The frontiers of biomedical science and its application to animal science in addressing the major challenges facing Australasian dairy farming
Murray D. Mitchell A E , Mallory A. Crookenden B , Kanchan Vaswani A , John R. Roche
A E , Mallory A. Crookenden B , Kanchan Vaswani A , John R. Roche  B C D and Hassendrini N. Peiris
B C D and Hassendrini N. Peiris  A
A
A Centre for Children’s Health Research, Institute of Health and Biomedical Innovation, Faculty of Health, The Queensland University of Technology, 62 Graham Street, South Brisbane, Qld 4101, Australia.
B DairyNZ Ltd, Private Bag 3221, Hamilton 3240, New Zealand.
C School of Biological Sciences, University of Auckland, Private Bag 92019, Auckland 1142, New Zealand.
D Present address: Ministry for Primary Industries, Pastoral House, Wellington 6140, New Zealand.
E Corresponding author. Email: murray.mitchell@qut.edu.au
Animal Production Science 60(1) 1-9 https://doi.org/10.1071/AN18579
Submitted: 10 September 2018 Accepted: 1 December 2018 Published: 21 December 2018
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Extraordinary advances are occurring in biomedical science that may revolutionise how we approach health and disease. Many have applications in the dairy industry. We have described one particular area of extracellular vesicles that have already proven to be of interest in diagnostics and prognostics for fertility and assessment of ‘transition’ cows (i.e. evaluation of the problems related to the risk of clinical diseases in dairy cows, such as mastitis and milk fever, during transition period). The addition of measurements of circulating RNA and DNA may prove of value in identifying dairy cows with higher risks of clinical diseases and potentially poor fertility. We describe the exciting opportunity provided by the possibility of generating exosomes to order as therapeutic agents to potentially enhance fertility. The even more radical concept of using exosomes to deliver a CRISPR-linked gene editing function is presented. Undoubtedly, the use of biomedical advances to assist the dairy industry is an obvious and practical approach that has significant merit.
Additional keywords: dairy cow, exosomes, fertility.
Introduction
Dairy cow health and fertility have declined over successive decades with genetic selection for increased milk production (Garnsworthy et al. 2008; Roche et al. 2011), while, at the same time, consumers have begun to focus more consistently on animal welfare and the desire to ensure that production animals have ‘a life worth living’ (Adamczyk 2018). In most industries, therefore, genetic-selection priorities have changed from productivity focussed to a better balance of production and functional traits, and management practices are being more intensely scrutinised for their effect on both the animal’s functional and affective states (Mellor 2016). Historically, many of the functional traits have been difficult to measure. However, with the development of new technologies for understanding the health and function of different tissues, there is a real opportunity to advance genetic selection for animal health and well-being and to individualise animal care through understanding the effect of farm management on each animal.
The use of non-invasive or minimally invasive methods for diagnosing or predicting the onset of disease or inflammation in real-time or categorising metabolic state patient-side is revolutionising human medicine (Mal 2016). New rapid and precise technologies for genomic, transcriptomic and metabolomic testing are constantly being developed and improved on. In medical practice, they offer clear advantages in patient comfort and well-being, due to either the immediacy of results (i.e. patient side and in real time) and the limited resources required that are declining in price exponentially, or by avoiding investigatory procedures that require periods of post-operative convalescence (Andión et al. 2014; Jo Delaney 2018).
These technologies and approaches can be transferred into animal science and veterinary clinical practice, enabling detailed tissue phenotyping without biopsies, and to better understand the effects of farm management on an animal’s functional and, possibly, affective states. Although blood sampling is at the core of these methods, there is also the potential use of milk in the case of dairy cows (Fricke et al. 2016). The use of saliva and urine, although possible, is more problematic in cattle.
At least two approaches to minimally invasive deep-tissue phenotyping have been advanced in recent years. One has been the use of circulating (free) DNA and RNA (Lo 2016; Lo and Lam 2016). In this, translation into clinical practice is occurring (Sun et al. 2017, 2018). In parallel, but perhaps slightly behind, is research into the use of exosomes as both diagnostic and therapeutic drug-delivery mechanisms. In the space available, we will focus in the potential uses of exosomes in dairy cow research.
Exosomes: the new frontier in patient care
Exosomes are small (40–120 nm), stable, lipid-bilayer nanovesicles that are formed by the inward budding of multivesicular bodies and are released into extracellular compartments, and identified in biological fluids (e.g. in milk, blood, urine and saliva; Mitchell et al. 2015; Koh et al. 2017). Through the analysis of tissue-specific markers on the exosomes, the tissue of origin can be determined (Salomon et al. 2014). They contain a diverse array of signalling molecules, including messenger RNA (mRNA), micro-RNA (miRNA), proteins, lipids and membrane receptors (Hata et al. 2010; Record et al. 2014; Gangoda et al. 2015; Mitchell et al. 2015), and they interact with target cells via multiple pathways. They can either directly activate target cell-membrane receptors, modify the extracellular milieu of the target cell, or fuse with the cell membrane and release their molecular cargo into the target cell (Cronin et al. 2012).
The cargo of circulating exosomes is indicative of the health status of a specific tissue, granting the capacity for use of exosomes as a tool for disease diagnosis without invasive and expensive biopsy techniques. The use of exosomes as diagnostic agents is particularly prevalent in the early detection of cancers (Melo et al. 2015; Kalluri 2016), although they offer considerable promise in the detection of other diseases also because differences in their cargo are being linked to disease states (Mitchell et al. 2015; Ngo et al. 2018). For example, exosomes have a role in the pathogenesis of neurodegenerative disease such as Alzheimer’s and Parkinson’s disease and exosomal content from tissues differs when cancerous tumours are present (Taylor and Gercel-Taylor 2008; Vella et al. 2016). Excitingly, exosomes have the potential to be ‘loaded’ with pharmaceutical agents or intracellular mediators including miRNAs (Lakhal and Wood 2011; Luan et al. 2017), which means that they could be prognostic, diagnostic or therapeutic agents.
The importance of exosomes in health and disease in humans has incited research into exosomes in dairy cattle and may lead to their application as a tool for diagnosing bovine health status (a summary of selected bovine exosome publications is provided in Table S1, available as supplementary material for this paper). With limited space to explore the role of these technologies in the animal sciences, we will focus on the role of exosomes as potential diagnostic and prognostic methods for fertility and transition cow health in dairy cows, as well as the use of exosomes for therapeutics. We will also incorporate, possibly, the most exciting advance in biomedical science in recent years, namely the CRISPR–Cas9 system for gene editing (Hille et al. 2018), with research in exosomes (Kim et al. 2017; Lin et al. 2018).
The decline in dairy cow fertility
The decline in dairy cow fertility has been attributed to the intensive genetic selection that has focussed primarily on milk-production traits. Until recently, very few selection indices have included functional traits, such as the health and reproduction of the dairy cows (Miglior et al. 2005; Walsh et al. 2011; Berry et al. 2016). As a result, milk-production capacity has increased dramatically, but fertility has steadily declined (Garnsworthy et al. 2008; Roche et al. 2011). Consequently, it has been estimated that 50% of the improved profitability from genetic selection for milk production has been lost, at least in grazing systems, due to the decreased productivity associated with reduced fertility (Evans et al. 2006). Poor reproductive efficiency in dairy herds results in longer inter-calving intervals, lower voluntary culling and greater replacement rates, increased cow maintenance costs, and slower genetic progress (Royal et al. 2000). Indications of poor fertility include the frequent occurrence of abnormal oestrus cycles, poor conception rates to first and successive inseminations and loss of pregnancies (Royal et al. 2000).
Fertilisation rates in single-ovulating dairy cows remain relatively high at above 80% and, as such, are not thought to be the issue associated with the production-related loss of fertility (Diskin et al. 2006; Sartori et al. 2010; Walsh et al. 2011). However, early embryonic loss is of concern, as greater rates of embryonic loss/fetal mortality and resulting lower calving rates occur to a greater extent in high-producing dairy cows than in moderate-producing dairy cows (Diskin et al. 2006; Diskin and Morris 2008; Walsh et al. 2011). The inability of the embryo to signal its existence (e.g. failure to initiate maternal recognition of pregnancy is one factor associated with early embryonic loss; Walker et al. 2010). A key component in this signalling is interferon tau, which is required to inhibit the release of anti-luteolytic prostaglandin F2α (PGF2α), and thus prevent the degradation of the corpus luteum and enable the continued release of progesterone, which is required for the maintenance of pregnancy (Diskin et al. 2006; Diskin and Morris 2008; Walsh et al. 2011).
Dairy cow health and the transition into lactation
A successful transition into lactation requires several metabolic, nutritional, physiological and immunological changes to occur in concert (i.e. homeorhesis). These changes make the transition period the time of greatest risk of metabolic and infectious disease for dairy cows, with an estimated third to a half of all cows succumbing to disease (LeBlanc 2010). In fact, in grazing systems, the risk of death during the first 3 weeks post-calving is three to six-fold greater than that at any other stage of lactation (Compton 2018).
Animals unable to meet the metabolic demands associated with the onset of lactation will experience metabolic disorders such as milk fever, ketosis, fatty liver, enteritis and displaced abomasum (Bigras-Poulin et al. 1990; Ingvartsen 2006). Likewise, bacteria-induced inflammatory disorders, such as mastitis and uterine infection, are most prevalent in the first few weeks post-calving (Sheldon and Dobson 2004; Østergaard et al. 2005) and can have profound effects not only on the animal’s health, but on the odds of successfully breeding subsequently (LeBlanc 2010). The severity of these inflammatory disorders and the ability to resolve their infections vary greatly among cows, suggesting that some cows are more at risk to the disease and the failure to adequately resolve (Formigoni and Trevisi 2003; Piccinini et al. 2004; McDougall et al. 2007; Fair 2015). For example, persistence of bacterial contamination in the uterus can result in inflammation, histological lesions of the endometrium, delayed involution and lower conception rates (i.e. negatively affect embryo survival; Sheldon et al. 2006; Herath et al. 2009).
There is a critical need to identify dairy cows with high fertility and those more vulnerable or resistant to disease. The identification of these animals using biomarkers could lead to early interventions and higher survival rates, resulting in reproductive success. Exosomes offer a potential route for the discovery of such markers.
Role of exosome in dairy cow fertility and health
Recently, we investigated the exosomal content of dairy cows differing in their metabolic health status during the onset of lactation (Crookenden et al. 2016b), their uterine health status (Almughlliq et al. 2018) and their fertility status (Mitchell et al. 2016; Koh et al. 2018). The exosomes isolated in each population of dairy cows demonstrated protein cargo profiles different from those of their control counterparts, indicating that the proteomic profile of an exosome is influenced by the ‘environment’ in which it is packaged. Still to be uncovered in these exosomes are the other signalling molecules which they carry, such as miRNA. The differences in exosomal cargo could be of utility in the development of biomarker panels for identification of health/disease status in dairy cows or, potentially, remedial therapy to improve health or the odds of a successful pregnancy.
The miRNA content of bovine milk exosomes differs depending on the stage of lactation (e.g. colostrum in contrast to mid-lactation milk; Hata et al. 2010). Several of the miRNAs and proteins identified in milk exosomes are those that have important roles in maintaining mammary gland health and initiating the immune response (Hata et al. 2010; Reinhardt et al. 2012; Koh et al. 2017). This implies that milk exosomes may have a role in protecting the mammary gland from infection and responding to bacterial pathogens. Indeed, exosomes isolated from bovine milk have unique cargo during mastitis when compared with milk from non-infected animals. For example, milk exosome miRNA profiles differ before, and 48 h after, infection of the mammary gland with Staphylococcus aureus, which may lead to the identification of biomarkers of subclinical mastitis (Sun et al. 2015). Sun et al. (2015) identified 14 miRNAs that were significantly differentially expressed between the control and infected animals and suggested that exosomal miRNAs miR-223 and miR-142-5p may be used as biomarkers of early mammary-gland infection. Furthermore, this gives insight into the host defence mechanism that could be utilised for development of novel treatments of bacterial infection without the need for antibiotics. MicroRNA-223 was identified only in milk exosomes from infected animals and miR-142–5p was upregulated over 250-fold in exosomes from S. aureus-infected animals compared with control animals (Sun et al. 2015). In concordance with this, Cai et al. (2018) also identified significant upregulation of miR-223 and miR-142-5p in milk exosomes of cows with experimentally induced S. aureus infection compared with control animals. This further highlighted these miRNAs as clinically important in mastitis and as potential targets for mastitis diagnosis (Cai et al. 2018).
The importance of exosomes in communication among cells, including those of the immune system, is well established (Théry et al. 2009). Interestingly, miR-223 is an important regulator of innate immune function and is involved in granulopoiesis, the haematopoietic process of granulocyte maturation (Fukao et al. 2007). Neutrophils are one such granulocyte and produce exosomes that have an important role in the inflammatory response (Gasser and Schifferli 2004). Neutrophil function is dampened over the calving period with the transition into lactation, which is evident by gene-expression changes that are likely to increase the risk of infectious disease (Detilleux et al. 1995; Crookenden et al. 2016a). Exosomes are involved in both immune stimulation and tolerance, depending on the cell origin (Raposo et al. 1996), and several studies have proposed the potential use of exosomes in immunotherapy (Pêche et al. 2003; Aline et al. 2004; Morse et al. 2005). Therefore, it is likely that exosomes could be used as a solution to improve immune function, which would be particularly useful during the challenging transition period and during the establishment of embryo within the uterus in early pregnancy.
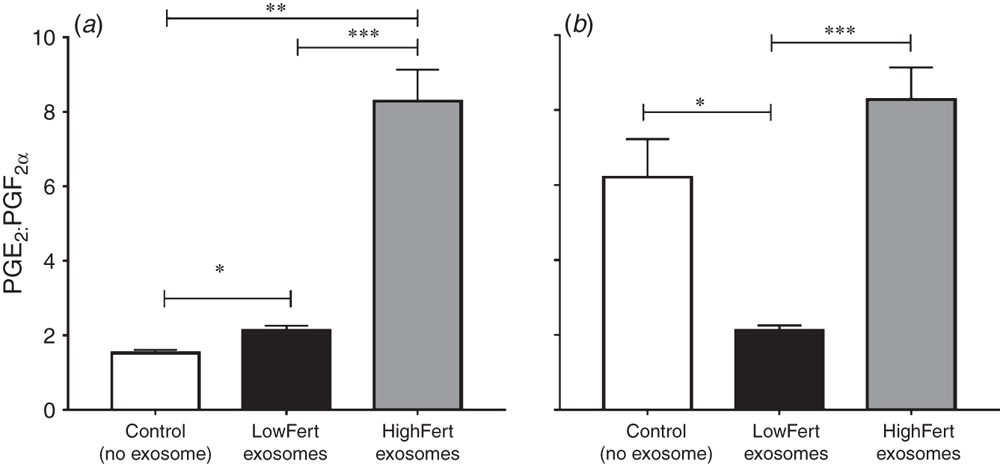
Roles for exosomes in communication during the development of gametes (Sullivan et al. 2005), culture of embryo in vitro (Qu et al. 2017) and between the embryo and mother (Cleys et al. 2014; Saadeldin et al. 2015) have also been described. Our recent investigations have focussed on the effects of exosomes isolated from cows with differing functional characteristics (i.e. divergent genetic merit for fertility, metabolically divergent and the presence or absence of subclinical endometritis on target-cell functions; e.g. cell proliferation and gene expression; Almughlliq et al. 2017; Crookenden et al. 2017). Our published findings have shown that endometrial gene expression is altered by exosomes from metabolically divergent animals and those with subclinical infection. Our, as yet, unpublished findings have also indicated a possible role for exosomes in the recovery of the uterus postpartum for subsequent reproduction (F. B. Almughlliq, Y. Q. Koh, H. N. Peiris, K. Vaswani, S. Meier, C. R. Burke, J. R. Roche, M. A. Crookenden, B. J. Arachchige, S. Reed and M. D. Mitchell, unpubl. data). Moreover, exosomes from the dairy cows genetically selected for inferior reproductive performance significantly altered the production of prostaglandins by endometrial cells towards a pattern negatively associated with the maintenance of pregnancy (Fig. 1; lower ratio of prostaglandin E2 (PGE2) to PGF2α). These findings suggest that low-fertility exosomes carry compounds that prevent the necessary suppression of the anti-luteolytic PGF2α (anti-luteolytic factor) and, therefore, potentially prevent the establishment/maintenance of pregnancy. The mechanism by which these low-fertility exosomes prevent this suppression is still unclear and needs further investigation. However, collectively, our research results have indicated that exosomes are able to package, protect and deliver content that is capable of altering the functions of peripheral tissues in dairy cows.
Exosomes, therefore, may provide a solution for (1) identifying cows that differ in their fertility function and/or their risk of developing disease during the transition period, enabling the identification of those animals that would benefit from early intervention, and (2) disease or subfertility treatment, utilising them as molecular delivery systems to deliver cargo to specific cell types. If we can achieve these solutions, we will be able to ameliorate diseases that create major challenges for dairy farmers and animal welfare.
Exosomes as a therapeutic drug-delivery vehicle
In human medicine, several molecules such as proteins, miRNA, mRNA, small interfering RNA (siRNA), and various chemical drugs and antivirals have been loaded into exosomes with the objective of using the exosomes as a drug-delivery vehicle. For example, Haney et al. (2015) introduced the protein catalase into exosomes, while miRNAs have also been encapsulated (Momen-Heravi et al. 2014).
An exosome may be an ideal candidate vehicle for delivering therapeutics. They have many of the desirable features of an ideal drug-delivery system, such as a long circulating half-life, the intrinsic ability to target tissues and biocompatibility (Ha et al. 2016), meaning that they are less likely to suffer immune rejection by the recipient’s body (Ha et al. 2016). For example, minimal adverse effects have been reported with the cross-species treatments (Zhu et al. 2017). Furthermore, encapsulating drugs or anti-inflammatory compounds in exosomes appears to increase the effectiveness of the delivered material. For example, the inflammatory activity of the phenolic compound curcumin is enhanced when it is encapsulated in exosomes (Sun et al. 2010). Recently, the anti-cancer drug paclitaxel displayed a 50-fold increase in efficacy when encapsulated within an exosome compared with when it was introduced directly (Kim et al. 2016). Exosomes encapsulating molecules may, therefore, serve as a more potent therapeutic delivery mechanism.
Milk exosomes, in particular, are ideal vehicles as they can deliver content across species with bovine milk exosomes known to be taken up by human phagocytes (Pitari et al. 2000; Izumi et al. 2015) and minimal adverse immune or inflammatory responses have been reported (Munagala et al. 2016). In the dairy industry, a major advantage of using milk to generate exosomes is that large volumes of milk are readily available. Collections can be made more frequently than with other fluids such as plasma. Large numbers of high-purity exosomes (fewer contaminating particles) can also be obtained from milk than from some other fluids such as saliva and urine (Koh et al. 2017; Vaswani et al. 2017).
Using exosomes as a vehicle is also advantageous as they can be introduced into the body via several routes. Some routes of administration include intranasal spray, intravenous injection and orally via injecting (i.e. by drinking fortified milk, among others). Zhuang et al. (2011) used curcumin encapsulated exosomes from the nasal region to the brain. Another research group has studied the effects of administering exosomes both intradermally and subcutaneously (Hao et al. 2006).
The development of targeted drug-delivery system for within the brain has been hindered by the inability of therapeutic molecules, with proven in vitro efficacy, to cross the blood brain barrier. A study by Alvarez-Erviti et al. (2011) highlighted the potential of exosomes to cross the blood–brain barrier. Here, GAPDH siRNA was loaded via electroporation into RVG exosomes, and delivered by intravenous injection into the tails of C57BL/6 mice (Alvarez-Erviti et al. 2011). Following which, a gene-specific knockdown as mediated by the exosomes was observed in the striatum, mid-brain and cortex of these mice (Alvarez-Erviti et al. 2011). Zhuang et al. (2011) used exosome-encapsulated curcumin for brain-related complications. Several other studies have reported similar abilities for exosomes to cross the blood–brain barrier and affect brain functions (Zhuang et al. 2011; Ridder et al. 2014; Haney et al. 2015).
Currently there are several methods of exosomal loading (i.e. incorporation of molecules into an intact exosome vesicle). These involve sonication, incubation, electroporation, blue light and chemical-based methodologies (Sun et al. 2010; Kim et al. 2016; Yim et al. 2016; Qu et al. 2017). Luan et al. (2017) suggested that passive loading via incubation may be less disruptive to exosomes; however, electroporation has proven successful in several studies and seems to be more widespread in its use (Alvarez-Erviti et al. 2011; Luan et al. 2017). To enable detection of loaded exosomes after delivery in in vivo models, fluorescent dyes labelling the exosome membrane are employed (Zhuang et al. 2011). DiO, DiR and PKH lipophilic dyes are commonly used in both in vitro and in vivo studies, as they have an affinity for the exosome membranes (Zhuang et al. 2011; Ohno et al. 2013; Tian et al. 2014).
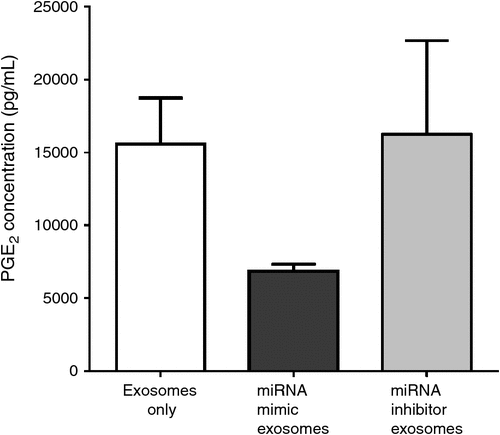
We recently evaluated the possibility of loading miRNA into milk exosomes (Fig. 2). The miRNA chosen was miR143 due to its ability to inhibit the action of cyclooxygenase (COX-2; also known as prostaglandin-endoperoxide synthase 2, prostaglandin H synthase; Ochs et al. 2011). COX-2 is an enzyme that catalyses the conversion of arachidonic acid to prostaglandins (e.g. PGE2 and PGF2α), which are crucial for the initiation and maintenance of pregnancy. Therefore, it serves as a potential miRNA of choice for loading into exosomes as a therapeutic agent for drug delivery, to improve reproductive function. The incubation methodology evaluated for the loading of mi143 and its inhibitor (specifically inhibit miR143 function) was that of 1 h at 37°C, as described by (Johnsen et al. 2014). The miR143 and miR143 inhibitor-loaded exosomes were co-incubated with bovine endometrial epithelial cells. Media was collected after 24 h of incubation and PGE2 production measured. The production of PGE2 by bovine endometrial epithelial cells decreased when treated with loaded miR143 milk exosomes. This inhibitory effect on PG production is in line with the expected result, as miR143 has been documented to supress Cox2 (Ochs et al. 2011). Still to be evaluated is the gene and protein expression of COX-2 in these cells. These results are promising and, with further development, the technique could be a potential candidate as a therapeutic agent to improve reproductive function.
|
The limitations currently arising in exosome applications are due to the infancy of this research field. As yet, the mechanisms by which exosomes target other cells (i.e. selectively deliver their content) are largely unknown. Another limitation is identifying the cell site of origin. By better understanding these two issues, both the sensitivity in diagnostics and therapeutics can be improved. Recent publications have shown an improvement in the ‘signal to noise ratio’ of knowing the cell site of origin, with examples being the use of cell-surface marker Glypican-1 for exosomes of cancer-cell origin, placental alkaline phosphatase for exosome from placental origin and Interferon tau for exosomes of bovine conceptus origin (Sarker et al. 2014; Melo et al. 2015; Nakamura et al. 2016). Several publications have also described ways of modifying the surface of an exosome in an attempt to target their delivery. These include both covalent and non-covalent strategies for the incorporation of target molecules on the surface of pre-isolated exosomes (Smyth et al. 2014; Wang et al. 2014; Nakase and Futaki 2015; Hood 2016; Armstrong et al. 2017; Luan et al. 2017) as well as using transfection to generate exosomes with targets on their surface. Through further development of methodologies to identify cells of origin and incorporate target structures, the potential of exosomes in diagnostics and therapeutics will greatly improve.
Conclusions and implications
The discovery of long-distance, inter-cellular nanoparticle messengers has provided a potential diagnostic and therapeutic technology. There is increasing evidence indicating that tissues differentially populate exosomal cargo, depending on their health status and functional state, providing a potential diagnostic platform for diseases that, historically, could have been detected only via invasive procedures, such as operations or biopsies. Furthermore, understanding the role of these molecular cargo components could provide new therapeutic drugs, but, more importantly, the exosome itself provides a vehicle for drug delivery that is targeted, long-lived, and, importantly, not prone to negative host reactions. As the physical technology that allows the measurement of exosomal cargo develops, such that it is easier, faster and less expensive to measure the contents of the exosomal cargo, it is plausible that in the next decade cow-side tests for the most common or important diseases and therapeutic solutions will be developed.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
Some studies described from our research group were funded via a partnership fund (DRCX1302) between the New Zealand Ministry of Business, Innovation and Employment and New Zealand dairy farmers through DairyNZ Inc. Laboratory experiments were also funded, in part, by the Australian Research Council (Grant Numbers ARC DP170104273 and ARC LP160101854) and the tissues investigated originated from a research platform funded by the aforementioned partnership fund (DRCX1302). Students in our laboratory have been supported by student scholarships from Shimadzu Corporation, Kyoto, Japan, and DairyNZ, Hamilton, New Zealand.
References
Adamczyk K (2018) Dairy cattle welfare as a result of human–animal relationship: a review. Annals of Animal Science 18, 601–622.| Dairy cattle welfare as a result of human–animal relationship: a review.Crossref | GoogleScholarGoogle Scholar |
Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I (2004) Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infection and Immunity 72, 4127–4137.
| Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection.Crossref | GoogleScholarGoogle Scholar |
Almughlliq FB, Koh YQ, Peiris HN, Vaswani K, McDougall S, Graham EM, Burke CR, Mitchell MD (2017) Effect of exosomes from plasma of dairy cows with or without an infected uterus on prostaglandin production by endometrial cell lines. Journal of Dairy Science 100, 9143–9152.
| Effect of exosomes from plasma of dairy cows with or without an infected uterus on prostaglandin production by endometrial cell lines.Crossref | GoogleScholarGoogle Scholar |
Almughlliq FB, Koh YQ, Peiris HN, Vaswani K, McDougall S, Graham EM, Burke CR, Arachchige BJ, Reed S, Mitchell MD (2018) Proteomic content of circulating exosomes in dairy cows with or without uterine infection. Theriogenology 114, 173–179.
| Proteomic content of circulating exosomes in dairy cows with or without uterine infection.Crossref | GoogleScholarGoogle Scholar |
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology 29, 341–345.
| Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes.Crossref | GoogleScholarGoogle Scholar |
Andión O, Canellas M, Banos JE (2014) Physical well-being in postoperative period: a survey in patients, nurses and physicians. Journal of Clinical Nursing 23, 1421–1429.
| Physical well-being in postoperative period: a survey in patients, nurses and physicians.Crossref | GoogleScholarGoogle Scholar |
Armstrong JPK, Holme MN, Stevens MM (2017) Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano 11, 69–83.
| Re-engineering extracellular vesicles as smart nanoscale therapeutics.Crossref | GoogleScholarGoogle Scholar |
Berry DP, Friggens NC, Lucy M, Roche JR (2016) Milk production and fertility in cattle. Annual Review of Animal Biosciences 4, 269–290.
| Milk production and fertility in cattle.Crossref | GoogleScholarGoogle Scholar |
Bigras-Poulin M, Meek AH, Martin SW (1990) Interrelationships among health problems and milk production from consecutive lactations in selected Ontario Holstein cows. Preventive Veterinary Medicine 8, 15–24.
| Interrelationships among health problems and milk production from consecutive lactations in selected Ontario Holstein cows.Crossref | GoogleScholarGoogle Scholar |
Cai M, He H, Jia X, Chen S, Wang J, Shi Y, Liu B, Xiao W, Lai S (2018) Genome-wide microRNA profiling of bovine milk-derived exosomes infected with Staphylococcus aureus. Cell Stress & Chaperones 23, 663–672.
| Genome-wide microRNA profiling of bovine milk-derived exosomes infected with Staphylococcus aureus.Crossref | GoogleScholarGoogle Scholar |
Cleys ER, Halleran JL, McWhorter E, Hergenreder J, Enriquez VA, da Silveira JC, Bruemmer JE, Winger QA, Bouma GJ (2014) Identification of microRNAs in exosomes isolated from serum and umbilical cord blood, as well as placentomes of gestational day 90 pregnant sheep. Molecular Reproduction and Development 81, 983–993.
| Identification of microRNAs in exosomes isolated from serum and umbilical cord blood, as well as placentomes of gestational day 90 pregnant sheep.Crossref | GoogleScholarGoogle Scholar |
Compton CWR (2018) The epidemiology of culling and mortality of New Zealand dairy cows. PhD Thesis, Veterinary Epidemiology, Massey University, Manawatu, New Zealand.
Cronin JG, Turner ML, Goetze L, Bryant CE, Sheldon IM (2012) Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biology of Reproduction 86, 51
| Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium.Crossref | GoogleScholarGoogle Scholar |
Crookenden MA, Heiser A, Murray A, Dukkipati VSR, Kay JK, Loor JJ, Meier S, Mitchell MD, Moyes KM, Walker CG, Roche JR (2016a) Parturition in dairy cows temporarily alters the expression of genes in circulating neutrophils. Journal of Dairy Science 99, 6470–6483.
| Parturition in dairy cows temporarily alters the expression of genes in circulating neutrophils.Crossref | GoogleScholarGoogle Scholar |
Crookenden MA, Walker CG, Peiris H, Koh Y, Heiser A, Loor JJ, Moyes KM, Murray A, Dukkipati VSR, Kay JK, Meier S, Roche JR, Mitchell MD (2016b) Short communication: proteins from circulating exosomes represent metabolic state in transition dairy cows. Journal of Dairy Science 99, 7661–7668.
| Short communication: proteins from circulating exosomes represent metabolic state in transition dairy cows.Crossref | GoogleScholarGoogle Scholar |
Crookenden MA, Walker CG, Peiris H, Koh Y, Almughlliq F, Vaswani K, Reed S, Heiser A, Loor JJ, Kay JK, Meier S, Donkin SS, Murray A, Dukkipati VSR, Roche JR, Mitchell MD (2017) Effect of circulating exosomes from transition cows on Madin–Darby bovine kidney cell function. Journal of Dairy Science 100, 5687–5700.
| Effect of circulating exosomes from transition cows on Madin–Darby bovine kidney cell function.Crossref | GoogleScholarGoogle Scholar |
Detilleux JC, Kehrli ME, Stabel JR, Freeman AE, Kelley DH (1995) Study of immunological dysfunction in periparturient Holstein cattle selected for high and average milk production. Veterinary Immunology and Immunopathology 44, 251–267.
| Study of immunological dysfunction in periparturient Holstein cattle selected for high and average milk production.Crossref | GoogleScholarGoogle Scholar |
Diskin MG, Morris DG (2008) Embryonic and early foetal losses in cattle and other ruminants. Reproduction in Domestic Animals 43, 260–267.
| Embryonic and early foetal losses in cattle and other ruminants.Crossref | GoogleScholarGoogle Scholar |
Diskin MG, Murphy JJ, Sreenan JM (2006) Embryo survival in dairy cows managed under pastoral conditions. Animal Reproduction Science 96, 297–311.
| Embryo survival in dairy cows managed under pastoral conditions.Crossref | GoogleScholarGoogle Scholar |
Evans RD, Wallace M, Shalloo L, Garrick DJ, Dillon P (2006) Financial implications of recent declines in reproduction and survival of Holstein–Friesian cows in spring-calving Irish dairy herds. Agricultural Systems 89, 165–183.
| Financial implications of recent declines in reproduction and survival of Holstein–Friesian cows in spring-calving Irish dairy herds.Crossref | GoogleScholarGoogle Scholar |
Fair T (2015) The contribution of the maternal immune system to the establishment of pregnancy in cattle. Frontiers in Immunology 6, 7
| The contribution of the maternal immune system to the establishment of pregnancy in cattle.Crossref | GoogleScholarGoogle Scholar |
Formigoni A, Trevisi E (2003) Transition cow: interaction with fertility. Veterinary Research Communications 27, 143–152.
| Transition cow: interaction with fertility.Crossref | GoogleScholarGoogle Scholar |
Fricke PM, Ricci A, Giordano JO, Carvalho PD (2016) Methods for and implementation of pregnancy diagnosis in dairy cows. The Veterinary Clinics of North America. Food Animal Practice 32, 165–180.
| Methods for and implementation of pregnancy diagnosis in dairy cows.Crossref | GoogleScholarGoogle Scholar |
Fukao T, Fukuda Y, Kiga K, Sharif J, Hino K, Enomoto Y, Kawamura A, Nakamura K, Takeuchi T, Tanabe M (2007) An evolutionarily conserved mechanism for microRNA-223 expression revealed by microRNA gene profiling. Cell 129, 617–631.
| An evolutionarily conserved mechanism for microRNA-223 expression revealed by microRNA gene profiling.Crossref | GoogleScholarGoogle Scholar |
Gangoda L, Boukouris S, Liem M, Kalra H, Mathivanan S (2015) Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic? Proteomics 15, 260–271.
| Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic?Crossref | GoogleScholarGoogle Scholar |
Garnsworthy PC, Sinclair KD, Webb R (2008) Integration of physiological mechanisms that influence fertility in dairy cows. Animal 2, 1144–1152.
| Integration of physiological mechanisms that influence fertility in dairy cows.Crossref | GoogleScholarGoogle Scholar |
Gasser O, Schifferli JA (2004) Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 104, 2543–2548.
| Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis.Crossref | GoogleScholarGoogle Scholar |
Ha D, Yang N, Nadithe V (2016) Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharmaceutica Sinica. B 6, 287–296.
| Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges.Crossref | GoogleScholarGoogle Scholar |
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV (2015) Exosomes as drug delivery vehicles for Parkinson’s disease therapy. Journal of Controlled Release 207, 18–30.
| Exosomes as drug delivery vehicles for Parkinson’s disease therapy.Crossref | GoogleScholarGoogle Scholar |
Hao S, Ye Z, Yang J, Bai O, Xiang J (2006) Intradermal vaccination of dendritic cell-derived exosomes is superior to a subcutaneous one in the induction of antitumor immunity. Cancer Biotherapy & Radiopharmaceuticals 21, 146–154.
| Intradermal vaccination of dendritic cell-derived exosomes is superior to a subcutaneous one in the induction of antitumor immunity.Crossref | GoogleScholarGoogle Scholar |
Hata T, Murakami K, Nakatani H, Yamamoto Y, Matsuda T, Aoki N (2010) Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochemical and Biophysical Research Communications 396, 528–533.
| Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs.Crossref | GoogleScholarGoogle Scholar |
Herath S, Lilly ST, Santos NR, Gilbert RO, Goetze L, Bryant CE, White JO, Cronin J, Sheldon IM (2009) Expression of genes associated with immunity in the endometrium of cattle with disparate postpartum uterine disease and fertility. Reproductive Biology and Endocrinology 7, 55
| Expression of genes associated with immunity in the endometrium of cattle with disparate postpartum uterine disease and fertility.Crossref | GoogleScholarGoogle Scholar |
Hille F, Richter H, Wong SP, Bratovic M, Ressel S, Charpentier E (2018) The biology of CRISPR–Cas: backward and forward. Cell 172, 1239–1259.
| The biology of CRISPR–Cas: backward and forward.Crossref | GoogleScholarGoogle Scholar |
Hood JL (2016) Post isolation modification of exosomes for nanomedicine applications. Nanomedicine 11, 1745–1756.
| Post isolation modification of exosomes for nanomedicine applications.Crossref | GoogleScholarGoogle Scholar |
Ingvartsen KL (2006) Feeding- and management-related diseases in the transition cow: physiological adaptations around calving and strategies to reduce feeding-related diseases. Animal Feed Science and Technology 126, 175–213.
| Feeding- and management-related diseases in the transition cow: physiological adaptations around calving and strategies to reduce feeding-related diseases.Crossref | GoogleScholarGoogle Scholar |
Izumi H, Tsuda M, Sato Y, Kosaka N, Ochiya T, Iwamoto H, Namba K, Takeda Y (2015) Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. Journal of Dairy Science 98, 2920–2933.
| Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages.Crossref | GoogleScholarGoogle Scholar |
Jo Delaney L (2018) Patient-centred care as an approach to improving health care in Australia. Collegian 25, 119–123.
| Patient-centred care as an approach to improving health care in Australia.Crossref | GoogleScholarGoogle Scholar |
Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M (2014) A comprehensive overview of exosomes as drug delivery vehicles: endogenous nanocarriers for targeted cancer therapy. Biochimica et Biophysica Acta 1846, 75–87.
Kalluri R (2016) The biology and function of exosomes in cancer. The Journal of Clinical Investigation 126, 1208–1215.
| The biology and function of exosomes in cancer.Crossref | GoogleScholarGoogle Scholar |
Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV (2016) Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine (London) 12, 655–664.
| Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells.Crossref | GoogleScholarGoogle Scholar |
Kim SM, Yang Y, Oh SJ, Hong Y, Seo M, Jang M (2017) Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. Journal of Controlled Release 266, 8–16.
| Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting.Crossref | GoogleScholarGoogle Scholar |
Koh YQ, Peiris HN, Vaswani K, Meier S, Burke CR, Macdonald KA, Roche JR, Almughlliq F, Arachchige BJ, Reed S, Mitchell MD (2017) Characterization of exosomes from body fluids of dairy cows. Journal of Animal Science 95, 3893–3904.
Koh YQ, Peiris HN, Vaswani K, Almughlliq FB, Meier S, Burke CR, Roche JR, Reed CB, Arachchige BJ, Reed S, Mitchell MD (2018) Proteome profiling of exosomes derived from plasma of heifers with divergent genetic merit for fertility. Journal of Dairy Science 101, 6462–6473.
| Proteome profiling of exosomes derived from plasma of heifers with divergent genetic merit for fertility.Crossref | GoogleScholarGoogle Scholar |
Lakhal S, Wood MJ (2011) Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. BioEssays 33, 737–741.
| Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers.Crossref | GoogleScholarGoogle Scholar |
LeBlanc S (2010) Monitoring metabolic health of dairy cattle in the transition period. The Journal of Reproduction and Development 56, S29–S35.
| Monitoring metabolic health of dairy cattle in the transition period.Crossref | GoogleScholarGoogle Scholar |
Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L, Tan J (2018) Exosome–liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Advanced Science 5, 1 700 611
| Exosome–liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs.Crossref | GoogleScholarGoogle Scholar |
Lo YMD (2016) Noninvasive prenatal testing complicated by maternal malignancy: new tools for a complex problem. NPJ Genomic Medicine 1, 15002
| Noninvasive prenatal testing complicated by maternal malignancy: new tools for a complex problem.Crossref | GoogleScholarGoogle Scholar |
Lo YM, Lam WK (2016) Tracing the tissue of origin of plasma DNA-feasibility and implications. Annals of the New York Academy of Sciences 1376, 14–17.
| Tracing the tissue of origin of plasma DNA-feasibility and implications.Crossref | GoogleScholarGoogle Scholar |
Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D (2017a) Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacologica Sinica 38, 754–763.
| Engineering exosomes as refined biological nanoplatforms for drug delivery.Crossref | GoogleScholarGoogle Scholar |
Mal M (2016) Noninvasive metabolic profiling for painless diagnosis of human diseases and disorders. Future Science OA 2, FSO106
| Noninvasive metabolic profiling for painless diagnosis of human diseases and disorders.Crossref | GoogleScholarGoogle Scholar |
McDougall S, Macaulay R, Compton C (2007) Association between endometritis diagnosis using a novel intravaginal device and reproductive performance in dairy cattle. Animal Reproduction Science 99, 9–23.
| Association between endometritis diagnosis using a novel intravaginal device and reproductive performance in dairy cattle.Crossref | GoogleScholarGoogle Scholar |
Mellor DJ (2016) Updating animal welfare thinking: moving beyond the ‘five freedoms’ towards ‘a life worth living’. Animals 6, 21
| Updating animal welfare thinking: moving beyond the ‘five freedoms’ towards ‘a life worth living’.Crossref | GoogleScholarGoogle Scholar |
Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R (2015) Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523, 177–182.
| Glypican-1 identifies cancer exosomes and detects early pancreatic cancer.Crossref | GoogleScholarGoogle Scholar |
Miglior F, Muir BL, Van Doormaal BJ (2005) Selection indices in Holstein cattle of various countries. Journal of Dairy Science 88, 1255–1263.
| Selection indices in Holstein cattle of various countries.Crossref | GoogleScholarGoogle Scholar |
Mitchell MD, Peiris HN, Kobayashi M, Koh YQ, Duncombe G, Illanes SE, Rice GE, Salomon C (2015) Placental exosomes in normal and complicated pregnancy. American Journal of Obstetrics and Gynecology 213, S173–S181.
| Placental exosomes in normal and complicated pregnancy.Crossref | GoogleScholarGoogle Scholar |
Mitchell MD, Scholz-Romero K, Reed S, Peiris HN, Koh YQ, Meier S, Walker CG, Burke CR, Roche JR, Rice G, Salomon C (2016) Plasma exosome profiles from dairy cows with divergent fertility phenotypes. Journal of Dairy Science 99, 7590–7601.
| Plasma exosome profiles from dairy cows with divergent fertility phenotypes.Crossref | GoogleScholarGoogle Scholar |
Momen-Heravi F, Bala S, Bukong T, Szabo G (2014) Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine 10, 1517–1527.
| Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages.Crossref | GoogleScholarGoogle Scholar |
Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK (2005) A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. Journal of Translational Medicine 3, 9
| A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer.Crossref | GoogleScholarGoogle Scholar |
Munagala R, Aqil F, Jeyabalan J, Gupta RC (2016) Bovine milk-derived exosomes for drug delivery. Cancer Letters 371, 48–61.
| Bovine milk-derived exosomes for drug delivery.Crossref | GoogleScholarGoogle Scholar |
Nakamura K, Kusama K, Bai R, Sakurai T, Isuzugawa K, Godkin JD, Suda Y, Imakawa K (2016) Induction of IFNT-stimulated genes by conceptus-derived exosomes during the attachment period. PLoS One 11, e0158278
| Induction of IFNT-stimulated genes by conceptus-derived exosomes during the attachment period.Crossref | GoogleScholarGoogle Scholar |
Nakase I, Futaki S (2015) Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Scientific Reports 5, 10112
Ngo TTM, Moufarrej MN, Rasmussen MH, Camunas-Soler J, Pan W, Okamoto J, Neff NF, Liu K, Wong RJ, Downes K, Tibshirani R, Shaw GM, Skotte L, Stevenson DK, Biggio JR, Elovitz MA, Melbye M, Quake SR (2018) Noninvasive blood tests for fetal development predict gestational age and preterm delivery. Science 360, 1133–1136.
| Noninvasive blood tests for fetal development predict gestational age and preterm delivery.Crossref | GoogleScholarGoogle Scholar |
Ochs MJ, Steinhilber D, Suess B (2011) MicroRNA involved in inflammation: control of eicosanoid pathway. Frontiers in Pharmacology 2, 39
| MicroRNA involved in inflammation: control of eicosanoid pathway.Crossref | GoogleScholarGoogle Scholar |
Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M (2013) Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Molecular Therapy 21, 185–191.
| Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells.Crossref | GoogleScholarGoogle Scholar |
Østergaard S, Chagunda MG, Friggens NC, Bennedsgaard TW, Klaas IC (2005) A stochastic model simulating pathogen-specific mastitis control in a dairy herd. Journal of Dairy Science 88, 4243–4257.
| A stochastic model simulating pathogen-specific mastitis control in a dairy herd.Crossref | GoogleScholarGoogle Scholar |
Pêche H, Heslan M, Usal C, Amigorena S, Cuturi MC (2003) Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation 76, 1503–1510.
| Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection.Crossref | GoogleScholarGoogle Scholar |
Piccinini R, Binda E, Belotti M, Casirani G, Zecconi A (2004) The evaluation of non-specific immune status of heifers in field conditions during the periparturient period. Veterinary Research 35, 539–550.
| The evaluation of non-specific immune status of heifers in field conditions during the periparturient period.Crossref | GoogleScholarGoogle Scholar |
Pitari G, Malergue F, Martin F, Philippe JM, Massucci MT, Chabret C, Maras B, Dupre S, Naquet P, Galland F (2000) Pantetheinase activity of membrane-bound Vanin-1: lack of free cysteamine in tissues of Vanin-1 deficient mice. FEBS Letters 483, 149–154.
| Pantetheinase activity of membrane-bound Vanin-1: lack of free cysteamine in tissues of Vanin-1 deficient mice.Crossref | GoogleScholarGoogle Scholar |
Qu P, Qing S, Liu R, Qin H, Wang W, Qiao F, Ge H, Liu J, Zhang Y, Cui W, Wang Y (2017) Effects of embryo-derived exosomes on the development of bovine cloned embryos. PLoS One 12, e0174535
| Effects of embryo-derived exosomes on the development of bovine cloned embryos.Crossref | GoogleScholarGoogle Scholar |
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. The Journal of Experimental Medicine 183, 1161–1172.
| B lymphocytes secrete antigen-presenting vesicles.Crossref | GoogleScholarGoogle Scholar |
Record M, Carayon K, Poirot M, Silvente-Poirot S (2014) Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochimica et Biophysica Acta (BBA): Molecular and Cell Biology of Lipids 1841, 108–120.
| Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies.Crossref | GoogleScholarGoogle Scholar |
Reinhardt TA, Lippolis JD, Nonnecke BJ, Sacco RE (2012) Bovine milk exosome proteome. Journal of Proteomics 75, 1486–1492.
| Bovine milk exosome proteome.Crossref | GoogleScholarGoogle Scholar |
Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Del Turco D, Starmann J, Macas J, Karpova D, Devraj K, Depboylu C, Landfried B, Arnold B, Plate KH, Hoglinger G, Sultmann H, Altevogt P, Momma S (2014) Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biology 12, e1001874
| Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation.Crossref | GoogleScholarGoogle Scholar |
Roche JR, Burke CR, Meier S, Walker CG (2011) Nutrition × reproduction interaction in pasture-based systems: is nutrition a factor in reproductive failure? Animal Production Science 51, 1045–1066.
| Nutrition × reproduction interaction in pasture-based systems: is nutrition a factor in reproductive failure?Crossref | GoogleScholarGoogle Scholar |
Royal M, Mann GE, Flint APF (2000) Strategies for reversing the trend towards subfertility in dairy cattle. Veterinary Journal 160, 53–60.
| Strategies for reversing the trend towards subfertility in dairy cattle.Crossref | GoogleScholarGoogle Scholar |
Saadeldin IM, Oh HJ, Lee BC (2015) Embryonic–maternal cross-talk via exosomes: potential implications. Stem Cells and Cloning: Advances and Applications 8, 103–107.
Salomon C, Torres MJ, Kobayashi M, Scholz-Romero K, Sobrevia L, Dobierzewska A, Illanes SE, Mitchell MD, Rice GE (2014) A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS One 9, e98667
| A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration.Crossref | GoogleScholarGoogle Scholar |
Sarker S, Scholz-Romero K, Perez A, Illanes SE, Mitchell MD, Rice GE, Salomon C (2014) Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. Journal of Translational Medicine 12, 204
Sartori R, Bastos MR, Wiltbank MC (2010) Factors affecting fertilisation and early embryo quality in single- and superovulated dairy cattle. Reproduction, Fertility and Development 22, 151–158.
| Factors affecting fertilisation and early embryo quality in single- and superovulated dairy cattle.Crossref | GoogleScholarGoogle Scholar |
Sheldon IM, Dobson H (2004) Postpartum uterine health in cattle. Animal Reproduction Science 82–83, 295–306.
| Postpartum uterine health in cattle.Crossref | GoogleScholarGoogle Scholar |
Sheldon IM, Lewis GS, LeBlanc S, Gilbert RO (2006) Defining postpartum uterine disease in cattle. Theriogenology 65, 1516–1530.
| Defining postpartum uterine disease in cattle.Crossref | GoogleScholarGoogle Scholar |
Smyth T, Petrova K, Payton NM, Persaud I, Redzic JS, Graner MW, Smith-Jones P, Anchordoquy TJ (2014) Surface functionalization of exosomes using click chemistry. Bioconjugate Chemistry 25, 1777–1784.
| Surface functionalization of exosomes using click chemistry.Crossref | GoogleScholarGoogle Scholar |
Sullivan R, Saez F, Girouard J, Frenette G (2005) Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells, Molecules & Diseases 35, 1–10.
| Role of exosomes in sperm maturation during the transit along the male reproductive tract.Crossref | GoogleScholarGoogle Scholar |
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG (2010) A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Molecular Therapy 18, 1606–1614.
| A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes.Crossref | GoogleScholarGoogle Scholar |
Sun J, Aswath K, Schroeder SG, Lippolis JD, Reinhardt TA, Sonstegard TS (2015) MicroRNA expression profiles of bovine milk exosomes in response to Staphylococcus aureus infection. BMC Genomics 16, 806
| MicroRNA expression profiles of bovine milk exosomes in response to Staphylococcus aureus infection.Crossref | GoogleScholarGoogle Scholar |
Sun K, Chan KC, Hudecova I, Chiu RW, Lo YM, Jiang P (2017) COFFEE: control-free noninvasive fetal chromosomal examination using maternal plasma DNA. Prenatal Diagnosis 37, 336–340.
| COFFEE: control-free noninvasive fetal chromosomal examination using maternal plasma DNA.Crossref | GoogleScholarGoogle Scholar |
Sun K, Lun FMF, Leung TY, Chiu RWK, Lo YMD, Sun H (2018) Noninvasive reconstruction of placental methylome from maternal plasma DNA: potential for prenatal testing and monitoring. Prenatal Diagnosis 38, 196–203.
| Noninvasive reconstruction of placental methylome from maternal plasma DNA: potential for prenatal testing and monitoring.Crossref | GoogleScholarGoogle Scholar |
Taylor DD, Gercel-Taylor C (2008) MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecologic Oncology 110, 13–21.
| MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer.Crossref | GoogleScholarGoogle Scholar |
Théry C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nature Reviews. Immunology 9, 581–593.
| Membrane vesicles as conveyors of immune responses.Crossref | GoogleScholarGoogle Scholar |
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G (2014) A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35, 2383–2390.
| A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy.Crossref | GoogleScholarGoogle Scholar |
Vaswani K, Koh YQ, Almughlliq FB, Peiris HN, Mitchell MD (2017) A method for the isolation and enrichment of purified bovine milk exosomes. Reproductive Biology 17, 341–348.
| A method for the isolation and enrichment of purified bovine milk exosomes.Crossref | GoogleScholarGoogle Scholar |
Vella LJ, Hill AF, Cheng L (2016) Focus on extracellular vesicles: exosomes and their role in protein trafficking and biomarker potential in Alzheimer’s and Parkinson’s disease. International Journal of Molecular Sciences 17, 173
| Focus on extracellular vesicles: exosomes and their role in protein trafficking and biomarker potential in Alzheimer’s and Parkinson’s disease.Crossref | GoogleScholarGoogle Scholar |
Walker CG, Meier S, Littlejohn MD, Lehnert K, Roche JR, Mitchell MD (2010) Modulation of the maternal immune system by the pre-implantation embryo. BMC Genomics 11, 474
| Modulation of the maternal immune system by the pre-implantation embryo.Crossref | GoogleScholarGoogle Scholar |
Walsh SW, Williams EJ, Evans AC (2011) A review of the causes of poor fertility in high milk producing dairy cows. Animal Reproduction Science 123, 127–138.
| A review of the causes of poor fertility in high milk producing dairy cows.Crossref | GoogleScholarGoogle Scholar |
Wang CF, Mäkilä EM, Kaasalainen MH, Liu D, Sarparanta MP, Airaksinen AJ, Salonen JJ, Hirvonen JT, Santos HA (2014) Copper-free azide-alkyne cycloaddition of targeting peptides toporous silicon nanoparticles for intracellular drug uptake. Biomaterials 35, 1257–1266.
| Copper-free azide-alkyne cycloaddition of targeting peptides toporous silicon nanoparticles for intracellular drug uptake.Crossref | GoogleScholarGoogle Scholar |
Yim N, Ryu SW, Choi K, Lee KR, Lee S, Choi H, Kim J, Shaker MR, Sun W, Park JH, Kim D, Heo WD, Choi C (2016) Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nature Communications 7, 12277
| Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module.Crossref | GoogleScholarGoogle Scholar |
Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z, Baek A, Jiang J, Elgamal OA, Mo X, Perle K, Chalmers J, Schmittgen TD, Phelps MA (2017) Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. Journal of Extracellular Vesicles 6, 1324730
| Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells.Crossref | GoogleScholarGoogle Scholar |
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, Miller D, Zhang HG (2011) Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Molecular Therapy 19, 1769–1779.
| Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain.Crossref | GoogleScholarGoogle Scholar |