Influence of temperature–humidity index and seasonal variations on semen quality, scrotal circumference, and hormones in Kail rams raised under subtropical climate
Nasir Hameed A B , Muhammad Zubair B , Nasim Ahmad A , Aneela Zameer Durrani C and Muhammad Irfan-ur-Rehman Khan
A B , Muhammad Zubair B , Nasim Ahmad A , Aneela Zameer Durrani C and Muhammad Irfan-ur-Rehman Khan  A *
A *
A
B
C
Abstract
Seasonality plays a vital part in determining the profitability of ovine farms, particularly in tropical and subtropical regions, where environmental factors influence reproductive efficiency. Understanding seasonal variations in semen quality, testicular parameters, and hormonal profiles is essential for optimizing breeding strategies in Kail rams.
The purpose of this study was to evaluate monthly variations in semen quality, scrotal circumference (SC), testicular echogenicity (TE), and plasma testosterone and melatonin concentrations in adult Kail rams.
Five adult Kail rams (2–3 years old) were monitored over 1 year. Semen was collected thrice monthly, testicular ultrasonography was performed, and blood samples were taken monthly. Semen parameters, SC, TE, and hormone concentrations were analyzed in relation to environmental variations, including temperature–humidity index (THI).
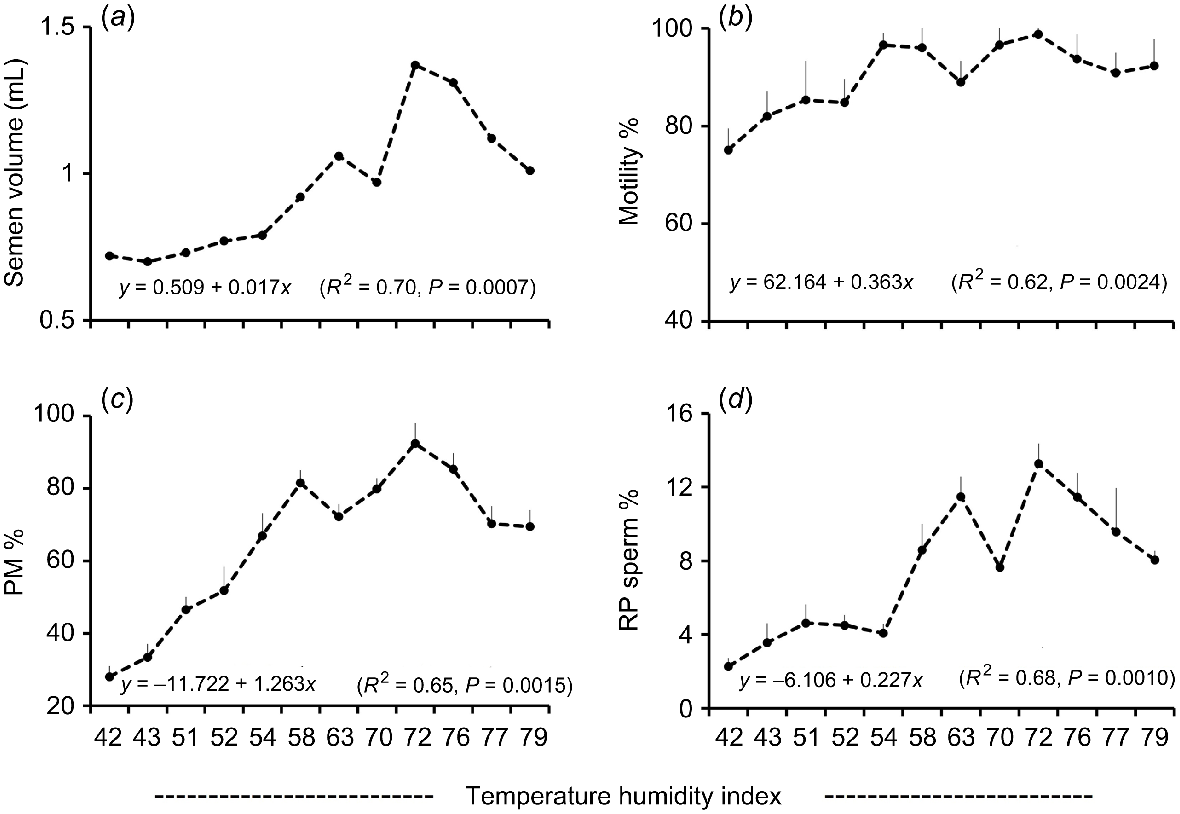
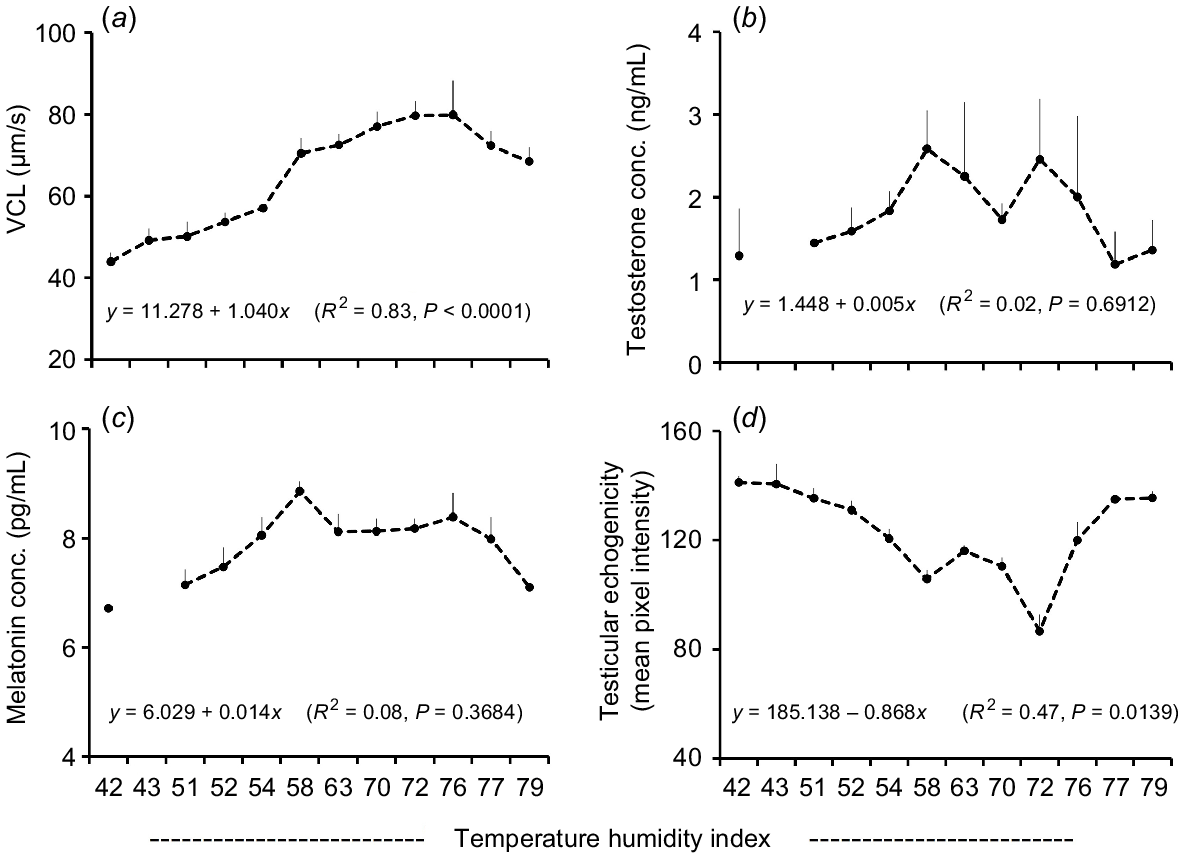
Semen volume (0.7 ± 0.03 mL) and SC (25.1 ± 0.6 cm) were lowest in January, peaking in September and July respectively. In December, sperm motility and kinematics were at their lowest. TE values were lowest in September and highest in January. No significant monthly variations were observed in testosterone and melatonin concentrations. Semen volume (R2 = 0.70), sperm total motility (R2 = 0.62), progressive motility (R2 = 0.65), rapid sperm (R2 = 0.68), curvilinear velocity (R2 = 0.83) and TE (R2 = 0.47) correlated significantly with THI. TE negatively correlated with sperm motility, kinematics, SC, and plasma testosterone and melatonin concentrations, whereas SC showed a positive correlation with sperm-quality parameters. No significant association was found between SC and hormonal concentrations.
Seasonal variations significantly influenced sperm motility, kinematics, SC, and TE, whereas testosterone and melatonin concentrations remained unaffected. The optimal THI range for semen production was 58–72.
These findings have highlighted the importance of seasonal reproductive management in tropical and subtropical ovine farming systems. TE could serve as a useful predictor of sperm quality, whereas SC may be a positive indicator of reproductive performance in Kail rams.
Keywords: hormones, Kail, scrotal circumference, seasonality, semen quality, testicular echogenicity, testosterone, THI.
Introduction
Seasonality plays a vital part in determining the profitability of ovine farms, particularly in tropical and subtropical regions. Although the seasonal influence on ram reproductive performance is often less evident than in ewes (Kridli et al. 2018), various factors can significantly influence ram semen quality. These factors include breed (Zamiri et al. 2010), photoperiod (Delgadillo et al. 2009), nutrition (Kafi et al. 2004), humidity (Malejane et al. 2014), and heat stress (Cárdenas-Gallegos et al. 2012). Additionally, latitudinal position affects testicular volume and semen output in rams (Blache et al. 2006).
Seasonal fluctuations influence several of ram’s reproductive characteristics, including scrotal circumference, sperm morphology, libido, and mating capacity. Some studies have reported significant seasonal variations in semen quality among rams in tropico–subtropical zones (Santos et al. 2015; Belkadi et al. 2017), whereas others have not (Malejane et al. 2014; Benmoula et al. 2017). Importantly, it seems that breeds differ in how the season influences semen quality (Oláh et al. 2013). Rams of different breeds, such as those from Morada Nova and Santa Ines (Kahwage et al. 2018), Chios (Ntemka et al. 2019), and Saint Croix (Sanchez-Davila et al. 2020) have shown negligible seasonal influence on the quality of their semen. Conversely, significant seasonal influences have been observed in Ghezel × Baluchi and Arkhar Merino × Ghezel rams (Moghaddam et al. 2012), as well as in other breeds such as Dorper (Malejane et al. 2014), Ouled Djellal (Belkadi et al. 2017), Zulu (Ngcobo et al. 2020), and Ile-de-France (Mamontova et al. 2021). Furthermore, the Assaf breed has shown better tolerance to adverse environmental conditions than has the Awassi breed (Zaher et al. 2020). Despite these findings, there is limited information on the reproductive patterns of native sheep breeds, highlighting the need for further research.
Seasonal variations in plasma testosterone and melatonin concentrations have also been explored in various sheep breeds. Both hormones exhibit marked seasonal variations, with lower concentrations during the off-season and higher concentrations during the breeding season, which influences fertility (Casao et al. 2010). Whereas melatonin concentration is primarily affected by season, both breed and season influence plasma testosterone concentrations in rams (Carvajal-Serna et al. 2019). Significant variations in plasma testosterone concentrations in relation to seasonal changes have been reported across different ram breeds (Mostafa and Farghal 2019). Furthermore, seasonal variations in testicular echogenicity and their association with semen quality have been assessed in various sheep breeds (Ntemka et al. 2018; Hedia et al. 2020; El-Shalofy et al. 2022).
The influence of heat stress on ram semen quality (Küçük and Aksoy 2020; Molina et al. 2024) and bull semen quality has been evaluated, documenting optimal temperature–humidity index (THI) ranges for semen production, depending on climatic conditions (Taylor et al. 1985; Fuerst-Waltl et al. 2006; Farooq et al. 2013). However, no study has documented the monthly variations in semen quality, testicular echogenicity, plasma concentrations of testosterone and melatonin, and the optimal THI range for rams in subtropical climates. Therefore, this study aims to evaluate the effect of monthly variations on semen quality, testicular echogenicity, and plasma concentrations of testosterone and melatonin, and to establish the correlation between testicular echogenicity, THI, and semen quality in Kail rams raised under a subtropical climate.
Materials and methods
Animals and location
Adult Kail rams (n = 5; age, 2–3 years; BCS, 3.5 ± 0.1) were used in this study. The Kail is a medium-sized sheep breed native to the Neelam and Leepa valleys of Azad Jammu and Kashmir (AJK), Pakistan. Adult rams average 41 kg in bodyweight and are characterized by a predominant white coat, with occasional black or brown head markings, medium ears, and a Roman nose. The breed is dual-purpose, raised for both meat and wool (fiber diameter, ~31 μm). All rams were housed at the experimental station of the University of Poonch (33.8584°N, 73.7654°E), Rawalakot, AJK, under a semi-intensive system. This included 6 h of grazing and access to water ad libitum. In winter, rams were provided hay feed alongside grazing and 400 g of concentrate consisting of soybean meal, canola meal, corn gluten and kernels, wheat bran, and maize grain. The Ethical Review Committee of the University of Veterinary and Animal Sciences, Lahore-Pakistan, approved all experimental protocols.
Meteorological data records
Monthly average of day length, relative humidity, temperature, THI, and day length from January to December 2022 are shown in Table 1. Seasons were defined as follows: winter: November to February; spring: March to 15 May; summer: 16 May to August; and autumn: September to October.
By using a previously published formula (Urie et al. 2018), THI was determined as follows:
where RH is the relative air humidity (%) and T is the dry bulb air temperature (°F).
Potential heat stress faced by the rams was assessed using the model outlined previously, which classifies THI values as follows: <72 (no stress), 72–78 (mild stress), 79–88 (moderate stress), and 89–98 (severe stress) (Armstrong 1994).
| Month | Temperature (°C) | Relative humidity (%) | THI | Day length | |
|---|---|---|---|---|---|
| January | 7.7 ± 2.3 | 59.2 ± 11.8 | 43.2 | 10 h 10 min | |
| February | 11.1 ± 2.7 | 68.9 ± 9.1 | 50.9 | 10 h 57 min | |
| March | 12.6 ± 2.4 | 62.9 ± 14.8 | 54.0 | 11 h 58 min | |
| April | 14.4 ± 3.1 | 56.3 ± 18.5 | 58.0 | 13 h 1 min | |
| May | 19.4 ± 2.4 | 34 ± 8.4 | 70.2 | 13 h 54 min | |
| June | 23.2 ± 2.8 | 38.6 ± 14.3 | 79.2 | 14 h 20 min | |
| July | 23.2 ± 1.8 | 65.8 ± 14.7 | 76.9 | 14 h 7 min | |
| August | 22.1 ± 1.1 | 48.4 ± 7.8 | 75.8 | 13 h 20 min | |
| September | 20.9 ± 1.0 | 70.4 ± 6.7 | 71.6 | 12 h 20 min | |
| October | 16.9 ± 3.5 | 65.9 ± 11.8 | 63.4 | 11 h 18 min | |
| November | 11.8 ± 1.3 | 70.9 ± 4.8 | 52.5 | 10 h 24 min | |
| December | 7.2 ± 2.5 | 61.5 ± 13 | 42.2 | 9 h 56 min |
THI, temperature–humidity index.
Semen processing and assessment
Semen from each ram (n = 5) was collected three times a month at 10-day intervals throughout 2022 via artificial vagina method (42°C). After measuring the volume of each ejaculate in a graduate collection vial, ejaculates of all five rams were pooled to mitigate individual variations. In total, 36 pooled ejaculates (3 pooled ejaculates per month × 12 months) were evaluated during this study. A computer-assisted sperm analyzer (CASA; SCA®, version 5.1, Microptic S.L., Spain) was used to evaluate the kinematics and motility of the sperm. In accordance with the manufacturer’s instructions, the CASA system made use of parameters that had already been verified (Hameed et al. 2023). These included the particle area: 20–60 μm2, with a drift of 5 μm/s for slow, 10 μm/s for medium, and >25 μm/s for rapid sperm motion at 37°C. A connectivity threshold of 10 was applied to identify a valid particle. Sperm total motility (TM), progressive motility (PM), rapid progressive (RP), and medium progressive (MP) motility were analyzed. Sperm motion kinematics including curvilinear velocity (VCL), average path velocity (VAP), straight-line velocity (VSL), amplitude of lateral head displacement (ALH), and beat cross frequency (BCF) were also analyzed. At least five different fields were observed and 500 sperm were assessed by placing a 5 μL drop of pooled semen on a pre-warmed (37°C) glass slide.
Blood collection and hormonal assays
To estimate plasma concentrations of testosterone and melatonin, monthly blood samples from five Kail rams were collected in EDTA vacutainers. Blood samples were centrifuged for 15 min at 3000g to harvest plasma. All samples were stored at −20°C until further analysis.
Plasma testosterone concentration was determined using a double-antibody radioimmunoassay (Immunotech, Beckman Coulter®, Czech Republic). With a minimum sensitivity of 0.04 ng/mL, the intra-assay and inter-assay coefficients of variation were 10.5% and 19% respectively. Melatonin plasma concentrations were measured using an enzyme-linked immunosorbent assay (ELISA)-based goat specific kit (KS18271, China) (Abbas et al. 2021). The intra-assay and inter-assay coefficients of variation in melatonin assay were 7% and 10% respectively.
Scrotal circumference (SC) and testicular echogenicity (TE)
Scrotal circumference (SC) was measured monthly in five rams over a period of 12 months by using a flexible scrotal tape (Barth tape). The testes were gently pulled to the bottom of the scrotum by grasping from neck and aligned side by side. The tape was snugly placed around the widest point of the scrotum without applying excess pressure and measurements were recorded in centimeters to ensure consistency and accuracy. Testicular echogenicity (TE) of both testes in each ram was assessed monthly for 12 months by using real-time B-mode ultrasound with a 7.5 MHz linear transducer (DRAMINSKI iScan®, Poland). Testicular ultrasonography was performed with the ram restrained in a standing position without sedatives. The transducer was placed parallel to the longitudinal axis of the testicle, scanning both the right and left testicles separately. The mediastinum of the testis served as a hyperechoic landmark for identifying the desired section of testicular parenchyma for pixel intensity (PI) calculation.
All testicular images were recorded at the same depth and focal point and then exported to a computer for further analysis. PI of each testicular sonogram was computed via Image J® software (version 1.46r; see https://ij.imjoy.io, National Institutes of Health, MD, USA) (Amjad et al. 2021). Six randomly placed squares of 2 mm size were selected on the testicular parenchyma, excluding the mediastinum. The PI of the image was calculated by averaging the estimates in six squares.
Statistical testing
All variables are expressed as means ± s.e.m. The influence of season and month on semen volume, quality, SC, TE, and plasma concentrations of testosterone and melatonin was analyzed through one-way ANOVA. To compare the months and seasons, Bonferroni’s posthoc test was employed. The association of THI with semen quality, hormones, and TE was evaluated using a linear regression model. The relationship between testicular echogenicity, and SC with semen picture and plasma concentration of testosterone and melatonin was analyzed using Pearson’s correlation coefficient. Statistical package SPSS (ver. 20.0, IBM Corporation, Armonk, NY, USA) was used to analyze the data, and statistical significance was considered at P ≤ 0.05.
Results
Seasonal and monthly variations in semen volume and SC
Semen volume peaked in autumn and summer (1.2 ± 0.15 and 1.15 ± 0.09 respectively), with the lowest value observed in winter (0.73 ± 0.01). Scrotal circumference followed a similar trend, being significantly larger in summer (34.1 ± 0.38) than in other seasons, with the smallest values recorded in winter (26.6 ± 0.51; Table 2). The influence of months on semen volume and SC is shown in Table 3. Semen volume remained statistically stable (P > 0.05) from January to May. It increased (P < 0.05) from June through September compared with the January–May interval, then declined (P < 0.05) from October to December, returning to levels statistically similar to those observed earlier in the year. Scrotal circumference showed no significant (P > 0.05) change from January to May, then increased (P < 0.05) during June to August compared with January–March and December). Values declined from September onward, with those in November and December being significantly lower than those in the summer peak.
| Variable | Autumn | Spring | Summer | Winter | |
|---|---|---|---|---|---|
| Semen volume (mL) | 1.2 ± 0.1a | 0.9 ± 0.1ab | 1.15 ± 0.1a | 0.7 ± 0.0b | |
| Motility (%) | 93.0 ± 3.0ab | 96.0 ± 0.0a | 93.6 ± 1.2a | 82.5 ± 2.9b | |
| PM (%) | 82.5 ± 9.5a | 75.3 ± 4.2a | 74.6 ± 6.2a | 41.0 ± 6.0b | |
| RP (%) | 13.5 ± 0.5a | 7.0 ± 1.5bc | 10.3 ± 1.2ab | 4.2 ± 0.5c | |
| MP (%) | 70.5 ± 9.5a | 65.3 ± 0.3a | 61.7 ± 6.7ab | 38.5 ± 4.7b | |
| VCL (μm/s) | 72.5 ± 2.5a | 65.0 ± 2.9ab | 75.0 ± 0.0a | 54.2 ± 3.6b | |
| VAP (μm/s) | 40.0 ± 2.0a | 36.0 ± 1.5ab | 41.3 ± 0.7a | 32.0 ± 1.2b | |
| VSL (μm/s) | 25.0 ± 2.0a | 20.0 ± 1.5ab | 23.0 ± 1.0ab | 18.0 ± 0.7b | |
| ALH (μm) | 3.45 ± 0.1ab | 3.5 ± 0.2a | 3.4 ± 0.0ab | 2.9 ± 0.1b | |
| BCF (Hz) | 6.3 ± 0.8ab | 4.7 ± 0.4bc | 6.5 ± 0.3a | 3.2 ± 0.1c | |
| Scrotal circumference (cm) | 30.2 ± 0.0b | 29.7 ± 0.1b | 34.1 ± 0.4a | 26.6 ± 0.5c | |
| Testicular echogenicity | 102.5 ± 12.50b | 120.0 ± 5.8ab | 130.0 ± 5.0ab | 137.5 ± 3.2a | |
| Testosterone (ng/mL) | 1.8 ± 0.3a | 2.1 ± 0.2a | 1.9 ± 0.3a | 1.5 ± 0.1a | |
| Melatonin (pg/mL) | 8.0 ± 0.05a | 8.3 ± 0.3a | 7.8 ± 0.3a | 7.1 ± 0.2a |
Values with different letters within a row are statistically significantly different among seasons (at P = 0.05), determined using one-way ANOVA, followed by Tukey’s posthoc test. Seasons are defined as winter (November–February), spring (March−15 May), summer (16 May–August), and autumn (September–October). VCL, curvilinear velocity, VAP, average path velocity, VSL, straight-line velocity, ALH, amplitude of lateral head displacement, BCF, beat-cross frequency.
| Month | Semen volume (mL) | Scrotal circumference (cm) | |
|---|---|---|---|
| January | 0.70 ± 0.03a | 25.1 ± 0.6a | |
| February | 0.73 ± 0.03ac | 27.2 ± 1.7ac | |
| March | 0.79 ± 0.05ac | 27.8 ± 1.5ac | |
| April | 0.92 ± 0.03ad | 30.7 ± 0.6ab | |
| May | 0.97 ± 0.04ad | 30.7 ± 0.5ab | |
| June | 1.01 ± 0.03bce | 33.3 ± 0.6bc | |
| July | 1.12 ± 0.05bd | 34.5 ± 0.7b | |
| August | 1.31 ± 0.12be | 34.4 ± 1.3b | |
| September | 1.37 ± 0.09b | 30.2 ± 1.0abc | |
| October | 1.06 ± 0.07a | 30.2 ± 0.7abc | |
| November | 0.77 ± 0.04a | 27.3 ± 1.1ac | |
| December | 0.72 ± 0.05a | 26.8 ± 0.7a |
Different letters within a column indicate a significant (P < 0.05) difference between the values. Data are means ± s.e.m.
Seasonal and monthly variation in sperm motility and kinematics
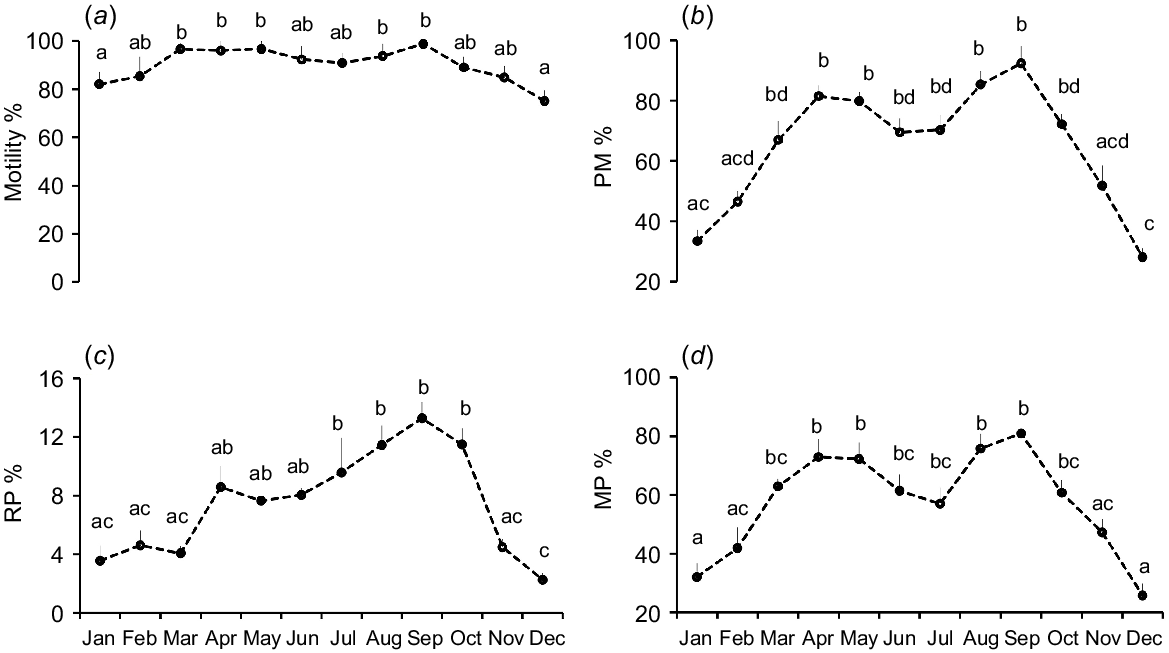
Significant variations were noted in sperm motility parameters, including TM, PM, RP, and MP sperm, across the seasons and different months. Sperm TM and PM percentages were reduced significantly (P < 0.05) in winter compared with other seasons (Table 2). The TM and PM of spermatozoa were significantly higher during March–May and August–September than in January and December (Fig. 1a, b). The percentages of RP and MP sperm were highest in autumn and significantly lower in winter (Table 2). The RP sperm percentage was significantly higher during July–October than in November–March (Fig. 1c). The percentage of MP sperm was also significantly (P < 0.05) higher during April–May and August–September than during November–February (Fig. 1d).
Monthly variations of (a) motility, (b) progressive motility (PM), (c) rapid progressive (RP), and (d) medium progressive (MP) sperm percentage of Kail rams (n = 5). Different letters indicate significant differences at P = 0.05 between different months. Data are means ± s.e.m.
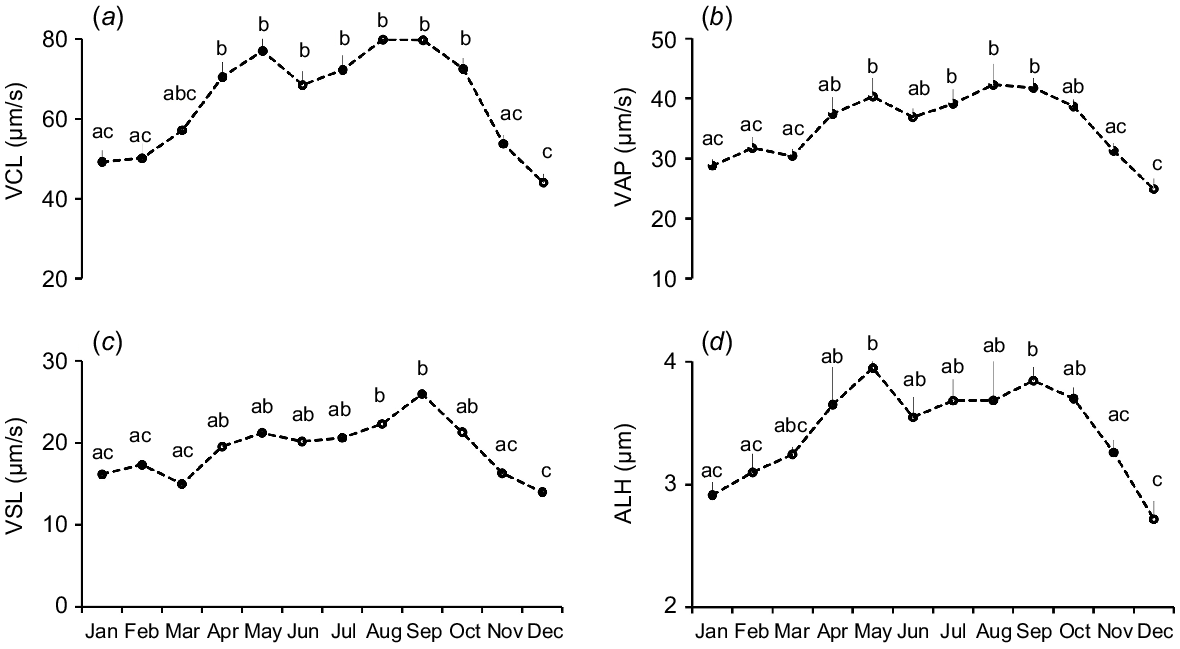
Among kinematic parameters, VCL, VAP, and VSL were significantly elevated in summer and autumn, whereas winter showed the lowest values. (Table 2). Sperm VCL values were significantly (P < 0.05) greater during April–October than November–February (Fig. 2a). Likewise, VAP values were significantly (P < 0.05) higher during May and July–September than in November–March (Fig. 2b). Sperm VSL values were higher during August through September than in November to March (Fig. 2c). Sperm ALH values were significantly lower in winter. Monthly analysis showed significantly (P < 0.05) higher ALH values during May and September than November to February (Fig. 2d). Likewise, sperm BCF values were lowest in winter and highest in summer. On a monthly basis, sperm BCF values were significantly greater during August–September than during November–March (Fig. 3a).
Monthly variations of sperm kinematics of Kail rams (n = 5). (a) Curvilinear velocity (VCL), (b) average path velocity (VAP), (c) straight-line velocity (VSL), and (d) amplitude of lateral head displacement (ALH). Different letters indicate significant differences at P = 0.05 between different months. Data are means ± s.e.m.
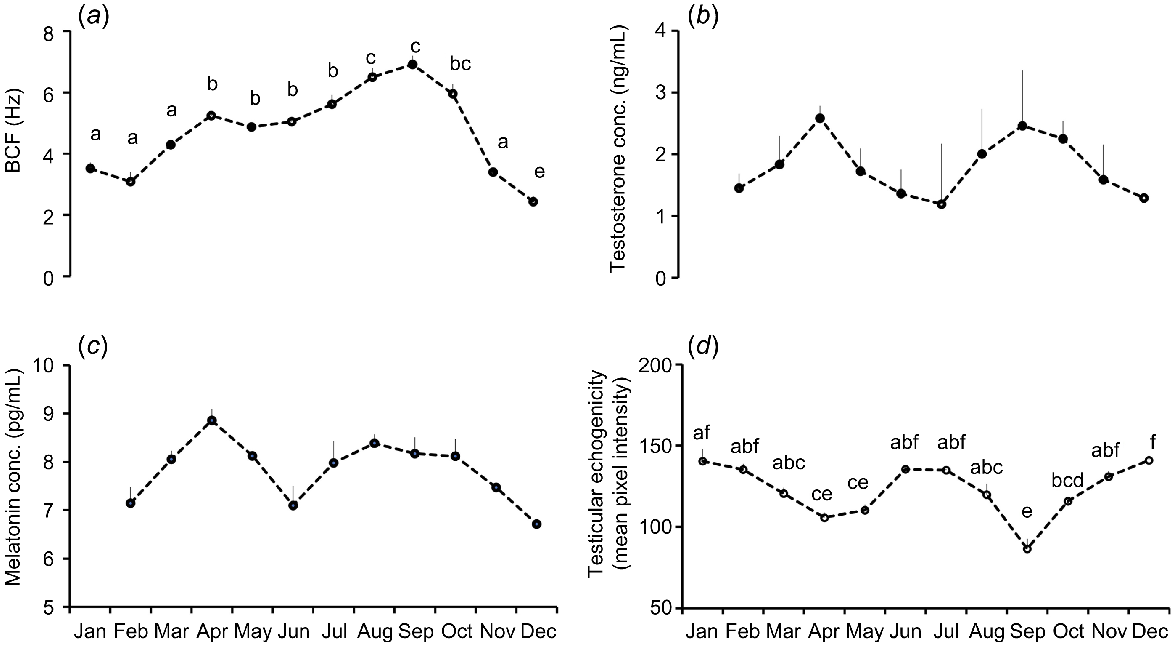
Monthly variations of (a) beat cross frequency of sperm (BCF), (b) plasma testosterone concentration, (c) melatonin concentration, and (d) testicular echogenicity of Kail rams (n = 5). Different letters indicate significant differences at P = 0.05 between different months. Data are means ± s.e.m.
Seasonal and monthly variation in testosterone and melatonin concentrations, and TE
No significant influence of seasons and months was observed on plasma concentrations of testosterone and melatonin (Table 2; Fig. 3b, c). TE values did not differ significantly among November, December, January, February, June, and July. The lowest values were observed in September, which were significantly lower than those in most other months (Fig. 3d). TE was significantly higher in winter (137.5 ± 3.2) than in autumn (102.5 ± 12.5), with spring (120.0 ± 5.8) and summer (130.0 ± 5.0) showing intermediate values that did not differ significantly from those of either winter or autumn (Table 2).
Effect of THI on sperm quality, hormonal profile, and TE
Regression analysis showed a significant (P < 0.05) positive association between THI and semen volume (R2 = 0.70; Fig. 4a), TM (R2 = 0.62; Fig. 4b), PM (R2 = 0.65; Fig. 4c), RP sperm (R2 = 0.68; Fig. 4d) and VCL (R2 = 0.83; Fig. 5a). In contrast, THI had no significant association with plasma concentration of testosterone (R2 = 0.02; Fig. 5b) and melatonin (R2 = 0.08; Fig. 5c). A significant negative association of THI and TE was found (R2 = 0.47; Fig. 5d).
Relationship between TE, SC, sperm motility and kinematics, testosterone, and melatonin
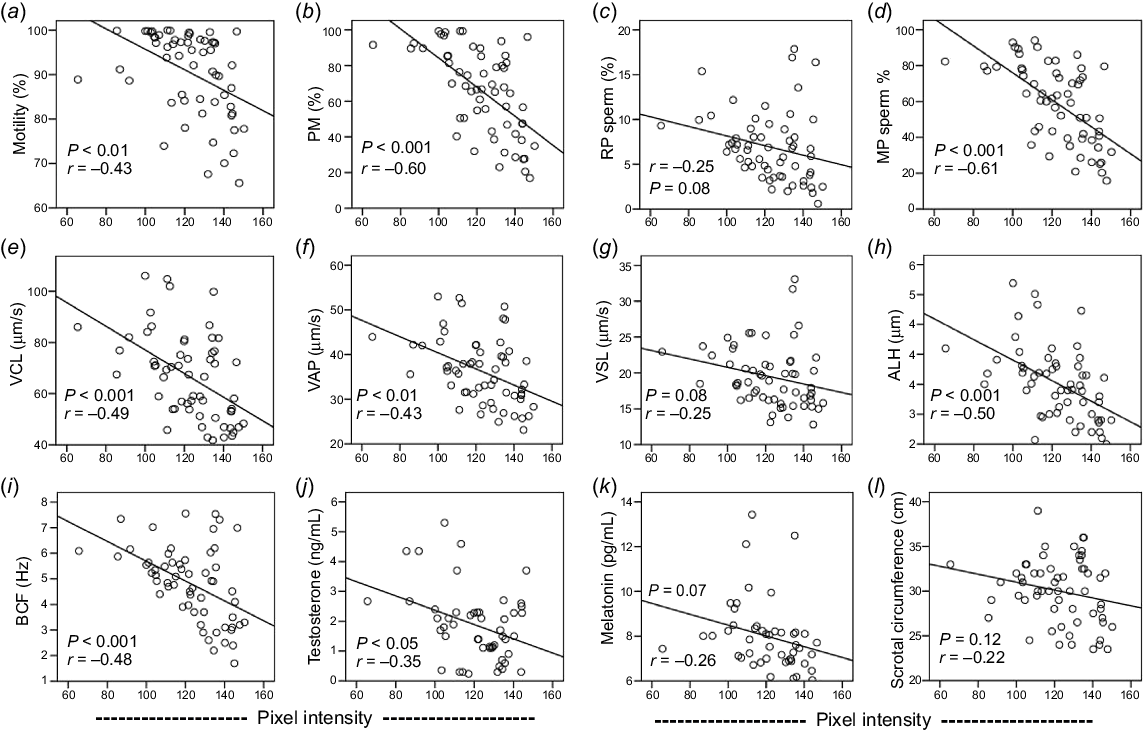
TE negatively correlated with TM, PM, and MP sperm. Likewise, TE had a negative correlation with VCL (r = −0.49), VAP (r = −0.43), ALH (r = −0.50), and BCF (r = −0.48). Non-significant correlations were found between TE, RP sperm (r = −0.25), VSL (r = −0.25), plasma concentration of melatonin (r = −0.26), and SC (r = −0.22). In contrast, TE had a significant negative correlation with plasma concentration of testosterone (r = −0.35; Figs 6a–l).
The relationship of (a) total motility, (b) progressive motility (PM), (c) rapid progressive sperm (RP), (d) medium progressive sperm (MP), (e) curvilinear velocity (VCL), (f) average path velocity (VAP), (g) straight-line velocity (VSL), (h) amplitude of lateral head displacement (ALH), (i) beat cross frequency (BCF) of sperm, (j) scrotal circumference (SC), plasma concertation of (k) melatonin and (l) testosterone, and testicular echogenicity of Kail rams (n = 5).
Relationship between SC, sperm motility and kinematics, testosterone, and melatonin
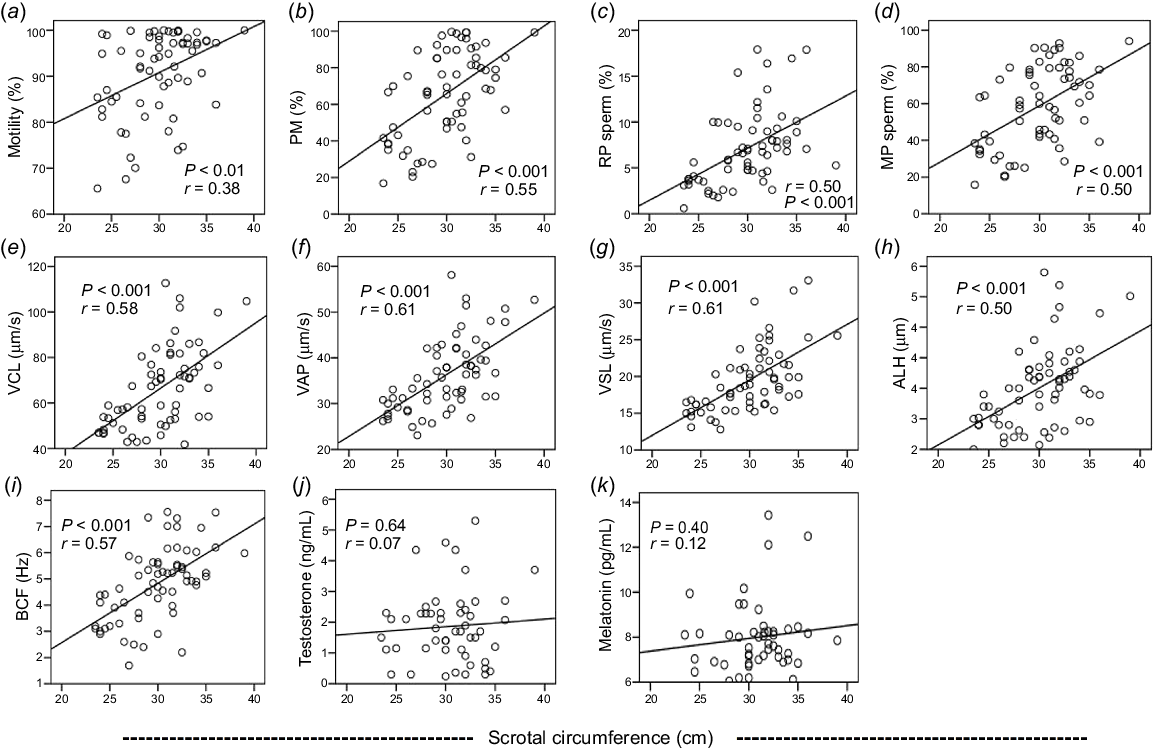
A significant positive correlation was found between SC and TM (r = 0.38), PM (r = 0.55), RP (r = 0.50) and MP sperm (r = 0.50), VCL (r = 0.58), VAP (r = 0.61), VSL (r = 0.61), ALH (r = 0.50), and BCF (r = 0.57). Correlations between SC and plasma concentrations of testosterone (r = 0.07) and melatonin (r = 0.12) were not significant (Figs 7a–k).
The relationship of (a) total motility, (b) progressive motility (PM), (c) rapid progressive sperm (RP), (d) medium progressive sperm (MP), (e) curvilinear velocity (VCL), (f) average path velocity (VAP), (g) straight-line velocity (VSL), (h) amplitude of lateral head displacement (ALH), (i) beat cross frequency (BCF) of sperm, and plasma concentation of (j) testosterone and (k) melatonin, and scrotal circumference of Kail rams (n = 5).
Discussion
As far as we are aware, this research is the first to document the seasonal and monthly variations in semen quality, testicular echogenicity, plasma testosterone and melatonin profiles, and the optimal THI range for ram semen production. Our findings showed a negative influence of the winter season on the semen quality and testicular echogenicity of Kail rams raised under a subtropical climate.
The observed monthly variations in semen volume and SC are consistent with earlier reports in rams (Menchaca et al. 2005; Oláh et al. 2013; Belkadi et al. 2017). The minimal semen volume during winter and maximal volume during autumn in Kail rams can be attributed to cold stress, which negatively affects libido and spermatogenesis, ultimately reducing semen volume (Benia et al. 2018). These findings align with the understanding that semen production in rams is highly correlated with temperature fluctuations (Demir et al. 2016). The peak semen volume during autumn is associated with optimal photoperiod, temperature, and the availability of lush green grazing fields. Similarly, the minimum SC recorded during the colder months is consistent with previous reports, as cold temperature prompts the testicles to pull up against the body, increasing the rugosity of the scrotum to limit heat loss (Kafi et al. 2004; Oláh et al. 2013; Pham and Schultz 2021).
The minimum values of sperm motility and kinematics during winter are well-aligned with the findings of previous reports in rams (Talebi et al. 2009; Malejane et al. 2014). Semen quality is known to be negatively influenced when temperatures fall below 12°C (Malejane et al. 2014; van Wettere et al. 2021). Continuous exposure to reduced temperatures and the unavailability of lush green grazing fields during winter negatively affect sperm quality in Kail rams. Although a downward trend was noted in semen attributes during June–July, it was not statistically significant, likely owing to the moderate summer conditions, with a maximum THI of 79 (Armstrong 1994).
Unfortunately, blood samples for January were unavailable for analysis in this study. The plasma concentrations of testosterone and melatonin showed a downward trend during winter and summer, although not being statistically significant, possibly owing to individual variation within the sample population. Combining data for testosterone and melatonin (winter + summer vs spring + autumn) also showed no significant differences in testosterone (1.7 ± 0.2 vs 1.2 ± 0.3) and melatonin (7.4 ± 0.3 vs 8.3 ± 0.3), suggesting subtle variation in plasma concentration of these hormones across the seasons, which may require a larger sample size to address biological variation.
In this study, THI significantly correlated with sperm quality parameters. Sperm quality improved within a THI range of 58–72, but was compromised at 76 and above, supporting the previous notion that a high THI negatively affects sperm quality (Llamas-Luceño et al. 2020). The findings also showed that both high and low THI reduce sperm quality in Kail rams, which is consistent with previous reports on bulls (Al-Kanaan et al. 2015). This study determined the lower and upper THI thresholds for optimal semen production in rams.
Seasonal variations in testicular pixel intensity showed markedly higher values during winter and lowest in autumn, potentially indicating seasonal testicular tissue changes. Moreover, higher TE is linked to a decline in spermatogenesis owing to cold stress (Brito et al. 2012). Thus, the lowest TE in autumn suggests peak testicular activity and spermatogenesis, whereas winter represents a regressive phase with diminished sperm production. Similar findings have been reported in previous studies on rams (Hedia et al. 2020; El-Shalofy et al. 2022). Furthermore, in our study, TE had a moderate negative correlation with sperm motility and kinematics, being consistent with previous reports in rams (Hedia et al. 2020; Montes-Garrido et al. 2022) and dogs (Moxon et al. 2015). In contrast, SC had a positive correlation with sperm-quality attributes, in agreement with findings in rams (Wahid and Yunus 1994) and bulls (Latif et al. 2009), indicating a positive relationship between testicle size and semen quality. A limitation of the present study is the lack of sperm concentration data, with ejaculates from individual rams being pooled at each collection time to ensure adequate volume for CASA and biochemical analyses. Although this approach provided consistency in sample processing, it did not account for individual-level variation of sperm concentration, which may vary seasonally.
Conclusions
In conclusion, monthly and seasonal fluctuations had a significant influence on semen volume, sperm motility, kinematic parameters, scrotal circumference, and testicular echogenicity in Kail rams, whereas testosterone and melatonin concentrations remained unaffected. Scrotal circumference showed a positive correlation with sperm-quality traits, whereas testicular echogenicity was negatively correlated. Semen quality was lowest in winter and improved significantly from late spring through autumn, with optimal THI for semen production spanning from 58 to 72. These findings have practical implications for local sheep producers because they indicate that breeding programs and semen collection for artificial insemination should be prioritized throughout the summer and autumn months to maximize reproductive efficiency.
Conflicts of interest
Authors declare that they have no conflicts of interest and have no financial or personal relationship(s) that may have inappropriately influenced the research and write-up of this paper.
Declaration of funding
This study was funded by the Pakistan Agriculture Research Council (PARC), Islamabad under Grant number AS-159.
Author contributions
The study was conceived planned, researched and written by M. I. R. Khan, N. Hameed and M. Zubair. M. Zubair and N. Hameed conducted the research and oversaw the feeding and management of animals. The manuscript was critically examined by N. Ahmad, A. Z. Durrani, and every other author.
References
Abbas M, Khan MI-u-R, Hameed N, Rehman A, Mohsin I, Bilal M, Shahzad M (2021) Melatonin along with eCG improves fresh semen quality and plasma concentrations of melatonin and testosterone during non-breeding season in Beetal bucks. Small Ruminant Research 205, 106569.
| Crossref | Google Scholar |
Al-Kanaan A, König S, Brügemann K (2015) Effects of heat stress on semen characteristics of Holstein bulls estimated on a continuous phenotypic and genetic scale. Livestock Science 177, 15-24.
| Crossref | Google Scholar |
Amjad M, Hameed N, Khan MIuR, Ullah F, Sattar A (2021) Effect of weaning method on body weight, testicular growth, sexual behavior, age of puberty, and testosterone concentration of Beetal bucks. Animal Science Journal 92, e13520.
| Crossref | Google Scholar | PubMed |
Armstrong D (1994) Heat stress interaction with shade and cooling. Journal of Dairy Science 77, 2044-2050.
| Crossref | Google Scholar | PubMed |
Belkadi S, Safsaf B, Heleili N, Tlidjane M, Belkacem L, Oucheriah Y (2017) Seasonal influence on sperm parameters, scrotal measurements, and serum testosterone in Ouled Djellal breed rams in Algeria. Veterinary World 10, 1486-1492.
| Crossref | Google Scholar | PubMed |
Benia AR, Saadi MA, Ait-Amrane A, Belhamiti TB, Selles SMA, Kaidi R (2018) Effect of season and age on main characteristics of sperm production in the Ouled-Djellal rams. Livestock Research for Rural Development 30, 1-14.
| Google Scholar |
Benmoula A, Badi A, El Fadili M, Khalil KE, Allai L, El Hilali A, El Amiri B (2017) Effect of season on scrotal circumference, semen characteristics, seminal plasma composition and spermatozoa motility during liquid storage in INRA180 rams. Animal Reproduction Science 180, 17-22.
| Crossref | Google Scholar | PubMed |
Blache D, Zhang S, Martin GB (2006) Dynamic and integrative aspects of the regulation of reproduction by metabolic status in male sheep. Reproduction Nutrition Development 46, 379-390.
| Crossref | Google Scholar | PubMed |
Brito LF, Barth AD, Wilde RE, Kastelic JP (2012) Testicular ultrasonogram pixel intensity during sexual development and its relationship with semen quality, sperm production, and quantitative testicular histology in beef bulls. Theriogenology 78, 69-76.
| Crossref | Google Scholar | PubMed |
Cárdenas-Gallegos MA, Aké-López JR, Centurión-Castro F, Magaña-Monforte JG (2012) The breed and season effects on scrotal circumference and semen characteristics of hair sheep rams under tropical conditions. Reproduction in Domestic Animals 47, e92-e94.
| Crossref | Google Scholar | PubMed |
Carvajal-Serna M, Torres-Ruda F, Cardozo JA, Grajales-Lombana H, Cebrián-Pérez JÁ, Muiño-Blanco T, Pérez-Pé R, Casao A (2019) Changes in melatonin concentrations in seminal plasma are not correlated with testosterone or antioxidant enzyme activity when rams are located in areas with an equatorial photoperiod. Animal Reproduction Science 200, 22-30.
| Crossref | Google Scholar | PubMed |
Casao A, Cebrián I, Asumpção ME, Pérez-Pé R, Abecia JA, Forcada F, Cebrián-Pérez JA, Muiño-Blanco T (2010) Seasonal variations of melatonin in ram seminal plasma are correlated to those of testosterone and antioxidant enzymes. Reproductive Biology and Endocrinology 8, 59.
| Crossref | Google Scholar | PubMed |
Delgadillo JA, Gelez H, Ungerfeld R, Hawken PAR, Martin GB (2009) The ‘male effect’ in sheep and goats—revisiting the dogmas. Behavioural Brain Research 200, 304-314.
| Crossref | Google Scholar | PubMed |
Demir A, Uslu M, Arslan OE (2016) The effect of seasonal variation on sexual behaviors in males and its correlation with hormone levels: a prospective clinical trial. Central European Journal of Urology 69, 285-289.
| Crossref | Google Scholar | PubMed |
El-Shalofy AS, Shahat AM, Hedia MG (2022) Effects of melatonin administration on testicular hemodynamics, echotexture, steroids production, and semen parameters during the non-breeding season in Ossimi rams. Theriogenology 184, 34-40.
| Crossref | Google Scholar | PubMed |
Farooq U, Ijaz A, Ahmad N, Rehman H, Zaneb H (2013) Seasonal variations in certain physical and biochemical attributes of semen from Cholistani bulls. Pakistan Veterinary Journal 33, 510-514.
| Google Scholar |
Fuerst-Waltl B, Schwarzenbacher H, Perner C, Sölkner J (2006) Effects of age and environmental factors on semen production and semen quality of Austrian Simmental bulls. Animal Reproduction Science 95, 27-37.
| Crossref | Google Scholar | PubMed |
Hameed N, Zubair M, Ahmad N, Durrani AZ, Khan MIUR (2023) Effects of extender type and storage time on sperm quality parameters of Kail ram semen stored at 5°C. Tropical Animal Health and Production 55(3), 171.
| Crossref | Google Scholar | PubMed |
Hedia MG, El-Belely MS, Ismail ST, Abo El-Maaty AM (2020) Seasonal changes in testicular ultrasonogram pixel-intensity and their association with semen characteristics in rams. Asian Pacific Journal of Reproduction 9, 49-54.
| Crossref | Google Scholar |
Kafi M, Safdarian M, Hashemi M (2004) Seasonal variation in semen characteristics, scrotal circumference and libido of Persian Karakul rams. Small Ruminant Research 53, 133-139.
| Crossref | Google Scholar |
Kahwage PR, Esteves SN, Jacinto MAC, Junior WB, Machado R, Romanello N, Passeri LF, de Mendonça KL, Garcia AR (2018) Assessment of body and scrotal thermoregulation and semen quality of hair sheep rams throughout the year in a tropical environment. Small Ruminant Research 160, 72-80.
| Crossref | Google Scholar |
Kridli RT, Abdullah AY, Obeidat BS, Qudsieh RI, Titi HH, Awawdeh MS (2018) Seasonal variation in sexual performance of Awassi rams. Animal Reproduction 4, 38-41.
| Google Scholar |
Küçük N, Aksoy M (2020) Effect of environmental heat stress on Kıvırcık ram sperm parameters. Journal of the Hellenic Veterinary Medical Society 71, 2073-2080.
| Crossref | Google Scholar |
Latif M, Ahmed J, Bhuiyan M, Shamsuddin M (2009) Relationship between scrotal circumference and semen parameters in crossbred bulls. Bangladesh Veterinarian 26, 61-67.
| Crossref | Google Scholar |
Llamas-Luceño N, Hostens M, Mullaart E, Broekhuijse M, Lonergan P, Van Soom A (2020) High temperature–humidity index compromises sperm quality and fertility of Holstein bulls in temperate climates. Journal of Dairy Science 103, 9502-9514.
| Crossref | Google Scholar | PubMed |
Malejane CM, Greyling JPC, Raito MB (2014) Seasonal variation in semen quality of Dorper rams using different collection techniques. South African Journal of Animal Science 44, 26-32.
| Crossref | Google Scholar |
Mamontova TV, Selionova MI, Aibazov A-MM (2021) Sexual activity and sperm production of charolais and Ile-De-France rams in different seasons of the year. Agricultural Biology 56, 752-762.
| Google Scholar |
Menchaca A, Pinczak A, Queirolo D (2005) Storage of ram semen at 5°C: effects of preservation period and timed artificial insemination on pregnancy rate in ewes. Animal Reproduction 2, 195-198.
| Google Scholar |
Moghaddam GH, Pourseif MM, Rafat SA (2012) Seasonal variation in semen quantity and quality traits of Iranian crossbred rams. Slovak Journal of Animal Science 45, 67-75.
| Google Scholar |
Molina JCJ, da Silva RS, Bidegain FA, Souza YB, Purdy PH, Blackburn HD, Azevedo HC (2024) Bioclimatic thermal stress indices and their relationships with andrological characteristics in hair rams. International Journal of Biometeorology 68, 253-261.
| Crossref | Google Scholar | PubMed |
Montes-Garrido R, Riesco MF, Anel-Lopez L, Neila-Montero M, Palacin-Martinez C, Boixo JC, de Paz P, Ortega-Ferrusola C, Hassan MAA, Anel L, Alvarez M (2022) Application of ultrasound technique to evaluate the testicular function and its correlation to the sperm quality after different collection frequency in rams. Frontiers in Veterinary Science 9, 1035036.
| Crossref | Google Scholar | PubMed |
Mostafa AS, Farghal M (2019) A study on the effect of age, breed and season on sexual behaviour, testosterone concentrations and scrotal circumference in Egyptian rams. Journal of Veterinary Medical Research 26, 91-100.
| Crossref | Google Scholar |
Moxon R, Bright L, Pritchard B, Bowen IM, de Souza MB, da Silva LDM, England GCW (2015) Digital image analysis of testicular and prostatic ultrasonographic echogencity and heterogeneity in dogs and the relation to semen quality. Animal Reproduction Science 160, 112-119.
| Crossref | Google Scholar | PubMed |
Ngcobo JN, Nephawe KA, Maqhashu A, Nedambale TL (2020) Seasonal variations in semen parameters of Zulu rams preserved at 10°C for 72 h during breeding and non-breeding season. American Journal of Animal and Veterinary Sciences 15, 226-239.
| Crossref | Google Scholar |
Ntemka A, Kiossis E, Boscos C, Theodoridis A, Kourousekos G, Tsakmakidis I (2018) Effects of testicular hemodynamic and echogenicity changes on ram semen characteristics. Reproduction in Domestic Animals 53, 50-55.
| Crossref | Google Scholar | PubMed |
Ntemka A, Kiossis E, Boscos C, Theodoridis A, Kourousekos G, Tsakmakidis I (2019) Impact of old age and season on Chios ram semen quality. Small Ruminant Research 178, 15-17.
| Crossref | Google Scholar |
Oláh J, Kusza S, Harangi S, Posta J, Kovács A, Pécsi A, Budai C, Jávor A (2013) Seasonal changes in scrotal circumference, the quantity and quality of ram semen in Hungary. Archives Animal Breeding 56, 102-108.
| Crossref | Google Scholar |
Pham S, Schultz JS (2021) Testicular thermoregulation with respect to spermatogenesis and contraception. Journal of Thermal Biology 99, 102954.
| Crossref | Google Scholar | PubMed |
Sanchez-Davila F, Bernal-Barragan H, Vazquez-Armijo JF, López-Villalobos N, Ledezma-Torres RA, Grizelj J, Brenner EG, Vasquez NA, Palomera CL (2020) Annual variation in reproductive parameters and sexual behaviour of Saint Croix rams in a semi-desert region in Mexico. Journal of Applied Animal Research 48, 499-506.
| Crossref | Google Scholar |
Santos SGCGd, Saraiva EP, Pimenta Filho EC, Santos LdFDd, Fonsêca VdFC, Veríssimo TNS, Almeida MEV, Pinheiro AdC (2015) Seasonal and circadian variation of the sexual behavior of Morada Nova rams in tropical environment. Revista Brasileira de Zootecnia 44, 8-14.
| Google Scholar |
Talebi J, Souri M, Moghaddam A, Karimi I, Mirmahmoodi M (2009) Characteristics and seasonal variation in the semen of Markhoz bucks in western Iran. Small Ruminant Research 85, 18-22.
| Crossref | Google Scholar |
Taylor JF, Bean B, Marshall CE, Sullivan JJ (1985) Genetic and environmental components of semen production traits of artificial insemination Holstein bulls. Journal of Dairy Science 68, 2703-2722.
| Crossref | Google Scholar |
Urie NJ, Lombard JE, Shivley CB, Adams AE, Kopral CA, Santin M (2018) Preweaned heifer management on US dairy operations: Part III. Factors associated with Cryptosporidium and Giardia in preweaned dairy heifer calves. Journal of Dairy Science 101, 9199-9213.
| Crossref | Google Scholar | PubMed |
van Wettere WHEJ, Kind KL, Gatford KL, Swinbourne AM, Leu ST, Hayman PT, Kelly JM, Weaver AC, Kleemann DO, Walker SK (2021) Review of the impact of heat stress on reproductive performance of sheep. Journal of Animal Science and Biotechnology 12, 26.
| Crossref | Google Scholar |
Wahid SA, Yunus JM (1994) Correlation between testicle measurements and libido and semen quality in rams. Asian–Australasian journal of animal sciences 7, 175-178.
| Crossref | Google Scholar |
Zaher HA, Alawaash SA, Swelum AA (2020) Effects of season and breed on the reproductive performance of sheep. Journal of Animal Reproduction and Biotechnology 35, 149-154.
| Crossref | Google Scholar |
Zamiri M, Khalili B, Jafaroghli M, Farshad A (2010) Seasonal variation in seminal parameters, testicular size. plasma testosterone concentration in Iranian Moghani rams. Small Ruminant Research 94, 132-136.
| Crossref | Google Scholar |