Dentition can predict maturity in young Merino sheep
Amy M. Bell A B , Sonja Dominik
A B , Sonja Dominik  A , Duncan Elks A , Graham Acton A and Jen Smith A
A , Duncan Elks A , Graham Acton A and Jen Smith A
A CSIRO Agriculture and Food, F.D. McMaster Laboratory, Chiswick, New England Highway, Armidale, NSW 2350, Australia.
B Corresponding author. Email: Amy.Bell@csiro.au
Animal Production Science - https://doi.org/10.1071/AN21099
Submitted: 19 February 2021 Accepted: 1 June 2021 Published online: 2 August 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC
Abstract
Context: A unique population of Merino sheep recorded for a range of production and reproduction traits presented an opportunity to calculate sire variation in dentition which may indicate maturity and influence marketing and selection decisions. A change in the definition of ‘lamb’ in the Australian sheep industry warranted an investigation of the relationship between production, reproduction and dentition.
Aims: To assess the variation in timing of dentition changes in Merino sheep and determine whether there are associations with key production and reproduction traits.
Methods: A population of 2150 pedigree-recorded Merino sheep were studied to analyse the sire variation in progeny for a range of dentition changes and production and reproduction traits. Dentition phenotypes included the age animals started to lose the deciduous lamb teeth, the age when one permanent incisor was in wear and the amount of time between these two events. Production records included bodyweight, fat and muscle traits. Reproduction records from the female progeny included the outcome of pregnancy scanning after the first joining opportunity. Sire variation for the age dentition changed was analysed. The effect of progeny age at hogget categorisation on production and reproduction was analysed.
Key results: Progeny that were heavier, fatter and with higher muscle measurements matured earlier. Female progeny were more likely to be pregnant if classed as hoggets earlier in life.
Conclusions: Dentition records provided useful indicators of maturity in Merino sheep in this study and can be used to inform decisions regarding the timing of marketing options and the likelihood of success when assessing female progeny for suitability to join at ~18 months of age.
Implications: Merino sires can exhibit a wide range of variation with respect to the age at which their progeny will mature, as indicated by their dentition. If animals are heavier at an earlier age, they are more likely to mature earlier, which has to be considered when planning nutritional requirements for growing out young male progeny, and females could be more successful as young breeders. Dentition is a useful tool to indicate maturity in young Merino sheep.
Keywords: Merino, dentition, maturity, reproduction.
Introduction
Dentition has been used in Australia and internationally to estimate and categorise the age and class of livestock (Faeber et al. 2014; Casburn 2016; McEachern 2017). The category an animal is assigned to can have large implications on the price of livestock at sale, with younger animals usually attracting a premium over older animals due to a potentially longer productive life for females and market specifications for animals going to slaughter. Australia recently changed the definition of ‘lamb’ from a sheep with 0 permanent incisor teeth to a sheep now being under 12 months of age or having no permanent incisor teeth in wear (Australian Meat Industry Council (AMIC) 2019). This change may present broader marketing opportunities for sheep producers and more closely align our livestock categories with our trading partners and competitors as the indication is that these animals carry optimal muscle without excess fat, and that eating quality is not negatively affected (Pethick et al. 2005). Previously, as soon as there was evidence of a lamb losing the deciduous milk teeth, it was classified as a ‘hogget’, and this typically happened anywhere between 12 and 19 months (Cocquyt et al. 2005; Casburn 2016). The age at which permanent incisor eruption commences, and the time between eruption and being in wear are not well described for Merino sheep. Estimates of the variation around the loss of milk teeth and the eruption of the permanent incisor teeth are present in literature for many breeds (Aitken and Meyer 1982; Meyer et al. 1982; Laws and Aitken 1988; Cocquyt et al. 2005), but there is not a wealth of knowledge relating to Merino sheep and sire variation within the Merino breed. Wiese et al. (2005) reported on teeth eruption in relation to eating quality across breeds, including Merinos; however, the dataset was small and did not include estimates of individual sire variation.
Economic, environmental and social challenges encourage livestock producers to maximise efficiencies when grazing livestock (Montossi et al. 2013a). Growth, production and reproduction targets are influenced by genetics and environment, resources available to achieve targets and an understanding of the variables able to be controlled or utilised. Knowledge of the timing of significant physiological changes in sheep can assist decision-making for replacement stock options and the timing of marketing animals. The transition from lamb to hogget status in sheep can represent a physical maturing, which can affect rates of production and reproduction, but it has not been explored whether the timing of, or the age at which the dentition changes, are associated with production or reproduction. Currently, there are no indicators for maturity to measure these changes and their variation and to provide an indication of maturity, associated production or carcass performance or reproductive success for females at their first joining opportunity. Knowledge of the variation around dentition changes and associations with other traits in Merinos may provide indications of how to expect progeny to perform if dentition is able to provide an estimate of maturity. Indicators of the timing of significant physiological changes in sheep may help inform husbandry events such as the first time of mating and when to expect male progeny to reach growth and/or targeted market specifications.
Reproductive efficiency in sheep enterprises is taking on higher importance with the size of the national flock, and environmental and economic stability (Ridoutt 2021). Sheep numbers are low in Australia compared with 20 years ago, having fallen from 118.6 million in 2000 (Montossi et al. 2013b) to 71 million by 2018 (MLA 2019) and the incentive to build the national flock is high. More sustainable and highly productive females are increasingly favoured, and the selection of sires whose progeny attain puberty, become pregnant and rear a lamb earlier in life increases in importance. There are many factors affecting the age at which puberty is reached for sheep (Valasi et al. 2012), including genetic factors. Measurements of variation in the age at which progeny attain puberty would enhance knowledge of what to expect when making selection decisions considering early or later-maturing breeds or individual sires. Changes in dentition and the age at which the change occurred may provide a suitable proxy for estimating puberty or maturity within a flock.
The present study explored the degree of variation in timing of dentition changes related to sires and the associations of dentition changes with key production and reproduction traits.
Materials and methods
Animals and phenotypes
The animals in the study were 2150 of the first generation (F1) progeny born in 2017 and 2018 at the New England site of the Merino Lifetime Productivity (MLP) project (Ramsay et al. 2019). Approval to conduct the experiment was granted by the CSIRO Chiswick Animal Ethics Committee (ARAs 16/33, 17/29, 18/29 and 19/29) and all procedures were conducted in accordance with the guidelines of the Australian Code for the Care and Use of Animals for Scientific Purposes; NHMRC 2013). In each year (2017 and 2018), 1350 ewes were artificially inseminated to 1 of 15 sires for a total of 28 unique sires across years, with two sires being used in both years to provide a genetic link. The sires used represented a wide range of phenotypic and genetic extremes for traits of interest in the sheep industry, and combinations of performance to allow assessments for lifetime productivity (https://merinosuperiorsires.com.au/mlp-project/sires/). Each sire was allocated to 90 ewes balanced for year of birth, bodyweight, condition score and genetic resource base. The genetic resource base refers to the dams used, with one-third of the ewes being from the CSIRO/AWI Breech Strike Genetics (BSG) flock (Smith et al. 2009) and the remaining two-thirds from the CSIRO Chiswick Station flock, which are typical New England Superfine Merino ewes. All foundation dams had an average fibre diameter of ~17 µm, greasy fleece weight of close to 4 kg and mature-ewe weight of 46–48 kg. Within each year, ewes were artificially inseminated in two consecutive weeks and therefore the lambs were born over ~2 weeks. Birth records for the resulting 2017 and 2018 F1 progeny collected during lambing included date of birth and maternal pedigree, sex, birthweight, birth and rear type (single or multiple), and other traits of interest relating to maternal behaviour and lamb survival scores. In each year, from weaning, the lambs were managed in two contemporary groups according to sex. Wether (male) progeny were also further divided (balanced for sire, birth-rearing type and bodyweight) at ~6 months of age into slaughter (feedlot finished and slaughtered as lambs) and retain groups (retained on-farm to adulthood for wool production). Only performance of retained wethers is reported in the present study, as the slaughter group were killed before eruption of their permanent teeth.
The bodyweight traits reported here are h1WT (~15–16 months, 1350 females and 800 retained males) and h2WT (~19 months, 1350 females only pre-mating). At the time of bodyweight measurement, ultrasound scanned fat (h1FAT and h2FAT) and eye-muscle depth (h1EMD and h2EMD) were both recorded at the ‘C’ site by a Sheep Genetics accredited scanner.
There were 713 and 637 2017 and 2018 drop F1 ewes respectively, in the study. The F1 ewes were naturally mated for their first lambing opportunity in April at ~19 months of age (median age up to the mid-point of joining was 589 days) for 35 days with 2.4 rams per 100 ewes. The ewes were routinely assessed by monitoring condition scores to ensure that target body condition scores of 3.2, on average, were achieved before joining, and before lambing. Supplementary feeding of grain was conducted if deemed necessary to achieve target body condition score. Ultrasound pregnancy scanning was conducted 86 days after commencement of mating. Litter size scanned and fetal age estimates were collected at pregnancy scanning. From pregnancy scanning, ewes were managed in contemporary groups according to the litter size scanned, with single-bearing and multiple-bearing ewes being separated and managed on the basis of condition score and nutritional requirements for the number of lambs scanned.
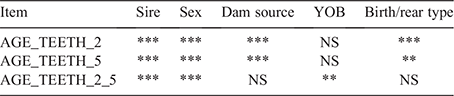
Measures of teeth eruption by physical observation commenced for all progeny from ~10 months of age. Animals were visually assessed every 2–4 weeks by using a 1–5 scoring system as outlined in Table 1. Traits reported here are age in days from birth to Teeth score 2 (AGE_TEETH_2, effectively the old definition of hogget) and from birth to Teeth score 5 (AGE_TEETH_5, the new definition of hogget) and days from Teeth score 2 to Teeth score 5 (AGE_DAYS_2_5).

|
Statistical analyses
Only data from the F1 progeny were analysed in the study. All data preparation and analyses were conducted using R (R Core Team 2013). Generalised linear mixed models (GLMM) were used to estimate differences and variation in the age in AGE_TEETH_2, AGE_TEETH_5 and AGE_DAYS_2_5. Fixed effects tested included sex, dam source, year of birth and a concatenation of birth type and rear type (BTRT). Sire was fitted as a random effect. Non-significant effects were sequentially excluded from the model.
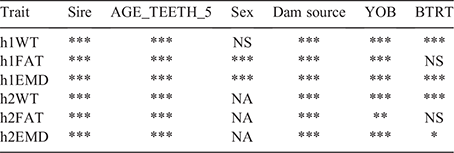
Generalised linear mixed models were also used to model the effect of AGE_TEETH_2 and AGE_TEETH_5 on hogget bodyweight (h1WT, h2WT), fat (h1FAT, h2FAT) and eye-muscle depth (h1EMD, h2EMD). Fixed effects included sex, dam source, year of birth and BTRT, and sire was fitted as a random effect. Sex was excluded from the second measurement taken at hogget age as only female progeny were assessed at that time. Analysis of the effect of AGE_TEETH_5 on h1WT, h1FAT and h1EMD was also conducted, and correlations were estimated between the traits using bivariate sire models. Non-significant effects were sequentially excluded from the models.
In a separate analysis, the relationship between dentition and conception rate for the F1 ewes was explored. Litter size scanned in the first joining season (LSS1) was used to generate a trait termed pregnancy success, with 0 recorded for ewes with LSS1 = 0, and 1 recorded for ewes with LSS1 > 0. A logistic regression to model the binary outcome of pregnancy success was used to assess sire variation. Fixed effects included birth type, rear type, dam source, year of birth, h2WT and AGE_TEETH_5. Sire was fitted as a random effect. Preliminary analysis showed a highly significant effect of premating bodyweight (h2WT) for LSS1 (P < 0.001) and for pregnancy success (P < 0.05). Premating bodyweight was pre-adjusted for the significant fixed effects of year of birth, dam source, birth type and rear type. The effect of hogget age (AGE_TEETH_5) and adjusted premating bodyweight on pregnancy success was then modelled including sire as a random term. Regression coefficients from significant effects of the logistic regression were stored and then expressed as relative odds ratios by calculating the exponent of the coefficient for each sire. Sires were ranked highest (Rank 1) to lowest by odds ratios and this ranking compared with rankings obtained from the output of GLMM models to calculate least-squares means for sire variation in traits.
To examine the relationship between AGE_TEETH_5 and pregnancy success, AGE_TEETH_5 was converted into ‘age status’, with the progeny being classified as ‘hogget’ if AGE_TEETH_5 ≤ 589 days in age, which was the mid-point of joining, and ‘lamb’ if AGE_TEETH_5 > 589 days. Generalised linear mixed models were also explored fitting adjusted premating weight, and age status (hogget or lamb). Sire means were predicted for pregnancy success and ranked from the highest to the lowest. The ranking of the predicted sire means using GLMM was compared with the ranking of the sires by using odds ratios from the logistic regression by way of correlating the two sets of values for each sire.
Results and discussion
The F1 wethers and ewes were, on average, 476 and 483 days of age respectively, when they initially lost their lamb teeth (AGE_TEETH_2), followed by 508 and 519 days of age when they reached Teeth score 5 or hogget age (AGE_TEETH_5; Fig. 1). The latter is approximately equivalent to 16–17 months. The minimum and maximum days to hogget age were 427 days and 671 days respectively. The average number of days from AGE_TEETH_2 to AGE_TEETH_5 was 34 days, with a range of 10–125 days, which agrees with Hongo et al. (2004). Under the new description of ‘lamb’ or ‘hogget’ for the Australian sheep industry, this provides at least one additional month or longer, compared with the previous classification system. Knowledge of this timeframe, and how it might vary with different sheep types, enables producers to manage and maximise liveweight targets for animals destined for sale, slaughter or joining. Marketing decisions for animals leaving the farm can be planned with more confidence, and producers can time planned joining events for maiden ewes to maximise conception rates. Knowledge of the timing of teeth eruption for Merino sheep and the variation within the trait will be an asset for management decisions and provide more objective information for forward planning in sheep enterprises.
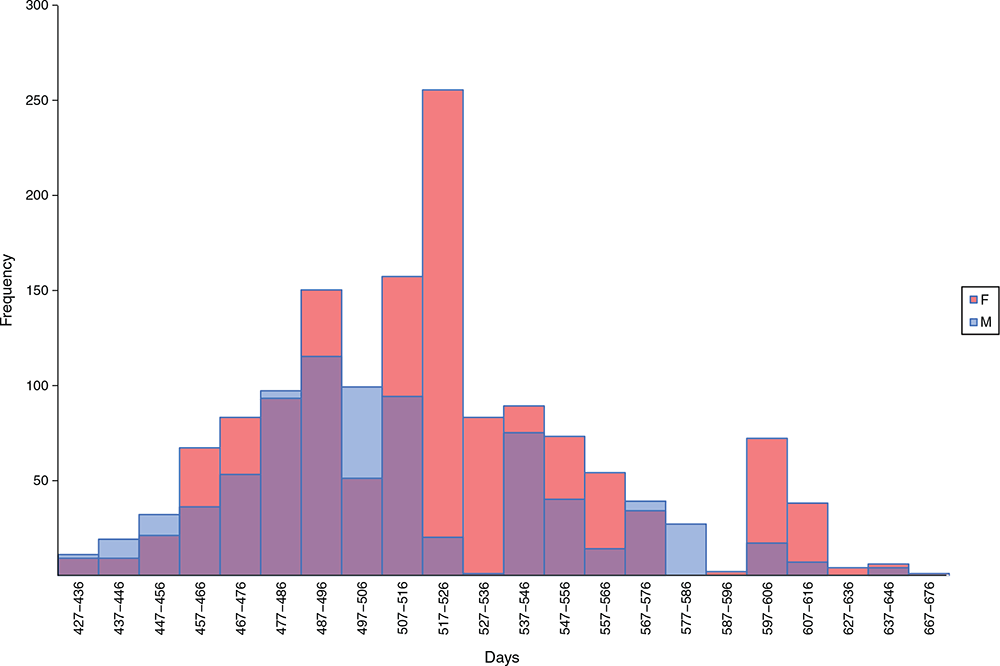
|
Linear models
Sire was highly significant for all three bodyweight and carcass traits in initial analyses, which indicated a wide degree of variation among the individual sires in terms of dentition changes. Table 2 outlines the significance of the effects on the dentition traits of interest, which are in agreement with published literature (Aitken and Meyer 1982; Meyer et al. 1982; Cocquyt et al. 2005). Estimates in the literature are common for breeds other than Merino sheep where strong differences exist across individual sires, particularly in British breeds. A high degree of sire variation was expected in the present study due to the broad range of sheep types represented by the sires used.
|
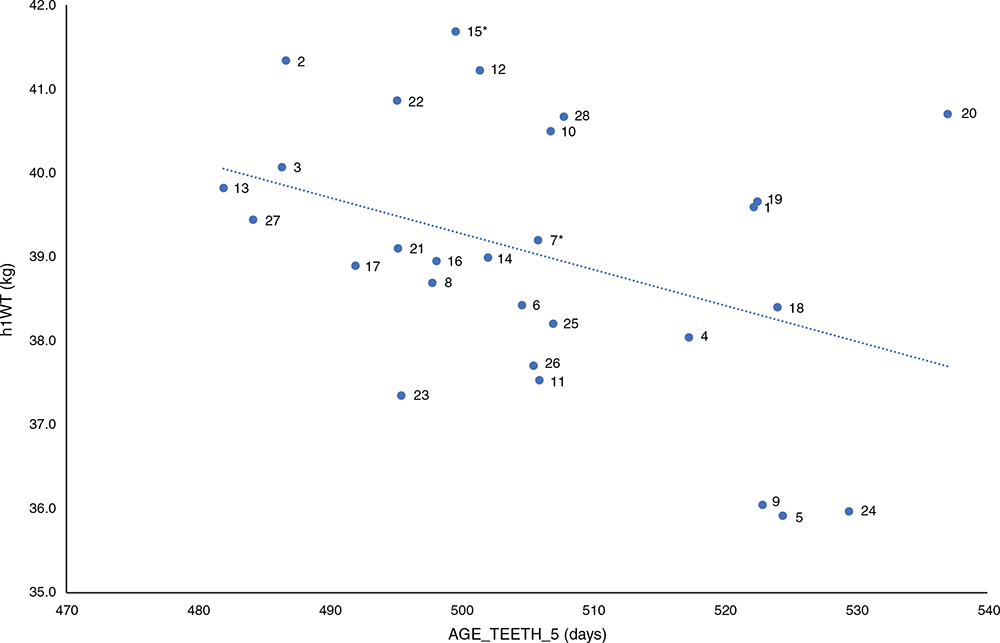
There was a highly significant (P < 0.001), negative phenotypic correlation (r = –0.28) between AGE_TEETH_5 and h1WT. The interaction of h1WT and sex was not significant for the AGE_TEETH_5. Figure 2 plots the sire predicted progeny-group means represented by sire number and shows a high degree of variation between the two traits. The trend was for heavier animals at h1WT to mature earlier, but there were exceptions. For example, progeny of Sire 20 were among the heaviest, but also had the highest AGE_TEETH_5; that is, it took progeny of this sire the longest time to reach the hogget stage. Conversely, progeny of Sires 5, 9 and 24 were also late to reach hogget stage, but had the lowest bodyweight estimates. Sire variation in reaching hogget stage indicated by dentition was significant, and there was a range of almost 60 days between sires. These results suggest that this knowledge can be used in decisions relating to sales to meet different target markets and specifications, with additional time to manage and plan operations for those that change later. For ewes, it may provide further information for the purchase of young replacement ewes, and timing of joining or selling decisions based on a more comprehensive assessment of maturity and the capacity to expect a successful joining at the first joining opportunity.
|
The significant fixed effects for hogget bodyweight (h1WT) are summarised in Table 3. A summary of the sire predicted progeny-group means for the production traits measured on all progeny, ordered by the AGE_TEETH_5, was recorded and can be found in Table 4. Sex may be expected to have a significant effect on h1WT; however, in the present study, sex was confounded with management group and they were managed quite differently. The ewes in this project were being carefully managed to target body condition scores to ensure the best chance of conception when joined at 19 months of age and this may have masked sex differences, with male progeny being quite often heavier at ages similar to their female half siblings, as found by Enser et al. (1998). With respect to the significance of the dam source on h1WT, progeny of dams from the BSG ewe base were, on average, significantly heavier than progeny of dams derived from the CSIRO Chiswick Station flock (LSM (±standard error)) of 38.65 (±0.4) kg, compared with 36.8 (±0.35) kg. Progeny born in 2017 were significantly heavier at h1WT than were progeny born in 2018, being 39.6 (±0.4) kg, on average, compared with 36.5 (±0.4) kg, which is a reflection of the environmental conditions of the time, specifically, severe drought. Progeny born and reared as singles were heavier than progeny born as multiples and either reared as singles or multiples.
|
|
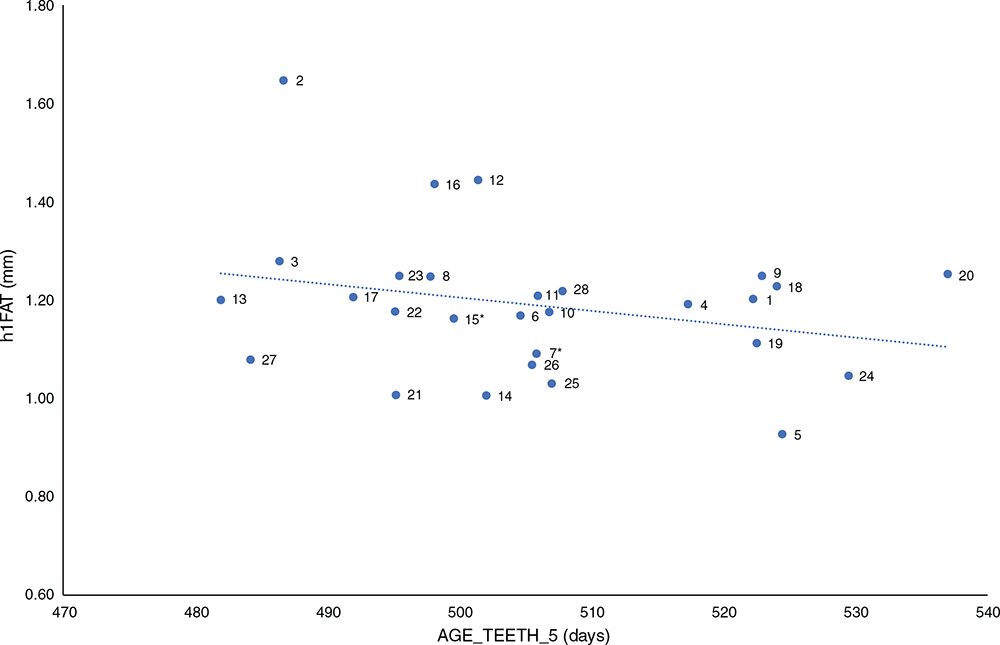
Figure 3 plots AGE_TEETH_5 versus hFAT1 measured at 15 months of age. There was a low negative (r = –0.08), but statistically significant (P < 0.001), correlation between AGE_TEETH_5 and h1FAT. Animals maturing younger, as indicated by their dentition, may have laid down more fat at the same age than did animals maturing later, as indicated by the negative correlation evident in the present study, and this is in agreement with the suggestion that while animals mature, growth is preferentially directed to bone first, then muscle and finally fat (Rouse et al. 1970). All effects tested for significance on h1FAT, including sex, were highly significant (P < 0.001), with the exception of BTRT (P = 0.109). Females, on average, had more fat cover than did males (1.80 ± 0.03 mm compared with 1.58 ± 0.03 mm), and progeny of dams from the BSG flock had more fat cover than did progeny of dams from the Chiswick Station flock (1.73 ± 0.04 mm versus 1.61 ± 0.03 mm). Like the h1WT result, progeny born in 2017 had significantly higher h1FAT measurements than did progeny born in 2018 (2.02 ± 0.05 mm versus 1.36 ± 0.04 mm), again reflecting the seasonal fluctuations and feeding levels precipitated by the prolonged drought.
|
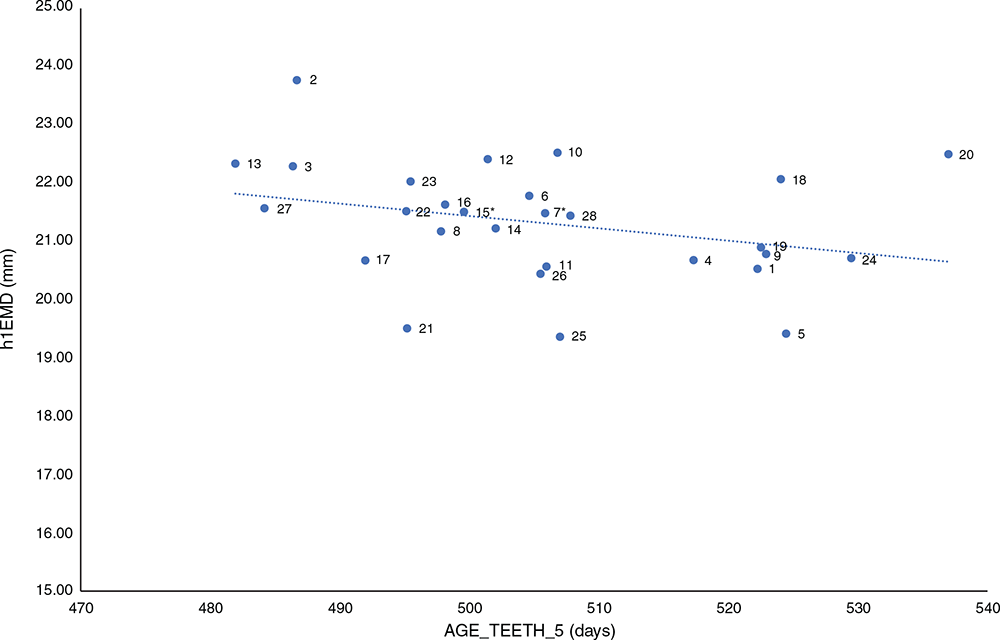
A similar pattern of earlier-maturing animals having higher values was evident for h1EMD (Fig. 4). There was a highly significant (P < 0.001), negative (r = –0.15) correlation between AGE_TEETH_5 and h1EMD. These results were complementary to those obtained for h1WT and h1FAT, and the h1EMD also reflected the suggestion that once the animals had matured to hogget status, muscle and fat were preferentially deposited, with many animals still physiologically maturing at the time of the first assessment. For h1EMD, all effects tested were highly significant (P < 0.001) and Fig. 4 plots sire predicted progeny-group means for h1EMD versus sire predicted progeny-group mean AGE_TEETH_5. Female progeny had a higher h1EMD than did male progeny (23.7 ± 0.22 mm versus 22.8 ± 0.23 mm), progeny of dams derived from the BSG flock had a higher h1EMD than did progeny of dams derived from the Chiswick Station flock (23.6 ± 0.24 mm versus 22.7 ± 0.22 mm), progeny born in 2017 had a higher h1EMD than did progeny born in 2018 (25.1 ± 0.25 mm versus 21.4 ± 0.25 mm), and single-born and reared progeny had a higher h1EMD than did progeny born and reared as multiples (23.4 ± 0.21 mm versus 23.0 ± 0.21 mm). Modelling the effect of h1EMD as a covariate on AGE_TEETH_5 showed h1EMD and all additional fixed effects and sire to be significant (P < 0.001 for all traits except BTRT, for which P = 0.18).
|
The second hogget age assessment conducted on the ewes alone before their first joining opportunity showed a marked improvement in bodyweight, particularly for the 2018 progeny. A summary of the significance of fitted effects for the second measurement in the female progeny can be found in Table 3. Seasonal conditions were vastly improved from January 2020 onward and this facilitated a significant increase in bodyweight (h2WT), and, on this occasion, the 2018 progeny were heavier than the 2017 drop progeny (49.0 ± 0.51 kg versus 45.6 ± 0.51 kg) at the same age. Dam source was highly significant, with the progeny of the BSG flock dams being heavier than the progeny of dams from the Chiswick Station flock (48.0 ± 0.50 kg versus 45.9 ± 0.44 kg). Birth and rear type were also highly significant, following a pattern similar to the h1WT measurements, where progeny born and raised as singles were heavier than were progeny born as multiples and then raised as either singles or multiples. When age status (either hogget or lamb by phenotypic assessment) was substituted in the model for hogget age (days), hogget progeny were significantly (P = 0.018) heavier at 47.5 ± 0.46 kg, than were the lamb progeny weighing 46.4 ± 0.60 kg.
The effects of sire, hogget age and dam source were highly significant for h2FAT. Progeny of dams from the BSG flock had more fat cover than did progeny of dams from the Chiswick Station flock (1.69 ± 0.04 mm versus 1.58 ± 0.03 mm). Year of birth was significant (P = 0.006), which again could reflect the prolonged drought conditions that improved only in the weeks leading up to joining for the 2018 progeny. The 2017 progeny had h2FAT measurements of 1.72 ± 0.04 mm and the 2018 progeny had h2FAT measurements of 1.59 ± 0.04 mm before their first joining opportunity. When age status (either hogget or lamb) was substituted in the model for AGE_TEETH_5, the effect of age status on h2FAT was not significant.
Analysis of h2EMD showed highly significant (P < 0.001) effects of sire, dam source and year of birth. Birth and rear type were significant (P = 0.013). Interestingly, the 2018 progeny showed a similar pattern for h2EMD and h2WT, whereby the second measurements for the traits conducted ~4 months after the first showed a dramatic improvement, beyond a normal daily gain for the class of livestock and this was likely to be attributed to the significant change in seasonal conditions and availability of pasture, in addition to supplementary feed.
Pregnancy and dentition analysis
Table 5 summarises the arithmetic means, minima and maxima for traits of interest in the dentition and pregnancy component of the present study. The raw means indicated an overall pregnancy success rate of 90% for all ewes at the first lambing opportunity. Using the classification of age status to categorise the progeny as either ‘hogget’ or ‘lamb’ showed that 91% of the progeny were hoggets and 9% were lambs at the mid-point of joining. Table 6 summarises the predicted sire progeny-group means for the second production measurements and reproduction measurements, which were recorded on the female progeny.
|
|
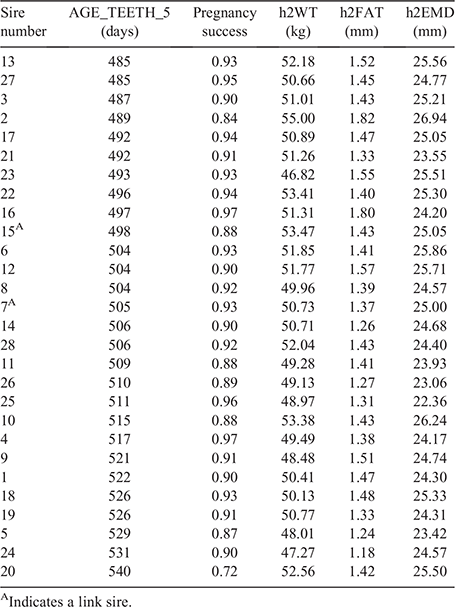
Sire had a significant effect on pre-mating bodyweight (h2WT; Fig. 5), potentially reflecting mature progeny and progeny still maturing in the lead up to joining. Sires with lower pregnancy success and heavier mean bodyweight values of their progeny (e.g. Sires 2 and 20) could indicate that animals may still be maturing physically and physiologically. There were four sires whose progeny showed a heavier mean pre-mating bodyweight (>50 kg) and lower conception rates (<83%) than did other sires. Coop (1973) estimated that reproductive maturity in females is often realised at 50–80% of mature body size, and the range of sheep types within the present study could allow for mature-ewe weights in excess of 70 kg. Further analysis will investigate mature-ewe body size, the relationship with h2WT and pregnancy success of the ewe progeny for each sire.
|
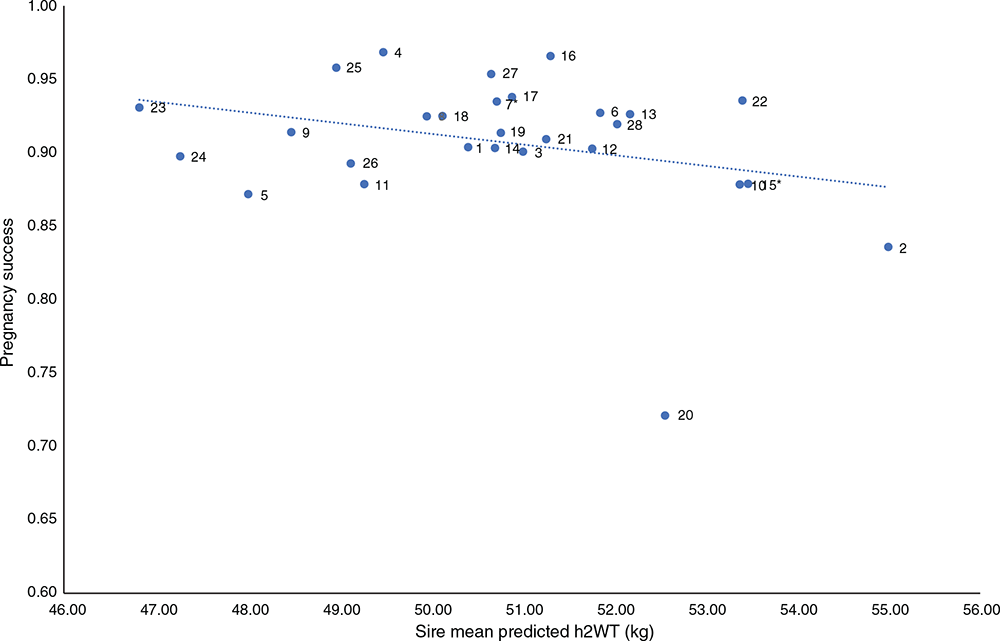
Figure 6 plots the sire predicted progeny-group mean values for AGE_TEETH_5 and pregnancy success. Sire variation for AGE_TEETH_5 was highly significant (P < 0.001), and there was a significant (P = 0.005) negative (r = –0.076) correlation, indicating that pregnancy success was affected by AGE_TEETH_5. There was a suggestion of sires with progeny reaching hogget age later also having a lower pregnancy success. The progeny of most of the sires achieved hogget status between ~485 and 531 days of age and achieved greater than 85% pregnancy success. However, progeny of Sire 20 had a mean hogget age of ~540 days and the pregnancy success was 72%. Progeny of this sire also showed a high h1WT. These observations may indicate that this sire could produce later-maturing progeny, which may affect pregnancy success at the first joining by increasing the age at puberty. This reinforces the idea that hogget age as measured by the change in dentition in the present study may be a reasonable indicator of a later-maturing sire. Further work will look at the conception rates in subsequent years and determine whether future joining events were successful.
|
For the binomial model, sire (P < 0.001) and adjusted premating bodyweight (P = 0.019) were significant effects for pregnancy success, whereas AGE_TEETH_5 was not. Fitting age status on pregnancy success showed a significant effect of sire (P < 0.001), adjusted pre-mating bodyweight (P = 0.037) and age status (hogget or lamb, P = 0.03). Fitting a GLMM for the effect of adjusted pre-mating bodyweight and age status on pregnancy success similarly found adjusted pre-mating bodyweight (P = 0.032) and age status (P = 0.018) to be significant. Correlations estimated between logistic regression and GLMM outputs indicated a very strong positive correlation of r = 0.995. The least-square mean estimated by GLMM for hogget pregnancy success was 0.910 (±0.013), and for lamb pregnancy success it was 0.842 (±0.029). This significant difference indicates that a lower proportion of progeny identified at the mid-point of joining as being lambs conceived at the first joining opportunity. Depending on the size and goals of a sheep enterprise, this may have implications for management decisions. Targeted joining of animals by age status as identified by dentition may be an option; for example, delaying joining of females identified as lambs for 1–2 months may increase conception rates as maturity progresses, but would add considerable complexity to the operation if more management groups are required. Knowledge of the variation in dentition at key ages in their particular animal’s productive life will provide more information to sheep producers. Targeted decisions in the selection of replacement breeding stock could be made by including information on sires for their propensity to have early maturing progeny via dentition records.
Further work using the MLP F1 progeny relating to young sheep dentition may focus on other production traits, including mature-ewe size and reproductive performance at the second mating opportunity. Adult sheep dentition in relation to reproductive performance and productivity has been investigated elsewhere (Richards et al. 2018) and will be a subject of ongoing research across the five sites that comprise the MLP project.
Conclusions
The variation evident in progeny of Merino sires for the age their progeny attained hogget status as assessed on dentition was highly significant in the present study, demonstrating up to a 2-month difference across sires when the progeny were categorised as hoggets. This presents an opportunity to optimise marketing strategies for male progeny or to appreciate differences in maturity rates and reproductive success in young female progeny. Sire variation in progeny hogget age and the effect on production and reproduction traits were significant. Hogget progeny had higher pregnancy success rates and there was a significant difference between pregnancy success in lambs and hoggets as classified by dentition. Dentition provided an estimate of progeny maturity and an estimate of variation around changes in dentition in Merinos was realised. Progeny of different sires or sheep types will mature at a range of ages and bodyweights and it is important to have an appreciation of these differences so that decisions can be made to suit individual sheep operations and target selection decisions relevant to the goals of independent operations.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
We acknowledge the support of AWI, who granted access to existing production data on the flock used in this study.
References
Aitken WM, Meyer HH (1982) Tooth eruption patterns in New Zealand sheep breeds. Proceedings of the New Zealand Society of Animal Production 42, 59–60.Australian Meat Industry Council (AMIC) (2019) Australia’s new definition of lamb – what you need to know. Factsheet–Lamb–Definition.pdf. Available at sheepproducers.com.au [Verified May 2021]
Casburn G (2016) How to tell the age of sheep. Primefact 1481, 2nd edn. Available at https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0004/179797/aging-sheep.pdf [Verified April 2021]
Cocquyt G, Driessen B, Simoens P (2005) Variability in the eruption of the permanent incisor teeth in sheep. The Veterinary Record 157, 619–623.
| Variability in the eruption of the permanent incisor teeth in sheep.Crossref | GoogleScholarGoogle Scholar | 16284330PubMed |
Coop IE (1973) Age and live weight in sheep. New Zealand Journal of Experimental Agriculture 1, 65–68.
| Age and live weight in sheep.Crossref | GoogleScholarGoogle Scholar |
Enser M, Hallett KG, Hewett B, Fursey GAJ, Wood JD, Harrington G (1998) Fatty acid content and composition of UK beef and lamb muscle in relation to production system and implications for human nutrition. Meat Science 49, 329–341.
| Fatty acid content and composition of UK beef and lamb muscle in relation to production system and implications for human nutrition.Crossref | GoogleScholarGoogle Scholar | 22060583PubMed |
Faeber CW, Durrant SM, Fishman Leon J, Day WE, Julen Day T, Merriam J, McNeal LG, Hill K, Harding RL (2014) Ageing sheep and goats by their teeth. Sheep and Goat Manual. Available at infovets.com [Verified April 2021]
Hongo A, Zhang J, Toukura Y, Akimoto M (2004) Changes in incisor dentition of sheep influence biting force. Grass and Forage Science 59, 293–297.
| Changes in incisor dentition of sheep influence biting force.Crossref | GoogleScholarGoogle Scholar |
Laws AJ, Aitken WM (1988) Dental configuration among different breeds of sheep. Research in Veterinary Science 45, 317–323.
| Dental configuration among different breeds of sheep.Crossref | GoogleScholarGoogle Scholar | 3212279PubMed |
McEachern S (2017) Review of the implications of changing the definition of lamb to allow eruption of permanent incisors, but without either incisor being in wear. Available at http://sheepproducers.com.au/wp-content/uploads/2017/11/McEachern-S-2017-Interim-Report-%E2%80%93-Review-of-the-implications-of-changing-definition-of-lamb-to-allow-eruption-of-permanent-incisors-but-without-either-incisor-being-in-wear.pdf [Verified April 2021]
Meyer HH, Aitken WM, Smeaton JE (1982) Inheritance of wear rate in the teeth of sheep. Proceedings of the New Zealand Society of Animal Production 43, 189–191.
MLA (2019) Sheep numbers – as at June 2018 Natural Resource Management Region. Available at https://www.mla.com.au/globalassets/mla-corporate/prices–markets/documents/trends–analysis/fast-facts–maps/sheep-numbers-map-2019-june-2018-1.pdf [Verified April 2021]
Montossi F, Font-i-Furnols M, del Campo M, San Julian R, Brito G, Sanudo C (2013a) Sustainable sheep production and consumer preference trends: compatibilities, contradictions, and unresolved dilemmas. Meat Science 95, 772–789.
| Sustainable sheep production and consumer preference trends: compatibilities, contradictions, and unresolved dilemmas.Crossref | GoogleScholarGoogle Scholar | 23769133PubMed |
Montossi F, De Barbieri I, Ciappesoni G, Ganzabal A, Banchero G, Luzardo S, San Julian R (2013b) Intensification, diversification and specialization to improve the competitiveness of sheep production systems under pastoral conditions: Uruguay’s case. Animal Frontiers 3, 28–35.
| Intensification, diversification and specialization to improve the competitiveness of sheep production systems under pastoral conditions: Uruguay’s case.Crossref | GoogleScholarGoogle Scholar |
NHMRC (2013) ‘Australian Code for the care and use of animals for scientific purposes.’ 8th edn. (National Health and Medical Research Council: Canberra, ACT, Australia)
Pethick DW, Hopkins DL, D’Souza DN, Thompson JM, Walker PJ (2005) Effects of animal age on the eating quality of sheep meat. Australian Journal of Experimental Agriculture 45, 491–498.
| Effects of animal age on the eating quality of sheep meat.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2013) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria)
Ramsay AMM, Swan AA, Swain BC (2019) Design and purpose of the Merino Lifetime Productivity project. Proc. Assoc. Advmt. Anim. Breed. Genet. 23, 512–515.
Richards JS, Sladek MA, Lee GJ (2018) Cumulative reproductive performance effect on overall lifetime productivity in Merino sheep. Animal Production Science 58, 1470–1480.
| Cumulative reproductive performance effect on overall lifetime productivity in Merino sheep.Crossref | GoogleScholarGoogle Scholar |
Ridoutt B (2021) Climate neutral livestock production: a radiative forcing-based climate footprint approach. Journal of Cleaner Production 291, 125260
| Climate neutral livestock production: a radiative forcing-based climate footprint approach.Crossref | GoogleScholarGoogle Scholar |
Rouse GH, Topel DG, Vetter RL, Rust RE, Wickersham TW (1970) Carcass composition of lambs at different stages of development. Journal of Animal Science 31, 846–855.
| Carcass composition of lambs at different stages of development.Crossref | GoogleScholarGoogle Scholar |
Smith JL, Brewer HG, Dyall T (2009) Heritability and phenotypic correlations for breech strike and breech strike resistance indicators in Merinos. Proceedings of the Association for the Advancement of Animal Breeding and Genetics 18, 334–337.
Valasi I, Chadio S, Fthenakis GC, Amiridis GS (2012) Management of pre-pubertal small ruminants: Physiological basis and clinical approach. Animal Reproduction Science 130, 126–134.
| Management of pre-pubertal small ruminants: Physiological basis and clinical approach.Crossref | GoogleScholarGoogle Scholar | 22326612PubMed |
Wiese SC, Pethick DW, Milton JTB, Davidson RH, McIntyre BL, D’Souza DN (2005) Effect of teeth eruption on growth performance and meat quality in sheep. Australian Journal of Experimental Agriculture 45, 509–515.
| Effect of teeth eruption on growth performance and meat quality in sheep.Crossref | GoogleScholarGoogle Scholar |


