Insights into the pathogenesis of catastrophic spontaneous humeral fractures in first-lactation dairy cows
A. S. Wehrle-Martinez A * , K. E. Lawrence A , P. J. Back A B , C. W. Rogers
A * , K. E. Lawrence A , P. J. Back A B , C. W. Rogers  A B , M. J. Gibson
A B , M. J. Gibson  A and K. E. Dittmer
A and K. E. Dittmer  A
A
A
B
Abstract
Spontaneous humeral fractures in first-lactation dairy cows have introduced significant challenges to the dairy industry in New Zealand, impacting animal welfare, farm economics, and veterinary practices. This review synthesizes current knowledge on the pathogenesis of these fractures and identifies potential key risk factors. The majority of bones from affected first-lactation dairy cows have osteoporosis, which is associated with inadequate bone formation and increased bone resorption. In addition, low total collagen content in bones from most affected dairy cows supports the hypothesis that inadequate bone formation is an important risk factor associated with humeral fractures in these cows. Spectroscopic analyses further confirmed a significant reduction in bone quality and strength. Novel findings suggest that low liver copper concentration in many of the affected cows’ results from the mobilisation of copper to the bone. Although limited, the accumulated evidence suggests that to mitigate the incidence of catastrophic fractures, adequate nutrition (especially protein-energy) should be supplied during important growth periods. While significant progress has been made in understanding the cause of these fractures, many uncertainties and areas requiring further research remain.
Keywords: bone, collagen, copper, dairy cow, heifer, humeral fracture, malnutrition, osteoporosis, protein/energy.
Introduction
The dairy sector in New Zealand is an important contributor to the country’s economy. The majority of milk solids produced are processed into products such as dried milk powder, butter or cheese for the export market rather than for domestic supply of fresh milk (Soutter 2019; Stats NZ 2024). The temperate climate is reflected in a dairy production system that is pasture-based and seasonal. In New Zealand, cows typically graze on pasture year-round, with varying levels of supplements provided, depending on the farm system and location (Luo and Ledgard 2021). Calving patterns are aligned with pasture growth, with most dairy cows calving in spring and twice-daily milking being the most common practice, although adoption of once-a-day and flexible milking practices are increasing (Lopez-Villalobos et al. 2023; Van der Zeijden et al. 2023). However, the heavy reliance on pasture supply to meet the nutritional demands can be detrimental to cow growth if the balance between the seasonal feed supply and the seasonal demands for growth and lactation is disrupted.
The high incidence of spontaneous humeral fractures in first-lactation dairy cows in the New Zealand dairy industry is a threat to cow welfare and the industry’s social licence to operate. All affected cows require prompt euthanasia, which has caused mental distress to farmers and veterinarians alike, and severe economic challenges on affected farms due to lost productivity arising from heifer losses. This has prompted investigations into the factors that might be contributing to fracture occurrence.
This review aims to synthesise current knowledge on the pathogenesis of humeral fractures in first-lactation dairy cows in New Zealand, highlighting possible key risk factors identified by research. By understanding the underlying causes and mechanisms of these fractures, it is hoped that we will help protect the health and productivity of young dairy cows, ensuring a more sustainable and profitable dairy industry.
Background
Bone fractures in ruminants are frequently observed in young animals following trauma from dystocia or handling (Gangl et al. 2006; Jean and Anderson 2014). In older cows, slipping or falling on hard surfaces and other causes of trauma are reported (Gangl et al. 2006; Kushwaha et al. 2011). The metacarpus, metatarsus, femur, and tibia bones are most frequently affected, with fewer occurrences described for the humerus (Crawford and Fretz 1985; Gangl et al. 2006; Kushwaha et al. 2011; Jean and Anderson 2014). A study of fractures in cattle from India reported that only 6 out of 160 (3.8%) cases of fractures in cattle occurred in the humerus, with most fractures occurring in the hindlimbs (Yadav et al. 2019). Similar results were reported by Crawford and Fretz (1985), with only 12 out of 213 (5.6%) cases affecting the humerus. The low incidence of humeral fractures in cows is attributed to the substantial protection provided to the bones by the strong surrounding muscle mass (Rakestraw 1996).
Fractures that are not the consequence of trauma are termed pathological fractures and are the result of underlying local or systemic disease that weaken bones (Williams and Streeter 2022). Since 2008, spontaneous humeral fractures in first-lactation dairy cows, particularly in 2-year-old first-lactation dairy cows (heifers), have been increasingly reported in New Zealand. The first major reported outbreak was on a farm in the Manawatū-Whanganui region of New Zealand in 2007, where 6 out of 200 (3%) first-lactation dairy cows experienced non-weight-bearing lameness in one of their forelimbs due to a fractured humerus (Weston 2008). Further testing revealed low serum and liver copper concentrations in three affected cows, and the fractures were considered to be the result of copper deficiency (Thompson et al. 2012).
Between 2007 and 2015, it was estimated that up to 12% of dairy farms in New Zealand were affected annually, involving approximately 4620 dairy cattle with humeral fractures (Hunnam et al. 2024). Most cases of spontaneous humeral fractures are reported in first-lactation dairy cows, with fewer occurring before first calving or in cows in their second lactation (Thompson et al. 2012; Wehrle-Martinez 2022). These fractures tend to occur up to 6-months post-partum, usually affect multiple first-lactation dairy cows on the farm, and occur over multiple seasons (Hunnam et al. 2024). This suggests a likely systemic issue affecting these animals, rather than isolated traumatic events.
Reports of spontaneous humeral fractures in dairy cows are now regularly featured in the quarterly report of diagnostic cases in the Surveillance Biosecurity magazine from the Ministry for Primary Industry, New Zealand (Ministry for Primary Industries 2024). Similar cases of spontaneous fractures of the humerus have also been reported on dairy farms in Victoria, Australia (Loughnan 2012).
The characteristic clinical sign in affected cows is a ‘hanging leg’ stance, where the affected limb is held up and hangs, colloquially named ‘dropped elbow’ or ‘dropped shoulder’ (Fig. 1).
Characteristic ‘hanging leg’ stance of cows affected with spontaneous humeral fracture (photo credit D. Butler).

Within the literature, the most commonly described location for humeral fractures in cattle is the diaphysis (the middle, narrower part of the bone) (Crawford and Fretz 1985). However, in more than 95% of the bones from cattle affected with spontaneous humeral fractures sent to the Massey University School of Veterinary Science heifer fracture research group (Palmerston North, New Zealand), the type of fracture was a complete, non-articular spiral fracture extending from the proximal metaphysis (the wider part near the joint) to the distal metaphysis spiralling along the diaphysis (Fig. 2) (Wehrle-Martinez et al. 2023a).
A spiral fracture extending from both ends of the of the humerus is typically seen in cases of spontaneous humeral fractures in New Zealand dairy cows.
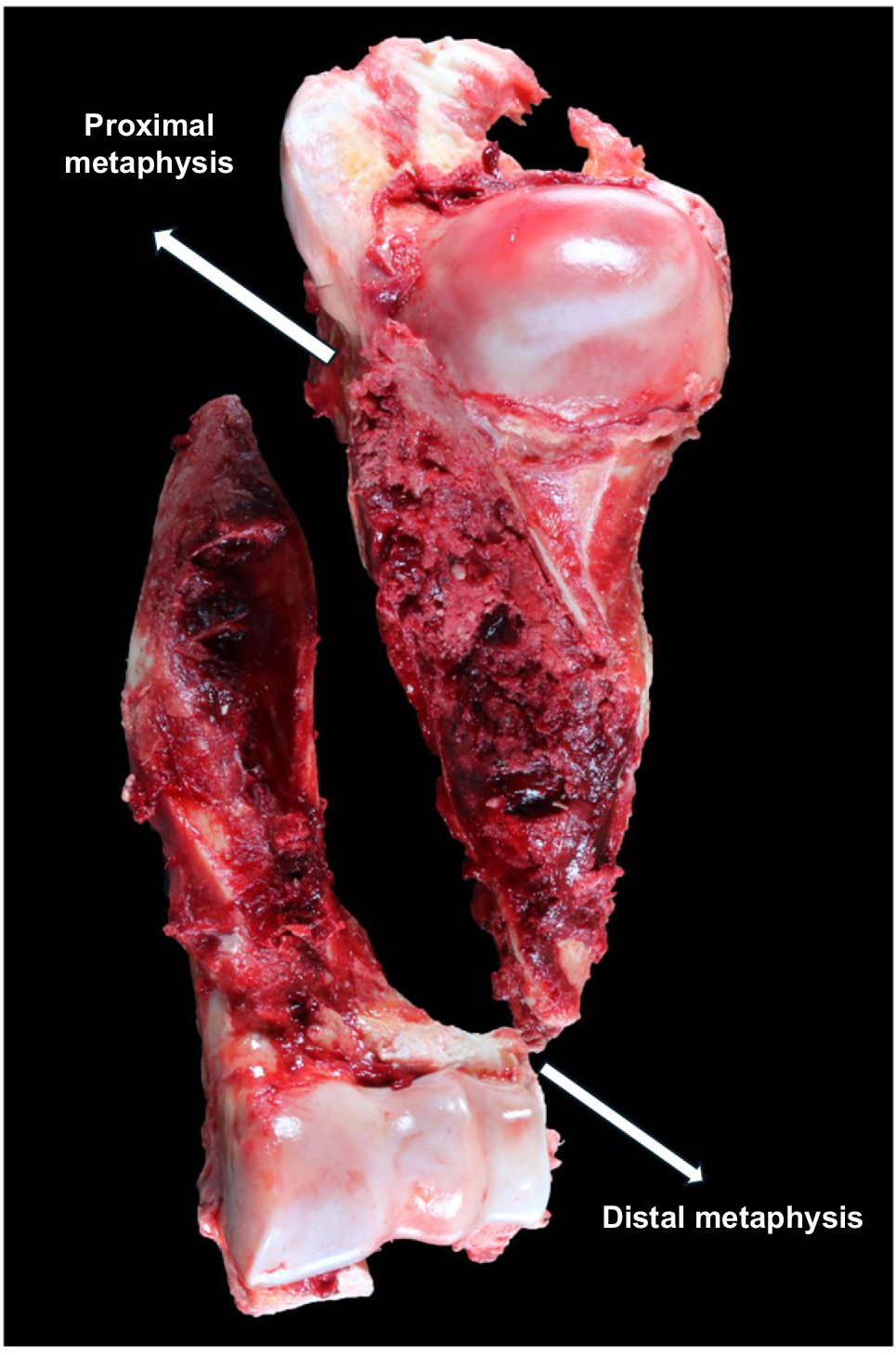
Initial case studies on spontaneous humeral fractures suggested that these fractures originated from the cut-back zone (area where the bone is reshaping itself) of the humeral proximal metaphysis (Dittmer et al. 2016). However, more extensive subsequent studies reported increased cortical resorption in the distal part of the humerus of first-lactation dairy cows with humeral fractures (Wehrle-Martinez et al. 2023a). It is now thought that spontaneous humeral fractures start from the distal metaphysis and spiral up to the proximal metaphysis (Wehrle-Martinez et al. 2023a). This theory is supported by the findings that the distal part of the bovine humerus has the highest stress concentration, making it a mechanically weaker zone of bone in cows with osteoporosis (Bouza-Rodríguez and Miramontes-Sequeiros 2014; Wehrle-Martinez et al. 2023a). More importantly, the presence of a fracture extending close to where the bone grows longitudinally (growth plate) suggests that the compromise in bone quality and strength occurred recently.
It has been reported that the humerus is the only bone that fractures due to uneven compressive and torsional forces applied to the distal diaphysis (Mulon 2013). Anecdotally, some farmers and veterinarians have mentioned that these fractures appear to occur when cows are standing up from lying down. The act of standing up may apply a significant twisting force, contributing to humeral fracture in these cows.
Investigating the pathogenesis of spontaneous humeral fractures
Investigations into outbreaks of pathologic fractures in production animals, regardless of the cause, need to prioritise examination of the environment, diet, and health status of affected animals. This approach is important for identifying potential causes or risk factors associated with fractures. However, pinpointing these causes or risk factors can be challenging, as changes in bone quantity and quality typically develop slowly and without symptoms, with bone fractures eventually resulting from compromised skeletal health (Craig et al. 2016).
Between 2019 and 2024, extensive research has been conducted by the heifer fracture research group at Massey University to identify potential causes and/or risk factors associated with spontaneous humeral fractures in dairy cows in New Zealand (Gibson 2021; Wehrle-Martinez 2022). A holistic and multidisciplinary approach was taken to evaluate, characterise, and test various aspects and theories related to the occurrence of humeral fractures. The approach included a comparison of farm management and husbandry practices between farms with and without cases of spontaneous humeral fractures, an analysis of clinical pathology data, as well as gross, histologic, and histomorphometric findings (Wehrle-Martinez et al. 2023a, 2023b, 2024; Hunnam et al. 2024). Furthermore, Raman and Fourier transform infrared spectroscopy was used to analyse the chemical composition and quality of bone from cows with and without humeral fractures (Wehrle-Martinez et al. 2023c). Finally, the bone collagen and collagen crosslinks content in the humerus of cows with and without humeral fractures were measured to understand the association of copper with spontaneous humeral fractures (Wehrle-Martinez et al. 2022).
Osteoporosis as a key risk factor
The first question that needed to be answered was: what is the main condition or disease compromising bone strength in dairy cows with spontaneous humeral fractures? The working hypothesis was that these fractures resulted from cumulative skeletal deficits caused by inadequate bone formation and increased resorption, ultimately leading to osteoporosis.
Initial studies on a small sample size (10 bones from cows with humeral fractures and 10 without) suggested that cows with humeral fractures have osteoporosis (Dittmer et al. 2016). A subsequent study with a larger sample size examined 80 humeri and 47 costochondral junctions from affected 2-year-old first-lactation dairy cows (referred to as affected cows from now in this manuscript) and compared those results with 22 humeri and 17 costochondral junctions from non-affected, age- and lactation-matched dairy cows (referred to as control cows from now in this manuscript). The study confirmed that most first-lactation dairy cows (81%) with humeral fractures have abnormal bone architecture and density linked to osteoporosis (Wehrle-Martinez et al. 2023a, 2023c). Osteoporosis is characterised by significant reduction in bone mass, mineral content, and bone matrix, which drastically compromises bone quality and strength, potentially leading to fractures (Raisz 2005; Nanes and Kallen 2014; Sözen et al. 2017).
The causes of osteoporosis are numerous, so the next step was to identify those that could be associated with humeral fractures in affected dairy cows (Craig et al. 2016). The same large study by Wehrle-Martinez et al. (2023a) showed that the changes in the humeri were associated with two main processes leading to osteoporosis: decreased or irregular bone formation and increased bone resorption. These two are the primary processes that compromise bone quality and quantity in affected cows, weakening the biomechanical strength of the humerus and leading to catastrophic spontaneous fractures (Wehrle-Martinez et al. 2023a).
Findings in bones that indicate issues with bone formation in affected cows include:
Thicker growth plates with abnormal architecture in 61% of affected cows.
Presence of growth arrest lines in 21% of affected cows.
Decreased amount of trabecular bone in 81% of affected cows and abnormal trabecular architecture in 78% of affected cows.
Thin cortex (mean of 220.1 ± 106.2 μm in affected cows compared to 374.8 ± 154.4 μm in control cows).
Decreased bone area (P < 0.005), trabecular width (P = 0.03) and decreased trabecular perimeter (P = 0.027) in affected cows compared to controls.
These findings suggest that affected cows may have failed to reach peak bone mass. Growth arrest lines were present in 21% of cows with humeral fractures. These can form when bone growth temporarily stops due to factors such as systemic illness, major stress, trauma or nutritional deprivation, leaving a transverse bone trabecula. Nutritional deprivation due to protein or energy restriction (undernutrition or inadequate feed quality) is the most likely contributor to the formation of growth arrest lines, considering the histological and histomorphometry findings reported in affected cows (Wehrle-Martinez et al. 2023a).
Subsequent bone resorption and remodelling, both observed in many affected humeri, can lead to the disappearance of these lines, likely obscuring their true prevalence (Wehrle-Martinez et al. 2023a). While the presence of growth arrest lines in 21% of affected cows provides convincing evidence of interrupted bone growth, their absence in other affected cows does not rule out similar periods of growth disruption, as remodelling may have removed these lines.
The presence of growth arrest lines in the examined sections also suggests that the changes leading to inadequate bone formation occurred recently. In New Zealand, the second winter of a heifer’s life, leading up to calving at 2 years of age in the early spring, coincides with a period of lower pasture quality and quantity and the potential for reduced growth rates if additional supplementary feed is not provided (Handcock et al. 2019). Therefore, it is plausible that this seasonal challenge contributes to skeletal growth deficits in cows that graze on pasture (Gibson et al. 2024). However, this hypothesis has not been prospectively studied and requires further investigation.
It is important to acknowledge that not all rising 2-year-old dairy cows experience poor pasture conditions, as many are fed on crop-based diets during their second winter. A recent study by Wehrle-Martinez et al. (2023a) found that osteoporosis and bone changes indicative of decreased and irregular bone formation and increased bone resorption were present in rising 2-year-old dairy cows regardless of winter-feeding strategy, including those grazing on fodder beet and those on other diets (swedes, maize, silage oats, and/or kale). However, the impact of specific crop-based diets on bone quality and fracture risk remains unclear and requires further investigation.
Moreover, studies investigating the relationship between bovine live weight, stature, and bone morphology have provided further evidence on why the humerus might be at risk of fractures, particularly following recent events such as the second winter nutrition. Notably, the humerus continues its longitudinal and appositional growth even after the metacarpus has ceased growing at 1 year of age, making it more susceptible to growth issues in the second winter (Gibson et al. 2019; Gibson et al. 2022; Gibson et al. 2025). These findings agree with measures of stature, whereby 90% of height is achieved in the first year but girth continues to grow after 2 years of age (Gibson et al. 2022). A case-control study using peripheral computed tomography to compare bone morphology between affected and control cows found that affected cows had a significant reduction in cortical bone mineral density. Additionally, there was a trend towards reduced bone length, cortical content, total bone content, and stress-strain index, suggesting these results reflect a recent period of compromised humeral bone growth, such as can occur during the second winter for New Zealand dairy cows (Gibson et al. 2021).
The second process observed in the bones from affected cows was increased bone resorption (high bone turnover). Findings associated with this process in affected cows included:
Decreased bone area (P < 0.005), trabecular density (in 81% of affected cows), trabecular width (P = 0.03) and trabecular perimeter (P = 0.027) in affected cows compared to control cows.
Thin cortex (mean of 220.1 ± 106.2 μm in affected cows compared to 374.8 ± 154.4 μm in control cows) with increased abnormal resorption (81% of affected cows).
Decreased trabecular architecture (in 78% of affected cows).
Marked bone resorption in the distal humerus in 76% of affected cows.
Bone resorption is a normal physiological process that occurs in cows during gestation and lactation, mainly due to increased demands for calcium (Rowland et al. 1972; Horst et al. 2005). This process is a significant risk factor for spontaneous humeral fractures, considering the reported three fold increase in bone resorption during lactation (Rowland et al. 1972; Horst et al. 2005), and the greater bone resorption observed in first-lactation cows compared to multiparous cows (Liesegang et al. 1998; Kim et al. 2010). In cows with spontaneous humeral fractures, the amount of bone resorption was found to be excessive and likely occurred for a longer time than in control cows (Wehrle-Martinez et al. 2023a). This is supported by the presence of abnormal cortical resorption, fewer, thinner, and less connected bone trabeculae, as well as reduced cortical thickness and the presence of large resorption canals in the cortex, including the distal humerus in affected cows (Wehrle-Martinez et al. 2023a). This finding raises questions about potential metabolic or nutritional imbalances exacerbating bone loss.
Further evidence of compromised bone strength was observed in bones from affected cows using Raman and Fourier transform infrared spectroscopy (FTIR) (Wehrle-Martinez et al. 2023c). These techniques are useful for the evaluation of bone chemical composition and hence can provide information about bone quality (Compston 2006; Paschalis et al. 2017). Raman and FTIR band ratios measured and studied for the first time in pooled bones from affected and control cows showed that the proximal humerus from affected cows, on average, is immature and more recently formed compared to the same bone from control cows (Wehrle-Martinez et al. 2023c). The formation of this new bone could also be a compensatory response for poor bone strength due to osteoporosis (Wehrle-Martinez et al. 2023c).
Copper deficiency has been proposed as a contributing factor to spontaneous humeral fractures, but the evidence is complex. Low liver copper concentrations are commonly measured in affected cows and consequently, copper deficiency is frequently suggested as a cause of humeral fractures in dairy cows (Suttle and Angus 1978; Grace and Knowles 2010; Thompson et al. 2012). This led to a second research question: what is the role of copper in humeral fractures in 2-year-old first-lactation dairy cows?
A significant number of affected cows have low liver copper concentrations (up to 80% of affected cows) and/or low serum copper concentrations (up to 21% of affected cows) at the time of fracture (Thompson et al. 2012; Dittmer et al. 2016; Wehrle-Martinez et al. 2023b). These findings suggest that copper deficiency may be a contributing factor to the occurrence of fractures (Thompson et al. 2012; Dittmer et al. 2016). During bone formation, copper plays a central role by facilitating collagen synthesis and crosslinking, both of which contribute to the mechanical properties of bone (Viguet-Carrin et al. 2006; Saito and Marumo 2010).
The role of copper deficiency in the pathogenesis of spontaneous humeral fractures in affected cows was investigated by assessing the total collagen and its crosslink content in the humerus of affected cows compared to control cows (Wehrle-Martinez et al. 2022). This study aimed to determine if there was a strong link between low liver copper concentrations and fracture risk, and to identify why affected cows have low liver copper concentrations. Twenty-six humeri from affected cows and 14 humeri from control cows were processed, and the total collagen and collagen crosslink content were quantified using mass spectrometry and the hydroxyproline assay, respectively (Wehrle-Martinez et al. 2022). This novel study showed that the low liver copper concentrations found in affected cows are likely the result of the mobilisation of stored copper (from the liver) to the bone. This was evidenced by the association of higher bone copper concentration (mean of 0.69 ± 0.27 mg/kg in affected cows compared to 0.41 ± 0.12 mg/kg in control cows; mean ± s.d.) and total collagen crosslink content (mean of 3979.07 ± 797.72 mg/mol in affected cows compared to 3058.71 ± 548.70 mg/mol in control cows; mean ± s.d.) observed in affected cows. More importantly, the lower total collagen content in humeri from affected cows (mean of 1.71 ± 0.43 mg in affected cows compared to 2.18 ± 0.46 mg in control cows; mean ± s.d.) could also evidence that periods of protein and/or energy deficiency during important growth periods are a significant contributory factor in the pathogenesis of spontaneous humeral fractures (Wehrle-Martinez et al. 2022). Protein provides the necessary amino acids for collagen synthesis, a key component of the bone matrix (Babraj et al. 2005), while sufficient caloric intake ensures the energy required for bone modelling and remodelling (Karsenty and Oury 2010). Although it is expected that bones with osteoporosis will have overall lower collagen content, these findings underscore the need for further research into the interplay between nutrition, copper metabolism, and bone integrity.
Liver samples from the same 26 affected and 14 control cows used for collagen and collagen crosslink quantification were analysed to determine the concentration of cadmium, iron, molybdenum and zinc, which are known to be antagonists of copper absorption (Grace and Knowles 2010). No significant accumulation of these were found in the liver of affected cows, suggesting a minimal antagonist impact to the liver copper concentrations (Wehrle-Martinez et al. 2022).
In recent years, a significant increase in fluoride concentrations in New Zealand topsoils due to the use of phosphate fertilisers has possibly resulted in an increase in ingestion of fluoride by grazing animals (Healy 1968; Hedley et al. 2007; Loganathan et al. 2008; Kim et al. 2016). We hypothesised that the use of phosphate fertilisers could have led to the accumulation of toxic amounts of fluoride in the bones of affected cows, significantly contributing to the decline in bone quality and strength (Ranjan and Ranjan 2015). This led to a third research question: Is fluoride toxicity a contributing factor to humeral fractures?
Bone fluoride concentration was measured in 35 affected cows and 21 control bones. The study concluded that although affected cows had significantly higher bone fluoride concentrations compared with control cows, the concentration remained well below the thresholds that cause significant adverse effects on bone (Grace 2010), making its role in fracture risk uncertain (Wehrle-Martinez et al. 2025). However, fluoride is reported to reduce collagen synthesis and collagen crosslink formation (Susheela and Mukerjee 1981; Ranjan and Ranjan 2015). While low total bone collagen is described in affected cows, there were no low collagen crosslinks, making bone fluoride concentration a less likely contributing risk factor in the pathogenesis of spontaneous humeral fractures.
The final question investigated whether there were any farm management and husbandry practices that were associated with humeral fractures. To do this, a case-control study approach was used to compare farm management and husbandry practices between farms across New Zealand with and without humeral fractures, using a farm-based questionnaire. To maximise the response rate, the authors employed diverse methods for questionnaire distribution (Wehrle-Martinez et al. 2024). Despite these efforts, responses were received from only 0.6% of dairy farms in New Zealand (68/11,179 farms for the 2019/2020 season), based on data from LIC, DairyNZ (2021). This was a major limitation of the study, as the low response rate may have introduced bias. The survey results suggested that Holstein-Friesian Jersey crossbred cows (KiwiCross) may be at higher risk of spontaneous humeral fractures. While fractures have also been reported in Holstein-Friesian, Jersey, and Ayrshire breeds, KiwiCross cows are the predominant breed in the New Zealand dairy herd, making up a significant proportion of the national herd (Wehrle-Martinez et al. 2024a). It is unclear whether this increased risk is due to breed susceptibility or simply reflects the higher proportion of crossbred cows in the national herd.
The survey also indicated that delaying calves’ access to pasture for a week after weaning appeared to reduce fracture risk (Wehrle-Martinez et al. 2024a). However, the specific reasons why some farms allowed or restricted calves’ access to pasture were not surveyed but are likely influenced by factors such as nutritional management and environmental conditions. Notably, case farms in the study were generally located further north, where calves may be placed on pasture earlier due to better weather. This regional variation could contribute to differences in the duration of milk feeding and may have implications for growth and health outcomes.
Is it important to note that while these findings offer preliminary insights, the study was underpowered to identify definitive risk factors and was limited by potential response bias. Furthermore, the proportion of fracture cases explained by the identified risk factors was not assessed, leaving significant gaps in our understanding of this condition. As highlighted by Hunnam et al. (2024), further research is urgently needed to clarify the aetiology and contributing factors of humeral fractures in dairy heifers.
Proposed pathogenesis of spontaneous humeral fractures
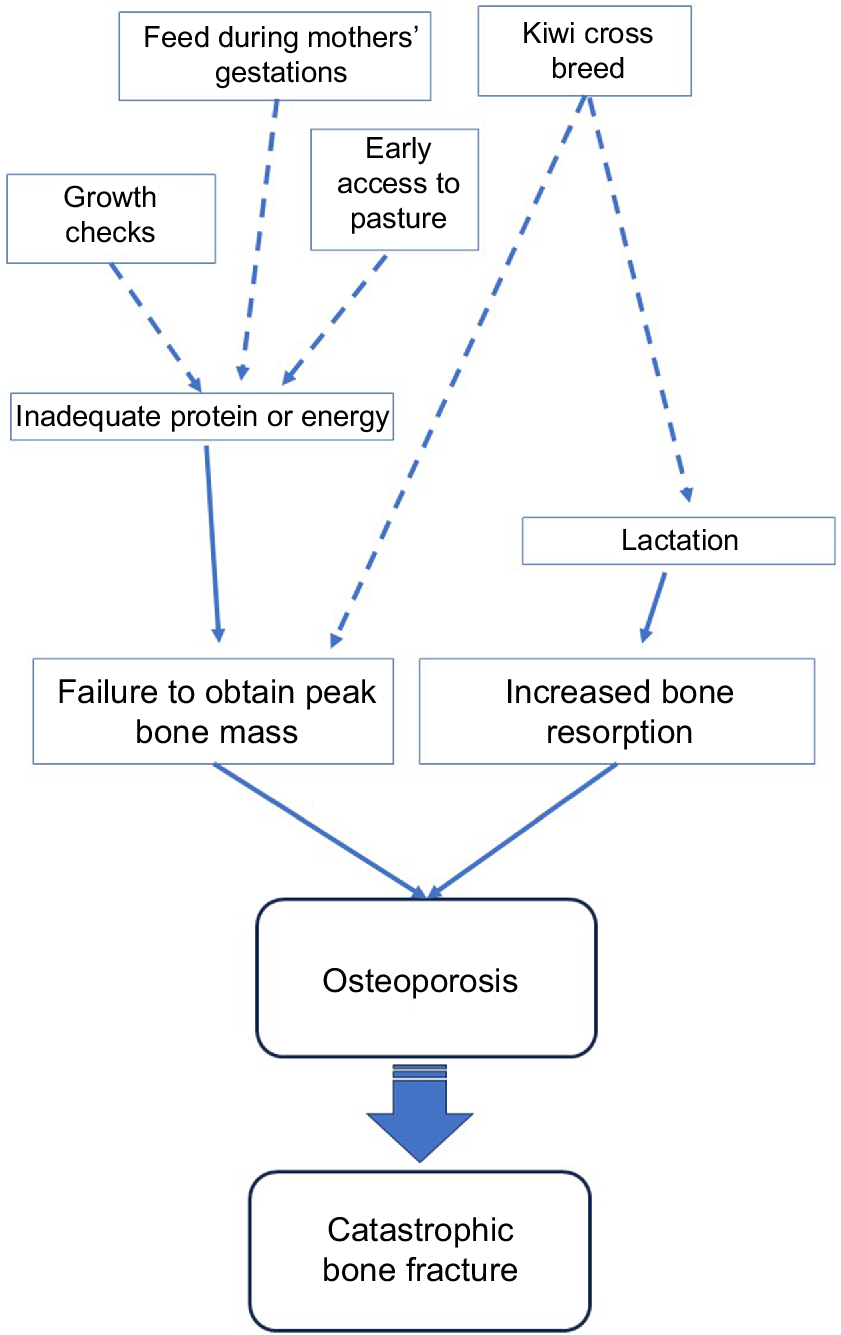
Current research evidence into the multiple factors potentially associated with the pathogenesis of spontaneous humeral fractures in first-lactation dairy cows from New Zealand strongly suggests that these fractures potentially result from multifactorial influences, with osteoporosis being the underlying pathology. Two primary factors have been identified:
periods of inadequate energy and/or protein nutrition, which prevent obtaining adequate bone mass, and
increased bone resorption associated with lactation (Fig. 3).
Ultimately these compromise bone quality and strength, predisposing cows to osteoporosis and bone fracture. Other risk factors (dotted lines, Fig. 3) may also influence energy/protein nutrition and/or bone resorption, but their precise roles require further investigation.
Proposed pathogenesis of catastrophic spontaneous humeral fractures in first-lactation cows from New Zealand. Solid arrows arise from primary factors identified in the pathogenesis of spontaneous humeral fractures, whereas dotted lines represent hypothesised effects that have not yet been confirmed through controlled research.
Significant gaps remain in our understanding of the pathogenesis of spontaneous humeral fractures in first-lactation dairy cows, particularly regarding why some farms experience frequent cases while others do not. Further epidemiological studies are needed to explore potential genetic predispositions, regional differences in nutrition, and management practices that influence bone health.
Data availability
The data supporting this review’s statements are available in the published articles cited within this manuscript. These articles can be accessed through their respective publishers.
Conflicts of interest
Penny Back and Chris Rogers are Associate Editors of Animal Production Science. To mitigate this potential conflict of interest, they had no editor-level access to this manuscript during peer review. The authors have no further conflicts of interest to declare.
Acknowledgements
The authors would like to thank all the farmers and veterinarians for sample collection and participation in completing questionaries.
References
Babraj JA, Smith K, Cuthbertson DJR, Rickhuss P, Dorling JS, Rennie MJ (2005) Human bone collagen synthesis is a rapid, nutritionally modulated process. Journal of Bone and Mineral Research 20(6), 930-937.
| Crossref | Google Scholar | PubMed |
Bouza-Rodríguez JB, Miramontes-Sequeiros LC (2014) Three-dimensional biomechanical analysis of the bovine humerus. Applied Bionics and Biomechanics 11(1–2), 13-24.
| Crossref | Google Scholar |
Compston J (2006) Bone quality: what is it and how is it measured? Arquivos Brasileiros de Endocrinologia e Metabologia 50(4), 579-585.
| Crossref | Google Scholar | PubMed |
Crawford WH, Fretz PB (1985) Long bone fractures in large animals a retrospective study. Veterinary Surgery 14(4), 295-302.
| Crossref | Google Scholar |
Dittmer KE, Hitchcock B, McDougall S, Hunnam JC (2016) Pathophysiology of humeral fractures in a sample of dairy heifers. New Zealand Veterinary Journal 64(4), 230-237.
| Crossref | Google Scholar | PubMed |
Gangl M, Grulke S, Serteyn D, Touati K (2006) Retrospective study of 99 cases of bone fractures in cattle treated by external coaptation or confinement. Veterinary Record 158(8), 264-268.
| Crossref | Google Scholar | PubMed |
Gibson MJ, Rogers CW, Dittmer K, Hickson RE, Pettigrew EJ, Back PJ (2019) Can bone measures of the bovine metacarpus predict humeral bone structure? New Zealand Journal of Animal Science and Production 79, 8-12.
| Google Scholar |
Gibson M, Dittmer K, Hickson R, Back P, Wehrle-Martinez A, Rogers C (2021) The mid-diaphysis is a poor predictor of humeral fracture risk indicating that predisposing factors are recent. Ruminants 1(1), 23-30.
| Crossref | Google Scholar |
Gibson MJ, Adams BR, Back PJ, Hickson RE, Dittmer KE, Rogers CW (2022) Live weight and bone growth from birth to 23 months of age in Holstein–Friesian, Jersey and Crossbred heifers. Dairy 3(2), 333-344.
| Crossref | Google Scholar |
Gibson MJ, Sneddon NW, Rogers CW, Back PJ, Dittmer KE, Martín NP (2025) The relationship between stature and live weight of dairy cows between birth and maturity. Ruminants 5(1), 7.
| Crossref | Google Scholar |
Handcock RC, Lopez-Villalobos N, McNaughton LR, Back PJ, Edwards GR, Hickson RE (2019) Live weight and growth of Holstein-Friesian, Jersey and crossbred dairy heifers in New Zealand. New Zealand Journal of Agricultural Research 62(2), 173-183.
| Crossref | Google Scholar |
Healy WB (1968) Ingestion of soil by dairy cows. New Zealand Journal of Agricultural Research 11(2), 487-499.
| Crossref | Google Scholar |
Horst RL, Goff JP, Reinhardt TA (2005) Adapting to the transition between gestation and lactation: differences between rat, human and dairy cow. Journal of Mammary Gland Biology and Neoplasia 10(2), 141-156.
| Crossref | Google Scholar | PubMed |
Hunnam JC, Lawrence K, Rashid ZBA, Hitchcock B, McDougall S, Wehrle-Martinez A, Weston JF (2024) An assessment of the epidemiology and herd-level impact of the fractured humerus epidemic in New Zealand dairy cattle, 2007–2015: results from four studies. Animals 14(3), 524.
| Crossref | Google Scholar | PubMed |
Jean GS, Anderson DE (2014) Decision analysis for fracture management in cattle. Veterinary Clinics of North America: Food Animal Practice 30(1), 1-10.
| Crossref | Google Scholar |
Karsenty G, Oury F (2010) The central regulation of bone mass, the first link between bone remodeling and energy metabolism. The Journal of Clinical Endocrinology & Metabolism 95(11), 4795-4801.
| Crossref | Google Scholar | PubMed |
Kim D, Yamagishi N, Ueki A, Miura M, Saito F, Sato S, Furuhama K (2010) Changes in plasma bone metabolic markers in periparturient dairy cows. Journal of Veterinary Medical Science 72(6), 773-776.
| Crossref | Google Scholar | PubMed |
Kim ND, Taylor MD, Drewry JJ (2016) Anthropogenic fluorine accumulation in the Waikato and Bay of Plenty regions of New Zealand: comparison of field data with projections. Environmental Earth Sciences 75, 147.
| Crossref | Google Scholar |
Kushwaha RB, Gupta AK, Bhadwal MS, Kumar S, Tripathi AK (2011) Incidence of fractures and their management in animals: a clinical study of 77 cases. Indian Journal of Veterinary Surgery 32(1), 54-56.
| Google Scholar |
Liesegang A, Sassi M-L, Risteli J, Eicher R, Wanner M, Riond J-L (1998) Comparison of bone resorption markers during hypocalcemia in dairy cows. Journal of Dairy Science 81(10), 2614-2622.
| Crossref | Google Scholar | PubMed |
Loganathan P, Hedley M, Grace ND (2008) Pasture soils contaminated with fertilizer-derived cadmium and fluorine: livestock effects. Reviews of Environmental Contamination and Toxicology 192, 29-66.
| Crossref | Google Scholar | PubMed |
Lopez-Villalobos N, Jayawardana JMDR, McNaughton LR, Hickson RE (2023) A review of once-a-day milking in dairy cow grazing systems. JDS Communications 4(4), 329-333.
| Crossref | Google Scholar | PubMed |
Loughnan T (2012) A case of copper deficiency in heifers with pathological fractures. The Australian Cattle Veterinarian 2012(65), 16-17.
| Google Scholar |
Luo J, Ledgard S (2021) New Zealand dairy farm systems and key environmental effects. Frontiers of Agricultural Science and Engineering 8(1), 148-158.
| Crossref | Google Scholar |
Ministry for Primary Industries (2024) Surveillance. Ministry for Primary Industries, Wellington, New Zealand. Available at https://www.sciquest.org.nz/browse/publications/view/106
Mulon P-Y (2013) Management of long bone fractures in cattle. InPractice 35(5), 265-271.
| Crossref | Google Scholar |
Nanes MS, Kallen CB (2014) Osteoporosis. Seminars in Nuclear Medicine 44(6), 439-450.
| Crossref | Google Scholar | PubMed |
Paschalis EP, Gamsjaeger S, Klaushofer K (2017) Vibrational spectroscopic techniques to assess bone quality. Osteoporosis International 28(8), 2275-2291.
| Crossref | Google Scholar | PubMed |
Raisz LG (2005) Pathogenesis of osteoporosis: concepts, conflicts, and prospects. Journal of Clinical Investigation 115(12), 3318-3325.
| Crossref | Google Scholar | PubMed |
Rakestraw PC (1996) Fractures of the humerus. Veterinary Clinics of North America: Food Animal Practice 12(1), 153-168.
| Crossref | Google Scholar | PubMed |
Rowland GN, Capen CC, Young DM, Black HE (1972) Microradiographic evaluation of bone from cows with experimental hypervitaminosis D, diet-induced hypocalcemia, and naturally occurring parturient paresis. Calcified Tissue Research 9(1), 179-193.
| Crossref | Google Scholar |
Saito M, Marumo K (2010) Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporosis International 21(2), 195-214.
| Crossref | Google Scholar | PubMed |
Soutter T (2019) NEW: an introduction to dairy – New Zealand. CZ insights. Available at https://www.czapp.com/analyst-insights/new-an-introduction-to-dairy-new-zealand/
Sözen T, Özışık L, Başaran NC (2017) An overview and management of osteoporosis. European Journal of Rheumatology 4(1), 46-56.
| Crossref | Google Scholar | PubMed |
Stats NZ (2024) Growth in export markets for New Zealand milk powder. Available at https://www.stats.govt.nz/news/growth-in-export-markets-for-new-zealand-milk-powder/
Susheela AK, Mukerjee D (1981) Fluoride poisoning and the effect on collagen biosynthesis of osseus and non-osseus tissues of rabbit. Toxicological European Research 3(2), 99-104.
| Google Scholar | PubMed |
Suttle NF, Angus KW (1978) Effects of experimental copper deficiency on the skeleton of the calf. Journal of Comparative Pathology 88(1), 137-148.
| Crossref | Google Scholar | PubMed |
Van der Zeijden M, Ellis A, Lopez-Villalobos N, Li S, Roy NC, McNabb W (2023) The protein composition of bovine milk from once-a-day and twice-a-day milking production systems in New Zealand. Dairy 4(4), 689-703.
| Crossref | Google Scholar |
Viguet-Carrin S, Garnero P, Delmas PD (2006) The role of collagen in bone strength. Osteoporosis International 17(3), 319-336.
| Crossref | Google Scholar | PubMed |
Wehrle-Martinez A, Naffa R, Back P, Rogers CW, Lawrence K, Loo T, Sutherland-Smith A, Dittmer K (2022) Novel assessment of collagen and its crosslink content in the humerus from primiparous dairy cows with spontaneous humeral fractures due to osteoporosis from New Zealand. Biology 11(10), 1387.
| Crossref | Google Scholar | PubMed |
Wehrle-Martinez A, Lawrence K, Back PJ, Rogers CW, Gibson M, Dittmer KE (2023a) Osteoporosis is the cause of spontaneous humeral fracture in dairy cows from New Zealand. Veterinary Pathology 60(1), 88-100.
| Crossref | Google Scholar | PubMed |
Wehrle-Martinez A, Dittmer KE, Back PJ, Rogers CW, Lawrence K (2023b) Biochemical profile of heifers with spontaneous humeral fractures suggest that protein-energy malnutrition could be an important factor in the pathology of this disease. New Zealand Veterinary Journal 71(1), 37-41.
| Crossref | Google Scholar | PubMed |
Wehrle-Martinez A, Waterland MR, Naffa R, Lawrence K, Back PJ, Rogers CW, Dittmer K (2023c) Bone quality changes as measured by Raman and FTIR spectroscopy in primiparous cows with humeral fracture from New Zealand. Frontiers in Veterinary Science 10, 106342.
| Crossref | Google Scholar |
Wehrle-Martinez A, Lawrence KE, Back PJ, Rogers CW, Dittmer KE (2024) Farm management and husbandry practices associated with spontaneous humeral fractures in New Zealand dairy heifers. New Zealand Veterinary Journal 72(2), 96-102.
| Crossref | Google Scholar | PubMed |
Wehrle-Martinez A, Dittmer KE, Back PJ, Rogers CW, Weston JF, Jeyakumar P, Pereira RV, Poppenga R, Taylor HS, Lawrence KE (2025) The association between fluoride concentrations and spontaneous humeral fracture in first-lactation dairy cows: results from two New Zealand studies. New Zealand Veterinary Journal 73(2), 143-147.
| Crossref | Google Scholar |
Williams NJ, Streeter RN (2022) Nonpathological phalangeal fractures in cattle: 17 cases (2004–2020). Journal of the American Veterinary Medical Association 260(3), 350-356.
| Crossref | Google Scholar |
Yadav GP, Sangwan V, Kumar A (2019) Comparative occurrence pattern of fractures in cattle and buffaloes. Veterinary World 12(7), 1154-1159.
| Crossref | Google Scholar | PubMed |


