Water quality and management in the Australian pig industry
Louise Edwards A C and Helen Crabb B
A C and Helen Crabb B
A Acqua by Davey Pty Ltd, 6 Lakeview Drive, Scoresby, Vic. 3179, Australia.
B Faculty of Veterinary and Agricultural Sciences, The University of Melbourne, Parkville, Vic. 3010, Australia.
C Corresponding author. Email: ledwards@acquabydavey.com
Animal Production Science 61(7) 637-644 https://doi.org/10.1071/AN20484
Submitted: 23 August 2020 Accepted: 10 December 2020 Published: 18 February 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
Context: Water is the first nutrient and an essential component of all agricultural production systems. Despite its importance there has been limited research on water, and in particular, the impact of its availability, management and quality on production systems.
Aims: This research sought to describe the management and quality of water used within the Australian pig industry. Specifically, the water sources utilised, how water was managed and to evaluate water quality at both the source and the point of delivery to the pig.
Methods: Fifty-seven commercial piggeries across Australia participated in this study by completing a written survey on water management. In addition, survey participants undertook physical farm parameter measurements including collecting water samples. Each water sample was tested for standard quality parameters including pH, hardness, heavy metals and microbiological status.
Key results: Responses were received from 57 farms, estimated to represent at least 22% of ‘large’ pig herds. Bore water was the most common water source being utilised within the farms surveyed. Management practices and infrastructure delivering water from the source to the point of consumption were found to differ across the farms surveyed. Furthermore, water was regularly used as a delivery mechanism for soluble additives such as antibiotics. The quality of water at the source and point of consumption was found to be highly variable with many parameters, particularly pH, hardness, salinity, iron, manganese and microbiological levels, exceeding the acceptable standard.
Conclusions: In general, water quality did not appear to be routinely monitored or managed. As a result, farm managers had poor visibility of the potential negative impacts that inferior water quality or management may be having on pig production and in turn the economics of their business. Indeed, inferior water quality may impact the delivery of antibiotics and in turn undermine the industry’s antimicrobial stewardship efforts.
Implications: The study findings suggest that water quality represents a significant challenge to the Australian pig industry. Access to drinking water of an acceptable quality is essential for optimal pig performance, health and welfare but also to ensure farm to fork supply chain integrity, traceability and food safety.
Keywords: animal production, antimicrobial stewardship, nutrition, pigs, water quality.
Introduction
Water is a fundamental component of all agricultural production systems with water being utilised from many different sources including rivers, irrigation channels, dams, ground water and mains supply in Australia. Due to geographical variation and climatic events, water source accessibility and its resulting quality can be highly variable. As such all water sources should be considered a farm biosecurity risk as they pose a viable entry point and conduit for transmission of water-borne pathogens (Department of Agriculture 2009).
Water is essential for most physiological functions (King 1999; Brumm 2010; Nyachoti and Kiarie 2010; National Research Council 2012; Sofi et al. 2014) and is commonly referred to as the first nutrient in animal nutrition. The volume of water consumed by a pig is influenced by several factors including, but not limited to, the environment, accessibility, animal physiology, health status and behaviour (Nyachoti and Kiarie 2010). Approximately 75% of pigs’ water intake is associated with their feed intake, and as pigs eat preferentially at certain times during the day, a ‘diurnal drinking pattern’ was observed (Bigelow and Houpt 1988). Indeed if monitored consistently, daily drinking water intake can be the first indication of emerging herd health issues (Brumm 2010; Andersen et al. 2014). The way in which water is provided to pigs is important to ensure optimal feed and water consumption (Brumm 2010; Australian Pork Limited 2016). The ambient temperature, the level of competition around the feeder, diet and water flow rate all influence water use and drinking behaviour in pigs (Adam and Voets 2006; Andersen et al. 2014). Water management systems including the number, type and height of drinkers, flow rates, water pressure and temperature may impact pig consumption, and must be adequate to cover the times of greatest demand (Brooks 1994; Li et al. 2005). Long-term inadequate or inconsistent water supply or flow rates can result in increased pig aggression, lowered growth rates and weaning weights, urinary-tract infections and gastric ulcers (Lumb et al. 2017). Several guidelines provide water management recommendations to Australian producers but the level of adoption is unknown (Brooks 1994; King 1999; Taylor et al. 2006; Brumm 2010; Australian Pork Limited 2016).
Access to drinking water of an appropriate physio-chemical and microbiological quality, free of deleterious or toxic substances is imperative for farm to fork supply chain traceability and food safety. The availability of a reliable water source is critical but an awareness of the quality of that water is of equal importance. When given poor quality water, pigs respond by consuming water in excess resulting in an increased urine output so diverting energy away from production (Nyachoti and Kiarie 2010). The available definitions of what constitutes poor water quality in pig production are as varied as they are limited. Typically, water quality is evaluated based on the analysis of its physical, chemical and microbiological composition. A challenge exists in that most published water quality surveys and resulting standards refer to water for human consumption as opposed to livestock and in turn as it relates specifically to pig production. A comprehensive review of published water quality standards including King (1999) and the National Research Council (2012) was completed by Edwards (2018) resulting in a recommended standard for the Australian pig industry.
Despite its importance, there appears to have been relatively limited research on the impact of water availability, quality and management on the performance, health and welfare of pigs. This is particularly important from an antimicrobial stewardship perspective when considering that drinking water is frequently used to administer water-soluble nutritional additives and veterinary chemical products (Little et al. 2019). The objectives of this study were to investigate the management and quality of water being utilised within the Australian pig industry from source to the point of consumption.
Materials and methods
A request for volunteers was made to members of the Australian pig industry. A total of 57 commercial piggeries responded with participants being provided a questionnaire, instructions and equipment to take representative water samples for quality assessment and physical measurements. In all cases, the survey was completed by the farm manager, veterinarian, nutritionist or personnel with technical expertise. A copy of the survey is provided as Supplementary Material to this paper.
The questionnaire was a qualitative paper-based self-assessment that sought to understand water management. Specifically, the sources of drinking water available and details of the water supply, water treatment and/or dosing systems in use were investigated. Participants were also asked to conduct several physical measurements such as drinking water temperature and flow rates on the same day that the survey was completed. All participating farms provided two water samples, one from the primary water source, and one from a water drinker within a single shed. The source collection point was defined as the point of entry, or the point where water entered the farm. The shed drinking water collection point was defined as the drinker furthest from the entry of water to the nominated shed. Where possible, participants were asked to select a grower shed as their nominated shed. Where practical, water was allowed to run freely before collection. Sterile water sample bottles and water quality analyses were provided by Eurofins Environment Testing Australia Pty Ltd (NATA Accreditation 1261). On collection, all water samples were chilled to 4°C and transported within 24 h for analysis.
Statistical analyses
Water test results were categorised as passing (1) or failing (0) the pig drinking water standard as defined in Tables 1 and 2 adapted from (Edwards 2018) taking into account residue limits imposed by Australian drinking water guidelines (NHMRC and NRMMC 2011) and pig drinking water standards (National Research Council 2012). Where chemical analyses results were below the Limit of Quantification (LOQ) the test result was reported as zero for the purposes of analysis. The LOQ is the lowest concentration of a specific chemical that can be detected and quantified, with an acceptable degree of certainty, using a specified analytical method and/or item of laboratory equipment as indicated for that test.

|
|
All statistical analyses were conducted using the R Statistical program (R Core Team 2013). Simple descriptive analyses using the Chi square test or t-test were conducted for binary or continuous variables respectively. Variables with P < 0.05 were considered statistically significant. Odds ratios and 95% confidence intervals were reported for logistic regression variables where appropriate. Tukey’s comparison of means was utilised to compare factor levels within a variable. The agreement between farmers reported responses and microbiologically assessed quality results was assessed used Cohen’s kappa.
Results
Fifty-seven pig producers participated in the survey, with all producers supplying a water sample from the water source and with one exception a water sample from a single shed for comparison. Not all questions were answered in the survey by all producers. The total number of responses to each question is shown by the denominator value with the numerator value representing the response given.
Source water
Bore water was the primary water source for most producers (60%, 34/57), followed by surface (28%, 16/57) and mains water (12%, 7/57). Surface water was sourced from river (5/16), lake (1/16), dam (8/16) or irrigation channel (2/16). Thirty-five percent of producers (20/57) used more than one primary source of water with surface (7/20) and bore water (12/20) the most frequently used secondary water source. Surface water sample sources were aggregated to a surface category for all subsequent analysis.
Water samples representative of the source was collected directly from the primary storage tank (44%, 25/57), bore (at the pump) (26%, 15/57), surface water (18%, 10/57), header tank (11%, 6/57), or the shed (2%, 1/57). The temperature of the water at the source was recorded by 74% of producers (42/57) with an average temperature at collection of 22.4°C (range 8–38°C). Participants were asked to qualitatively describe their water source quality with 74% (31/42) describing it as ‘good’ or ‘very good’. In terms of quantitative water testing, 12% (6/52) tested water every 6 months, 35% (18/52) annually, 31% (16/52) sporadically and 23% (12/52) never.
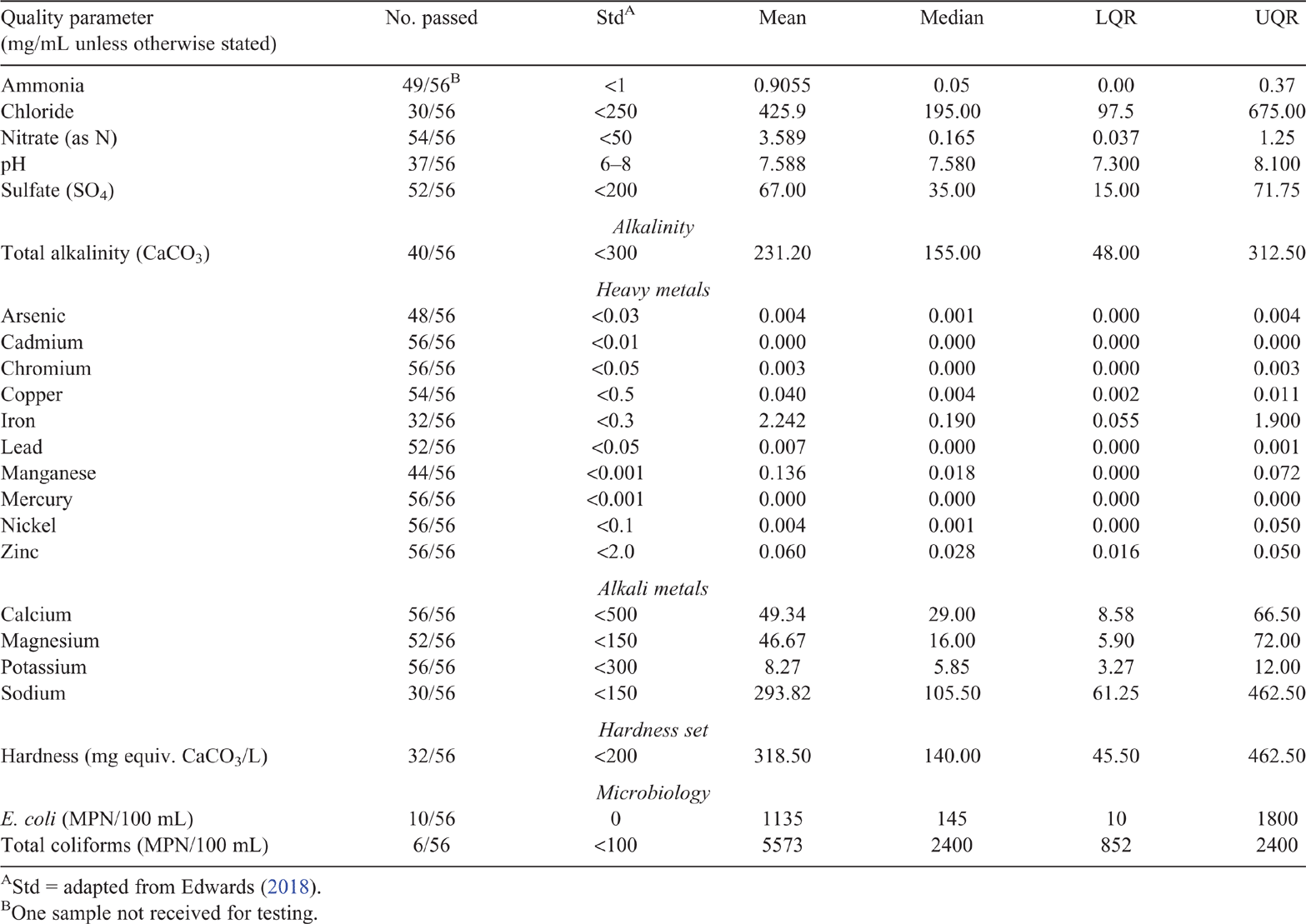
Source water quality
Five (9%, 5/57) water source samples were below all the maximum acceptable water quality standards as described by the pig drinking water standard (Edwards 2018). Source water quality results are presented in Table 1. All source water samples were within the standard for cadmium, chromium, copper, lead, magnesium, nickel, zinc, calcium and potassium. Those samples outside the standard did not exceed the recommended maximum (Edwards 2018) for any one element.
Source water was more likely to contain sodium (27/57), chloride (28/57), iron (24/57) and manganese (15/57) in quantities higher than the standard. The sodium (χ2 (2) = 14.02, P = 0.0009) and chloride (χ2 (2) = 11.98, P = 0.002) content was significantly higher in bore water (bore > mains = surface). There was no significant difference in iron (χ2 (2) = 1.7, P = 0.41) or manganese (χ2 (2) = 2.03, P = 0.36) content between water sources. Sulfate levels were higher than the standard in six water samples, all from bore water, but none were high enough for significant health impact (>7000 mg/L; National Research Council 2012).
Source water pH
The mean water pH was 7.7 (s.d. ± 0.6), and within the acceptable range in 74% (42/57) of samples (Table 1). All samples that were outside the acceptable range were alkaline (pH range 8.1–9.3), but no difference in pH between water sources was observed pH (R2 = 0.003, F(2, 54) = 0.09, P = 0.9).
Source water microbiological criteria
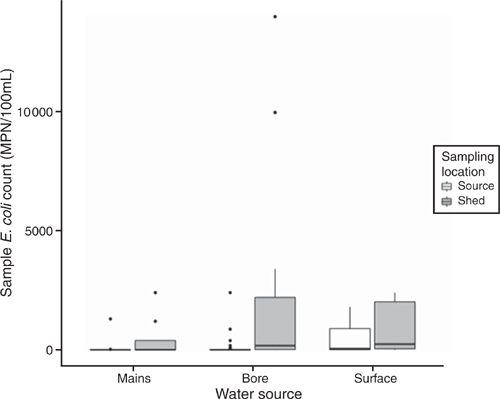
The microbiological quality of water was assessed using Escherichia coli and total coliform counts; most probable number per 100 mL (MPN/100 mL). Both the quantity of bacteria and the number of samples passing the acceptable standard were assessed (Table 1). E. coli was detected in more than half the source water samples collected (57%, 32/56) with E. coli detected in all surface water samples (15/15), 45% of bore water samples (15/33) and 25% of mains water samples (2/8), (χ2 (2) = 16.4, P = 0.0002). There was no statistically significant difference between water source types in the quantity of E. coli detected (R2 = 2.39, F(2,53) = 2.39, P = 0.10), but E. coli counts were higher in surface water compared with bore and mains water (surface (P = 0.08) > bore = mains, Fig. 1).
|
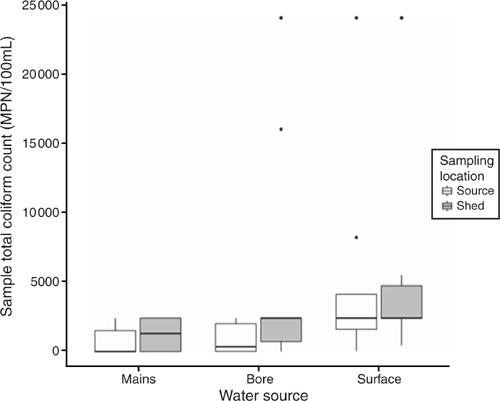
Coliforms were detected in 66% (37/56) of the source water samples (Table 1). As with the E. coli counts, surface water (13/15) and bore water (21/33) were more likely to fail the standard than mains water (3/8), (χ2 (2) = 5.84, P = 0.05). Total coliform counts in surface water (R2 = 0.197, F(2, 53) = 6.59, P = 0.003) were significantly higher than either bore or mains water (surface > bore = mains); dam water (P < 0.001) had significantly poorer microbiological quality than any other surface source (lake, river or irrigation channel) despite the smaller numbers of samples obtained from other surface water sources (R2 = 0.29, F(6,49) = 3.49, P = 0.005, Fig. 2).
|
Water temperature measured at the time of collection (42 samples) did not have a significant effect on E. coli counts (R2 = 0.03, F(1, 39) = 1.34, P = 0.25) or total coliform counts (R2 = –4.5 × 10−5, F(1, 39) = 0.002 P = 0.96).
The overall agreement between producer assessment of water quality and the quantified microbiological quality of the water was poor for both E. coli (k = 0.25, 95% CI (0.16, 0.34)) and total coliforms (k = 0.14, 95% CI (0.07, 0.21)). Producers rating their water quality as poor or average were more likely to have E. coli (odds ratio OR) = 8.4, 95% CI (0.93, 76.15), χ2 = 4.58, P = 0.03) but not coliforms (OR = 4.55, 95% CI (0.5, 41.42), χ2 = 2.07, P = 0.15) present in the water.
Most producers did not treat their drinking water before use by pigs (78.6%, 44/56). Those producers that reported water treatment (21.4%, 12/56) sourced their water from either bore or mains supply (75%, 9/12). Where water treatment details were reported sediment filtration (3/7) was most frequent, with chlorination (1/7), reverse osmosis (1/7), ultraviolet radiation (1/7), or magnets (1/7) also used. Producers reporting some form of water treatment were no more likely to meet the microbiological quality criteria for either E. coli (χ2 (1) = 0.25, P = 0.61) or total coliforms (χ2 (1) = 0.009, P = 0.92). The one producer who was chlorinating drinking water, did not pass any microbiological criteria (E. coli, total coliforms) in either the source or shed water samples tested, indicating that in this instance chlorination was not adequate to render drinking water safe for consumption.
Drinking water supply to pigs
Management practices and infrastructure delivering water from the source to the point of consumption were found to be variable across the farms surveyed. Drinking water was provided to pigs in pens via bowl (13/57), nipple drinker (nipple 10/57 or bite 10/57) or via a combination of both drinker types (20/57). Most producers self-rated the level of drinker cleanliness as good (23/57) or very good (14/57), and none as poor. Of the producers using bowls or troughs to supply drinking water most (26/33) cleaned the drinkers between batches of pigs. Sixty percent of producers (35/57) indicated that drinkers were cleaned between batches with drinkers and pens cleaned using high pressure water by most producers (26/57) with half of those using a disinfectant post wash (14/57). Several participants responded that no cleaning occurred between batches (14/57). The complete drinker system (drinkers, pipes, header tank) was cleaned only sporadically by most producers (24/57) and as never by some survey respondents (13/57). The method of cleaning drinking systems most frequently employed was flushing (18/57), with a few using chlorine (4/57) or pH (9/57) treatments.
The frequency that the flow rates of drinkers were checked ranged from daily (17/57) to never (7/57). The median drinker flow rate was reported as 2550 mL/min (range 300 to 6300 mL/min), with the median water temperature at the point of collection 24°C (range 9.0–29.5°C).
Shed water quality
Test results for each water quality element are presented in Table 2. In general, shed water quality was poorer across all the parameters tested compared with the source from which it came (Tables 1 and 2). All samples tested from the source and shed passed the standard for mercury, nickel, zinc, calcium, potassium, chromium and cadmium. More than half the source and shed samples tested above the standard for iron (60%, 57%) and manganese (73%, 78%). The quantity of iron (t(84) = 0.87, P = 0.43) or manganese (t(95) = –0.78, P = 0.43) did not differ between the source and shed samples.
The number of water samples that tested outside the recommended range increased from source to shed for arsenic (8/56), ammonia (5/56), lead (4/56) and copper (2/56). There was no statistically significant difference between source and shed in the water quantity of arsenic (t(106) = 0.21, P = 0.83), ammonia (t(59) = –1.5, P = 0.12), lead (t(59) = –0.72, P = 0.47) or copper (t(56) = –1.41, P = 0.16).
There was no statistically significant difference between source and shed water quality for sulfate (t(92) = 0.94, P = 0.35), chloride (t(92) = 0.97, P = 0.33), nitrate (t(111) = 0.097, P = 0.92), or sodium (t(98) = 0.79, P = 0.42).
Shed water pH
Shed water pH was not affected by source water pH (R2 = 0.004, F(2,53) = 1.126, P = 0.33) however there was a moderate correlation between the source and shed pH (R2 = 0.34, F(1,54) = 28.33, P < 0.001). Fewer shed samples (37/56) tested within the recommended range but there was no significant difference between source and shed water pH (t(97) = 0.90, P = 0.37). Samples outside the reference range were more likely to be alkaline (n = 17, pH range 8.1–9.0) than acidic (n = 2, pH range 3.9–4.2). Several producers (22/57) indicated the use of an acidifier (18/22) or an alkali (5/22) to clean drinkers or drinker lines. The reported use of a pH modifier had no significant effect on the pH of the shed water (χ2(2) = 3.2, P = 0.2) at the point of sampling compared with the pH of the water source at the time of testing.
Shed water microbiological criteria
A small number of producers passed the shed microbiological criteria for E. coli (17%, 10/56) and coliforms (11%, 6/56). Both E. coli (t(60.5) = 2.82, P = 0.006) and coliform counts (t(84.18) = 2.75, P = 0.007) were significantly higher in water collected in the shed than from the water source. Shed water coliform counts but not E. coli counts (χ2(2) = 3.2, P = 0.2) were significantly associated with the water source. Mains water was six times more likely to pass the coliform count (OR = 6.2, 95% CI (0.93, 43.1), χ2(2) = 7.81, P = 0.02) than bore or surface water. In summary, water samples collected at the source had a lower microbiological load compared with those collected in the shed. Furthermore, microbiological load increased as water was obtained from less controlled water supply sources with mains water being the most controlled and surface water the least (Figs 1, 2).
Seventy-five percent of responses (43/57) indicated that cleaning of drinkers, drinker lines and equipment occurred between batches of pigs using physical scrubbing (5/43) or flushing (18/43). A smaller number of participants used chemicals such as acidifiers (9/43) or chlorine (4/43). Where some form of water treatment of the source water and hygiene measures such as cleaning bowls, lines or header tanks between batches were undertaken, both the E. coli (OR = 0.79, 95% CI (0.1, 3.79), χ2(2) = 19.1, P < 0.001) and the total coliform (OR = 1.73, 95% CI (0.21, 10.15), χ2(2) = 23.3, P < 0.001) counts were more likely to meet the microbiological standard. In the absence of water treatment, individual cleaning activities such as cleaning bowls (χ2(1) = 3.8 × 10−5, P = 1.0) or drinker lines (regardless of method) (χ2(4) = 4 × 10−5, P = 1.0) had minimal effect on microbiological quality of the water at the shed sampling point.
Discussion
This study aimed to develop a greater understanding of the management and quality of drinking water being delivered to pigs on Australian pig farms. A survey of 57 industry participants, estimated to represent at least 22% of ‘large’ pig herds (Australian Pork Limited 2018) demonstrated that despite the critical role water plays in animal health, production and welfare, water is largely the ‘forgotten nutrient’ with the potential impacts and consequences largely unknown.
The survey provided an overview of the types of water sources being utilised on Australian piggeries and subsequent management to the point of the consumption. Typically, producers were reliant on their local water source with bore water being the most common available source. Of the 57 piggeries surveyed, only 7 had access to mains water supply which would suggest that the majority of water sources being utilised are impacted by many factors such as local topography, seasonal fluctuations and weather events. A diverse range of infrastructure and water management practices were found to be in place, consistent with observations made in the Belgian pig industry (Vandeel et al. 2019). Indeed, drinker flow rates were found to range from 300 to 6300 mL/min yet the recommended minimum flow rate for growers is 1000 mL/min (Australian Pork Limited 2016). These subtle differences in water management may further exacerbate the inherent variability in source water quality and in turn at the point of consumption. Indeed, the quality of water at both the source and that of the shed drinking water was found to be suboptimal in one water quality parameter or more.
In the present study, a total of 23 parameters were used to determine water quality and compared with the maximum acceptable standard (Edwards 2018). The most common water parameters to exceed the acceptable standard were pH, hardness (mg equivalent to CaCO3/L), salinity, iron, manganese and microbiological levels, namely E. coli and total coliforms. Although not a focus of this study, it would be valuable to quantify the impacts on animal health including effects on water palatability, water infrastructure and solubility of products being delivered via the drinking water. Where a quality parameter was observed to exceed the acceptable standard in the source water, it was typically found to be outside the acceptable standard and by the same quantum at the point of consumption, being the shed drinker. In contrast, microbiological levels (E. coli, total coliforms) were higher in the shed drinking water compared with the source, irrespective of its origin or drinker type employed. This result was in contrast with the ‘good-to-very good’ subjective rating given by participants of water hygiene at the source and at the drinker. It is important to note that the water quality assessment was reliant on the participants following all instructions to ensure representative water samples were taken. An additional constraint to the study was the inability to take replicate water samples. Despite these limitations, the assessment of water quality provided valuable insights into the variability of water quality across the industry. The survey revealed inconsistencies in infrastructure and water management including cleaning, sanitisation and water testing practices which all have the potential to contribute to suboptimal water quality. It was interesting to note that even where a water treatment system was in use that the microbiological levels at the drinker remained consistently higher than at the source. Indeed, pH modifiers (acid or alkali) added for the purposes of cleaning did not significantly impact water pH when comparing the pH of the water source to that of the shed drinking water (P = 0.2) suggesting that this may not be a suitable approach on these particular farms. It is feasible that the higher levels may have been due to sample contamination when collecting water at the drinker, perhaps through inoculation from the pig itself given its foraging feeding behaviour; however, the observation was consistent irrespective of drinker type or infrastructure at the point of consumption. Poor and/or infrequent cleaning practices of old or difficult to clean infrastructure can in particular lead to bio-film build-up and in turn microbiological contamination (Lumb et al. 2017).
An understanding of water quality through regular testing was found to be lacking in the current study consistent with observations made in the Belgian pig industry where only 30.7% of the study participants evaluating water quality at least once a year (Vandeel et al. 2019). It is recommended that water is tested at least annually at the source and the drinker (Lumb et al. 2017; Edwards 2018). A lack of frequent testing suggests that many producers may not be aware of water quality issues and therefore the potential impact on the health and well-being of their livestock and in turn, the economic value of their business. Aside from being a health risk, water is also a biosecurity risk. The high microbiological levels reported in this study are concerning particularly not just for animal consumption but also in instances where water may be utilised for cleaning and spray cooling of animals. E. coli and total coliforms should be considered as water quality parameters of concern and their regular monitoring is recommended. Other parameters to consider for regular monitoring include pH, hardness, salinity, Fe and Mn.
A lack of awareness of water quality was concerning when considering that 79% of participants (45/57) were utilising drinking water as a conduit for the delivery of antibiotics and that inferior water quality can negatively impact the solubility, stability and in turn efficacy of antibiotic treatments (Dorr et al. 2009; Felix et al. 2016; Edwards 2018; Little et al. 2019). Several survey participants commented on the value of water medication but also on the challenges of preparing stock solutions but did not appear to link this difficulty with water quality issues or other constraints such as drug dose, ambient temperature or water consumption. One wonders whether the antibiotics administered via drinking water are treating the microbiological load in the medium in which they are being delivered rather than actually treating the animal intended. The cost of poor water quality may not just be on feed intake and production efficiency (McLeese et al. 1992; Nyachoti and Kiarie 2010) but also antimicrobial resistance (Little et al. 2019; Lees et al. 2020). The lack of consistent water testing may manifest itself in the observation that only 12 participants had implemented water treatment systems. In contrast, most farms had a system in place to add water medication.
Further research is needed to understand water management best practices and determine the most cost-effective water treatments in response to common water quality issues. This information can then be used to establish best management practices which if followed will in turn maximise production system efficiency and welfare. Furthermore, this information can then be used to ensure appropriate administration of antibiotics in the form of a stewardship decision-making tool. Good water quality and its management are critical for the appropriate delivery of water-soluble antibiotics. Indeed, by optimising water quality and management, industry will be ensuring antibiotics are being used in line with the industry Antimicrobial Stewardship strategy (Australian Eggs Ltd 2018).
In conclusion, the study findings suggest that water accessibility, management on farm and its ensuing quality represents a significant challenge to the Australian pig industry. Access to a clean and plentiful water supply of optimal mineral and chemical composition with a negligible microbiological load is fundamental to optimising animal production. Further research is required to understand the impact of suboptimal water quality and in turn the most cost-effective water treatment and management practices to ensure that pig performance, health and welfare is optimised.
Conflicts of interest
While conducting this study, Dr Edwards was an employee of Ridley Corporation a partner organisation of the Co-operative Research Centre for High Integrity Australian Pork.
Acknowledgements
The authors express their gratitude to all industry participants for their voluntary contribution to this study. The authors acknowledge the contributions made by Dr Steve Little (Capacity+ Ag Consulting), Ms Molly Coleman, Mr Joe Edwards, Mr Robert Parkes, Ms Jill Ramsey, Mr Greg Stuart and Mr Andrew Younger (all of Ridley Corporation). This study was funded by the Co-operative Research Centre for High Integrity Australian Pork (Grant No 2A-118). The funding sources had no role in the collection or preparation of the data, manuscript or the decision to submit this research for publication.
References
Adam M, Voets H, Nielsen JP, Jorsal SE (Eds) (2006) Rearing piglets on nipple drinkers using a proportionner and medicated water system: a study of drinking behaviour of piglets. In ‘19th International Pig Veterinary Society Congress’, Copenhagen, Denmark, 16–19 July. (International Pig Veterinary Society, Available at https://www.theipvs.com/)Andersen HML, Dybkjær L, Herskin MS (2014) Growing pigs’ drinking behaviour: number of visits, duration, water intake and diurnal variation Animal 8, 1881–1888.
Australian Eggs Ltd (2018) Antimicrobial stewardship in Australian livestock industries. Animal Health Australia, Canberra, Australia. Available at https://www.animalhealthaustralia.com.au/antimicrobial-stewardship-in-australian-livestock-industries/ [Verified 16 December 2020]
Australian Pork Limited (2016) Fact sheet. Water supply to pigs. 3. Available at https://australianpork.com.au/wp-content/uploads/2013/09/Fact-sheet-water.pdf [Verified 16 December 2020]
Australian Pork Limited (2018) Annual report 2017–2018. Barton, ACT. Available at https://australianpork.com.au/wp-content/uploads/2019/11/Annual-Report-2017-2018_web.pdf
Bigelow JA, Houpt TR (1988) Feeding and drinking patterns in young pigs. Physiology & Behavior 43, 99–109.
| Feeding and drinking patterns in young pigs.Crossref | GoogleScholarGoogle Scholar |
Brooks PH, Lyons TP, Jaques KA (Eds) (1994) Water – forgotten nutrient and novel delivery system. In ‘10th Annual symposium, biotechnology in the feed industry’. (Alltech: Nottingham University Press: Nottingham, UK)
Brumm M (2010) ‘Water recommendations and systems for swine.’ (US Pork Center of Excellence, Pork Information Gateway: Clive, IA, USA)
Department of Agriculture (2009) ‘National water biosecurity manual – poultry production.’ (Commonwealth of Australia: Canberra, ACT, Australia)
Dorr PM, Madson D, Spencer W, Scheidt AB, Almond GW (2009) Impact of pH modifiers and drug exposure on the solubility of pharmaceutical products commonly administered through water delivery systems. Journal of Swine Health and Production 17, 217–222.
Edwards L (2018) Drinking water quality and its impact on the health and performance of pigs. Final report prepared for the cooperative research centre for High Integrity Australian Pork. No. 2A–118A, Canberra, Australia. Available at http://porkcrc.com.au/wp-content/uploads/2018/08/2A-118-Drinking-Water-Quality-Final-Report.pdf [Verified 16 December 2020]
Felix LMB, Moreira LC, Chlavone-Filho O, Mattedi S (2016) Solubility measurements of amoxicillin in mixtures of water and ethanol from 283.15 to 298.15 K. Fluid Phase Equilibria 422, 78–86.
| Solubility measurements of amoxicillin in mixtures of water and ethanol from 283.15 to 298.15 K.Crossref | GoogleScholarGoogle Scholar |
King RK (1999) ‘Water supply to pigs. A report prepared for the Pig Research and Development Corporation.’ (PRDC: Roseworthy, SA, Australia)
Lees P, Pelligand L, Giraud E, Toutain P-L (2020) A history of antimicrobial durgs in animals: evolution and revolution. Journal of Veterinary Pharmacology and Therapeutics 00, 1–35.
Li YZ, Chénard L, Lemay SP, Gonyou HW (2005) Water intake and wastage at nipple drinkers by growing-finishing pigs. Journal of Animal Science 83, 1413–1422.
| Water intake and wastage at nipple drinkers by growing-finishing pigs.Crossref | GoogleScholarGoogle Scholar | 15890820PubMed |
Little SB, Crabb H, Woodward AP, Browning GF, Billman-Jacobe H (2019) Review: water medication of growing pigs: sources of between-animal variability in systemic exposure to antimicrobials. Animal 13, 3031–3040.
| Review: water medication of growing pigs: sources of between-animal variability in systemic exposure to antimicrobials.Crossref | GoogleScholarGoogle Scholar | 31475656PubMed |
Lumb KR, Robertson J, Scott HE, Woolfenden NJ (2017) Optimising the use of antimicrobials: preparing the industry for in-water delivery in the short term and improving hygiene and more effectively targeting medication in the longer term., Kenilworth, Warwickshire. Available at https://pork.ahdb.org.uk/media/274247/51510014_ft014_raft-solutions-ltd-water-report_approved_september-2017.pdf [Verified 16 December 2020]
McLeese JM, Tremblay ML, Patience JF, Christison GI (1992) Water intake patterns in the weanling pig: effect of water quality, antibiotics and probiotics. Animal Science 54, 135–142.
| Water intake patterns in the weanling pig: effect of water quality, antibiotics and probiotics.Crossref | GoogleScholarGoogle Scholar |
National Research Council (2012) Water. In ‘Nutrient requirements of swine’. pp. 66–74. (National Academies Press: Washington DC, USA)
NHMRC and NRMMC (2011) ‘Australian drinking water guidelines paper 6. National water quality management strategy.’ (Commonwealth of Australia: Canberra, ACT, Australia)
Nyachoti M, Kiarie E (2010) Water in swine production: a review of its significance and conservation strategies. In ‘Sharing ideas and information for efficient pork production. Manitoba Swine Seminar. Manitoba, Canada. Vol. 24’. Available at https://thepigsite.com/articles/water-in-swine-production-a-review-of-its-significance-and-conservation-strategies [Verified 16 December 2020]
R Core Team (2013) ‘R: A language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria)
Sofi MH, Gudi R, Karamuthil-Melethil S, Perez N, Johnson BM, Vasu C (2014) pH of drinking water influences the composition of gut microbiome and type 1 diabetes incidence. Diabetes 63, 632–644.
| pH of drinking water influences the composition of gut microbiome and type 1 diabetes incidence.Crossref | GoogleScholarGoogle Scholar | 24194504PubMed |
Taylor G, Roese G, Brewster C (2006) ‘Primefacts 108: Water medication for pigs.’ (NSW DPI: Sydney, NSW, Australia)
Vandeel F, Filippitzi M-E, Dewulf J, Daeseleire E, Eeckhout M, Devreese M, Croubels S (2019) Oral group medication in pig production: characterising medicated feed and drinking systems. Vet Record 185, 1–9.
| Oral group medication in pig production: characterising medicated feed and drinking systems.Crossref | GoogleScholarGoogle Scholar |


