A copy number variant near KITLG is associated with the roan pattern in alpacas
Ishani Shah A , Naomi Gray A , David Groth A , Samantha Brooks B and Kylie Munyard
B and Kylie Munyard  A *
A *
A Curtin Medical School and Curtin Health Innovation Research Institute, Faculty of Health Sciences, Curtin University, Perth, WA, Australia.
B Animal Sciences, University of Florida, Gainesville, FL, USA.
Animal Production Science 63(11) 1008-1016 https://doi.org/10.1071/AN22463
Submitted: 15 December 2022 Accepted: 13 March 2023 Published: 4 April 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: The alpaca roan pattern is characterised by white and coloured fibre interspersed together, with a distinctive lighter body and darker extremities, and commonly is believed to be inherited in an autosomal dominant manner. It is of interest to the alpaca fibre industry as it causes ‘contamination’ of coloured fibre with white fibres, but cannot be detected in white or light fawn animals. Other livestock species, such as horses, cattle, goats, and pigs, exhibit comparable phenotypes, which are associated with candidate variant(s) in either KIT or KITLG.
Aims: To identify a region or regions of the genome that is/are causative of the roan pattern in alpacas.
Methods: We conducted a genome-wide association study (GWAS) by using 13 roan and 14 non-roan alpacas sampled from the USA, Australia, and New Zealand. Regions of genome-wide significance were examined for variants that correlated with the roan phenotype.
Key results: A novel candidate single-nucleotype polymorphism (SNP; Super-Scaffold_15:39 742 851T > A), located 272 kb upstream of KITLG, was identified in 1 of 12 regions with genome-wide significant association (P ≤ 5 × 10−8). We identified the candidate SNP-containing region (Super-Scaffold_15:39 742 096–39 887 419) to be a 145 kb copy number variant (CNV) that is likely to be a tandem duplication. All 13 roan alpacas had one or two copies of the roan-associated T allele and all except three non-roans had zero copies. Furthermore, we determined the Mendelian inheritance of copy number haplotypes and their allelic composition in a roan and a non-roan family.
Conclusions: Our data support the hypothesised autosomal incomplete dominant mode of inheritance of the roan pattern in alpacas and suggests that the effect of the T allele CNV version is likely to be suppressed when in cis with the A allele CNV version. However, additional verification is required to validate the finding and determine the functional effect.
Implications: Identification of the cause, or a marker for roan pattern will allow alpaca breeders to select for or against the roan pattern, even when the phenotype is hidden, and therefore increase production output and profitability.
Keywords: alpaca, CNV, colour, fibre, genotyping by sequencing, GWAS, KITLG, pattern, roan, SNP.
Introduction
Coat colours and patterns in livestock species have been artificially selected for thousands of years, giving rise to numerous colour phenotypes. Alpacas (Vicugna pacos) produce over 20 colours of base fibre and exhibit a range of patterns, such as classic grey, roan (aka modern grey in the USA and UK), blue-eyed white, tuxedo, piebald and appaloosa (Munyard 2013; Thangavel et al. 2015). The large variety of high-quality, naturally coloured fibre is gaining popularity within the ‘green’, environmentally conscious market and so many alpaca breeders are selecting for coloured fibres instead of the traditional white (Thangavel et al. 2015). For most domesticated species, selective breeding practices have significantly improved since the development and commercialisation of genetic screening (Toro 2010). Similarly, the Alpaca Coat Colour DNA test (Neogen Australasia) was developed to report the so-far known genotypes for the three loss-of-function MC1R alleles (no black pigment able to be produced) and three loss-of-function ASIP genes (only black pigment able to be produced; Feeley and Munyard 2009; Feeley et al. 2011; Munyard 2013), and the presence or absence of the classic grey pattern (Jones et al. 2019), the only colours with known genetic causes.
The roan coat pattern in alpacas presents as depigmented fibres interspersed within the base colour, generally being concentrated to the central body and with darker extremities (Fig. 1). In the fibre industry, roan fibre is considered a contaminant to coloured fibre and undesirable for commercial processing, whereas hobby spinners and knitters often prefer such variety (Mathews et al. 2019). Genetic testing is required for the early and accurate detection of roan animals as the pattern is highly variable in its expressivity, distribution, and age of onset. Roan commonly appears at birth or early in life (Munyard 2013) and develops over time, whereas late-onset depigmentation is classed as age-related greying. However, in practice, distinguishing between the two traits is difficult due to the phenotypic variability of roan (Goldleaf and Windella alpacas, Dr C. Oddie pers. comm.). Detecting the pattern on white or light fawn animals is very difficult, and these so-called cryptic roans are typically responsible for the hidden dissemination of the trait within herds.
A similar autosomal dominant (or incomplete dominant) roan phenotype has also been described in other species. Genetic studies in horses (Marklund et al. 1999; Grilz-Seger et al. 2020; Voß et al. 2020), cattle (Charlier et al. 1996; Seitz et al. 1999), pigs (Cho et al. 2011; Lim et al. 2011; Fontanesi and Russo 2013), and goats (Talenti et al. 2018) have shown a shared association between roan and the candidate genes KIT proto-oncogene, receptor tyrosine kinase (KIT) or KIT ligand (KITLG). The biological cause of roan is predicted to be the premature depletion of melanocyte stem-cells (melanoblasts) within hair follicles, leading to gradual and permanent depigmentation of individual fibres (Hachiya et al. 2009; Endou et al. 2014; Bian et al. 2019; Qiu et al. 2019). KIT and KITLG are essential signalling proteins involved in migration, differentiation, maturation, and survival of melanoblasts (Besmer et al. 1993; Wehrle-Haller 2003). Apart from melanogenesis, KIT and KITLG are also vital for gametogenesis (Besmer et al. 1993) and haematopoiesis (Zsebo et al. 1990). Thus, loss-of-function mutations in either gene are associated with patterns of depigmentation (Hemesath et al. 1998; Cieslak et al. 2011) as well as pleiotropic effects such as subfertility in homozygous roan cattle (Charlier et al. 1996) and homozygous lethality in some roan horses (Hintz and Van Vleck 1979).
Although mammalian pigmentation is highly conserved, genetic heterogeneity exists for many interspecies colour phenotypes (Cieslak et al. 2011; Brancalion et al. 2022). Over 688 pigmentation-associated genes are known (Baxter et al. 2019). We hypothesised that an autosomal incompletely dominant regulatory variant in or around a gene involved in pigmentation was responsible for the roan coat pattern in alpacas. The aim was to identify a candidate gene and associated variant(s) through use of a genome-wide association study (GWAS), and thereby improve our knowledge of the genetic basis of the roan coat pattern in alpacas.
Materials and methods
Samples
Whole-genome sequence data (WGS) were generated from blood samples of 46 white, fawn, bay, black, white spotted, classic grey, blue eyed white and roan (modern grey) alpacas. Animals were a mix of closely and distantly related individuals, and were sampled from Australian, New Zealand and American herds. Sequences were generated using Illumina Novaseq 150 bp paired-end reads with approximately 30× depth-of-coverage per sample. Phenotype data, in the form of photographs, and pedigree information were available for 35 of the sampled animals.
Genotyping by sequencing
All fastq files were processed through an in-house genotyping by sequencing (GBS) bioinformatics workflow, by using bash script on cloud computing infrastructure (Nimbus Pawsey), providing two instances with eight cores and 32 Gb of RAM. The GBS workflow was based on the best practices for variant-discovery analysis guide by the Broad institute (Van der Auwera and O’Connor 2020) and used open-source software. Raw FASTQ format reads were trimmed (polyG minimum length = 5) and quality filtered via Fastp v.0.23.2 (Chen et al. 2018) by using default parameters. Quality-controlled reads were mapped (with local alignment) to the draft VicPac4 reference genome (Brian Davis, Texas A&M, pers. comm.) via Bowtie2 v2.3.5.1, by using default parameters (Langmead and Salzberg 2012). Mapped reads were sorted and indexed via Samtools v1.10 (Danecek et al. 2021), followed by formatting (AddOrReplaceReadGroups) and quality control (MarkDuplicates) using Picard v2.27.1 (Broad Institute 2019). Individual BAM (binary alignment map) files were processed with GATK v4.2.6.1 HaplotypeCaller (Poplin et al. 2018) to produce GVCF (genomic-variant call format) files, which were subsequently merged using GATK GenomicsDBImport. Joint genotyping was performed with GATK GenotypeGVCFs by using the GenomicsDBImport workspace as input. Single-nucleotide polymorphisms (SNP) were extracted from the VCF file via GATK SelectVariants.
Genome-wide association analysis
The unfiltered VCF file containing only SNP data was processed using PLINK v1.9 (Purcell et al. 2007). SNPs with a minor allele frequency of <0.05 and those missing a genotype for >5% of animals were removed. Animals missing genotypes for >5% of SNPs were also excluded. Dark roan alpacas (n = 13) were selected as cases and dark non-roans (n = 14) as controls. The white and light fawn animals (n = 19) were excluded from the GWAS due to the ambiguity of their observed phenotype. Quality-controlled genome-wide SNPs were included in a case-control association analysis using the Fisher’s exact test model in PLINK (Purcell et al. 2007). A genome-wide significance threshold of P = 5 × 10−8 was applied, and Manhattan plots were generated using SNPEVG v3.2 (Wang et al. 2012).
Candidate variants
All genomic regions with genome-wide significant association (P ≤ 5 × 10−8) and with underlying support observed on the Manhattan plot, were manually assessed for potential candidate variants. Both SNP and indel genotypes, within the complete VCF file, were considered and viewed on Integrated Genomic Viewer (IGV) v2.12.3 (Robinson et al. 2011). Variant search was additionally extended to the core pigment genes KIT, MITF, ASIP, MC1R, and those significantly downregulated in prematurely greying human hair follicles (Bian et al. 2019), namely, SOX10, TYR, TYRP1, MLANA, PMEL, MATP and GPR143. Gene sequences were retrieved from the VicPac3.1 gene database on NCBI (https://www.ncbi.nlm.nih.gov/gene/) and aligned to the candidate VicPac4 (draft) regions via the BLASTn v2.9.0+ command line tool (Camacho et al. 2009) to identify genes in those regions. Where possible, pedigree data were checked for Mendelian inheritance of variant genotype and all animals were checked for potential misclassification of phenotype through examination of available photographs and personal communication with breeders.
PCR and sequencing of the candidate SNP
To validate the candidate SNP (Super-Scaffold_15:39 742 851T > A), 10 additional dark roans plus four animals from the GWAS were genotyped through targeted polymerase chain reaction (PCR) and subsequent DNA sequencing. Genomic DNA was extracted from blood samples following the PureLink Genomic DNA Mini Kit (Invitrogen) protocol. Forward (5′-TCATGCTCCCCTGAACAACA-3′) and reverse (5′-TCTCGTTGATGATGGTGGGAG-3′) PCR primers were designed using Primer3Plus (https://www.primer3plus.com/), having a predicted product size of 541 bp. PCR was performed using a T100 Thermal Cycler (Kyratec) in a total volume of 10 μL, containing ~100 ng genomic DNA, 2.5 U MyTaq HS DNA polymerase (Bioline), 2 μL MyTaq reaction buffer (5×) (Bioline) and 3 pmol of each primer. The amplification conditions were as follows: 1 min at 95°C, 30 cycles of 15 s at 95°C, 15 s at 60°C, 30 s at 72°C, then 5 min at 72°C. For each sample, the above PCR was performed in triplicate and the pooled products were visualised on a 2% w/v SB buffer agarose gel, followed by purification by using a FavorPrep PCR Clean-Up Mini Kit (Favorgen). Two separate sequencing reactions (forward and reverse) were performed by the Australian Genome Research Facility, by using the respective PCR primers, and following the ABI Prism BigDye Terminator Cycle Sequencing protocol. Sequence data were visualised and manually analysed using FinchTV v1.4.0 (Team Giospiza 2004).
Copy-number variant analysis
Analysis of copy number was limited to the scaffold that contained the candidate variant and was conducted using CNVpytor v1.2.1 (Suvakov et al. 2021), following the developers’ guide. Briefly, depth-of-coverage of mapped reads was corrected for GC bias and normalised to a diploid copy number for each sample, complemented by B-allele (referring to the non-reference or variant allele) frequency analysis of SNPs and small indels. Read-pair orientation of mapped reads was assessed in the candidate region using IGV (Robinson et al. 2011) to view BAM files. The proportion of roan and non-roan associated allele at the candidate SNP, and variants in visual linkage disequilibrium (LD) with it, were considered when manually determining the roan:non-roan copy-number ratio for each sample.
Results
In total, 28 296 476 SNPs and 4 191 807 indels were detected. Following SNP extraction and quality control, 16 863 747 SNPs were retained, and all samples had a genotyping rate of >95%. The GWAS showed 12 regions with association greater than P ≤ 5 × 10−8 (Fig. 2a). However, only one region contained variant genotypes that were concordant with phenotypes (Supplementary material Fig. S1).
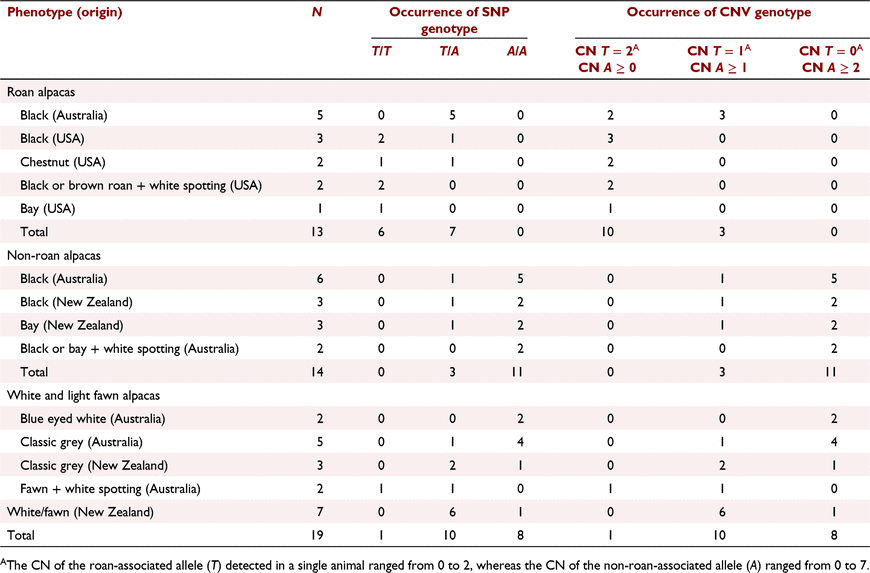
A candidate SNP (Super-Scaffold_15:39 742 851T > A) was identified 272 kb upstream of KITLG (Fig. 2b). All 13 roan alpacas were genotyped as either homozygous or heterozygous for the reference allele (T), and all but 3 of 14 non-roans were homozygous for the variant allele (A). It is interesting to note that the alpaca used to generate the reference genome is light fawn in colour. Almost half of the white and light fawn animals (n = 19) were heterozygous for the roan-associated allele, and one was homozygous (Table 1). Mendelian inheritance of the SNP genotype was concordant for the 12 parent-offspring trios included in the GWAS (data not shown). Additionally, pedigree analyses of all animals supported the dominant mode of inheritance of roan (or cryptic roan in the white and light fawn animals; data not shown).
On inspection of raw read depth at the candidate SNP, an allelic imbalance was observed for all except five heterozygotes (n = 20). Sanger sequencing of the four re-sequenced exemplar samples (T/T, A/A, T/A balanced, T/A unbalanced) was consistent with the WGS genotypes. Three of ten additional (i.e. Sanger sequence only) roan animals were genotyped A/A and the remaining seven had an unreliable T/A genotype, which supported a hypothesis of allelic imbalance at this locus.
In the Super-Scaffold_15:39 742 096–39 887 419 region, which contained the Super-Scaffold_15:39 742 851T > A candidate, an abrupt increase in depth-of-coverage was observed in all except 8 of the 46 of the BAM files, suggestive of a CNV. Subsequently, the presence of a CNV 127–272 kb upstream of KITLG was validated (Fig. 3a, b). The copy number ratio of the roan (T):non-roan (A) associated allele was estimated for all samples (Table 1). All eight US roan alpacas were closely related, and each had two copies of T and zero or one copies of A. Of the five Australian roan alpacas, only two were related (second generation) and all had one or two copies of T and one to four copies of A. All except three (one Australian and two New Zealand) non-roan animals (n = 14) had no copies of T and three to seven copies of A.
Read-pair orientation analysis of individual BAM files demonstrated a trend of right–left oriented discordant paired-end reads mapped at the boundaries of the CNV, indicative of a tandem duplication (Fig. 3c). A smaller 40 kb CNV region (Super-Scaffold_15:39 763 502–39 804 057) was predicted to occur in tandem within the 145 kb (Super-Scaffold_15:39 742 096–39 887 419) tandem duplication.
All progeny T:A counts were concordant with those expected through Mendelian inheritance. We could unambiguously infer the CNV haplotypes within two nuclear families by using Mendelian inheritance principles (Fig. 4). These were the only families in which at least one individual had a normal diploid copy count (CN = 2) in the homozygous state. All US roan animals within a single family had at least one haplotype with just the T allele version. The other family of four consisted of two non-roan and two classic grey animals, and all except the dam (non-roan) had only haplotypes composed of the A allele copies. Possible misclassification of the dam’s phenotype was checked, and she did not roan at a later stage in life.
|
|
Discussion
These data suggest an association of the T allele version of a 145 kb tandem CNV upstream of KITLG with the roan phenotype in alpacas. It is also tempting to speculate that the roaning effect of T is suppressed when in cis with the non-roan associated version A. In support of this concept, one non-roan animal had a haplotype composed of one T and two A copies (cn = AAT; Fig. 4). Similarly, two roan animals, each with cn = TA and cn = T haplotypes, expressed roan to a lesser extent than did their roan relatives with only the cn = T haplotype (Fig. 4).
The 145 kb candidate CNV ends 127 kb upstream of KITLG. HapMap data have shown that 90% of CNVs have two or more allelic states (McCarroll et al. 2008; Handsaker et al. 2015) and that 66.7% of haplotypes consisting of copy-number gains are composed of alternative allelic copies (Palta et al. 2015), which reflects what we have discovered here. The association of the genes KITLG or its receptor KIT with the roan phenotype has been well established in other species (Charlier et al. 1996; Marklund et al. 1999; Cho et al. 2011; Lim et al. 2011; Fontanesi and Russo 2013; Talenti et al. 2018; Grilz-Seger et al. 2020; Voß et al. 2020). More compelling evidence that this CNV could be associated with the roan trait in alpacas is that KITLG expression in mice is regulated by a ~200 kb region 100 kb upstream of the gene (Bedell et al. 1995), and mutations in this region cause depigmentation, anaemia and embryonic death (Bedell et al. 1996). Similarly, a study comparing the parallel evolution of colour variation in human and stickleback fish has attributed variation in KITLG expression to cis-regulatory changes in a large region upstream of the gene (Miller et al. 2007). Therefore, a potential mechanism of action for roan in alpacas is disruption to a cis-regulatory element involved in KITLG regulation, such as an enhancer domain, caused by the T-allele version, which could be rescued when the functional A-allele version is in cis.
An allele dosage effect may also contribute to the expression of roan. The observed copy-number counts varied both among and between roan and non-roan animals. The variation in copy number was mostly attributed to the A-allele version (Table 1). This not only reflects the strong (artificial) selection for non-roan alpacas but also suggests that the dosage of A may be associated with the extent or onset of roan. A study in dogs identified a 6 kb CNV located 152 kb upstream of KITLG that was associated with pigment intensity, with a higher copy number correlating to higher intensity (Weich et al. 2020). Since the early depletion of hair follicle melanoblasts, thus causing gradual and permanent depigmentation, is the probable cause of roan (Hachiya et al. 2009; Endou et al. 2014; Bian et al. 2019; Qiu et al. 2019), it is plausible that a related mechanism may also contribute to pigment intensity. Although we did not account for variation in pigment intensity in this work, the observed A copy-version counts were higher among the Australian (unrelated) roans than the US roans (related). Unlike the Australian roans, the US roans were selectively bred for the roan pattern. This information, in addition to the fact that the US alpacas displayed the pattern earlier than did the Australian roans, suggests that a higher copy number of the A version may reduce, or delay, the expression of roan.
Interestingly, the alpaca that is the source of the reference genome also had a cn = T haplotype. Considering her light fawn base colour and darker extremities, it is plausible that she was a cryptic roan. Almost half of the sampled white or light fawn animals had one copy of T, and although unphased, this observation is consistent with the knowledge that cryptic roan animals often go undetected until mated with dark base-colour animals. All but three dark non-roan animals had varying copy numbers of A alone (Table 1), indicating homozygosity at the putative functionally important site. An autosomal incomplete dominant inheritance pattern can be inferred from these data, whereby a single haplotype consisting solely of the T allele copy version likely confers the expression of roan. There is no evidence of homozygous lethality as evidenced by six roan alpacas (USA) that are homozygous for the T allele version of the CNV (Table 1).
Breed specific allelic heterogeneity owing to potential founder effect is known in roan horses (Grilz-Seger et al. 2020; Voß et al. 2020) and pigs (Cho et al. 2011; Lim et al. 2011). Therefore, we cannot exclude the possibility of multiple causative variants for roan in alpacas or that the candidate SNP may be in LD with the true casual variant(s). This may explain why 3 of the 10 additional roan animals genotyped A/A at the candidate SNP. However, all 10 animals were sampled from the same farm and the three non-concordant animals developed the roan pattern at a much later age than did the rest (Windella and Goldleaf alpacas, Dr C. Oddie and Mr B. Fallon pers. comm.). It is possible that the late onset of pattern development was age-related, which highlights the importance of recording the onset and progression of the roan phenotype.
We hypothesise that a cis-regulatory mechanism alters the expression of KITLG and causes premature depigmentation of individual fibres, which may be further influenced by allelic dosage effects. This study has provided novel insights into the genetic basis of the roan coat pattern in alpacas and suggests that the KITLG CNV influences both pheomelanic and eumelanic base-colour phenotypes.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was funded by the Morris Animal Foundation (Grant # D18LA-002).
Acknowledgements
This research was supported by funding from the Morris Animal Foundation (D18LA-002) and the Curtin Medical School. This work was also supported by resources provided by the Pawsey Supercomputing Research Centre with funding from the Australian Government and the Government of Western Australia. Thanks go to all of the alpaca owners and breeders who provided access to their animals, with special mention to Dr Carolyn Oddie (Windella alpacas), Brett Fallon and Tyler Bennie (Goldleaf alpacas) for their help with sample collection and wisdom on alpaca breeding practices.
References
Baxter LL, Watkins-Chow DE, Pavan WJ, Loftus SK (2019) A curated gene list for expanding the horizons of pigmentation biology. Pigment Cell & Melanoma Research 32, 348–358.| A curated gene list for expanding the horizons of pigmentation biology.Crossref | GoogleScholarGoogle Scholar |
Bedell MA, Brannan CI, Evans EP, Copeland NG, Jenkins NA, Donovan PJ (1995) DNA rearrangements located over 100 kb 5’ of the Steel (Sl)-coding region in Steel-panda and Steel-contrasted mice deregulate Sl expression and cause female sterility by disrupting ovarian follicle development. Genes & Development 9, 455–470.
| DNA rearrangements located over 100 kb 5’ of the Steel (Sl)-coding region in Steel-panda and Steel-contrasted mice deregulate Sl expression and cause female sterility by disrupting ovarian follicle development.Crossref | GoogleScholarGoogle Scholar |
Bedell MA, Cleveland LS, O’Sullivan TN, Copeland NG, Jenkins NA (1996) Deletion and interallelic complementation analysis of Steel mutant mice. Genetics 142, 935–944.
| Deletion and interallelic complementation analysis of Steel mutant mice.Crossref | GoogleScholarGoogle Scholar |
Besmer P, Manova K, Duttlinger R, Huang EJ, Packer A, Gyssler C, Bachvarova RF (1993) The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Development 119, 125–137.
| The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis.Crossref | GoogleScholarGoogle Scholar |
Bian Y, Wei G, Song X, Yuan L, Chen H, Ni T, Lu D (2019) Global downregulation of pigmentation-associated genes in human premature hair graying. Experimental and Therapeutic Medicine 18, 1155–1163.
| Global downregulation of pigmentation-associated genes in human premature hair graying.Crossref | GoogleScholarGoogle Scholar |
Brancalion L, Haase B, Wade CM (2022) Canine coat pigmentation genetics: a review. Animal Genetics 53, 3–34.
| Canine coat pigmentation genetics: a review.Crossref | GoogleScholarGoogle Scholar |
Broad Institute (2019) ‘Picard tools.’ Broad Institute, GitHub repository. http://broadinstitute.github.io/picard/
Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL (2009) BLAST+: architecture and applications. BMC Bioinformatics 10, 421
| BLAST+: architecture and applications.Crossref | GoogleScholarGoogle Scholar |
Charlier C, Denys B, Belanche JI, Coppieters W, Grobet L, Mni M, Womack J, Hanset R, Georges M (1996) Microsatellite mapping of the bovine roan locus: a major determinant of White Heifer Disease. Mammalian Genome 7, 138–142.
| Microsatellite mapping of the bovine roan locus: a major determinant of White Heifer Disease.Crossref | GoogleScholarGoogle Scholar |
Chen S, Zhou Y, Chen Y, Gu J (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890.
| fastp: an ultra-fast all-in-one FASTQ preprocessor.Crossref | GoogleScholarGoogle Scholar |
Cho I-C, Zhong T, Seo B-Y, Jung E-J, Yoo C-K, Kim J-H, Lee J-B, Lim H-T, Kim B-W, Lee J-H, Ko M-S, Jeon J-T (2011) Whole-genome association study for the roan coat color in an intercrossed pig population between Landrace and Korean native pig. Genes & Genomics 33, 17–23.
| Whole-genome association study for the roan coat color in an intercrossed pig population between Landrace and Korean native pig.Crossref | GoogleScholarGoogle Scholar |
Cieslak M, Reissmann M, Hofreiter M, Ludwig A (2011) Colours of domestication. Biological Reviews 86, 885–899.
| Colours of domestication.Crossref | GoogleScholarGoogle Scholar |
Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, Whitwham A, Keane T, McCarthy SA, Davies RM, Li H (2021) Twelve years of SAMtools and BCFtools. GigaScience giab008
| Twelve years of SAMtools and BCFtools.Crossref | GoogleScholarGoogle Scholar |
Endou M, Aoki H, Kobayashi T, Kunisada T (2014) Prevention of hair graying by factors that promote the growth and differentiation of melanocytes. The Journal of Dermatology 41, 716–723.
| Prevention of hair graying by factors that promote the growth and differentiation of melanocytes.Crossref | GoogleScholarGoogle Scholar |
Feeley NL, Munyard KA (2009) Characterisation of the melanocortin-1 receptor gene in alpaca and identification of possible markers associated with phenotypic variations in colour. Animal Production Science 49, 675–681.
| Characterisation of the melanocortin-1 receptor gene in alpaca and identification of possible markers associated with phenotypic variations in colour.Crossref | GoogleScholarGoogle Scholar |
Feeley NL, Bottomley S, Munyard KA (2011) Three novel mutations in ASIP associated with black fibre in alpacas (Vicugna pacos). The Journal of Agricultural Science 149, 529–538.
| Three novel mutations in ASIP associated with black fibre in alpacas (Vicugna pacos).Crossref | GoogleScholarGoogle Scholar |
Fontanesi L, Russo V (2013) Molecular genetics of coat colour in pigs. Acta Agriculturae Slovenica 16
Grilz-Seger G, Reiter S, Neuditschko M, Wallner B, Rieder S, Leeb T, Jagannathan V, Mesarič M, Cotman M, Pausch H, Lindgren G, Velie B, Horna M, Brem G, Druml T (2020) A genome-wide association analysis in Noriker horses identifies a SNP associated with roan coat color. Journal of Equine Veterinary Science 88, 102950
| A genome-wide association analysis in Noriker horses identifies a SNP associated with roan coat color.Crossref | GoogleScholarGoogle Scholar |
Hachiya A, Sriwiriyanont P, Kobayashi T, Nagasawa A, Yoshida H, Ohuchi A, Kitahara T, Visscher MO, Takema Y, Tsuboi R, Boissy RE (2009) Stem cell factor-KIT signalling plays a pivotal role in regulating pigmentation in mammalian hair. The Journal of Pathology 218, 30–39.
| Stem cell factor-KIT signalling plays a pivotal role in regulating pigmentation in mammalian hair.Crossref | GoogleScholarGoogle Scholar |
Handsaker RE, Van Doren V, Berman JR, Genovese G, Kashin S, Boettger LM, McCarroll SA (2015) Large multiallelic copy number variations in humans. Nature Genetics 47, 296–303.
| Large multiallelic copy number variations in humans.Crossref | GoogleScholarGoogle Scholar |
Hemesath TJ, Price ER, Takemoto C, Badalian T, Fisher DE (1998) MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature 391, 298–301.
| MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes.Crossref | GoogleScholarGoogle Scholar |
Hintz HF, Van Vleck LD (1979) Lethal dominant roan in horses. Journal of Heredity 70, 145–146.
| Lethal dominant roan in horses.Crossref | GoogleScholarGoogle Scholar |
Jones M, Sergeant C, Richardson M, Groth D, Brooks S, Munyard K (2019) A non-synonymous SNP in exon 3 of the KIT gene is responsible for the classic grey phenotype in alpacas (Vicugna pacos). Animal Genetics 50, 493–500.
| A non-synonymous SNP in exon 3 of the KIT gene is responsible for the classic grey phenotype in alpacas (Vicugna pacos).Crossref | GoogleScholarGoogle Scholar |
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nature Methods 9, 357–359.
| Fast gapped-read alignment with Bowtie 2.Crossref | GoogleScholarGoogle Scholar |
Lim HT, Zhong T, Cho IC, Seo BY, Kim JH, Lee SS, Ko MS, Park HB, Kim BW, Lee JH, Jeon JT (2011) Novel alternative splicing by exon skipping in KIT associated with whole-body roan in an intercrossed population of Landrace and Korean Native pigs. Animal Genetics 42, 451–455.
| Novel alternative splicing by exon skipping in KIT associated with whole-body roan in an intercrossed population of Landrace and Korean Native pigs.Crossref | GoogleScholarGoogle Scholar |
Marklund S, Moller M, Sandberg K, Andersson L (1999) Close association between sequence polymorphism in the KIT gene and the roan coat color in horses. Mammalian Genome 10, 283–288.
| Close association between sequence polymorphism in the KIT gene and the roan coat color in horses.Crossref | GoogleScholarGoogle Scholar |
Mathews M, Mathews T, Kostiakos ECK (2019) Gorgeous Greys. Camelid Connections Magazine – Specialty Publications. Candelo New South Wales, Australia. https://camelidconnections.com.au
McCarroll SA, Kuruvilla FG, Korn JM, Cawley S, Nemesh J, Wysoker A, Shapero MH, de Bakker PIW, Maller JB, Kirby A, Elliott AL, Parkin M, Hubbell E, Webster T, Mei R, Veitch J, Collins PJ, Handsaker R, Lincoln S, Nizzari M, Blume J, Jones KW, Rava R, Daly MJ, Gabriel SB, Altshuler D (2008) Integrated detection and population-genetic analysis of SNPs and copy number variation. Nature Genetics 40, 1166–1174.
| Integrated detection and population-genetic analysis of SNPs and copy number variation.Crossref | GoogleScholarGoogle Scholar |
Miller CT, Beleza S, Pollen AA, Schluter D, Kittles RA, Shriver MD, Kingsley DM (2007) cis-regulatory changes in kit ligand expression and parallel evolution of pigmentation in sticklebacks and humans. Cell 131, 1179–1189.
| cis-regulatory changes in kit ligand expression and parallel evolution of pigmentation in sticklebacks and humans.Crossref | GoogleScholarGoogle Scholar |
Munyard K (2013) ‘Inheritance of white colour in alpacas: identifying the genes involved.’ (Rural Industries Research and Development Corporation: Wagga Wagga, NSW, Australia)
Palta P, Kaplinski L, Nagirnaja L, Veidenberg A, Möls M, Nelis M, Esko T, Metspalu A, Laan M, Remm M (2015) Haplotype phasing and inheritance of copy number variants in nuclear families. PLoS ONE 10, e0122713
| Haplotype phasing and inheritance of copy number variants in nuclear families.Crossref | GoogleScholarGoogle Scholar |
Poplin R, Ruano-Rubio V, DePristo MA, Fennell TJ, Carneiro MO, Van der Auwera GA, Kling DE, Gauthier LD, Levy-Moonshine A, Roazen D, Shakir K, Thibault J, Chandran S, Whelan C, Lek M, Gabriel S, Daly MJ, Neale B, MacArthur DG, Banks E (2018) Scaling accurate genetic variant discovery to tens of thousands of samples. BioRxiv: The Preprint Server for Biology 201178
| Scaling accurate genetic variant discovery to tens of thousands of samples.Crossref | GoogleScholarGoogle Scholar |
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. The American Journal of Human Genetics 81, 559–575.
| PLINK: a tool set for whole-genome association and population-based linkage analyses.Crossref | GoogleScholarGoogle Scholar |
Qiu W, Chuong C-M, Lei M (2019) Regulation of melanocyte stem cells in the pigmentation of skin and its appendages: biological patterning and therapeutic potentials. Experimental Dermatology 28, 395–405.
| Regulation of melanocyte stem cells in the pigmentation of skin and its appendages: biological patterning and therapeutic potentials.Crossref | GoogleScholarGoogle Scholar |
Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP (2011) Integrative genomics viewer. Nature Biotechnology 29, 24–26.
| Integrative genomics viewer.Crossref | GoogleScholarGoogle Scholar |
Seitz JJ, Schmutz SM, Thue TD, Buchanan FC (1999) A missense mutation in the bovine MGF gene is associated with the roan phenotype in Belgian Blue and Shorthorn cattle. Mammalian Genome 10, 710–712.
| A missense mutation in the bovine MGF gene is associated with the roan phenotype in Belgian Blue and Shorthorn cattle.Crossref | GoogleScholarGoogle Scholar |
Suvakov M, Panda A, Diesh C, Holmes I, Abyzov A (2021) CNVpytor: a tool for copy number variation detection and analysis from read depth and allele imbalance in whole-genome sequencing. GigaScience giab074
| CNVpytor: a tool for copy number variation detection and analysis from read depth and allele imbalance in whole-genome sequencing.Crossref | GoogleScholarGoogle Scholar |
Talenti A, Bertolini F, Williams J, Moaeen-ud-Din M, Frattini S, Coizet B, Pagnacco G, Reecy J, Rothschild MF, Crepaldi P, Italian Goat Consortium (2018) Genomic analysis suggests KITLG is responsible for a roan pattern in two Pakistani goat breeds. Journal of Heredity 109, 315–319.
| Genomic analysis suggests KITLG is responsible for a roan pattern in two Pakistani goat breeds.Crossref | GoogleScholarGoogle Scholar |
Team Geospiza (2004) ‘FinchTV 1.4.0.’ (Geospiza, Inc.: Seattle, WA, USA)
Thangavel K, Rathinamoorthy R, Ganesan P (2015) Sustainable luxury natural fibers – production, properties, and prospects. In ‘Handbook of sustainable luxury textiles and fashion. Vol. 1’. (Eds MA Gardetti, SM Subramanian) pp. 59–98. (Springer: Singapore)
Toro MA (2010) Future trends in animal breeding due to new genetic technologies. Advances in Animal Biosciences 1, 546–557.
| Future trends in animal breeding due to new genetic technologies.Crossref | GoogleScholarGoogle Scholar |
Van der Auwera GA, O’Connor BD (2020) ‘Genomics in the cloud: using Docker, GATK, and WDL in terra.’ 1st edn. (O’Reilly Media: Sebastopol, CA, USA)
Voß K, Tetens J, Thaller G, Becker D (2020) Coat color roan shows association with KIT variants and no evidence of lethality in Icelandic horses. Genes 11, 680
| Coat color roan shows association with KIT variants and no evidence of lethality in Icelandic horses.Crossref | GoogleScholarGoogle Scholar |
Wang S, Dvorkin D, Da Y (2012) SNPEVG: a graphical tool for GWAS graphing with mouse clicks. BMC Bioinformatics 13, 319
| SNPEVG: a graphical tool for GWAS graphing with mouse clicks.Crossref | GoogleScholarGoogle Scholar |
Wehrle-Haller B (2003) The role of kit-ligand in melanocyte development and epidermal homeostasis. Pigment Cell Research 16, 287–296.
| The role of kit-ligand in melanocyte development and epidermal homeostasis.Crossref | GoogleScholarGoogle Scholar |
Weich K, Affolter V, York D, Rebhun R, Grahn R, Kallenberg A, Bannasch D (2020) Pigment intensity in dogs is associated with a copy number variant upstream of KITLG. Genes 11, 75
| Pigment intensity in dogs is associated with a copy number variant upstream of KITLG.Crossref | GoogleScholarGoogle Scholar |
Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu R-Y, Birkett NC, Okino KH, Murdock DC, et al. (1990) Stem cell factor is encoded at the SI locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell 63, 213–224.
| Stem cell factor is encoded at the SI locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor.Crossref | GoogleScholarGoogle Scholar |