediction of accuracy and maximisation of long term response. Genetica 136, 245–257.
| Genomic selection: prediction of accuracy and maximisation of long term response.Crossref | GoogleScholarGoogle Scholar |
Graser HU, Tier B, Johnston DJ, Barwick SA (2005) Genetic evaluation for the beef industry in Australia. Australian Journal of Experimental Agriculture 45, 913–921.
| Genetic evaluation for the beef industry in Australia.Crossref | GoogleScholarGoogle Scholar |
Hayes BJ, Bowman PJ, Chamberlain AC, Goddard ME (2009) Invited review: genomic selection in dairy cattle: progress and challenges. Journal of Dairy Science 92, 433–443.
| Invited review: genomic selection in dairy cattle: progress and challenges.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXit1Kju7s%3D&md5=23939241311e2729579adc8fdcbcfdabCAS |
Johnston DJ, Jeyaruban MG, Graser H-U (2010) Evaluation of Pfizer Animal Genetics HD 50K MVP calibration. Available at http://agbu.une.edu.au/pdf/Pfizer_50K_September%202010.pdf [Verified 25 August 2011]
Johnston DJ, Tier B, Graser HU (2011) Beef cattle genetic evaluation in the genomics era. Association for the Advancement of Animal Breeding and Genetics 19, 279–286.
Kinghorn BP (1997) An index of information content for genotype probabilities derived from segregation analysis. Genetics 145, 479–483.
Kolath W (2009) Feedlot marker assisted management. In ‘Beef Improvement Federation 41st annual research symposium’. (Ed. AL Van Eenennaam) pp. 103–106. Available at http://www.bifconference.com/bif2009/proceedings/G4_pro_Kolath.pdf [Verified 25August 2011]
Kononoff PJ, Deobald HM, Stewart EL, Laycock AD, Marquess FLS (2005) The effect of a leptin single nucleotide polymorphism on quality grade, yield grade, and carcass weight of beef cattle. Journal of Animal Science 83, 927–932.
Lambert DK (2008) The expected utility of genetic information in beef cattle production. Agricultural Systems 99, 44–52.
| The expected utility of genetic information in beef cattle production.Crossref | GoogleScholarGoogle Scholar |
Lusk JL (2007) Association of single nucleotide polymorphisms in the leptin gene with body weight and backfat growth curve parameters for beef cattle. Journal of Animal Science 85, 1865–1872.
| Association of single nucleotide polymorphisms in the leptin gene with body weight and backfat growth curve parameters for beef cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXot1Oht7Y%3D&md5=3068e392aef92c2c0a37c6c2d8b509cbCAS |
MacNeil MD, Northcutt SL (2008) National cattle evaluation system for combined analysis of carcass characteristics and indicator traits recorded using ultrasound in Angus cattle. Journal of Animal Science 86, 2518–2524.
| National cattle evaluation system for combined analysis of carcass characteristics and indicator traits recorded using ultrasound in Angus cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1ams7rK&md5=36b4ffcaaf194224c488f52237580bffCAS |
MacNeil MD, Northcutt SL, Schnable RD, Garrick DJ, Woodward BW, Taylor JF (2010) Genetic correlations between carcass traits and molecular breeding values in Angus cattle. In ‘Proceedings of the 9th world congress of genetics applied to livestock production’. Available at http://www.kongressband.de/wcgalp2010/assets/pdf/0482.pdf [Verified 25 August 2011]
Marquez GC, Speidel SE, Enns RM, Garrick DJ (2010) Genetic diversity and population structure of American Red Angus cattle. Journal of Animal Science 88, 59–68.
| Genetic diversity and population structure of American Red Angus cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXls1yksw%3D%3D&md5=df992a8ca9c0e3cf4ed8d6945a6c107cCAS |
McCann LP (1974) ‘The battle of bull runts.’ (North Plains Books & Art: Columbus, OH)
Melton BE (1995) Conception to consumption: the economics of genetic improvement. In ‘Proceedings of the Beef Improvement Federation 27th annual research symposium and annual meeting’. Sheridan, Wyoming. pp. 40–87.
NASS (2011) ‘Cattle.’ (Agricultural Statistics Board, United States Department of Agriculture: Washington, DC). Available at http://www.vdacs.virginia.gov/livestock/textfiles/Catt-01-28-2011.txt [Verified 25 August 2011]
Northcutt SL (2011) Genomic choices. American Angus Association®/AngusGenetics Inc. release. Available at http://www.angus.org/AGI/GenomicChoice11102011.pdf [Verified 10 December 2011]
PAG (Pfizer Animal Genetics) (2010) ‘Technical summary.’ Available at https://animalhealth.pfizer.com/sites/pahweb/US/EN/PublishingImages/Genetics%20Assets/HD50K/50K%20Tech%20Summary%204-13-10.pdf [Verified 25 August 2011]
Pollak EJ (2005) Application and impact of new genetic technologies on beef cattle breeding: a ‘real world’ perspective. Australian Journal of Experimental Agriculture 45, 739–748.
| Application and impact of new genetic technologies on beef cattle breeding: a ‘real world’ perspective.Crossref | GoogleScholarGoogle Scholar |
Roughsedge T, Am PR, Thompson R, Simm G (2005) Development of a maternal breeding goal and tools to select for this goal in UK beef production. Animal Science (Penicuik, Scotland) 81, 221–232.
| Development of a maternal breeding goal and tools to select for this goal in UK beef production.Crossref | GoogleScholarGoogle Scholar |
Sinha V (2009) Lost contract takes major bite out of MetaMorphix. Washington Business Journal, 2 November 2009. Available at http://www.bizjournals.com/washington/stories/2009/11/02/story5.html [Verified 25 August 2011]
Sunyaev S, Ramensky V, Koch I, Lathe W, Kondrashov AS, Bork P (2001) Prediction of deleterious human alleles. Human Molecular Genetics 10, 591–597.
| Prediction of deleterious human alleles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXit1ehtbs%3D&md5=c72a0f6be47964fb45f4c6c40be52d6cCAS |
Teseling CF, Parnell PF (2011) The effective management of deleterious genetic conditions of cattle. Association for the Advancement of Animal Breeding and Genetics 19, 131–134.
Van Eenennaam AL (2011) Beef translational genomics: lessons from the literature. Association for the Advancement of Animal Breeding and Genetics 19, 271–278.
Van Eenennaam AL, Li J, Thallman RM, Quaas RL, Dikeman ME, Gill CA, Franke DE, Thomas AG (2007a) Validation of commercial DNA tests for quantitative beef quality traits. Journal of Animal Science 85, 891–900.
| Validation of commercial DNA tests for quantitative beef quality traits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjslWlu7o%3D&md5=185ded947a2309529cd86c8e06d17acdCAS |
Van Eenennaam AL, Weaber RL, Drake DJ, Penedo MCT, Quaas RL, Garrick DJ, Pollak EJ (2007b) DNA-based paternity analysis and genetic evaluation in a large, commercial cattle ranch setting. Journal of Animal Science 85, 3159–3169.
| DNA-based paternity analysis and genetic evaluation in a large, commercial cattle ranch setting.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtl2ru7jF&md5=9ae22235db1b411d3f7aa434c97743cbCAS |
Van Eenennaam AL, van der Werf JHJ, Goddard ME (2011) The value of using DNA markers for beef bull selection in the seedstock sector. Journal of Animal Science 89, 307–320.
| The value of using DNA markers for beef bull selection in the seedstock sector.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhvFSntb0%3D&md5=e5e80496b989f4adbda2021a961016aeCAS |
Whitlock BK, Kaiser L, Maxwell HS (2008) Heritable bovine fetal abnormalities. Theriogenology 70, 535–549.
| Heritable bovine fetal abnormalities.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cvhs1Kmtg%3D%3D&md5=f346ea270e1385584c0672cf8aed05d2CAS |


