Effects of hormonal growth promotants (HGP) on growth, carcass characteristics, the palatability of different muscles in the beef carcass and their interaction with aging
J. M. Thompson A G , B. M. McIntyre B , G. D. Tudor C , D. W. Pethick D , R. Polkinghorne E and R. Watson FA Cooperative Research Centre for Beef Genetic Technologies, School of Environmental and Rural Sciences, University of New England, NSW 2351, Australia.
B Department of Agriculture, Baron-Hay Court, South Perth, WA 6151, Australia.
C Department of Agriculture, PO Box 1231, Bunbury, WA 6231, Australia.
D Murdoch University, Murdoch, WA 6150, Australia.
E Marrinya Agricultural Enterprises, 70 Vigilantis Road, Wuk Wuk, Vic. 3875, Australia.
F Department of Mathematics and Statistics, University of Melbourne, Parkville, Vic. 3010, Australia.
G Corresponding author. Email: jthompso@une.edu.au
Australian Journal of Experimental Agriculture 48(11) 1405-1414 https://doi.org/10.1071/EA07131
Submitted: 8 May 2007 Accepted: 20 June 2008 Published: 16 October 2008
Abstract
Effects of hormonal growth promotant (HGP) implantation on liveweight, carcass and meat quality measurements were examined using 80 Angus yearling cattle. After entry to the feedlot, 40 steers and 40 heifers were implanted with Revalor-S (28 mg oestradiol and 140 mg trenbolone acetate) and Revalor-H (20 mg oestradiol, 200 mg trenbolone acetate), respectively. Cattle were slaughtered after 55 and 65 days on feed. Samples from the Mm. longissimus dorsi, biceps femoris (the cap and body portions), gluteus medius (the eye and D portions), infraspinatus and triceps brachii were prepared for sensory testing after aging for 5 and 21 days after slaughter. A total of 854 muscle samples were cooked by grill (601) or roast (253) methods and served to consumers using the Meat Standards Australia taste panel protocols.
When adjusted to the same initial liveweight, implantation with Revalor-H and Revalor-S resulted in a 4 and 7% increase in slaughter weight, respectively. Implantation resulted in an increased ossification score in steers (P < 0.05), but not in heifers. There was a significant interaction (P < 0.05) between HGP implantation and days aged for shear force. There was a small effect of HGP implants on compression (P < 0.05), but not on cook loss and intramuscular fat percentage. Muscles differed in their response to HGP implantation (P < 0.05) for tenderness, overall liking and palatability scores. Muscles also differed in their aging rates after slaughter (P < 0.05). The greatest response in sensory scores to HGP implantation was found in those muscles that had the highest aging rates. Possible mechanisms by which muscles differed in their response to HGP implantation are discussed.
Introduction
Hormonal growth promotants (HGPs) are used in Australia to increase liveweight and feed efficiency of cattle, both on pasture and in the feedlot. Although there has been considerable research quantifying the benefits of HGP implants in terms of their effect on liveweight and carcass yield (Sawyer and Barker 1988), less attention has been paid to the effect of HGP implantation on the carcass traits and beef palatability.
As early as 1991 questions were raised in the National Beef Quality Audit in the United States as to the potential for HGP implants to lower marbling scores and reduce beef tenderness (Smith et al. 1992). Even today there is still much debate on the subject, to the extent that the conclusions of recent reviews have varied, with Nichols et al. (2002) stating that HGPs had only limited, if any, effect on beef palatability. In contrast, Dikeman (2007) concluded that whilst tenderness was reduced in implanted compared with non-implanted cattle these effects on tenderness were probably not of commercial importance. While there is variation in the interpretation of the literature, a recent meta-analysis of available published results and unpublished data by Watson (2008) clearly showed that when the results from different experiments were considered collectively, M. longissimus dorsi samples from cattle implanted with HGP had greater shear forces and lower tenderness scores than carcasses from non-implanted cattle.
There is mounting evidence that HGPs increase efficiency and growth rates of cattle by modifying protein turnover rates in the body (Kerth et al. 2003; Dunshea et al. 2005). In reviewing the interaction between protein turnover and eating quality, Koohmaraie et al. (2002) proposed that if in part an increase in net protein in the live animal was achieved by a decrease in protein turnover (i.e. as a function of increased calpastatin activity in the muscle), this would reduce post-mortem aging rates, resulting in tougher meat. As differences in aging rates between muscles appear to be a function of the activity of the calpain/calpastatin system (Geesink et al. 1995), it is possible that the magnitude of the HGP effect on palatability may interact with muscle. Most of the studies that have examined the effect of HGP implantation on meat quality have only focussed on the M. longissimus dorsi and generally have only sampled one aging time.
The magnitude of the effect of HGP implants on palatability is particularly important in Australia with the recent implementation of Meat Standards Australia (MSA) beef grading scheme. The MSA scheme is a beef quality grading system focussed on accurately describing palatability of individual beef cuts to the consumer (Polkinghorne et al. 1999, 2008; Thompson 2002), which uses critical control points from all sectors of the beef supply chain to predict consumer palatability of individual muscles in the carcass. If the impact of HGP implants interacts with muscle, then unlike other grading schemes around the world, the MSA system is well placed to incorporate HGP effect as an input into its prediction model.
This study investigated the impact of Revalor-H and Revalor-S implants on carcass traits and meat quality in young, growing Bos taurus steers and heifers finished in a feedlot production system typical of that used to prepare beef for the Australian market. The research also examined the HGP effects on the palatability of several muscles, aged for 5 and 21 days after slaughter.
Materials and methods
Animals and experimental design
The experiment used 40 Angus yearling steers and 40 Angus yearling heifers, which at the commencement of the experiment had mean (±s.e.) liveweights of 310 (±13.0) kg and 260 (±12.8) kg, respectively. Within sex, cattle were stratified on liveweight and allocated to either control or treatment groups, within which animals were allocated to slaughter at either 55 or 65 days after implantation.
On commencement of feeding, steers in the treatment group were implanted with Revalor-S (28 mg oestradiol and 140 mg trenbolone acetate), while heifers in the treatment group were implanted with Revalor-H (20 mg oestradiol, 200 mg trenbolone acetate) via subcutaneous ear implants. Cattle were fed a starter ration for 14 days and then a high concentrate ration until slaughter. Cattle were weighed without fasting at the commencement of the experiment, and thereafter at ~14-day intervals until slaughter.
Slaughter
Fifty-five days after implantation, one pen of heifers and one pen of steers (both containing implanted and control animals) were slaughtered, with the remaining pens slaughtered 10 days later (65 days after implantation). These slaughter times ensured that the HGP implants were still within the effective payout period of 80 days.
Before slaughter, animals were weighed full and transported to a local abattoir for slaughter the following day. Bodies were stimulated using a low voltage system directly after knocking. Following dressing, hot carcass weight and P8 fat depth were measured according to AUS-MEAT procedures (Anon. 1992). Carcass sides were then placed in a chiller, and pH, temperature and time measured in the M. longissimus dorsi at the 12/13th rib junction at approximately hourly intervals for the first 6–8 h after slaughter. After an overnight chill, carcasses were quartered and measured by MSA graders for 10/11th rib fat depth, ossification score, AUS-MEAT marbling score and ultimate pH (Perry et al. 2001). AUS-MEAT marbling scores were recorded in increments of 0.1 units to provide a more continuous scale within the AUS-MEAT chips. The ossification scoring system was based on the USDA system (Romans et al. 1994). At boning, the striploin, silverside, rump and blade primals were removed from both sides of each carcass, vacuum packed and transported at 1°C for preparation of sensory and objective samples.
Sample preparation
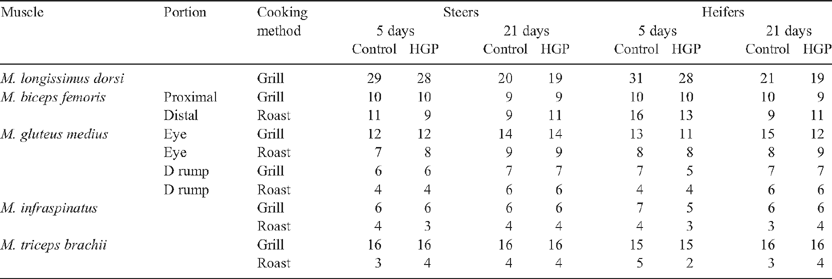
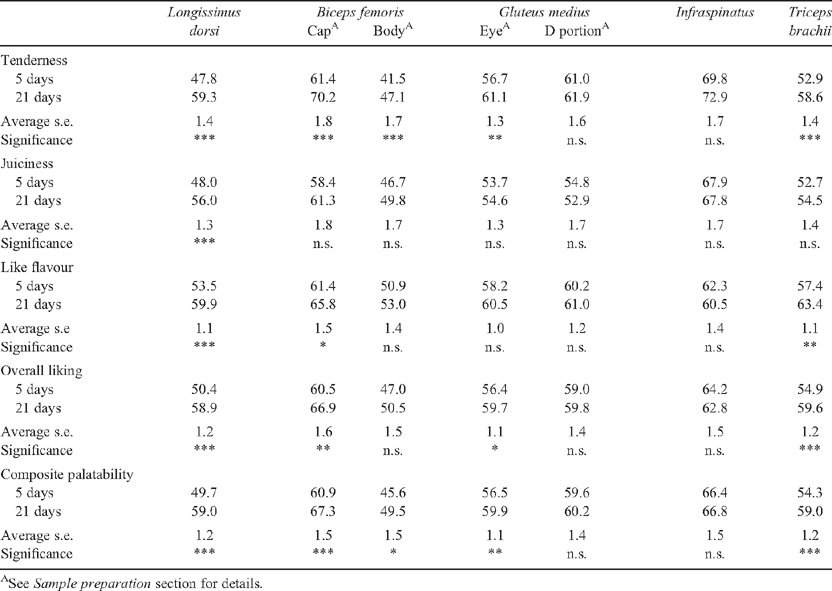
Five days after slaughter the primals were separated into the following muscles for preparation of grilling or roasting samples. The M. longissimus dorsi (both the anterior and posterior portions of the striploin were prepared for grilling), M. biceps femoris (the proximal portion called the cap from the rump primal was prepared for grilling, while the distal portion called the body from the silverside primal was prepared for roasting), M. gluteus medius (separated into two portions along the natural seam with the larger ventral portion termed the ‘D’ and the remaining dorsal portion termed the ‘eye’, both of which were prepared for grilling or roasting), M. infraspinatus (prepared for grilling or roasting), M. triceps brachii (prepared for grilling or roasting) and M. semitendinosus (prepared for roasting).
The preparation of sensory samples has been described by Watson et al. (2008). Briefly, muscles were denuded of all fat and epimysium. Samples for the 5- and 21-day aging periods alternated between carcass side. Five 25-mm-thick steaks were prepared for each grill sample and blocks (~60 by 60 by 150 mm long) prepared for roast samples. Further samples of the anterior and posterior portions of the M. longissimus were taken for objective laboratory measurements (~250-g blocks). These were vacuum packed and aged for 5 or 21 days before freezing at –20°C. A 150-g sample was also collected and frozen from the anterior portion of the M. longissimus dorsi for intramuscular fat measurements.
Objective measurements
Shear force and compression blocks were cooked in a water bath for 1 h at 70°C. The methodology for the preparation and measurement of shear force and compression has been described in detail by Perry et al. (2001). Cooking loss was calculated as a percentage of weight change of the objective block during cooking. Intramuscular fat percentage was determined on freeze-dried samples of the M. longissimus using near-infrared spectrophotometry (Perry et al. 2001).
Sensory design and cooking protocol
The taste panel design has been described by Watson et al. (2008). Briefly, grill samples were tested using a Latin square design which comprised 108 samples, allocated to nine sessions and tasted by 180 consumers. The five steaks from each sample were tested in five separate sessions. After cooking, each steak was halved and served to two consumers. In total, each muscle sample was tasted by 10 different consumers. The roast taste panels were a modification of the above design, where each taste panel evaluated 36 samples which were tasted by 60 consumers.
For both steak and roast panels, each consumer tested a starter sample, followed by six experimental samples. Consumers were asked to score either grill steaks and roast slice samples for tenderness, juiciness, like flavour and overall liking by placing marks on 100-mm lines anchored with the following definitions: tenderness (very tough to very tender); juiciness (very dry to very juicy); like flavour (dislike extremely to like extremely); overall liking (dislike extremely to like extremely).
Individual sensory scores for tenderness, juiciness, like flavour and overall liking were weighted by 0.4, 0.1, 0.2 and 0.3, respectively and summed to calculate a palatability score (MQ4, Polkinghorne et al. 1999; Watson et al. 2008). Before calculating the mean sensory scores for each sample, the 10 individual scores for each sample were ranked and the two highest and two lowest scores clipped to reduce the variance of the mean sensory scores (Polkinghorne et al. 1999; Watson et al. 2008).
The numbers of sensory samples tested from each muscle and cooking protocol are shown in Table 1. There were a total of 854 samples tested by the grill and roast cooking methods. This included several duplicate M. longissimus dorsi samples from the anterior position aged for 5 days.
|
Statistical analysis
One treated heifer lost its implant soon after induction. Therefore, its liveweight, carcass traits, and meat quality data were excluded from subsequent analyses.
Calculation of glycolytic rate
Changes in both pH and temperature as functions of time were modelled in individual carcasses using the exponential function

where yt is pH, or temperature at time t; au is ultimate pH, or temperature; ai is initial pH, or temperature; k is the exponential rate of pH or temperature change and t is time after slaughter. This equation was fitted using PROC NLIN (SAS 1997). Parameters from the pH v. time equation was used to predict the time to achieve pH 6.0, which was then used in the temperature v. time equation to predict temperature at pH 6.0 (temp@pH6).
Liveweight and carcass measurements
The four liveweights recorded after implantation were analysed in a repeated-measurements analysis (SAS 1997), which included terms for sex, HGP nested within sex [written as HGP(sex)], and liveweight at implantation as a covariate. The sex effect was tested on pen, while other effects were tested on the between animal term. Interactions were not significant (P > 0.05).
Final liveweight and carcass weight were analysed using PROC GLM (SAS 1997). Models included fixed effects for sex, HGP(sex) and liveweight at implantation as a covariate. Carcass measurements (ossification and marble scores, fat depth at the P8 and 12/13th sites, ultimate pH and temperature at pH 6) were analysed using similar GLM models, but including a covariate for carcass weight, rather than liveweight at implantation. Again, sex was tested against pen.
Objective meat quality
Objective meat quality measurements (shear force, compression, cooking loss and intramuscular fat percentage) of anterior and posterior portions of the M. longissimus dorsi were analysed using mixed models (PROC MIXED, SAS 1997), which contained terms for sex, HGP(sex), position, days aged and the interaction days aged × HGP(sex). Other interactions were tested but were found to be not significant (P > 0.05). Sex was tested on pen and HGP(sex) on the between animal term.
Sensory scores
The final model for sensory scores was developed using a multivariate analysis, where the dependent variables comprised the four sensory scores. Initially, the multivariate model contained fixed effects for sex, HGP(sex), cooking method, muscle, position(muscle) and days aged, which examined the effects on tenderness, juiciness, like flavour and overall liking scores, simultaneously. First and second order interactions were tested and, if significant (P < 0.05), were included in the model.
PROC MIXED was then used to examine the effects of the final multivariate model for each of the four sensory variables and the MQ4 score individually. The final model contained fixed effects for sex, HGP(sex), cook, muscle, position(muscle), days aged, muscle × days aged and muscle × HGP(sex). Sex was tested on pen and HGP(sex) on the between animal term.
Results
Liveweight changes during feedlotting
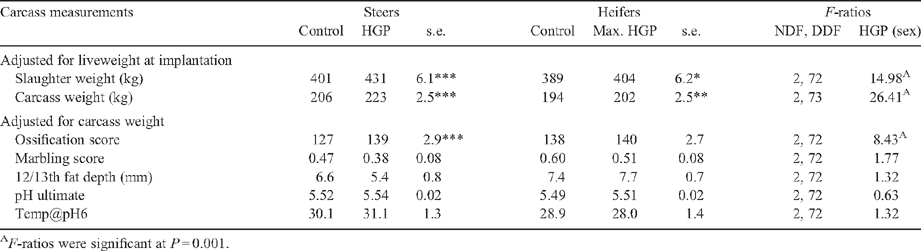
Table 2 shows the F-ratios for the repeated-measurements analysis of liveweight during feedlotting. Steers were heavier than heifers (P < 0.05), and this difference was constant over the feedlotting period. The HGP(sex) term was highly significant (P < 0.001), both as a main effect and the interaction with time. The covariate for initial liveweight was significant (P < 0.05).
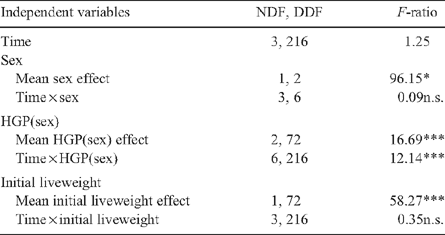
|
Figure 1 shows the increase in liveweight in heifers and steers for the HGP and control treatments over the feedlotting period. Within both the steer and heifer groups, differences between HGP and controls groups increased with time, with a larger HGP implant response in the steers than in the heifers.
|
Final liveweight and carcass traits
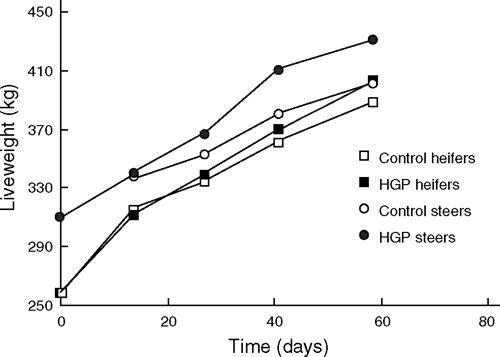
Predicted means for the HGP implant effects on slaughter and carcass weights within sex class are shown in Table 3, after adjustment for initial liveweight. There was almost a 2-fold increase in liveweight due to the HGP implanted in steers, compared with the heifers (30 kg v. 15 kg for steers and heifers, respectively). As expected a similar trend was evident for carcass weight, where HGP-implanted steer carcasses were 17 kg heavier than the controls, compared with only an 8-kg advantage for the heifers.
There was a significant (P < 0.05) HGP(sex) effect on ossification score. Within the steers, HGP treatment resulted in a higher ossification score than the controls (P < 0.01), although there was no effect of HGP treatment on ossification score in the heifers (Table 3). There was no effect of HGP(sex) treatment on marbling score, 12/13th rib fat depth, ultimate pH or temp@pH6 (P > 0.05).
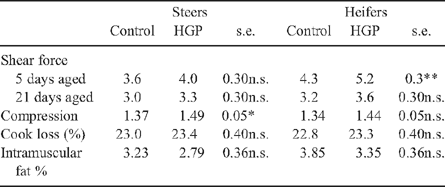
Objective meat quality
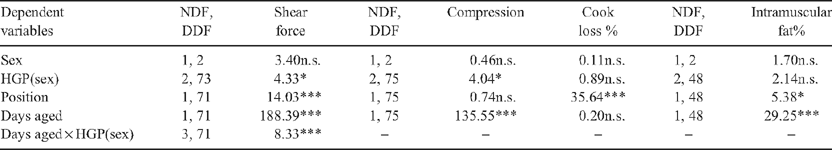
There was a significant (P < 0.001) interaction between HGP(sex) and days aged (Table 4) whereby the HGP(sex) effect decreased in samples which were aged for longer (Table 5). Compression values for M. longissimus dorsi samples from HGP-implanted steer carcasses were greater than the controls (P < 0.05). The magnitude of the HGP(sex) effect on compression values did not interact with aging (P > 0.05). There was no effect of HGP(sex) treatment on cooking loss or intramuscular fat % (P > 0.05, Table 5).
|
Within the M. longissimus dorsi a position effect was significant for shear force, cook loss and intramuscular fat percentage (P < 0.05, Table 4) whereby the caudal portion of the M. longissimus dorsi had 0.3 kg greater shear force, 1.4% greater cooking loss and 0.17% greater intramuscular fat percentage than the cranial portion.
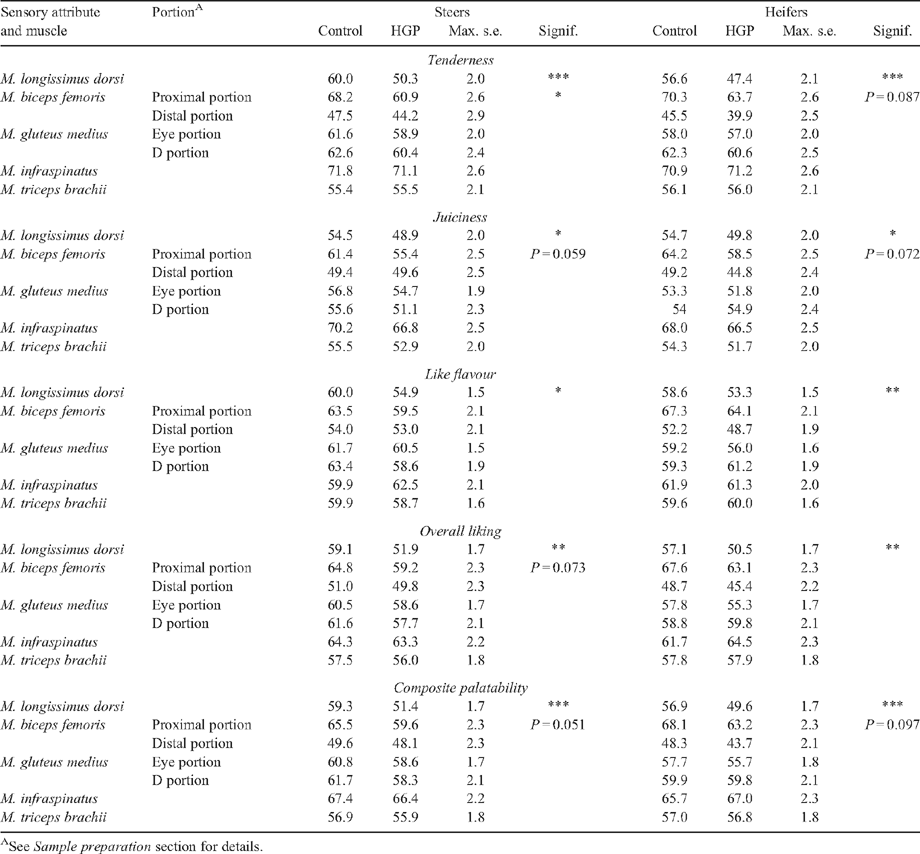
Sensory scores
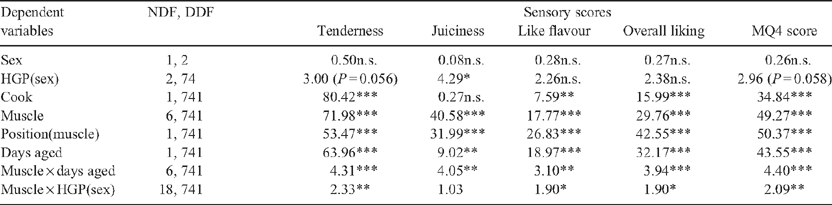
The magnitude of the HGP(sex) effect interacted with muscle for tenderness, overall liking and MQ4 scores (P < 0.05, Table 6). Predicted means in Table 7 showed a large decrease in the sensory scores for the M. longissimus dorsi (P < 0.05) in HGP-implanted steer and heifer carcasses compared with the respective controls. There was a similar trend in the M. biceps femoris, although this did not always attain significance. Differences in sensory scores between HGP-implanted carcasses and controls were not significant (P > 0.05) for the various portions of the Mm. gluteus medius, infraspinatus and triceps brachii.
|
There was a significant interaction between the change in sensory scores with aging and muscle for all sensory scores (P < 0.05, Table 8). The greatest increase in sensory scores due to aging occurred in the M. longissimus dorsi (P < 0.05), with very little change in the M. infraspinatus. The ranking of the muscles on the changes in the different sensory scores with aging from 5 to 21 days was relatively consistent.
|
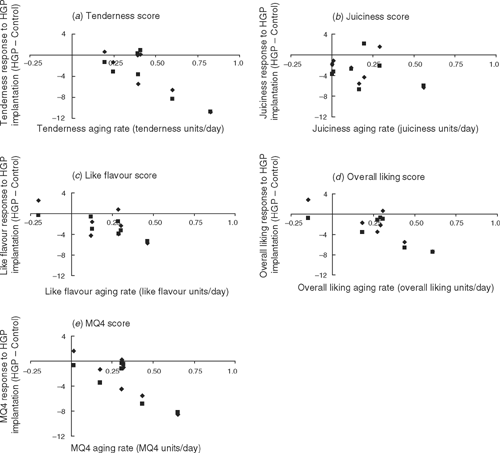
Aging rates for the different sensory scores (i.e. 21-day minus 5-day sensory scores expressed as the change in score per day) were plotted against the mean change in sensory scores due to HGP implantation for the different muscles from heifer and steer carcasses (Fig. 2). For all sensory scores there was a clear trend for muscles with the greatest increase in sensory scores between days 5 and 21 to have the greatest HGP effect on sensory scores.
|
Cooking method had a significant effect on tenderness, like flavour, overall liking and MQ4 scores (P < 0.05, Table 6). Although not tabulated, roasting resulted in greater tenderness, like flavour, overall liking and MQ4 scores than grilling.
Position within muscle was significant for all sensory variables (P < 0.001, Table 7). The cranial portion of the M. longissimus dorsi had greater sensory scores than the caudal portion (the anterior portion had 10.9, 8.0, 5.3, 7.9 and 8.2 greater taste panel scores than the posterior portion for tenderness, juiciness, like flavour, overall liking and MQ4 scores, respectively). Position effects within the M. gluteus medius eye and D portions were not significant (P > 0.05).
Discussion
Effect of HGP implantation on liveweight and carcass measurements
The application of Revalor-S and Revalor-H in steers and heifers resulted in a 30- and 15-kg increase in liveweight over the feeding period. This compared favourably with the review of Australian literature by Sawyer and Barker (1988) who concluded that the gains from a single implant were of the order of 10–20 kg over a 100- to 150-day feeding period. When expressed as a percentage increase, our results were less than Dikeman (2003) who concluded that United States results generally show a 10–20% increase in growth rate due to implants. The smaller increase may reflect the shorter feeding period commonly used in Australia. The formulations of Revalor-S and Revalor-H given to steers and heifers differed slightly in the active ingredients, so it was not possible to directly compare their performance. Montgomery et al. (2001) commented that androgen based and combination implants were required to ensure a liveweight response in heifers.
The increase in ossification score in HGP-treated steers reported in this study agreed with other studies (e.g. Apple et al. 1991). There was no effect of HGP implantation on ossification score in heifers, which was consistent with the results of Crouse et al. (1987). Implantation of steers or heifers with Revalor-S or Revalor-H did not significantly decrease marbling score, or intramuscular fat percentage in the present study. Other reviews have reported a trend, albeit often small, for HGP implants to decrease marbling score (Hunter et al. 2001b; Dikeman 2003). Johnson et al. (1996) and Dunshea et al. (2005) concluded that the effect of implants on marbling was via an increase in lean, rather than fat deposition. Duckett et al. (1999) concluded that the HGP effect was most likely through dilution of the fat, rather than a direct effect on marbling per se. This present study also showed no effect of HGP implants on subcutaneous fat thickness, which again was consistent with other studies (Duckett et al. 1996; Hunter et al. 2001a).
Effect of HGP implants on sensory and objective meat quality
In this study, heifer or steer carcasses which had been implanted with Revalor-S or Revalor-H and slaughtered within the payout period, showed lower sensory scores and greater shear force and compression measurements than carcasses from control animals. There was a clear difference between HGP-implanted carcasses and controls for the M. longissimus dorsi and to a lesser extent the proximal portion of the M. biceps femoris. For the other muscles tested in this study, the HGP effect failed to achieve significance when viewed as a simple contrast between implanted and control carcasses.
The M. longissimus dorsi from HGP-implanted carcasses initially had a greater shear force after 5 days aging, whereas after 21 days aging, the difference had halved. Platter et al. (2003) found no interaction between HGP treatment and aging time for shear force of the M. longissimus dorsi aged 14 or 21 days. Perhaps this was not surprising, given that aging is most rapid immediately after rigor, and by 14 and 21 days most of the changes in shear force would have already occurred.
The smaller differences between HGP and the control groups in compression compared with shear force and the lack of an aging × treatment interaction would suggest that most of the effect was being exhibited via the myofibre axis and decreased with aging. The general consensus is that HGP implants result in muscle hypertrophy via a net increase in net protein accretion (e.g. Vernon and Buttery 1978; Johnson et al. 1996; Dunshea et al. 2005), although the relative contributions of muscle synthesis and muscle degradation varies between studies. Some studies have reported no effect of testosterone and oestradiol on protein synthesis or degradation rates (Roeder et al. 1986; Desler et al. 1996), although others have shown an increase in protein synthesis alone (Martinez et al. 1984; Hayden et al. 1992). Kerth et al. (2003) concluded that implantation of cattle with trenbolone acetate impacted both synthesis and degradation, with perhaps the strongest effect on protein synthesis.
Several studies have shown reduced protease activity in post-mortem muscle of implanted animals. In an earlier review, Dransfield (1994) concluded that implanted animals showed increases in calpastatin, an inhibitor to the calpain enzymes in the live animal. This was supported by Gerken et al. (1995) who showed that implants with oestradiol and trenolone acetate resulted in an increase in calpaststin activity. Dransfield (1994) proposed a model for tenderisation in which he hypothesised that the use of growth promoters were likely to decrease protein turnover in the live animal, which if carried through to the post-mortem carcass would impact on the aging rates and result in tougher meat. Questions on how changes in protein turnover in the live animal could impact on tenderness were reviewed by Koohmaraie et al. (2002). They concluded that increases in protein synthesis per se were unlikely to impact tenderness, because the factors that regulate protein synthesis were not directly involved in the regulation of meat tenderness and tenderisation. Rather, it was the calpastatin activity that was important in determining post-mortem tenderness. Koohmaraie et al. (2002) commented that most of the examples, where increased net protein accretion has been achieved in the live animal, have generally been via decreased protein degradation and, therefore, would potentially have a negative impact on meat quality.
Ouali and Talmant (1990) reported a range of calpain to calpastatin ratios in the muscles of the beef carcass which contributed to differences in aging rates across the musculature of the carcass. Those muscles with the greater aging rates had lower levels of calpastatin. Presumably, the increase in calpastatin due to HGP implants (Gerken et al. 1995) occurred across the musculature and, therefore, those muscles with the highest calpain to calpastatin ratio (or greatest aging rate) would have the greatest response to HGP application and, therefore, potentially the greatest impact on post-mortem tenderness. While protease activity was not measured in this study, aging rates for individual muscles were estimated. Figure 2 clearly showed that those muscles with the greatest aging rates also had the greatest increase in toughness to HGP implants.
Although the higher calpastatin levels in the muscles from HGP implanted animals would presumably lead to slower aging rates after slaughter, this study failed to show any interaction between HGP treatment and aging rate for sensory scores, although this interaction did achieve significance for shear force. However, it was likely that by 5 days of aging, differences in enzyme activity due to HGP implantation would have largely been exhausted (Koohmaraie and Geesink 2006). Therefore, in the present study, it would be difficult to detect differences in aging rate between HGP-treated and control samples. Gerken et al. (1995) investigated the effect of several HGP treatments on sensory and objective traits in three different muscles at 7, 14 and 21 days. While they reported a HGP implant effect for the top round (i.e. M. gluteus medius), the interaction between HGP implant and aging was not significant for objective or sensory traits in the three muscles tested.
Conclusion
Steers and heifers implanted with Revalor-S or Revalor-H and slaughtered within the payout period resulted in tougher meat in some muscles. The greatest response was in those muscles that had the greatest aging rates after slaughter. This result was consistent with a mechanism for the increased lean due to HGP implants being in part due to reduced protein degradation, possibly as a result of increased calpastatin activity in the live animal, which resulted in lower aging rates and less tender meat after the animal had been slaughtered. This effect was most evident in those muscles with the greatest aging rates, as presumably these muscles would respond most to increased calpastatin activity. The MSA system, which predicts palatability of individual muscles from a range of production and processing inputs, is well placed to incorporate the HGP by muscle interaction into the muscle-based palatability model.
Acknowledgements
Thanks are due to the technical assistance of the staff at Vasse Research Station for the feeding and care of the animals. Cosign Pty Ltd prepared the samples for sensory testing and in conjunction with Sensory Solutions Pty Ltd, conducted the sensory testing.
Apple JK,
Dikeman ME,
Simms DD, Kulh G
(1991) Effect of synthetic hormone implants, singularly or in combinations, on performance carcass traits and longissimus palatability of Holstein steers. Journal of Animal Science 69, 4437–4448.
|
CAS |
PubMed |

Crouse JD,
Schanbacher BD,
Cross HR,
Seideman SC, Smith SB
(1987) Growth and carcass traits of heifers as affected by hormonal treatment Journal of Animal Science 64, 1434–1440.
|
CAS |
PubMed |

Desler MM,
Jones SJ,
Smith CW, Woods TL
(1996) Effects of dexamethasone and anabolic agents on proliferation and protein synthesis and degradation in C2C12 myogenic cells. Journal of Animal Science 74, 1265–1273.
|
CAS |
PubMed |

Dikeman ME
(2003) Metabolic modifiers and genetics: effects on carcass traits and meat quality. Brazilian Journal of Food Technology 6(Special issue), 1–38.

Dikeman ME
(2007) Effect of metabolic modifiers on carcass traits and meat quality. Meat Science 77, 121–135.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Dransfield E
(1994) Optmisation of tenderization, ageing and tenderness. Meat Science 36, 105–121.
| Crossref | GoogleScholarGoogle Scholar |

Duckett SK,
Wagner DG,
Owens FN,
Dolezal HG, Gill DR
(1996) Effects of estrogenic and androgenic implants on performance, carcass traits, and meat tenderness in feedlot steers: a review. The Professional Animal Scientist 12, 205–214.

Duckett SK,
Wagner DG,
Owens FN,
Dolezal HG, Gill DR
(1999) Effect of anaobolic implants on beef intramuscular fat lipid content. Journal of Animal Science 77, 1100–1104.
|
CAS |
PubMed |

Dunshea FRD,
Souza DN,
Pethick DW,
Harper GS, Warner RD
(2005) Effects of dietary factors and other metabolic modifiers on quality and nutritional value of meat. Meat Science 71, 8–38.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Geesink GH,
Koolmees PA,
van Laack HLJM, Smulders EJM
(1995) Determinants of tenderisation in beef longissimus dorsi and triceps brachi muscles. Meat Science 41, 7–17.
| Crossref | GoogleScholarGoogle Scholar |

Gerken CL,
Tatum JD,
Morgan JB, Smith GC
(1995) Use of genetically identical (clone) steers to determine the effects of estrogenic and androgenic implants on beef quality and palatability characteristics. Journal of Animal Science 73, 3317–3324.
|
CAS |
PubMed |

Hayden JM,
Bergen WG, Merkel RA
(1992) Skeletal muscle protein metabolism and serum growth hormone, insulin, and cortisol concentrations in growing steers implanted with estradiol-17β, trenbolone acetate, or estradiol-17β plus trenbolone acetate. Journal of Animal Science 70, 2109–2119.
|
CAS |
PubMed |

Hunter RA,
Burrow HM, McCrabb GJ
(2001a) Sustained growth promotion, carcass weight and meat quality of steers slaughtered at three liveweights. Australian Journal of Experimental Agriculture 41, 1033–1040.
| Crossref | GoogleScholarGoogle Scholar |

Johnson BJ,
Anderson PT,
Meiske JC, Dayton WR
(1996) Effects of combined trenbolone acetate and estradiol implant of feedlot performance carcass characteristics and carcass composition of feedlot steers. Journal of Animal Science 74, 363–371.
|
CAS |
PubMed |

Kerth CR,
Montgomery JL,
Morrow KJ,
Galyean ML, Miller MF
(2003) Protein turnover and sensory traits of longissimus muscle from implanted and non-implanted heifers. Journal of Animal Science 81, 1728–1735.
|
CAS |
PubMed |

Koohmaraie M, Geesink GH
(2006) Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Science 74, 34–43.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Koohmaraie M,
Kent MP,
Shackelford SD,
Veieth E, Wheeler TL
(2002) Meat tenderness and muscle growth: is there any relationship? Meat Science 62, 345–352.
| Crossref | GoogleScholarGoogle Scholar |

Martinez JA,
Buttery PJ, Pearson JT
(1984) The mode of action of anabolic agents: the effect of testosterone on muscle protein metabolism in the female rat. The British Journal of Nutrition 52, 515–521.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Montgomery TH,
Dew PF, Brown MS
(2001
) Optimizing carcass value and the use of anabolic implants in beef cattle. Journal of Animal Science 79(E. Suppl.), E296–E306.

Nichols WT,
Galyean ML,
Thomson DU, Hutcheson JP
(2002) Review: effects of steroid implants on the tenderness of beef. The Professional Animal Scientist 18, 202–210.

Ouali A, Talmant A
(1990) Calpains and calpastatin distribution in bovine, porcine and ovine skeletal muscles. Meat Science 28, 331–348.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Perry D,
Shorthose WR,
Ferguson DM, Thompson JM
(2001) Methods used in the CRC program for the determination of carcass yield and beef quality. Australian Journal of Experimental Agriculture 41, 953–958.
| Crossref | GoogleScholarGoogle Scholar |

Platter WJ,
Tatum JD,
Belk KE,
Scanga JA, Smith GC
(2003) Effects of repetitive use of hormonal implants on beef carcass quality, tenderness, and consumer ratings of beef palatability. Journal of Animal Science 91, 984–996.

Polkinghorne R,
Thompson JM,
Watson R,
Gee A, Porter M
(2008) Evolution of the Meat Standards Australia (MSA) beef grading system. Australian Journal of Experimental Agriculture 48, 1351–1359.

Roeder RA,
Thorpe SD,
Byers FM,
Schelling GT, Gunn JM
(1986) Influence of anabolic agents on protein synthesis and degradation in muscle cells grown in culture. Growth 50, 485–495.
|
CAS |
PubMed |

Sawyer GJ, Barker DJ
(1988) Growth promotants in cattle in Australia. Australian Veterinary Journal 65, 101–108.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Thompson J
(2002) Managing meat tenderness. Meat Science 62, 295–308.
| Crossref | GoogleScholarGoogle Scholar |

Vernon GG, Buttery PJ
(1978) The effect of trenbolone acetate with time on the various responses of protein synthesis of the rat. The British Journal of Nutrition 40, 563–572.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Watson R
(2008) Meta-analysis of the published effects of HGP use on beef palatability in steers as measured by objective and sensory testing. Australian Journal of Experimental Agriculture 48, 1425–1433.
|
CAS |

Watson R,
Gee A,
Polkinghorne R, Porter M
(2008) Consumer assessment of eating quality – development of protocols for Meat Standards Australia (MSA) testing. Australian Journal of Experimental Agriculture 48, 1360–1367.