Overcoming nature’s paradox in skeletal muscle to optimise animal production
Gordon S. Lynch A B and René Koopman A
A B and René Koopman A
A Centre for Muscle Research, Department of Physiology, The University of Melbourne, Vic. 3010, Australia.
B Corresponding author. Email: gsl@unimelb.edu.au
Animal Production Science 59(11) 1957-1969 https://doi.org/10.1071/AN19361
Submitted: 25 June 2019 Accepted: 5 July 2019 Published: 16 September 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Nature’s paradox in skeletal muscle describes the seemingly mutually exclusive relationship between muscle fibre size and oxidative capacity. In mammals, there is a constraint on the size at which mitochondria-rich, high O2-dependent oxidative fibres can attain before they become anoxic or adapt to a glycolytic phenotype, being less reliant on O2. This implies that a muscle fibre can hypertrophy at the expense of its endurance capacity. Adaptations to activity (exercise) generally obey this relationship, with optimal muscle endurance generally being linked to an enhanced proportion of small, slow oxidative fibres and muscle strength (force and/or power) being linked to an enhanced proportion of large, fast glycolytic fibres. This relationship generally constrains not only the physiological limits of performance (e.g. speed and endurance), but also the capacity to manipulate muscle attributes such as fibre size and composition, with important relevance to the livestock and aquaculture industries for producing specific muscle traits such as (flesh) quality, texture and taste. Highly glycolytic (white) muscles have different traits than do highly oxidative (red) muscles and so the ability to manipulate muscle attributes to produce flesh with specific traits has important implications for optimising meat production and quality. Understanding the biological regulation of muscle size, and phenotype and the capacity to manipulate signalling pathways to produce specific attributes, has important implications for promoting ethically sustainable and profitable commercial livestock and aquaculture practices and for developing alternative food sources, including ‘laboratory meat’ or ‘clean meat’. This review describes the exciting potential of manipulating muscle attributes relevant to animal production, through traditional nutritional and pharmacological approaches and through viral-mediated strategies that could theoretically push the limits of muscle fibre growth, adaptation and plasticity.
Additional keywords: muscle fibre, muscle fiber, muscle growth, muscle plasticity, oxidative, glycolytic.
Introduction
Skeletal muscles are remarkably plastic, capable of modifying their phenotype according to functional demand and perturbations in innervation, load, hormones and other regulating factors. Muscle fibres can be defined physiologically by their speed of contraction and resistance to fatigue, which are properties that are determined by contractile and regulatory protein isoforms and metabolism. Quickly contracting muscles are composed predominantly of fast (myosin) isoforms; slowly contracting muscles are composed of slow (myosin) isoforms. Metabolic properties provide the capacity for sustained contraction, with muscles contracting forcefully but infrequently relying on anaerobic metabolism, and more frequently contracting muscles relying on oxidative metabolism (Pette 2001; Schiaffino 2007; Schiaffino and Reggiani 2011; Lynch 2017).
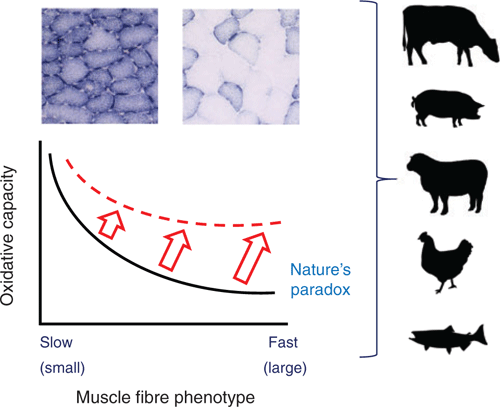
While skeletal muscles can adapt to imposed demands, there are physiological constraints on muscle fibre size and composition that ultimately limit adaptation. An intriguing and unresolved question in muscle biology is what governs and limits adaptation and plasticity, which is often described as the ‘muscle paradox’ (van Wessel et al. 2010). This implies that muscle fibres challenged to simultaneously increase their size/mass/strength (hypertrophy) and fatigue resistance (oxidative capacity) will increase strength or fatigue resistance to a lesser extent than do fibres increasing either of these attributes alone (Fig. 1; van der Laarse et al. 1998; van Wessel et al. 2010; van der Zwaard et al. 2018). Adaptations to activity (exercise) generally obey this relationship, with optimal muscle endurance being generally linked to an enhanced proportion of small, slow oxidative fibres, and muscle strength (force and/or power) being linked to an enhanced proportion of large, fast glycolytic fibres. The paradox has (until now) constrained not only the physiological limits of performance (e.g. speed and endurance), but also the capacity to manipulate muscle attributes such as fibre size and composition, with potential application to the livestock and aquaculture industries for producing specific muscle traits relevant to flesh quality, texture and taste. Muscle quality and quantity are strongly influenced by environmental factors (e.g. nutrition and exercise) and manipulating pathways regulating muscle protein and fat composition can alter texture and fat deposition. Genetic selection to promote muscle size has been negatively correlated with textural quality in fish and livestock (Chen and Lee 2016; Moghadam et al. 2017; Robinson et al. 2017), such that selecting for muscle growth typically leads to more glycolytic (‘white’), tougher meat. The robustness of this paradoxical relationship is unclear, because sophisticated molecular tools, including viral-vector technologies, can now be applied to force expression of specific muscle attributes in ways not examined previously.
|
Significance of skeletal muscle for animal production
Skeletal muscle is essential for life. We need muscles to breathe, to eat and to interact with the environment. Building and maintaining healthy muscles is needed for body heat, metabolism and movement throughout life. Yet, in later life, muscles of all mammals begin to shrink and weaken, threatening independence, productivity and quality of life. Skeletal muscle is also food, with a considerable proportion of the global population relying on muscle from livestock and fish for the best sources of high-quality proteins (Lynch and Koopman 2018). Understanding how muscles develop, grow and adapt is important not just for enhancing athletic performance or improving safety and productivity in the work place, but for livestock and aquaculture production, including optimising farming practices, nutritional feeding strategies, and meat production and quality for lucrative domestic and export markets (Lee et al. 2010; Lefaucheur 2010; Astruc 2014; Listrat et al. 2016; Parr et al. 2016).
Skeletal muscle fibres
Muscle comprises functionally diverse fibre types ranging in size, metabolism and contractility (Burke et al. 1973; Larsson et al. 1991; Schiaffino 2007; Schiaffino and Reggiani 2011; Blaauw et al. 2013). Muscle phenotype is largely defined by fibre number, fibre cross-sectional area, muscle architecture and fibre-type distribution. On the basis of myosin heavy-chain protein isoforms, which largely dictate the rate of force development, velocity of shortening and rate of cross-bridge cycling, mammalian muscle fibres are broadly classified as slow-twitch (Type I) or fast-twitch (Types IIa, IId/x and IIb). While Type I and Type IIa fibres primarily generate ATP via oxidative metabolism and Type IId/x and IIb fibres generate energy mostly through glycolysis (Schiaffino 2007; Murgia et al. 2015), fibre classification based on myosin heavy-chain isoform composition does not necessarily correlate well with oxidative capacity, and variation among species and even among mammals (e.g. the absence of Type IIb fibres in humans compared with mice and rats) should also be considered (Gouspillou et al. 2014). However, on the basis of an extensive body of literature examining relationships between muscle fibre cross-sectional area (fibre size) and oxidative capacity, it is generally accepted that larger fibres have relatively lower oxidative capacities than do smaller fibres, regardless of the type. In fact, both Type I and Type IIa fibres have a large oxidative capacity and are usually smaller than Type IIb and Type IIx fibres (van Wessel et al. 2010).
Signalling pathways regulating muscle adaptation and plasticity
Muscle fibres are highly plastic and capable of altering their properties in response to contractile demand or other perturbations of signalling pathways that regulate isoform composition. The cellular, biochemical and molecular processes governing fibre identity and regulating adaptation and plasticity, are becoming clearer. The slow-muscle fibre phenotype is controlled by biochemical signalling related to protein kinase C, calcineurin–nuclear factor of activated T-cells (NFAT), AMP-activated protein kinase, estrogen-related receptor gamma, sex-determining region Y-box 6, and peroxisome proliferator-activated receptor gamma co-activator 1-α (Tong et al. 2009; Ljubicic et al. 2014; Omairi et al. 2016). Calcineurin–NFAT (nuclear factor of activated T-cells) signalling plays an important role in regulating fast-to-slow muscle phenotypic adaptations (Chin et al. 1998). Calcineurin (gene name: Ppp3ca) is a Ca2+/calmodulin-dependent phosphatase that dephosphorylates NFAT, resulting in its nuclear translocation and binding to specific sequences on the promoters of target genes that induce slow oxidative muscle fibre programming (Olson and Williams 2000; Dunn et al. 2001; Allen and Leinwand 2002; Parsons et al. 2003; Stupka et al. 2006, 2007, 2008). As a key master regulator of slow-muscle programming and muscle oxidative capacity, manipulating calcineurin expression is one approach to interrogate the muscle paradox and selectively alter muscle fibre composition. Each of these master regulators of muscle phenotype could be similarly explored for their ability to selectively alter muscle phenotype.
Fast, glycolytic muscles are regulated by activation of Akt signalling, and removing inhibition of Akt signalling through myostatin potently induces formation of glycolytic fibres (Trendelenburg et al. 2009). Myostatin, a negative regulator of skeletal muscle mass that inhibits muscle-cell differentiation, requires Smad2 and Smad3 downstream of the activin receptor II (ActRII)/activin receptor-like kinase receptor complex; Sartori et al. 2009). Other transforming growth-factor β (TGF-β)-like molecules can also block differentiation, including TGF-β1, growth-differentiation factor 11 (GDF-11), activins and bone morphogenetic proteins 2 and 7. These signalling pathways for muscle growth and those for muscle atrophy that ultimately regulate muscle fibre size have been described in detail elsewhere (Glass 2010; Bodine and Baehr 2014; Rom and Reznick 2016; Winbanks et al. 2016).
Myostatin, also known as growth/differentiation factor-8 (GDF8), is a member of the TGF-β superfamily of secreted proteins. It is highly expressed in muscle and negatively regulates proliferation. Deletion leads to a well described hypermuscular phenotype in mice, cattle and humans, with muscles being glycolytic with reduced numbers of mitochondria (McPherron and Lee 1997; McPherron et al. 1997; Schuelke et al. 2004). Myostatin inhibitors include the myostatin propeptide, 1 follistatin and the follistatin-related gene, as well as growth and differentiation factor-associated serum protein-1 (Hill et al. 2003). The most potent inhibitor is follistatin and its overexpression has powerful growth-promoting effects in skeletal muscle (Zheng et al. 2017). Follistatin is an endogenous ligand-binding partner for myostatin and activin A (a growth factor implicated in reducing muscle fibre size; Trendelenburg et al. 2009; Chen et al. 2014; Davey et al. 2016; Winbanks et al. 2016). Myostatin antagonists are being developed as therapies for muscle-wasting diseases such as Duchenne muscular dystrophy (DMD) due to their strong hypertrophic effects on skeletal muscle (Whittemore et al. 2003; Murphy et al. 2010). Strategies to engineer (and upregulate) follistatin also have potential for these conditions, by combining the hypertrophic actions of myostatin antagonism with the anti-inflammatory and anti-fibrotic effects of activin A antagonism (Rodino-Klapac et al. 2009; Iskenderian et al. 2018; Schumann et al. 2018). Using viral vectors to force expression of follistatin in mouse skeletal muscles can produce phenomenal 100% (and greater) increases in skeletal muscle mass (Winbanks et al. 2012; Sepulveda et al. 2015). Strategies to inhibit myostatin or overexpress follistatin for application to animal production will be discussed later in this review.
Significance of the muscle paradox
The concept of the muscle paradox and its important physiological consequences in animals have been described in detail elsewhere (van der Laarse et al. 1998; Kinsey et al. 2007; Jimenez et al. 2013; Omairi et al. 2016; van der Zwaard S et al. 2018). In mammals, the paradox represents a constraint on the size at which mitochondria-rich, high O2-dependent oxidative fibres can attain before they become anoxic or adapt to a glycolytic phenotype, less reliant on O2. This means that a muscle fibre can hypertrophy at the expense of its endurance capacity (Fig. 1; van Wessel et al. 2010). Despite having an inherent capacity to alter their attributes, the extent of change or adaptation is influenced by the number and/or magnitude of different perturbing stimuli, especially those affecting muscle fibre size and muscle fibre composition. These signalling pathways can be complementary to effect considerable change in phenotype or they may compete or interfere with each other to limit adaptations. An often-described example is that of exercising humans training simultaneously for both strength and endurance who experience less of an adaptation than if they trained just for one outcome; this is a phenomenon called the ‘interference effect’ or ‘concurrent training effect’ (Baar 2014; Fyfe et al. 2014; Coffey and Hawley 2017).
Typically, when muscles are loaded during resistance training, muscle fibres hypertrophy, leading to an increase in mass. Conversely, with endurance exercise, muscles adapt by increasing their oxidative metabolism facilitated through increased mitochondrial enzymes and capillary density, not through hypertrophy. The underlying mechanisms responsible for limiting adaptation (in either direction) in the face of competing stimuli remain unresolved, in part because current understanding has relied on physical activity (exercise) as the perturbing stimulus. During exercise/physical activity, only a small fraction of the total number of fibres within muscles are recruited to complete specific tasks and usually for only brief periods, such as, for example, a few seconds for maximal sprinting or powerlifting, to a few hours with endurance activities, such as marathon running. To properly interrogate the limitations of muscle adaptation and plasticity requires driving expression of key attributes using viral-vector gene-delivery tools to maximise muscle size or oxidative capacity, and so rigorously test hypotheses about the muscle paradox. Viral-vector technologies that permit direct targeting of specific growth and oxidative pathways can facilitate extremes of muscle hypertrophy and adaptive potential, with superior interrogation of the biological signalling pathways that can maximise muscle attributes singly or in combination. Overcoming nature’s limits on muscle attributes would have broad application to all aspects of skeletal muscle biology, but especially to better understand muscle development and growth to optimise meat production and quality, which is relevant to animal production and livestock and aquaculture.
While viral-vector technologies provide a powerful approach for testing the limits of skeletal muscle adaptation, relevant to optimising muscle attributes from animal production, signalling pathways regulating muscle size, and phenotype can also be manipulated through nutritional and pharmacological strategies. A brief overview of selected nutritional and drug approaches for altering muscle attributes is provided.
Nutritional strategies to alter skeletal muscle attributes and phenotype
The growth of animals and their body composition (muscle, fat and bone content) can be manipulated through the energy and protein content provided in the diet. Indeed, intensive (ad libitum) feeding of beef cattle can improve animal growth rates, final bodyweights and feed efficiencies compared with their pasture-fed counterparts (Vestergaard et al. 2000a, 2000b). Here, we focus on the fundamental principles that underpin skeletal muscle adaptation to changes in dietary intake and how these principles can be applied to enhance muscle growth and metabolism.
Protein
The most intensively studied dietary components in relation to growth of animals are protein and total energy content that can be metabolised (carbohydrate, lipids and protein). As amino acids are the building blocks for producing new protein, it is not surprising that adequate dietary protein intake is the main driver of muscle growth. Indeed, classical studies performed in growing pigs in the 1980s showed that at equivalent levels of energy intake, pigs provided a diet with adequate protein exhibited more rapid and efficient growth than did those on a protein-deficient diet (Campbell and Dunkin 1983). A more recent study in broiler chickens examined 14 different iso-energetic diets with varying macro-nutrient compositions to assess the relative importance of protein, lipid and starch on growth performance (Liu et al. 2017). The study confirmed that energy derived from protein was more important than non-protein energy in terms of weight gain, and that a balance between protein and energy supplies was required for efficient muscle-protein deposition (Liu et al. 2017). To enhance protein utilisation (nitrogen retention), digestion rate of different protein sources and amino acid composition need to be considered. For example, studies in humans have established that proteins more rapidly digested and absorbed (i.e. whey and casein hydrolysates compared with casein) result in enhanced amino acid delivery to the muscle with higher rates of protein synthesis (Koopman et al. 2009; Pennings et al. 2011).
Supplementation with animal-derived protein is more effective in stimulating protein synthesis (Tang et al. 2009) and promoting hypertrophy in humans than is supplementation with plant-based proteins (Wilkinson et al. 2007). Studies in pigs have confirmed that addition of animal-derived protein enhances performance, nutrient digestibility and gut morphology more than does addition of plant-derived protein sources (Yun et al. 2005). Interestingly, the majority of an animal’s dietary intake of protein/amino acids is through intake of plant-based proteins. In contrast to animal-based proteins, which have a well balanced amino acid composition essential for growth and development, plant-based proteins are nutritionally unbalanced and deficient in some essential amino acids. Therefore, plant-based protein diets require higher crude protein intake or supplementation with an animal protein source (derived from meat/fish and diary processing) or specific amino acids (Beski et al. 2015). There are considerable advantages of reducing dietary crude protein with supplementation of free amino acids for sustainable livestock production, including saving on protein ingredients, reducing nitrogen excretion, feed costs and the risk of gut disorders, without impairing growth performance compared with traditional diets (Wang et al. 2018). Some amino acids with beneficial effects on skeletal muscle growth will now be discussed in detail.
Leucine
Muscle cells are highly sensitive to changes in amino acid availability, which plays a major role in the regulation of protein synthesis and breakdown. Amino acid abundance results in enhanced activity of the mechanistic target of rapamycin complex 1 (mTORC1), which is one of the key regulators of protein turnover that drives protein synthesis and growth (Ham et al. 2014a). Of all amino acids, the branched-chain amino acid, leucine, is the most potent stimulator of mTORC1 and protein synthesis in vitro and in vivo (Ham et al. 2014a). As such, leucine has received considerable attention as a potential pharmaconutrient to enhance growth. Multiple studies have shown that administration of leucine or leucine-rich supplements acutely increases protein synthesis in mice and rats (Anthony et al. 2000), pigs (Murgas Torrazza et al. 2010), sheep (Schaefer et al. 1986) and healthy humans (Wall et al. 2013). Interestingly, long-term, placebo-controlled, isocaloric studies in adult humans have consistently shown no beneficial effect of leucine supplementation on skeletal muscle mass or function (Verhoeven et al. 2009). We have critically evaluated the therapeutic potential of leucine to attenuate the skeletal muscle wasting associated with ageing, cancer and immobilisation/bed rest (Ham et al. 2014a) and highlighted the impact of inflammation on amino acid sensing, mTOR activation and stimulation of protein synthesis (Ham et al. 2016). Leucine, as a standalone nutritional intervention, is not effective in preventing muscle wasting. In contrast, some studies in rapidly growing young pigs fed a protein-restricted diet have shown that feeding with leucine (or its metabolite β-hydroxymethylbutyrate) can enhance growth (Wan et al. 2016; Zheng et al. 2016). Using porcine myoblasts, in vitro studies have suggested that leucine induces a fast-to-slow fibre-type transition via AMP-activated protein kinase/SIRT1-mediated (Chen et al. 2019) or FOXO1-mediated (Zhang et al. 2019) signalling. In contrast, recent in vivo studies demonstrated that leucine feeding in piglets suppressed oxidative phosphorylation and fatty acid β-oxidation, with activation of glycolysis and slow-to-fast fibre-type transition (Fan et al. 2017). More detailed studies are needed to elucidate the effect of leucine feeding on muscle phenotype in animals used for meat production.
Arginine and citrulline
Citrulline is a non-proteinogenic amino acid (i.e. an amino acid not incorporated into protein with a unique inter-organ metabolism) and it plays a central role in the delivery of arginine to skeletal muscle (Moinard and Cynober 2007). Since citrulline is not metabolised in the gut, oral citrulline administration is more efficient in increasing plasma and muscle concentrations of arginine than is arginine feeding. The semi-essential amino acid arginine is critically involved in numerous physiological functions, including providing substrate to produce creatine, urea and nitric oxide (NO). NO is a key signalling molecule that stimulates release of growth factors such as insulin and growth hormone and plays a role in vasodilation (and, thus, nutrient delivery to the muscle), satellite cell activation, myoblast fusion and overload-induced skeletal-muscle hypertrophy (Ham et al. 2014b). Arginine availability clearly plays a role in the regulation of protein synthesis and skeletal muscle mass in both NO-dependent and NO-independent ways (Ham et al. 2014b).
Citrulline supplementation reduces muscle wasting in conditions of arginine deficiency. In rats, massive intestinal resection results in skeletal muscle arginine deficiency and muscle atrophy, while restoration of skeletal muscle arginine pools with citrulline improves muscle protein metabolism and attenuates muscle wasting (Osowska et al. 2004). We have demonstrated a direct role for arginine in the protection of skeletal muscle cells from cachectic stimuli in C2C12 myotubes in vitro (Ham et al. 2014a). Arginine reduced muscle wasting in a dose-dependent manner and modulated protein synthesis rates in a mTORC1-dependent manner (Ham et al. 2014a). We have also demonstrated a novel direct protective effect of L-citrulline on protein metabolism and skeletal muscle cell size that is not mediated by signalling through mTORC1 (Ham et al. 2015a). Interestingly, studies performed in vivo have demonstrated that citrulline treatment has no effect on therapeutically relevant outcome measures such as skeletal muscle mass and peak muscle force after 14 days of hind-limb immobilisation (Ham et al. 2015b). The effect of citrulline feeding on muscle growth in pigs, sheep and beef cattle has not been investigated in detail. In contrast, many studies have examined the effect of arginine supplementation in pigs and, although capable of improving some aspects of meat quality, arginine does not affect growth performance and carcass yield in growing–finishing pigs (Madeira et al. 2014, 2015, 2016; Hu et al. 2017).
Glycine and related compounds
Glycine is one of the non-essential amino acids often considered to be biologically neutral. However, studies have indicated that glycine exerts a range of physiological effects in numerous tissues and cell types in vitro and in vivo (Koopman et al. 2017). Glycine is a substrate for the production of glutathione, heme and creatine and, therefore, plays a role in overall antioxidant defence and metabolism. Glycine administration also modulates homeostasis by activating glycine-gated chloride channels in inflammatory cells, thereby effectively reducing [Ca2+]i cytokine production, and whole-body (systemic) inflammation in several models (Zhong et al. 2003). Because inflammation plays a key role in the aetiology of many muscle-wasting conditions, we have tested the hypothesis that glycine supplementation represents a simple, safe and promising nutritional intervention for tackling skeletal-muscle wasting in many diseases and conditions. We have shown that glycine protects from wasting in mouse models of cancer cachexia (Ham et al. 2014c) and enhances the anabolic response to leucine during inflammatory conditions (Ham et al. 2016). Our observations are consistent with other studies, showing that glycine supplementation attenuated the inflammatory response to lipopolysaccharide in broiler chicks and enhanced average daily gains in bodyweight (Takahashi et al. 2008). A glycine-related compound that has received considerable interest is guadinoacetic acid (GAA), a precursor of creatine. GAA is synthesised from arginine and glycine, and GAA supplementation improves growth performance (DeGroot et al. 2019), breast meat yield (Córdova-Noboa et al. 2018a) and reduces the severity of wooden breast myopathy in broilers (Córdova-Noboa et al. 2018b). An amino acid such as glycine or its derivatives that modulate inflammation and metabolism will be valuable additions to nutritional interventions for livestock and aquaculture.
Choline
Choline is an essential water-soluble nutrient with multiple biological roles, including countering inflammation and oxidative stress, promoting neurotransmission and membrane composition and enhancing lipogenesis. One of the ways in which choline, and its derivative betaine, can modulate muscle homeostasis is by serving as a methionine precursor (via one-carbon metabolism) and in the regulation of methylation of DNA, histones and other proteins (Abbasi et al. 2017).
Choline deficiency is implicated in neurological disorders, fatty liver disease, atherosclerosis and muscle wasting (Zeisel et al. 1991). Choline supplementation can combat deficiencies and complications (Fischer et al. 2007). We recently tested the hypothesis that choline supplementation would be beneficial in mdx dystrophic mice, the most widely used murine model of DMD, which is the most severe of the muscular dystrophies (Alves et al. 2019). Choline administration attenuated the dystrophic pathology, with reductions in the expression of inflammatory markers, macrophages and collagen infiltration (Alves et al. 2019). Choline supplementation in broiler chickens during the grower and finisher period effectively improved the feed conversion ratio, carcass yield and moisture content of leg muscle (Jahanian and Ashnagar 2018). Similarly, betaine feeding improved growth performance in broiler chickens (Chen et al. 2018; Shakeri et al. 2018) and was effective for the resynthesis of methionine to sustain protein synthesis in pigs fed a methionine-restricted diet (McBreairty et al. 2016).
Pharmacological strategies to alter muscle attributes and phenotype
Because muscle mass relies on myoblast proliferation during prenatal (or prehatch) stages and fibre hypertrophy through protein synthesis and nuclei donation by satellite cells after birth (or hatch), pharmacological approaches to optimise cellular and molecular mechanisms of myogenesis and muscle development are important (Chen and Lee 2016). Technologies to control fat and muscle composition in livestock were reviewed by Sillence (2004) who provided a comprehensive evaluation of pharmacological strategies, including anabolic steroids, corticosteroid suppressors, β-adrenoceptor agonists (β-agonists), growth hormone (GH), insulin-like growth factor-I (IGF-I), adipokines, myostatin inhibition and selective androgen receptor modulators (Cesbron et al. 2017). Various combinations of these approaches can also be employed to promote and sustain muscle growth and alter lean meat-yield production, and marbling (Boles et al. 2009).
Hormonal growth-promoting agents (‘promoters’ or ‘promotants’) have been used for promoting muscle growth in farm animals (including cattle and pigs) and have been reported as beneficial for production efficiency, profit and reduced environmental effects, yet their effects on meat quality (particularly on measures of toughness) have yet to be resolved (Lean et al. 2018). The purpose of hormonal growth promotants, as described (somewhat cheekily) by Stephany (2010). is ‘to obtain more edible muscle meat for less money’ but essentially to shift nutrient use towards carcass lean tissue deposition at the expense of adipose tissue (Johnson and Chung 2007). However, different countries around the world have enacted total bans or restricted the use of specific growth-promoting/growth-partitioning drugs because of their potential toxicity and carcinogenic properties (Leporati et al. 2014). Other countries still allow their use in animal production, reflecting changes in consumer preferences and international politics (Higgins 2004; Stephany 2010; Bonny et al. 2018; Farmer and Farrell 2018).
Testosterone (anabolic steroids) and growth hormone (GH)
The ability of the sex steroids testosterone, oestrogen and progesterone and their synthetic derivatives (nandrolone, trenbolone, melenogestrol and hexoestrol) to increase lean tissue growth in ruminants is undisputed (Sillence 2004; Dayton and White 2014). Although their use remains controversial, anabolic implants (containing estrogenic and trenbolone acetate combinations) are used routinely in some countries during the finishing phase of beef production to improve animal performance and feed efficiency (Reinhardt 2007; Duckett and Pratt 2014), despite implants having potentially adverse effects on carcass quality and eating quality, depending on dose and frequency (Garmyn and Miller 2014). Similarly, GH and recombinant GH can change carcass composition, especially in pigs, but only to a mild extent in cattle (Sillence 2004). Steroid-based growth promoters generally elevate local and circulating IGF-I concentrations through activation of steroid receptors and downstream signalling pathways, which influence proliferation and myogenic differentiation of muscle stem cells (satellite cells), increasing protein synthesis, and reducing protein degradation, with net protein accretion and muscle hypertrophy (Du 2014). Other studies have argued that GH stimulates muscle growth in cattle, in part, by stimulating protein synthesis in muscle through a GH receptor-mediated, IGF-I-independent mechanism, with liver-derived circulating IGF-I being the major mechanism mediating the growth-stimulatory effect of GH on muscle in cattle and other domestic animals (Jiang and Ge 2014).
β-adrenoceptor agonists
Although traditionally used for treating bronchospasm in animals and humans, it became apparent that stimulation of the β-adrenergic system with β-adrenoceptor agonists (particularly β2-agonists) had the ability to increase skeletal-muscle mass and decrease body fat. These so-called ‘repartitioning effects’ proved desirable for the livestock industry with the intention of improving feed efficiency and meat quality (Lynch and Ryall 2008). Although protein turnover rates can be augmented by β-agonists in humans (Hostrup et al. 2018), their muscle anabolic effects appear to be much less pronounced than those observed in livestock. Studies on cattle, sheep and pigs have shown that the tissue responsiveness to β-agonists varies from species to species, and even among different tissues within a species, primarily because of differences in the density of β-receptor subtypes (Lynch and Ryall 2008). Many studies have examined the use of β-agonists in livestock, especially with respect to their potential to improve meat quantity and, to a lesser extent, quality, because they can increase toughness in beef loin (Sillence 2004; Dunshea et al. 2005). The anabolic effects of β-agonists attenuate as β-adrenoceptors in skeletal muscle downregulate, and, in some cases, sudden withdrawal (of some β-agonists) can result in a marked catabolic response (Sillence 2004). These factors influence how β-agonists might be used commercially to maximise muscle growth but also limit tissue residues of these compounds that may produce off-target (adrenaline-like) effects in consumers.
Two β-adrenergic agonists are approved for use in cattle fed in confinement for slaughter in the United States, namely, zilpaterol hydrochloride and ractopamine hydrochloride, with the purpose of increasing the rate of gain, improving feed efficiency and increasing carcass leanness (Delmore et al. 2010; Brown et al. 2014; Martin et al. 2014). Maximum residue limits for ractopamine determined by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) were adopted by the Codex Alimentarius Commission (Codex), although no such limits have been determined for zilpaterol (Centner et al. 2014). Many countries disagree with the Codex standards and maintain a policy of restricting or banning meat products containing β-agonists (Centner et al. 2014), whereas other countries have fewer concerns. Methods are being developed to manage the safety of imported meat products from countries where zilpaterol use is still permitted, to prevent β-agonist poisoning due to secondary contamination (Sung et al. 2015). The consumption of contaminated meat products can lead to potentially serious side effects, including palpitations, peripheral vasodilatation, headache and cardiovascular complications, as well as tremor and muscle cramps (Lynch 2002).
On the basis of their growth-promoting effects on skeletal muscle, we have examined the therapeutic potential of several β-agonists (fenoterol, clenbuterol, formoterol) for muscle-wasting conditions, in animal (mouse, rat) models of muscular dystrophies and sarcopenia (Beitzel et al. 2007; Lynch and Ryall 2008; Ryall and Lynch 2008; Koopman et al. 2010). From a ‘muscle paradox’ perspective and attempting to elicit maximal muscle hypertrophy, we have found that these β-agonists typically cause a (dose-dependent) 10–20% increase in muscle mass within 2–4 weeks, after which the muscle-mass response reaches a plateau due to receptor downregulation and desensitisation. Because the heart also contains β-adrenoceptors (mainly β1-adrenoceptors but also some β2-adrenoceptors), β-agonist administration even with highly selective β2-agonists can trigger off-target complications such as cardiac hypertrophy (Ryall and Lynch 2008). From a therapeutic perspective, obviating the deleterious cardiovascular side effects of β-agonists remains an important challenge (Ryall and Lynch 2008).
Therefore, we investigated whether β-adrenoceptor-mediated signalling could be modulated in skeletal muscle via gene delivery to the target tissue, thus avoiding risks associated with β-agonists. In mice, intramuscular administration of a recombinant adeno-associated virus-based vector expressing the β2-adrenoceptor increased muscle mass by more than 20% within 4 weeks, a hypertrophic response comparable to that of administration of formoterol for 4 weeks. Recombinant adeno-associated virus-based vectors are emerging tools for therapeutic gene delivery because of their capacity for efficacious and targeted delivery of transgenes to mammalian skeletal muscles (Hagg et al. 2016). The study showed that gene therapy-based interventions targeting the β2-adrenoceptor pathway could promote skeletal muscle hypertrophy independent of ligand administration, highlighting how these methods could be utilised for altering muscle mass, being relevant to treating muscle-wasting conditions, but also for livestock production.
Myostatin and follistatin
As described in the introductory sections of this review (see Signalling pathways regulating muscle adaptation and plasticity), β-agonist administration can increase skeletal-muscle mass in mammals, but the magnitude of hypertrophy is much less than what can be elicited with manipulation of the TGF-β superfamily signalling pathway, such as myostatin inhibition or increasing follistatin expression. Myostatin (GDF-8), a member of the TGF-β superfamily, is a negative regulator of myogenesis and suppresses myoblast proliferation and myogenic differentiation. Several animals, including cattle, sheep, dogs and humans, display the ‘double-muscled’ phenotype due to mutations in the myostatin gene and understanding of different null alleles and polymorphisms in the myostatin gene could be applied to improving meat production in livestock animals (Aiello et al. 2018). Myostatin positively regulates slow but negatively regulates fast myosin, such that in transgenic myostatin null mice, there is a shift towards faster isoforms (Wang et al. 2012). Even heterozygous myostatin-knockout pigs exhibit a disproportionate increase in muscle mass and more fast glycolytic muscle fibres than do wild type pigs (Xing et al. 2017).
Chen and Lee (2016) reviewed inhibitors of myostatin as methods of enhancing muscle growth and development for animal production, indicating that there are currently no commercial myostatin inhibitors for agriculture or biomedical purposes because safe and effective options are yet to be identified. They suggested that further investigation of myostatin inhibitors and administration strategies may revolutionise animal production and the medical field. For example, because myostatin exerts its actions on skeletal muscle via interaction with ActRIIB, inhibition of this receptor is an attractive therapeutic avenue for attenuating muscle wasting (Swiderski and Lynch 2015) but also for animal production. Blocking myostatin signalling through genetic and pharmacological approaches induces skeletal-muscle hypertrophy, whereas overexpression or systemic administration causes muscle atrophy (Lee et al. 2012). Myostatin signalling can be disrupted by neutralising antibodies to myostatin (Whittemore et al. 2003; Murphy et al. 2010), a modified myostatin propeptide to block myostatin (Bogdanovich et al. 2005), and a soluble ActRIIB receptor Fc fusion protein (Lee et al. 2005; Tsuchida 2008; Zhou et al. 2010; Attie et al. 2013). With respect to therapeutic applications, it should be noted that a randomised, double-blind, placebo-controlled, ascending-dose trial of the fusion protein myostatin inhibitor, ACE-031, in DMD patients, although not associated with serious or severe adverse events, was stopped after the second dosing regimen due to potential safety concerns of epistaxis (nosebleeds) and telangiectasias (‘spider veins’; Campbell et al. 2017).
Of most relevance to interrogating the ‘muscle paradox’ perspective, we undertook a series of investigations in mice on the effect of a myostatin-inhibiting antibody (PF-354) on skeletal muscle wasting, in settings of sarcopenia, unloading, muscular dystrophy and cancer cachexia (Murphy et al. 2011). Myostatin inhibition not only attenuated muscle atrophy in these wasting settings, but in otherwise healthy mice, it increased muscle fibre cross-sectional area by 12% and enhanced maximum force (function) of mouse tibialis anterior muscles by 35% (Murphy et al. 2010). Compared with transgenic myostatin null mice that exhibit a shift in muscle phenotype to having a larger proportion of fast Type II (glycolytic) fibres and a smaller proportion of slow Type I (oxidative) fibres than do wild-type mice (Girgenrath et al. 2005), we found that myostatin antibody (PF-354) treatment increased the proportion of type IIa (fast oxidative) fibres by 114% and enhanced the activity of oxidative enzymes (e.g. succinate dehydrogenase) by 39% (Murphy et al. 2010). Therefore, the effects of myostatin inhibition vary depending on the mode of intervention, indicating that it is possible (at least through an inhibitory antibody) to produce larger, more oxidative skeletal muscles; these findings were not predicted on the basis of the muscle paradox. Producing larger, more oxidative muscle fibres is favourable for animal production, ultimately producing greater yields and potentially superior flesh qualities.
Follistatin is a potent myostatin antagonist that acts via a pathway independent of the myostatin signalling cascade by inhibiting binding of myostatin to the ActRIIB. Therefore, administration of follistatin can enhance skeletal muscle mass with fewer off-target effects compared with administration of other myostatin inhibitors trialled previously (Swiderski and Lynch 2015; Hardee and Lynch 2019). Mice genetically engineered to overexpress follistatin, specifically in skeletal muscle, had at least twice the amount of muscle mass of control mice (Chang et al. 2017) and viral vector-mediated expression of follistatin in mouse skeletal muscles produced similar (or even greater) increases in muscle mass (Winbanks et al. 2012; Sepulveda et al. 2015; Davey et al. 2016). This makes follistatin attractive for studying the relationship between muscle growth and muscle phenotype, especially for exploring the limits of muscle fibre size.
Less is known about the role of follistatin in skeletal muscle development of livestock, but in pigs, muscle-specific follistatin overexpression enhanced skeletal muscle growth, highlighting its potential for increasing muscle mass in pigs and other livestock species (Chang et al. 2017). Transgenic rainbow trout overexpressing follistatin exhibited increased total muscle surface area with epaxial and hypaxial muscling similar to that observed in double-muscled cattle and myostatin null mice, being attributed to inhibition of myostatin and possibly other growth factors (Medeiros et al. 2009). The hypaxial muscling generated a phenotype with well developed abdominal and intercostal muscles (as in athletic humans) and was dubbed ‘six pack’! (Medeiros et al. 2009). With respect to understanding the muscle paradox and limitations on muscle fibre size, the increased muscling in the transgenic rainbow trout was attributed to hyperplasia, with more fibres per unit area and increases in the percentage of smaller fibres and the number of total fibres (Medeiros et al. 2009).
In addition to systemic or intramuscular adeno-associated virus follistatin delivery, nanoparticle-mediated delivery of follistatin mRNA to the liver after subcutaneous administration (Schumann et al. 2018) has emerged as an effective way to increase muscle mass, with potential relevance to animal production. After subcutaneous injection of mRNA-loaded nanoparticles, the mRNA accumulates and internalises in the liver, where the hepatic cellular machinery produces follistatin. Serum concentrations of follistatin remained elevated for 72 h after injection and reduced concentrations of activin A and myostatin, with repeated injections over 8 weeks being required to increase lean muscle mass by 10% compared with controls (Schumann et al. 2018). The nanoparticle delivery of follistatin, while not as efficacious for increasing muscle mass as a single adeno-associated virus injection, provides a way to transiently manipulate follistatin concentration that may prove desirable for animal-production applications.
Conclusions
The muscle paradox suggests fibre size and oxidative capacity are mutually exclusive, such that muscle fibres can hypertrophy at the expense of their endurance capacity. While skeletal muscle adaptations to perturbing stimuli generally obey this relationship, there are some situations (including genetic manipulation and pharmacological interventions) where this limitation can be overcome to produce larger, more oxidative muscle fibres.
On the basis of the evidence provided herein, extremes of muscle hypertrophy can be achieved, especially through manipulation of TGF-β signalling, including strategies that decrease myostatin and increase follistatin. Genetic selection of myostatin increases muscle fibre size but shifts muscle phenotype to being more glycolytic, which are attributes associated with tougher flesh and less desirable meat quality. Antibody-directed suppression of myostatin has, in some mammals (such as mice), caused muscle fibre hypertrophy and a concomitant increase in the overall muscle oxidative capacity, attributes that would not be predicted based on the muscle paradox. Viral vector methods and nanoparticle-mediated delivery of mRNA are powerful tools for manipulating biochemical signalling to direct muscle growth and phenotype. Combinatorial approaches may have potentially greater efficacy in animal production for selectively altering muscle attributes, ultimately to produce larger, more oxidative muscles with more desirable flesh qualities. Such strategies are theoretically applicable to farm animals from chickens to free range ruminants, recognising, of course, that safety concerns would need to be interrogated rigorously for the health and safety of both the animals being farmed and the consumers eating the meat products. This is especially relevant to some societal views and restrictions on the use of drugs, hormones or engineering methods to alter animal growth trajectories and flesh quality.
The capacity to overcome the paradox to enhance specific muscle attributes has important implications for agriculture and aquaculture; for ageing, occupational/work physiology, and sports performance; for the development of ‘laboratory meat’ or ‘clean meat’ and other synthetic foods; and for the engineering of bioartificial muscles and tissues with attributes that confer functionality and biological purpose.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We are grateful for research grant support from the Australian Research Council (DP190101937, DP150100206), the National Health and Medical Research Council (GNT1144772, GNT1124474, GNT1120714, GNT1065456), the Duchenne Parent Project, The Netherlands (18.015), and Cancer Council Victoria (APP1120752).
References
Abbasi IHR, Abbasi F, Soomro RN, Abd El-Hack ME, Abdel-Latif MA, Li W, Hao R, Sun F, Bodinga BM, Hayat K, Yao J, Cao Y (2017) Considering choline as methionine precursor, lipoproteins transporter, hepatic promoter and antioxidant agent in dairy cows. AMB Express 7, 214| Considering choline as methionine precursor, lipoproteins transporter, hepatic promoter and antioxidant agent in dairy cows.Crossref | GoogleScholarGoogle Scholar | 29178045PubMed |
Aiello D, Patel K, Lasagna E (2018) The myostatin gene: an overview of mechanisms of action and its relevance to livestock animals. Animal Genetics 49, 505–519.
| The myostatin gene: an overview of mechanisms of action and its relevance to livestock animals.Crossref | GoogleScholarGoogle Scholar | 30125951PubMed |
Allen DL, Leinwand LA (2002) Intracellular calcium and myosin isoform transitions. Calcineurin and calcium-calmodulin kinase pathways regulate preferential activation of the IIa myosin heavy chain promoter. The Journal of Biological Chemistry 277, 45323–45330.
| Intracellular calcium and myosin isoform transitions. Calcineurin and calcium-calmodulin kinase pathways regulate preferential activation of the IIa myosin heavy chain promoter.Crossref | GoogleScholarGoogle Scholar | 12235157PubMed |
Alves FM, Caldow MK, Trieu J, Naim T, Montgomery MK, Watt MJ, Lynch GS, Koopman R (2019) Choline administration attenuates aspects of the dystrophic pathology in mdx mice. Clinical Nutrition Experimental 24, 83–91.
| Choline administration attenuates aspects of the dystrophic pathology in mdx mice.Crossref | GoogleScholarGoogle Scholar |
Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS (2000) Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. The Journal of Nutrition 130, 139–145.
| Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation.Crossref | GoogleScholarGoogle Scholar | 10720160PubMed |
Astruc T (2014) Muscle fiber types and meat quality. In ‘Encyclopedia of meat sciences’. 2nd edn. (Ed. CDM Dikeman) pp. 442–448. (Elsevier: Oxford, UK)
Attie KM, Borgstein NG, Yang Y, Condon CH, Wilson DM, Pearsall AE, Kumar R, Willins DA, Seehra JS, Sherman ML (2013) A single ascending-dose study of muscle regulator ACE-031 in healthy volunteers. Muscle & Nerve 47, 416–423.
| A single ascending-dose study of muscle regulator ACE-031 in healthy volunteers.Crossref | GoogleScholarGoogle Scholar |
Baar K (2014) Using molecular biology to maximize concurrent training. Sports Medicine 44, 117
| Using molecular biology to maximize concurrent training.Crossref | GoogleScholarGoogle Scholar |
Beitzel F, Sillence MN, Lynch GS (2007) β-Adrenoceptor signaling in regenerating skeletal muscle after β-agonist administration. American Journal of Physiology. Endocrinology and Metabolism 293, E932–E940.
| β-Adrenoceptor signaling in regenerating skeletal muscle after β-agonist administration.Crossref | GoogleScholarGoogle Scholar | 17623752PubMed |
Beski SSM, Swick RA, Iji PA (2015) Specialized protein products in broiler chicken nutrition: a review. Animal Nutrition 1, 47–53.
| Specialized protein products in broiler chicken nutrition: a review.Crossref | GoogleScholarGoogle Scholar | 29766993PubMed |
Blaauw B, Schiaffino S, Reggiani C (2013) Mechanisms modulating skeletal muscle phenotype. Comprehensive Physiology 3, 1645–1687.
| Mechanisms modulating skeletal muscle phenotype.Crossref | GoogleScholarGoogle Scholar | 24265241PubMed |
Bodine SC, Baehr LM (2014) Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. American Journal of Physiology. Endocrinology and Metabolism 307, E469–E484.
| Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1.Crossref | GoogleScholarGoogle Scholar | 25096180PubMed |
Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS (2005) Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. The FASEB Journal 19, 543–549.
| Myostatin propeptide-mediated amelioration of dystrophic pathophysiology.Crossref | GoogleScholarGoogle Scholar | 15791004PubMed |
Boles JA, Boss DL, Neary KI, Davis KC, Tess MW (2009) Growth implants reduced tenderness of steaks from steers and heifers with different genetic potentials for growth and marbling. Journal of Animal Science 87, 269–274.
| Growth implants reduced tenderness of steaks from steers and heifers with different genetic potentials for growth and marbling.Crossref | GoogleScholarGoogle Scholar | 18791138PubMed |
Bonny SPF, Hocquette JF, Pethick DW, Legrand I, Wierzbicki J, Allen P, Farmer LJ, Polkinghorne RJ, Gardner GE (2018) The variability of the eating quality of beef can be reduced by predicting consumer satisfaction. Animal 12, 2434–2442.
| The variability of the eating quality of beef can be reduced by predicting consumer satisfaction.Crossref | GoogleScholarGoogle Scholar | 29606159PubMed |
Brown TR, Sexten AK, Lawrence TE, Miller MF, Thomas CL, Yates DA, Hutcheson JP, Hodgen JM, Brooks JC (2014) Comparative effects of zilpaterol hydrochloride and ractopamine hydrochloride on live performance and carcass characteristics of calf-fed Holstein steers. Journal of Animal Science 92, 4217–4222.
| Comparative effects of zilpaterol hydrochloride and ractopamine hydrochloride on live performance and carcass characteristics of calf-fed Holstein steers.Crossref | GoogleScholarGoogle Scholar | 25006068PubMed |
Burke RE, Levine DN, Tsairis P, Zajac FE (1973) Physiological types and histochemical profiles in motor units of the cat gastrocnemius. The Journal of Physiology 234, 723–748.
| Physiological types and histochemical profiles in motor units of the cat gastrocnemius.Crossref | GoogleScholarGoogle Scholar | 4148752PubMed |
Campbell RG, Dunkin AC (1983) The effects of energy intake and dietary protein on nitrogen retention, growth performance, body composition and some aspects of energy metabolism of baby pigs. British Journal of Nutrition 49, 221–230.
| The effects of energy intake and dietary protein on nitrogen retention, growth performance, body composition and some aspects of energy metabolism of baby pigs.Crossref | GoogleScholarGoogle Scholar | 6830750PubMed |
Campbell C, McMillan HJ, Mah JK, Tarnopolsky M, Selby K, McClure T, Wilson DM, Sherman ML, Escolar D, Attie KM (2017) Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: results of a randomized, placebo-controlled clinical trial. Muscle & Nerve 55, 458–464.
| Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: results of a randomized, placebo-controlled clinical trial.Crossref | GoogleScholarGoogle Scholar |
Centner TJ, Alvey JC, Stelzleni AM (2014) Beta agonists in livestock feed: status, health concerns, and international trade. Journal of Animal Science 92, 4234–4240.
| Beta agonists in livestock feed: status, health concerns, and international trade.Crossref | GoogleScholarGoogle Scholar | 25057027PubMed |
Cesbron N, Sydor A, Penot M, Prevost S, Le Bizec B, Dervilly-Pinel G (2017) Analytical strategies to detect enobosarm administration in bovines. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment 34, 632–640.
| Analytical strategies to detect enobosarm administration in bovines.Crossref | GoogleScholarGoogle Scholar |
Chang F, Fang R, Wang M, Zhao X, Chang W, Zhang Z, Li N, Meng Q (2017) The transgenic expression of human follistatin-344 increases skeletal muscle mass in pigs. Transgenic Research 26, 25–36.
| The transgenic expression of human follistatin-344 increases skeletal muscle mass in pigs.Crossref | GoogleScholarGoogle Scholar | 27787698PubMed |
Chen PR, Lee K (2016) Inhibitors of myostatin as methods of enhancing muscle growth and development. Journal of Animal Science 94, 3125–3134.
| Inhibitors of myostatin as methods of enhancing muscle growth and development.Crossref | GoogleScholarGoogle Scholar | 27695802PubMed |
Chen JL, Walton KL, Winbanks CE, Murphy KT, Thomson RE, Makanji Y, Qian H, Lynch GS, Harrison CA, Gregorevic P (2014) Elevated expression of activins promotes muscle wasting and cachexia. The FASEB Journal 28, 1711–1723.
| Elevated expression of activins promotes muscle wasting and cachexia.Crossref | GoogleScholarGoogle Scholar | 24378873PubMed |
Chen R, Zhuang S, Chen YP, Cheng YF, Wen C, Zhou YM (2018) Betaine improves the growth performance and muscle growth of partridge shank broiler chickens via altering myogenic gene expression and insulin-like growth factor-1 signaling pathway. Poultry Science 97, 4297–4305.
| Betaine improves the growth performance and muscle growth of partridge shank broiler chickens via altering myogenic gene expression and insulin-like growth factor-1 signaling pathway.Crossref | GoogleScholarGoogle Scholar | 30085311PubMed |
Chen X, Xiang L, Jia G, Liu G, Zhao H, Huang Z (2019) Leucine regulates slow-twitch muscle fibers expression and mitochondrial function by Sirt1/AMPK signaling in porcine skeletal muscle satellite cells. Animal Science Journal 90, 255–263.
| Leucine regulates slow-twitch muscle fibers expression and mitochondrial function by Sirt1/AMPK signaling in porcine skeletal muscle satellite cells.Crossref | GoogleScholarGoogle Scholar | 30523660PubMed |
Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS (1998) A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes & Development 12, 2499–2509.
| A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type.Crossref | GoogleScholarGoogle Scholar |
Coffey VG, Hawley JA (2017) Concurrent exercise training: do opposites distract? The Journal of Physiology 595, 2883–2896.
| Concurrent exercise training: do opposites distract?Crossref | GoogleScholarGoogle Scholar | 27506998PubMed |
Córdova-Noboa HA, Oviedo-Rondón EO, Sarsour AH, Barnes J, Ferzola P, Rademacher-Heilshorn M, Braun U (2018a) Performance, meat quality, and pectoral myopathies of broilers fed either corn or sorghum based diets supplemented with guanidinoacetic acid. Poultry Science 97, 2479–2493.
| Performance, meat quality, and pectoral myopathies of broilers fed either corn or sorghum based diets supplemented with guanidinoacetic acid.Crossref | GoogleScholarGoogle Scholar | 29669056PubMed |
Córdova-Noboa HA, Oviedo-Rondón EO, Sarsour AH, Barnes J, Sapcota D, López D, Gross L, Rademacher-Heilshorn M, Braun U (2018b) Effect of guanidinoacetic acid supplementation on live performance, meat quality, pectoral myopathies and blood parameters of male broilers fed corn-based diets with or without poultry by-products. Poultry Science 97, 2494–2505.
| Effect of guanidinoacetic acid supplementation on live performance, meat quality, pectoral myopathies and blood parameters of male broilers fed corn-based diets with or without poultry by-products.Crossref | GoogleScholarGoogle Scholar | 29669035PubMed |
Davey JR, Watt KI, Parker BL, Chaudhuri R, Ryall JG, Cunningham L, Qian H, Sartorelli V, Sandri M, Chamberlain J, James DE, Gregorevic P (2016) Integrated expression analysis of muscle hypertrophy identifies Asb2 as a negative regulator of muscle mass. JCI Insight 1, e85477
| Integrated expression analysis of muscle hypertrophy identifies Asb2 as a negative regulator of muscle mass.Crossref | GoogleScholarGoogle Scholar | 27182554PubMed |
Dayton WR, White ME (2014) Role of satellite cells in anabolic steroid-induced muscle growth in feedlot steers. Journal of Animal Science 92, 30–38.
| Role of satellite cells in anabolic steroid-induced muscle growth in feedlot steers.Crossref | GoogleScholarGoogle Scholar | 24166993PubMed |
DeGroot AA, Braun U, Dilger RN (2019) Guanidinoacetic acid is efficacious in improving growth performance and muscle energy homeostasis in broiler chicks fed arginine-deficient or arginine-adequate diets. Poultry Science 9, pez036
Delmore RJ, Hodgen JM, Johnson BJ (2010) Perspectives on the application of zilpaterol hydrochloride in the United States beef industry. Journal of Animal Science 88, 2825–2828.
| Perspectives on the application of zilpaterol hydrochloride in the United States beef industry.Crossref | GoogleScholarGoogle Scholar | 20382871PubMed |
Du M (2014) Implants, muscle development, and meat quality. Journal of Animal Science 92, 1–2.
| Implants, muscle development, and meat quality.Crossref | GoogleScholarGoogle Scholar | 24249800PubMed |
Duckett SK, Pratt SL (2014) Anabolic implants and meat quality. Journal of Animal Science 92, 3–9.
| Anabolic implants and meat quality.Crossref | GoogleScholarGoogle Scholar | 24243897PubMed |
Dunn SE, Simard AR, Bassel-Duby R, Williams RS, Michel RN (2001) Nerve activity-dependent modulation of calcineurin signaling in adult fast and slow skeletal muscle fibers. The Journal of Biological Chemistry 276, 45243–45254.
| Nerve activity-dependent modulation of calcineurin signaling in adult fast and slow skeletal muscle fibers.Crossref | GoogleScholarGoogle Scholar | 11555650PubMed |
Dunshea FR, D’Souza DN, Pethick DW, Harper GS, Warner RD (2005) Effects of dietary factors and other metabolic modifiers on quality and nutritional value of meat. Meat Science 71, 8–38.
| Effects of dietary factors and other metabolic modifiers on quality and nutritional value of meat.Crossref | GoogleScholarGoogle Scholar | 22064049PubMed |
Fan Q, Long B, Yan G, Wang Z, Shi M, Bao X, Hu J, Li X, Chen C, Zheng Z, Yan X (2017) Dietary leucine supplementation alters energy metabolism and induces slow-to-fast transitions in longissimus dorsi muscle of weanling piglets. British Journal of Nutrition 117, 1222–1234.
| Dietary leucine supplementation alters energy metabolism and induces slow-to-fast transitions in longissimus dorsi muscle of weanling piglets.Crossref | GoogleScholarGoogle Scholar | 28643619PubMed |
Farmer LJ, Farrell DT (2018) Beef-eating quality: a European journey. Animal 12, 2424–2433.
| Beef-eating quality: a European journey.Crossref | GoogleScholarGoogle Scholar | 30004320PubMed |
Fischer LM, daCosta KA, Kwock L, Stewart PW, Lu TS, Stabler SP, Allen RH, Zeisel SH (2007) Sex and menopausal status influence human dietary requirements for the nutrient choline. The American Journal of Clinical Nutrition 85, 1275–1285.
| Sex and menopausal status influence human dietary requirements for the nutrient choline.Crossref | GoogleScholarGoogle Scholar | 17490963PubMed |
Fyfe JJ, Bishop DJ, Stepto NK (2014) Interference between concurrent resistance and endurance exercise: molecular bases and the role of individual training variables. Sports Medicine (Auckland, N.Z.) 44, 743–762.
| Interference between concurrent resistance and endurance exercise: molecular bases and the role of individual training variables.Crossref | GoogleScholarGoogle Scholar |
Garmyn AJ, Miller MF (2014) Implant and beta agonist impacts on beef palatability. Journal of Animal Science 92, 10–20.
| Implant and beta agonist impacts on beef palatability.Crossref | GoogleScholarGoogle Scholar | 24158364PubMed |
Girgenrath S, Song K, Whittemore LA (2005) Loss of myostatin expression alters fiber-type distribution and expression of myosin heavy chain isoforms in slow- and fast-type skeletal muscle. Muscle & Nerve 31, 34–40.
| Loss of myostatin expression alters fiber-type distribution and expression of myosin heavy chain isoforms in slow- and fast-type skeletal muscle.Crossref | GoogleScholarGoogle Scholar |
Glass DJ (2010) PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Current Topics in Microbiology and Immunology 346, 267–278.
| PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.Crossref | GoogleScholarGoogle Scholar | 20593312PubMed |
Gouspillou G, Sgarioto N, Norris B, Barbat-Artigas S, Aubertin-Leheudre M, Morais JA, Burelle Y, Taivassalo T, Hepple RT (2014) The relationship between muscle fiber type-specific PGC-1α content and mitochondrial content varies between rodent models and humans. PLoS One 9, e103044
| The relationship between muscle fiber type-specific PGC-1α content and mitochondrial content varies between rodent models and humans.Crossref | GoogleScholarGoogle Scholar | 25121500PubMed |
Hagg A, Colgan TD, Thomson RE, Qian H, Lynch GS, Gregorevic P (2016) Using AAV vectors expressing the β2-adrenoceptor or associated Gα proteins to modulate skeletal muscle mass and muscle fibre size. Scientific Reports 6, 23042
| Using AAV vectors expressing the β2-adrenoceptor or associated Gα proteins to modulate skeletal muscle mass and muscle fibre size.Crossref | GoogleScholarGoogle Scholar | 26972746PubMed |
Ham DJ, Caldow MK, Lynch GS, Koopman R (2014a) Leucine as a treatment for muscle wasting: a critical review. Clinical Nutrition 33, 937–945.
| Leucine as a treatment for muscle wasting: a critical review.Crossref | GoogleScholarGoogle Scholar | 25444557PubMed |
Ham DJ, Caldow MK, Lynch GS, Koopman R (2014b) Arginine protects muscle cells from wasting in vitro in an mTORC1-dependent and NO-independent manner. Amino Acids 46, 2643–2652.
| Arginine protects muscle cells from wasting in vitro in an mTORC1-dependent and NO-independent manner.Crossref | GoogleScholarGoogle Scholar | 25096520PubMed |
Ham DJ, Murphy KT, Chee A, Lynch GS, Koopman R (2014c) Glycine administration attenuates skeletal muscle wasting in a mouse model of cancer cachexia. Clinical Nutrition (Edinburgh, Lothian) 33, 448–458.
| Glycine administration attenuates skeletal muscle wasting in a mouse model of cancer cachexia.Crossref | GoogleScholarGoogle Scholar |
Ham DJ, Gleeson BG, Chee A, Baum DM, Caldow MK, Lynch GS, Koopman R (2015a) L–Citrulline protects skeletal muscle cells from cachectic stimuli through an iNOS-dependent mechanism. PLoS One 10, e0141572
| L–Citrulline protects skeletal muscle cells from cachectic stimuli through an iNOS-dependent mechanism.Crossref | GoogleScholarGoogle Scholar | 26513461PubMed |
Ham DJ, Kennedy TL, Caldow MK, Chee A, Lynch GS, Koopman R (2015b) Citrulline does not prevent skeletal muscle wasting or weakness in limb-casted mice. The Journal of Nutrition 145, 900–906.
| Citrulline does not prevent skeletal muscle wasting or weakness in limb-casted mice.Crossref | GoogleScholarGoogle Scholar | 25740910PubMed |
Ham DJ, Caldow MK, Chhen V, Chee A, Wang X, Proud CG, Lynch GS, Koopman R (2016) Glycine restores the anabolic response to leucine in a mouse model of acute inflammation. American Journal of Physiology. Endocrinology and Metabolism 310, E970–E981.
| Glycine restores the anabolic response to leucine in a mouse model of acute inflammation.Crossref | GoogleScholarGoogle Scholar | 27094036PubMed |
Hardee JP, Lynch GS (2019) Current pharmacotherapies for sarcopenia. Expert Opinion on Pharmacotherapy 23, 1–13.
| Current pharmacotherapies for sarcopenia.Crossref | GoogleScholarGoogle Scholar |
Higgins AJ (2004) Fat versus lean: the quest for beautiful buttocks. Veterinary Journal 167, 217–218.
| Fat versus lean: the quest for beautiful buttocks.Crossref | GoogleScholarGoogle Scholar |
Hill JJ, Qiu Y, Hewick RM, Wolfman NM (2003) Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: a novel protein with protease inhibitor and follistatin domains. Molecular Endocrinology 17, 1144–1154.
| Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: a novel protein with protease inhibitor and follistatin domains.Crossref | GoogleScholarGoogle Scholar | 12595574PubMed |
Hostrup M, Reitelseder S, Jessen S, Kalsen A, Nyberg M, Egelund J, Kreiberg M, Kristensen CM, Thomassen M, Pilegaard H, Backer V, Jacobson GA, Holm L, Bangsbo J (2018) Beta2-adrenoceptor agonist salbutamol increases protein turnover rates and alters signalling in skeletal muscle after resistance exercise in young men. The Journal of Physiology 596, 4121–4139.
| Beta2-adrenoceptor agonist salbutamol increases protein turnover rates and alters signalling in skeletal muscle after resistance exercise in young men.Crossref | GoogleScholarGoogle Scholar | 29968301PubMed |
Hu CJ, Jiang QY, Zhang T, Yin YL, Li FN, Deng JP, Wu GY, Kong XF (2017) Dietary supplementation with arginine and glutamic acid modifies growth performance, carcass traits, and meat quality in growing-finishing pigs. Journal of Animal Science 95, 2680–2689.
Iskenderian A, Liu N, Deng Q, Huang Y, Shen C, Palmieri K, Crooker R, Lundberg D, Kastrapeli N, Pescatore B, Romashko A, Dumas J, Comeau R, Norton A, Pan J, Rong H, Derakhchan K, Ehmann DE (2018) Myostatin and activin blockade by engineered follistatin results in hypertrophy and improves dystrophic pathology in mdx mouse more than myostatin blockade alone. Skeletal Muscle 8, 34
| Myostatin and activin blockade by engineered follistatin results in hypertrophy and improves dystrophic pathology in mdx mouse more than myostatin blockade alone.Crossref | GoogleScholarGoogle Scholar | 30368252PubMed |
Jahanian R, Ashnagar M (2018) Effects of dietary supplementation of choline and carnitine on growth performance, meat oxidative stability and carcass composition of broiler chickens fed diets with different metabolisable energy levels. British Poultry Science 59, 470–476.
| Effects of dietary supplementation of choline and carnitine on growth performance, meat oxidative stability and carcass composition of broiler chickens fed diets with different metabolisable energy levels.Crossref | GoogleScholarGoogle Scholar | 29856245PubMed |
Jiang H, Ge X (2014) Mechanism of growth hormone stimulation of skeletal muscle growth in cattle. Journal of Animal Science 92, 21–29.
| Mechanism of growth hormone stimulation of skeletal muscle growth in cattle.Crossref | GoogleScholarGoogle Scholar | 24166991PubMed |
Jimenez AG, Dillaman RM, Kinsey ST (2013) Large fibre size in skeletal muscle is metabolically advantageous. Nature Communications 4, 2150
| Large fibre size in skeletal muscle is metabolically advantageous.Crossref | GoogleScholarGoogle Scholar | 23851638PubMed |
Johnson BJ, Chung KY (2007) Alterations in the physiology of growth of cattle with growth-enhancing compounds. The Veterinary Clinics of North America. Food Animal Practice 23, 321–332.
| Alterations in the physiology of growth of cattle with growth-enhancing compounds.Crossref | GoogleScholarGoogle Scholar | 17606154PubMed |
Kinsey ST, Hardy KM, Locke BR (2007) The long and winding road: influences of intracellular metabolite diffusion on cellular organization and metabolism in skeletal muscle. The Journal of Experimental Biology 210, 3505–3512.
| The long and winding road: influences of intracellular metabolite diffusion on cellular organization and metabolism in skeletal muscle.Crossref | GoogleScholarGoogle Scholar | 17921152PubMed |
Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK, Lemosquet S, Saris WH, Boirie Y, van Loon LJ (2009) Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. The American Journal of Clinical Nutrition 90, 106–115.
| Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein.Crossref | GoogleScholarGoogle Scholar | 19474134PubMed |
Koopman R, Gehrig SM, Léger B, Trieu J, Walrand S, Murphy KT, Lynch GS (2010) Cellular mechanisms underlying temporal changes in skeletal muscle protein synthesis and breakdown during chronic β-adrenoceptor stimulation in mice. The Journal of Physiology 588, 4811–4823.
| Cellular mechanisms underlying temporal changes in skeletal muscle protein synthesis and breakdown during chronic β-adrenoceptor stimulation in mice.Crossref | GoogleScholarGoogle Scholar | 20937713PubMed |
Koopman R, Caldow MK, Ham DJ, Lynch GS (2017) Glycine metabolism in skeletal muscle: implications for metabolic homeostasis. Current Opinion in Clinical Nutrition and Metabolic Care 20, 237–242.
| Glycine metabolism in skeletal muscle: implications for metabolic homeostasis.Crossref | GoogleScholarGoogle Scholar | 28375879PubMed |
Larsson L, Ansved T, Edström L, Gorza L, Schiaffino S (1991) Effects of age on physiological, immunohistochemical and biochemical properties of fast-twitch single motor units in the rat. The Journal of Physiology 443, 257–275.
| Effects of age on physiological, immunohistochemical and biochemical properties of fast-twitch single motor units in the rat.Crossref | GoogleScholarGoogle Scholar | 1668338PubMed |
Lean IJ, Golder HM, Lees NM, McGilchrist P, Santos JEP (2018) Effects of hormonal growth promotants on beef quality: a meta-analysis. Journal of Animal Science 96, 2675–2697.
| Effects of hormonal growth promotants on beef quality: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 29659862PubMed |
Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN, Wright JF, Barker C, Ehrmantraut G, Holmstrom J, Trowell B, Gertz B, Jiang MS, Sebald SM, Matzuk M, Li E, Liang LF, Quattlebaum E, Stotish RL, Wolfman NM (2005) Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proceedings of the National Academy of Sciences, USA 102, 18117–18122.
| Regulation of muscle growth by multiple ligands signaling through activin type II receptors.Crossref | GoogleScholarGoogle Scholar |
Lee SH, Joo ST, Ryu YC (2010) Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Science 86, 166–170.
| Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality.Crossref | GoogleScholarGoogle Scholar | 20605337PubMed |
Lee SJ, Huynh TV, Lee YS, Sebald SM, Wilcox-Adelman SA, Iwamori N, Lepper C, Matzuk MM, Fan CM (2012) Role of satellite cells versus myofibers in muscle hypertrophy induced by inhibition of the myostatin/activin signaling pathway. Proceedings of the National Academy of Sciences, USA 109, E2353–E2360.
| Role of satellite cells versus myofibers in muscle hypertrophy induced by inhibition of the myostatin/activin signaling pathway.Crossref | GoogleScholarGoogle Scholar |
Lefaucheur L (2010) A second look into fibre typing: relation to meat quality. Meat Science 84, 257–270.
| A second look into fibre typing: relation to meat quality.Crossref | GoogleScholarGoogle Scholar | 20374784PubMed |
Leporati M, Bergoglio M, Capra P, Bozzetta E, Abete MC, Vincenti M (2014) Development, validation and application to real samples of a multiresidue LC–MS/MS method for determination of β2 -agonists and anabolic steroids in bovine hair. Journal of Mass Spectrometry 49, 936–946.
| Development, validation and application to real samples of a multiresidue LC–MS/MS method for determination of β2 -agonists and anabolic steroids in bovine hair.Crossref | GoogleScholarGoogle Scholar | 25230191PubMed |
Listrat A, Lebret B, Louveau I, Astruc T, Bonnet M, Lefaucheur L, Picard B, Bugeon J (2016) How muscle structure and composition influence meat and flesh quality. The Scientific World Journal 2016, 3182746
| How muscle structure and composition influence meat and flesh quality.Crossref | GoogleScholarGoogle Scholar | 27022618PubMed |
Liu SY, Selle PH, Raubenheimer D, Gous RM, Chrystal PV, Cadogan DJ, Simpson SJ, Cowieson AJ (2017) Growth performance, nutrient utilisation and carcass composition respond to dietary protein concentrations in broiler chickens but responses are modified by dietary lipid levels. British Journal of Nutrition 118, 250–262.
| Growth performance, nutrient utilisation and carcass composition respond to dietary protein concentrations in broiler chickens but responses are modified by dietary lipid levels.Crossref | GoogleScholarGoogle Scholar | 28875867PubMed |
Ljubicic V, Burt M, Jasmin BJ (2014) The therapeutic potential of skeletal muscle plasticity in Duchenne muscular dystrophy: phenotypic modifiers as pharmacologic targets. The FASEB Journal 28, 548–568.
| The therapeutic potential of skeletal muscle plasticity in Duchenne muscular dystrophy: phenotypic modifiers as pharmacologic targets.Crossref | GoogleScholarGoogle Scholar | 24249639PubMed |
Lynch GS (2002) Beta-2 agonists. In ‘Performance Enhancing Substances in Sport and Exercise’. (Eds MS Bahrke, CE Yesalis) pp. 47–64. (Human Kinetics: Champaign, IL)
Lynch GS (2017) Therapeutic potential of skeletal muscle plasticity and slow muscle programming for muscular dystrophy and related muscle conditions. In ‘Plasticity of skeletal muscle’. (Ed. K Sakuma) pp. 277–292. (Springer: Singapore)
Lynch GS, Koopman R (2018) Dietary meat and protection against sarcopenia. Meat Science 144, 180–185.
| Dietary meat and protection against sarcopenia.Crossref | GoogleScholarGoogle Scholar | 29941158PubMed |
Lynch GS, Ryall JG (2008) Role of β-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiological Reviews 88, 729–767.
| Role of β-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease.Crossref | GoogleScholarGoogle Scholar | 18391178PubMed |
Madeira MS, Pires VM, Alfaia CM, Luxton R, Doran O, Bessa RJ, Prates JA (2014) Combined effects of dietary arginine, leucine and protein levels on fatty acid composition and gene expression in the muscle and subcutaneous adipose tissue of crossbred pigs. British Journal of Nutrition 111, 1521–1535.
| Combined effects of dietary arginine, leucine and protein levels on fatty acid composition and gene expression in the muscle and subcutaneous adipose tissue of crossbred pigs.Crossref | GoogleScholarGoogle Scholar | 24502766PubMed |
Madeira MS, Alfaia CM, Costa P, Lopes PA, Martins SV, Lemos JP, Moreira O, Santos-Silva J, Bessa RJ, Prates JA (2015) Effect of betaine and arginine in lysine-deficient diets on growth, carcass traits, and pork quality. Journal of Animal Science 93, 4721–4733.
| Effect of betaine and arginine in lysine-deficient diets on growth, carcass traits, and pork quality.Crossref | GoogleScholarGoogle Scholar | 26523565PubMed |
Madeira MS, Rolo ES, Alfaia CM, Pires VR, Luxton R, Doran O, Bessa RJ, Prates JA (2016) Influence of betaine and arginine supplementation of reduced protein diets on fatty acid composition and gene expression in the muscle and subcutaneous adipose tissue of cross-bred pigs. British Journal of Nutrition 115, 937–950.
| Influence of betaine and arginine supplementation of reduced protein diets on fatty acid composition and gene expression in the muscle and subcutaneous adipose tissue of cross-bred pigs.Crossref | GoogleScholarGoogle Scholar | 26819073PubMed |
Martin JN, Garmyn AJ, Miller MF, Hodgen JM, Pfeiffer KD, Thomas CL, Rathmann RJ, Yates DA, Hutcheson JP, Brooks JC (2014) Comparative effects of beta-adrenergic agonist supplementation on the yield and quality attributes of selected subprimals from calf-fed Holstein steers. Journal of Animal Science 92, 4204–4216.
| Comparative effects of beta-adrenergic agonist supplementation on the yield and quality attributes of selected subprimals from calf-fed Holstein steers.Crossref | GoogleScholarGoogle Scholar | 25006060PubMed |
McBreairty LE, Robinson JL, Harding SV, Randell EW, Brunton JA, Bertolo RF (2016) Betaine is as effective as folate at re-synthesizing methionine for protein synthesis during moderate methionine deficiency in piglets. European Journal of Nutrition 55, 2423–2430.
| Betaine is as effective as folate at re-synthesizing methionine for protein synthesis during moderate methionine deficiency in piglets.Crossref | GoogleScholarGoogle Scholar | 26419586PubMed |
McPherron AC, Lee SJ (1997) Double muscling in cattle due to mutations in the myostatin gene. Proceedings of the National Academy of Sciences, USA 94, 12457–12461.
| Double muscling in cattle due to mutations in the myostatin gene.Crossref | GoogleScholarGoogle Scholar |
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387, 83–90.
| Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member.Crossref | GoogleScholarGoogle Scholar | 9139826PubMed |
Medeiros EF, Phelps MP, Fuentes FD, Bradley TM (2009) Overexpression of follistatin in trout stimulates increased muscling. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 297, R235–R242.
| Overexpression of follistatin in trout stimulates increased muscling.Crossref | GoogleScholarGoogle Scholar | 19474387PubMed |
Moghadam HK, Johnsen H, Robinson N, Andersen Ø, Jørgensen EH, Johnsen HK, Bæhr VJ, Tveiten H (2017) Impacts of early life stress on the methylome and transcriptome of Atlantic salmon. Scientific Reports 7, 5023
| Impacts of early life stress on the methylome and transcriptome of Atlantic salmon.Crossref | GoogleScholarGoogle Scholar | 28694447PubMed |
Moinard C, Cynober L (2007) Citrulline: a new player in the control of nitrogen homeostasis. The Journal of Nutrition 137, 1621S–1625S.
| Citrulline: a new player in the control of nitrogen homeostasis.Crossref | GoogleScholarGoogle Scholar | 17513438PubMed |
Murgas Torrazza R, Suryawan A, Gazzaneo MC, Orellana RA, Frank JW, Nguyen HV, Fiorotto ML, El-Kadi S, Davis TA (2010) Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. The Journal of Nutrition 140, 2145–2152.
| Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation.Crossref | GoogleScholarGoogle Scholar | 20962152PubMed |
Murgia M, Nagaraj N, Deshmukh AS, Zeiler M, Cancellara P, Moretti I, Reggiani C, Schiaffino S, Mann M (2015) Single muscle fiber proteomics reveals unexpected mitochondrial specialization. EMBO Reports 16, 387–395.
| Single muscle fiber proteomics reveals unexpected mitochondrial specialization.Crossref | GoogleScholarGoogle Scholar | 25643707PubMed |
Murphy KT, Koopman R, Naim T, Léger B, Trieu J, Ibebunjo C, Lynch GS (2010) Antibody-directed myostatin inhibition in 21-mo-old mice reveals novel roles for myostatin signaling in skeletal muscle structure and function. The FASEB Journal 24, 4433–4442.
| Antibody-directed myostatin inhibition in 21-mo-old mice reveals novel roles for myostatin signaling in skeletal muscle structure and function.Crossref | GoogleScholarGoogle Scholar | 20624929PubMed |
Murphy KT, Chee A, Gleeson BG, Naim T, Swiderski K, Koopman R, Lynch GS (2011) Antibody-directed myostatin inhibition enhances muscle mass and function in tumor-bearing mice. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 301, R716–R726.
| Antibody-directed myostatin inhibition enhances muscle mass and function in tumor-bearing mice.Crossref | GoogleScholarGoogle Scholar | 21677277PubMed |
Olson EN, Williams RS (2000) Calcineurin signaling and muscle remodeling. Cell 101, 689–692.
| Calcineurin signaling and muscle remodeling.Crossref | GoogleScholarGoogle Scholar | 10892739PubMed |
Omairi S, Matsakas A, Degens H, Kretz O, Hansson KA, Solbrå AV, Bruusgaard JC, Joch B, Sartori R, Giallourou N, Mitchell R, Collins-Hooper H, Foster K, Pasternack A, Ritvos O, Sandri M, Narkar V, Swann JR, Huber TB, Patel K (2016) Enhanced exercise and regenerative capacity in a mouse model that violates size constraints of oxidative muscle fibres. eLife 5, e16940
| Enhanced exercise and regenerative capacity in a mouse model that violates size constraints of oxidative muscle fibres.Crossref | GoogleScholarGoogle Scholar | 27494364PubMed |
Osowska S, Moinard C, Neveux N, Loï C, Cynober L (2004) Citrulline increases arginine pools and restores nitrogen balance after massive intestinal resection. Gut 53, 1781–1786.
| Citrulline increases arginine pools and restores nitrogen balance after massive intestinal resection.Crossref | GoogleScholarGoogle Scholar | 15542514PubMed |
Parr T, Mareko MHD, Ryan KJP, Hemmings KM, Brown DM, Brameld JM (2016) The impact of growth promoters on muscle growth and the potential consequences for meat quality. Meat Science 120, 93–99.
| The impact of growth promoters on muscle growth and the potential consequences for meat quality.Crossref | GoogleScholarGoogle Scholar | 27179582PubMed |
Parsons SA, Wilkins BJ, Bueno OF, Molkentin JD (2003) Altered skeletal muscle phenotypes in calcineurin Aα and Aβ gene-targeted mice. Molecular and Cellular Biology 23, 4331–4343.
| Altered skeletal muscle phenotypes in calcineurin Aα and Aβ gene-targeted mice.Crossref | GoogleScholarGoogle Scholar | 12773574PubMed |
Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ (2011) Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. The American Journal of Clinical Nutrition 93, 997–1005.
| Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men.Crossref | GoogleScholarGoogle Scholar | 21367943PubMed |
Pette D (2001) Historical Perspectives: plasticity of mammalian skeletal muscle. Journal of Applied Physiology 90, 1119–1124.
| Historical Perspectives: plasticity of mammalian skeletal muscle.Crossref | GoogleScholarGoogle Scholar | 11181628PubMed |
Reinhardt C (2007) Growth-promotant implants: managing the tools. The Veterinary Clinics of North America. Food Animal Practice 23, 309–319.
| Growth-promotant implants: managing the tools.Crossref | GoogleScholarGoogle Scholar | 17606153PubMed |
Robinson NA, Timmerhaus G, Baranski M, Andersen Ø, Takle H, Krasnov A (2017) Training the salmon’s genes: influence of aerobic exercise, swimming performance and selection on gene expression in Atlantic salmon. BMC Genomics 18, 971
| Training the salmon’s genes: influence of aerobic exercise, swimming performance and selection on gene expression in Atlantic salmon.Crossref | GoogleScholarGoogle Scholar | 29246115PubMed |
Rodino-Klapac LR, Haidet AM, Kota J, Handy C, Kaspar BK, Mendell JR (2009) Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle & Nerve 39, 283–296.
| Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease.Crossref | GoogleScholarGoogle Scholar |
Rom O, Reznick AZ (2016) The role of E3 ubiquitin-ligases MuRF-1 and MAFbx in loss of skeletal muscle mass. Free Radical Biology & Medicine 98, 218–230.
| The role of E3 ubiquitin-ligases MuRF-1 and MAFbx in loss of skeletal muscle mass.Crossref | GoogleScholarGoogle Scholar |
Ryall JG, Lynch GS (2008) The potential and the pitfalls of β-adrenoceptor agonists for the management of skeletal muscle wasting. Pharmacology & Therapeutics 120, 219–232.
| The potential and the pitfalls of β-adrenoceptor agonists for the management of skeletal muscle wasting.Crossref | GoogleScholarGoogle Scholar |
Sartori R, Milan G, Patron M, Mammucari C, Blaauw B, Abraham R, Sandri M (2009) Smad2 and 3 transcription factors control muscle mass in adulthood. American Journal of Physiology. Cell Physiology 296, C1248–C1257.
| Smad2 and 3 transcription factors control muscle mass in adulthood.Crossref | GoogleScholarGoogle Scholar | 19357234PubMed |
Schaefer AL, Davis SR, Hughson GA (1986) Estimation of tissue protein synthesis in sheep during sustained elevation of plasma leucine concentration by intravenous infusion. British Journal of Nutrition 56, 281–288.
| Estimation of tissue protein synthesis in sheep during sustained elevation of plasma leucine concentration by intravenous infusion.Crossref | GoogleScholarGoogle Scholar | 3676202PubMed |
Schiaffino S (2007) Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology (Bethesda, MD) 22, 269–278.
| Activity-dependent signaling pathways controlling muscle diversity and plasticity.Crossref | GoogleScholarGoogle Scholar |
Schiaffino S, Reggiani C (2011) Fiber types in mammalian skeletal muscles. Physiological Reviews 91, 1447–1531.
| Fiber types in mammalian skeletal muscles.Crossref | GoogleScholarGoogle Scholar | 22013216PubMed |
Schuelke M, Wagner KR, Stolz LE, Hübner C, Riebel T, Kömen W, Braun T, Tobin JF, Lee SJ (2004) Myostatin mutation associated with gross muscle hypertrophy in a child. The New England Journal of Medicine 350, 2682–2688.
| Myostatin mutation associated with gross muscle hypertrophy in a child.Crossref | GoogleScholarGoogle Scholar | 15215484PubMed |
Schumann C, Nguyen DX, Norgard M, Bortnyak Y, Korzun T, Chan S, Lorenz AS, Moses AS, Albarqi HA, Wong L, Michaelis K, Zhu X, Alani AWG, Taratula OR, Krasnow S, Marks DL, Taratula O (2018) Increasing lean muscle mass in mice via nanoparticle-mediated hepatic delivery of follistatin mRNA. Theranostics 8, 5276–5288.
| Increasing lean muscle mass in mice via nanoparticle-mediated hepatic delivery of follistatin mRNA.Crossref | GoogleScholarGoogle Scholar | 30555546PubMed |
Sepulveda PV, Lamon S, Hagg A, Thomson RE, Winbanks CE, Qian H, Bruce CR, Russell AP, Gregorevic P (2015) Evaluation of follistatin as a therapeutic in models of skeletal muscle atrophy associated with denervation and tenotomy. Scientific Reports 5, 17535
| Evaluation of follistatin as a therapeutic in models of skeletal muscle atrophy associated with denervation and tenotomy.Crossref | GoogleScholarGoogle Scholar | 26657343PubMed |
Shakeri M, Cottrell JJ, Wilkinson S, Ringuet M, Furness JB, Dunshea FR (2018) Betaine and antioxidants improve growth performance, breast muscle development and ameliorate thermoregulatory responses to cyclic heat exposure in broiler chickens. Animals 8, 162
| Betaine and antioxidants improve growth performance, breast muscle development and ameliorate thermoregulatory responses to cyclic heat exposure in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Sillence MN (2004) Technologies for the control of fat and lean deposition in livestock. Veterinary Journal 167, 242–257.
| Technologies for the control of fat and lean deposition in livestock.Crossref | GoogleScholarGoogle Scholar |
Stephany RW (2010) Hormonal growth promoting agents in food producing animals. In ‘Doping in sports, handbook of experimental pharmacology’. (Eds D Thieme, P Hemmersbach) pp. 355–367. (Springer-Verlag: Heidelberg, Germany)
Stupka N, Plant DR, Schertzer JD, Emerson TM, Bassel-Duby R, Olson EN, Lynch GS (2006) Activated calcineurin ameliorates contraction-induced injury to skeletal muscles of mdx dystrophic mice. The Journal of Physiology 575, 645–656.
| Activated calcineurin ameliorates contraction-induced injury to skeletal muscles of mdx dystrophic mice.Crossref | GoogleScholarGoogle Scholar | 16793906PubMed |
Stupka N, Schertzer JD, Bassel-Duby R, Olson EN, Lynch GS (2007) Calcineurin-Aα activation enhances the structure and function of regenerating muscles after myotoxic injury. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 293, R686–R694.
| Calcineurin-Aα activation enhances the structure and function of regenerating muscles after myotoxic injury.Crossref | GoogleScholarGoogle Scholar | 17475677PubMed |
Stupka N, Schertzer JD, Bassel-Duby R, Olson EN, Lynch GS (2008) Stimulation of calcineurin Aα activity attenuates muscle pathophysiology in mdx dystrophic mice. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 294, R983–R992.
| Stimulation of calcineurin Aα activity attenuates muscle pathophysiology in mdx dystrophic mice.Crossref | GoogleScholarGoogle Scholar | 18199592PubMed |
Sung IK, Park SJ, Kang K, Kim MY, Cho S (2015) Development and application of a method for rapid and simultaneous determination of three β-agonists (clenbuterol, ractopamine, and zilpaterol) using liquid chromatography-tandem mass spectrometry. Han-gug Chugsan Sigpum Hag-hoeji 35, 121–129.
| Development and application of a method for rapid and simultaneous determination of three β-agonists (clenbuterol, ractopamine, and zilpaterol) using liquid chromatography-tandem mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
Swiderski K, Lynch GS (2015) Therapeutic potential of orphan drugs for the rare skeletal muscle diseases. Expert Opinion on Orphan Drugs 3, 1397–1425.
| Therapeutic potential of orphan drugs for the rare skeletal muscle diseases.Crossref | GoogleScholarGoogle Scholar |
Takahashi K, Aoki A, Takimoto T, Akiba Y (2008) Dietary supplementation of glycine modulates inflammatory response indicators in broiler chickens. British Journal of Nutrition 100, 1019–1028.
| Dietary supplementation of glycine modulates inflammatory response indicators in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 18377692PubMed |
Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM (2009) Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. Journal of Applied Physiology 107, 987–992.
| Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men.Crossref | GoogleScholarGoogle Scholar | 19589961PubMed |
Tong JF, Yan X, Zhu MJ, Du M (2009) AMP-activated protein kinase enhances the expression of muscle-specific ubiquitin ligases despite its activation of IGF-1/Akt signaling in C2C12 myotubes. Journal of Cellular Biochemistry 108, 458–468.
| AMP-activated protein kinase enhances the expression of muscle-specific ubiquitin ligases despite its activation of IGF-1/Akt signaling in C2C12 myotubes.Crossref | GoogleScholarGoogle Scholar | 19639604PubMed |
Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ (2009) Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. American Journal of Physiology. Cell Physiology 296, C1258–C1270.
| Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size.Crossref | GoogleScholarGoogle Scholar | 19357233PubMed |
Tsuchida K (2008) Targeting myostatin for therapies against muscle-wasting disorders. Current Opinion in Drug Discovery & Development 11, 487–494.
van der Laarse WJ, Des Tombe AL, Lee-De Groot MBE, Diegenbach PC (1998) Size principle of striated muscle cells. Netherlands Journal of Zoology 48, 213–223.
van der Zwaard S, van der Laarse WJ, Weide G, Bloemers FW, Hofmijster MJ, Levels K, Noordhof DA, de Koning JJ, de Ruiter CJ, Jaspers RT (2018) Critical determinants of combined sprint and endurance performance: an integrative analysis from muscle fiber to the human body. The FASEB Journal 32, 2110–2123.
| Critical determinants of combined sprint and endurance performance: an integrative analysis from muscle fiber to the human body.Crossref | GoogleScholarGoogle Scholar | 29217665PubMed |
van Wessel T, de Haan A, van der Laarse WJ, Jaspers RT (2010) The muscle fiber type–fiber size paradox: hypertrophy or oxidative metabolism? European Journal of Applied Physiology 110, 665–694.
| The muscle fiber type–fiber size paradox: hypertrophy or oxidative metabolism?Crossref | GoogleScholarGoogle Scholar | 20602111PubMed |
Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, van Loon LJ (2009) Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. The American Journal of Clinical Nutrition 89, 1468–1475.
| Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men.Crossref | GoogleScholarGoogle Scholar | 19321567PubMed |
Vestergaard M, Oksbjerg N, Henckel P (2000a) Influence of feeding intensity, grazing and finishing feeding on muscle fibre characteristics and meat colour of semitendinosus, longissimus dorsi and supraspinatus muscles of young bulls. Meat Science 54, 177–185.
| Influence of feeding intensity, grazing and finishing feeding on muscle fibre characteristics and meat colour of semitendinosus, longissimus dorsi and supraspinatus muscles of young bulls.Crossref | GoogleScholarGoogle Scholar | 22060614PubMed |
Vestergaard M, Therkildsen M, Henckel P, Jensen LR, Andersen HR, Sejrsen K (2000b) Influence of feeding intensity, grazing and finishing feeding on meat and eating quality of young bulls and the relationship between muscle fibre characteristics, fibre fragmentation and meat tenderness. Meat Science 54, 187–195.
| Influence of feeding intensity, grazing and finishing feeding on meat and eating quality of young bulls and the relationship between muscle fibre characteristics, fibre fragmentation and meat tenderness.Crossref | GoogleScholarGoogle Scholar | 22060615PubMed |
Wall BT, Hamer HM, de Lange A, Kiskini A, Groen BB, Senden JM, Gijsen AP, Verdijk LB, van Loon LJ (2013) Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clinical Nutrition (Edinburgh, Lothian) 32, 412–419.
| Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men.Crossref | GoogleScholarGoogle Scholar |
Wan H, Zhu J, Su G, Liu Y, Hua L, Hu L, Wu C, Zhang R, Zhou P, Shen Y, Lin Y, Xu S, Fang Z, Che L, Feng B, Wu D (2016) Dietary supplementation with β-hydroxy-β-methylbutyrate calcium during the early postnatal period accelerates skeletal muscle fibre growth and maturity in intra-uterine growth-retarded and normal-birth-weight piglets. British Journal of Nutrition 115, 1360–1369.
| Dietary supplementation with β-hydroxy-β-methylbutyrate calcium during the early postnatal period accelerates skeletal muscle fibre growth and maturity in intra-uterine growth-retarded and normal-birth-weight piglets.Crossref | GoogleScholarGoogle Scholar | 26917333PubMed |
Wang M, Yu H, Kim YS, Bidwell CA, Kuang S (2012) Fiber-type distribution and expression of myosin heavy chain isoforms in newborn heterozygous myostatin-knockout pigs. Biochemical and Biophysical Research Communications 426, 83–88.
| Fiber-type distribution and expression of myosin heavy chain isoforms in newborn heterozygous myostatin-knockout pigs.Crossref | GoogleScholarGoogle Scholar | 22910409PubMed |
Wang Y, Zhou J, Wang G, Cai S, Zeng X, Qiao S (2018) Advances in low-protein diets for swine. Journal of Animal Science and Biotechnology 9, 60
| Advances in low-protein diets for swine.Crossref | GoogleScholarGoogle Scholar | 30034802PubMed |
Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, Hill JJ, Jalenak M, Kelley P, Knight A, Maylor R, O’Hara D, Pearson A, Quazi A, Ryerson S, Tan XY, Tomkinson KN, Veldman GM, Widom A, Wright JF, Wudyka S, Zhao L, Wolfman NM (2003) Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochemical and Biophysical Research Communications 300, 965–971.
| Inhibition of myostatin in adult mice increases skeletal muscle mass and strength.Crossref | GoogleScholarGoogle Scholar | 12559968PubMed |
Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM (2007) Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. The American Journal of Clinical Nutrition 85, 1031–1040.
| Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage.Crossref | GoogleScholarGoogle Scholar | 17413102PubMed |
Winbanks CE, Weeks KL, Thomson RE, Sepulveda PV, Beyer C, Qian H, Chen JL, Allen JM, Lancaster GI, Febbraio MA, Harrison CA, McMullen JR, Chamberlain JS, Gregorevic P (2012) Follistatin-mediated skeletal muscle hypertrophy is regulated by Smad3 and mTOR independently of myostatin. The Journal of Cell Biology 197, 997–1008.
| Follistatin-mediated skeletal muscle hypertrophy is regulated by Smad3 and mTOR independently of myostatin.Crossref | GoogleScholarGoogle Scholar | 22711699PubMed |
Winbanks CE, Murphy KT, Bernardo BC, Qian H, Liu Y, Sepulveda PV, Beyer C, Hagg A, Thomson RE, Chen JL, Walton KL, Loveland KL, McMullen JR, Rodgers BD, Harrison CA, Lynch GS, Gregorevic P (2016) Smad7 gene delivery prevents muscle wasting associated with cancer cachexia in mice. Science Translational Medicine 8, 348ra98
| Smad7 gene delivery prevents muscle wasting associated with cancer cachexia in mice.Crossref | GoogleScholarGoogle Scholar | 27440729PubMed |
Xing XX, Xuan MF, Jin L, Guo Q, Luo ZB, Wang JX, Luo QR, Zhang GL, Cui CD, Cui ZY, Kang JD, Yin XJ (2017) Fiber-type distribution and expression of myosin heavy chain isoforms in newborn heterozygous myostatin-knockout pigs. Biotechnology Letters 39, 1811–1819.
| Fiber-type distribution and expression of myosin heavy chain isoforms in newborn heterozygous myostatin-knockout pigs.Crossref | GoogleScholarGoogle Scholar | 28861647PubMed |
Yun JH, Kwon IK, Lohakare JD, Choi JY, Yong JS, Zheng J, Cho WT, Chae BJ (2005) Comparative efficacy of plant and animal protein sources on the growth performance, nutrient digestibility, morphology and caecal microbiology of early-weaned pigs. Asian-Australasian Journal of Animal Sciences 18, 1285–1293.
| Comparative efficacy of plant and animal protein sources on the growth performance, nutrient digestibility, morphology and caecal microbiology of early-weaned pigs.Crossref | GoogleScholarGoogle Scholar |
Zeisel SH, Da Costa KA, Franklin PD, Alexander EA, Lamont JT, Sheard NF, Beiser A (1991) Choline, an essential nutrient for humans. The FASEB Journal 5, 2093–2098.
| Choline, an essential nutrient for humans.Crossref | GoogleScholarGoogle Scholar | 2010061PubMed |
Zhang S, Chen X, Huang Z, Chen D, Yu B, Chen H, He J, Luo J, Zheng P, Yu J, Luo Y (2019) Leucine promotes porcine myofibre type transformation from fast-twitch to slow-twitch through the protein kinase B (Akt)/forkhead box 1 signalling pathway and microRNA-27a. British Journal of Nutrition 121, 1–8.
| Leucine promotes porcine myofibre type transformation from fast-twitch to slow-twitch through the protein kinase B (Akt)/forkhead box 1 signalling pathway and microRNA-27a.Crossref | GoogleScholarGoogle Scholar | 30449288PubMed |
Zheng L, Wei H, Cheng C, Xiang Q, Pang J, Peng J (2016) Supplementation of branched-chain amino acids to a reduced-protein diet improves growth performance in piglets: involvement of increased feed intake and direct muscle growth-promoting effect. British Journal of Nutrition 115, 2236–2245.
| Supplementation of branched-chain amino acids to a reduced-protein diet improves growth performance in piglets: involvement of increased feed intake and direct muscle growth-promoting effect.Crossref | GoogleScholarGoogle Scholar | 27079773PubMed |
Zheng H, Qiao C, Tang R, Li J, Bulaklak K, Huang Z, Zhao C, Dai Y, Li J, Xiao X (2017) Follistatin N terminus differentially regulates muscle size and fat in vivo. Experimental & Molecular Medicine 49, e377
| Follistatin N terminus differentially regulates muscle size and fat in vivo.Crossref | GoogleScholarGoogle Scholar |
Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, Bunzendaul H, Bradford B, Lemasters JJ (2003) L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Current Opinion in Clinical Nutrition and Metabolic Care 6, 229–240.
| L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent.Crossref | GoogleScholarGoogle Scholar | 12589194PubMed |
Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, Goldberg AL, Han HQ (2010) Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142, 531–543.
| Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival.Crossref | GoogleScholarGoogle Scholar | 20723755PubMed |


