The contribution of pathogenic soil microbes to ring formation in an iconic Australian arid grass, Triodia basedowii (Poaceae)
Neil D. Ross A and Angela T. Moles A B
A B
A Evolution & Ecology Research Centre, School of Biological, Earth and Environmental Sciences, UNSW Sydney, NSW 2052, Australia.
B Corresponding author. Email: a.moles@unsw.edu.au
Australian Journal of Botany 69(3) 113-120 https://doi.org/10.1071/BT20122
Submitted: 22 September 2020 Accepted: 23 February 2021 Published: 29 March 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Ring-forming species of spinifex grasses (Triodia spp.) are a dominant feature across much of Australia’s arid and semi-arid zone. Researchers have long been curious about the mechanisms underpinning their striking growth form. However, none of the factors investigated to date provide a convincing explanation for ring formation. Here, we asked whether an accumulation of pathogenic soil microbes might impede seedling emergence and subsequent growth in the centre of Triodia basedowii rings. We collected soil from inside and outside naturally occurring spinifex rings and compared plants grown in soil with live microbes to plants grown in sterilised soil. Consistent with our hypothesis, we found that emergence of T. basedowii seedlings was lower in live soil from inside the rings than in live soil from outside the rings. Further, seedling emergence in soil from inside the rings increased significantly in response to soil sterilisation. We found no significant difference in growth between sterile and live soils. However, this might be due to a lack of power caused by high rates of seedling mortality in all treatments. Overall, our study provides evidence for the role of soil pathogens in shaping this iconic Australian grass.
Keywords: arid zone, Australia, culm, fire, hummock grassland, obligate seeder, seedling emergence, semi-arid zone, spinifex, soil pathogens, Triodia basedowii, clonal plants.
Introduction
Ring formation is a curiosity observed among many species of clonal plants (Bonanomi et al. 2014; Watt 1947). Growth in ring-forming clonal plants is characterised by outward radial expansion and establishment of individual ramets followed by progressive die-back of inner, older roots and shoots (Bonanomi et al. 2005, 2014; Sheffer et al. 2007; Cartenì et al. 2012). Ring-forming species are often found in grassland communities (Bonanomi et al. 2014) and semi-arid or arid regions (Sheffer et al. 2007; Ravi et al. 2008) including Australian hummock grasslands (Beadle 1981; Specht 1981). In this study, we investigated ring formation in the Australian grass Triodia basedowii E.Pritz. We asked whether soil microbes might contribute to the distinctive ring growth form of T. basedowii which is commonly seen in Australia’s arid regions.
The hummock grasslands of Australia’s arid and semi-arid interior are dominated by species in the genus Triodia R.Br. (spinifex) and cover at least 1.3 × 106 km2 or 18% of the continent (Griffin 1984; National Vegetation Information System 2007). Although Triodia species are a grass, their ‘hummock’ growth form, consisting of a stiff tangle of culms (hollow stems) and pungent leaves (Burbidge 1945), is functionally similar to that of a shrub (Rice and Westoby 1999; Nicholas et al. 2011), where large individuals can form domes up to 2 m high and more than 2 m in diameter (Burbidge 1945; Lazarides 1997). Triodia hummocks form habitat and provide food for small mammals, lizards and birds (Murray and Dickman 1994; Daly et al. 2007; Brown et al. 2009) and accumulated biomass fuels cyclical wildfires initiating landscape regeneration (Nicholas et al. 2009, 2011; Gamage et al. 2012). Triodia species are valued by Australian indigenous people, providing resins for use in traditional tool making and medicine as well as food and fibre (Gamage et al. 2012), and pastoralists use some species for grazing (Lazarides 1997). As a result of their geographic extent, Triodia grasses are of great ecological, cultural and economic importance.
Ring formation occurs in several Triodia species (Burbidge 1945; Lazarides 1997). Of the known ring formers, Triodia basedowii is widespread across the interior of Australia, growing among sand dunes from 19 to 30°S (Lazarides 1997; Grigg et al. 2008). T. basedowii is killed by fire and is therefore an obligate seeder, relying on an accumulated soil seed bank for regeneration (Casson and Fox 1987; Westoby et al. 1988; Rice and Westoby 1999). Hummocks comprise a collection of individually rooted culms linked by stolons, where the death of roots in older culms is thought to initiate die-back within the centre of ring (Burbidge 1945). Westoby et al. (1988) found that recruitment in T. basedowii was concentrated along the outer edges of burnt hummocks despite similar densities of live seeds under hummocks and around their edges. This outward bias in seedling recruitment could explain ring-shaped hummocks in second-generation plants, but does not explain death and collapse within older hummocks (Burbidge 1946). Thus, although outward radial growth with central die-back is an established phenomenon in Triodia species (Burbidge 1945; Beard 1984), little is known of what causes the death of older culms.
The fact that many ring-forming species occur in arid ecosystems has led some researchers to suggest that water availability might drive ring-formation in some species (Getzin et al. 2016; Yizhaq et al. 2019; Herooty et al. 2020). Although models and observational data are consistent with this idea in some ecosystems, other researchers have noted that ring-formation also occurs in several ecosystems in which water limitation is highly unlikely to be important, including salt-marshes and alluvial grasslands (Cartenì et al. 2012). Further, although the presence of plants can affect soil moisture, the soil in the centre of Asphodelus ramosus rings in Israel have a comparable water content to the soil in the surrounding matrix (Yizhaq et al. 2019). Thus, in this system, water availability does not seem to provide a good explanation for ring-forming plants expanding into the matrix while dying back inside rings. Getzin et al. (2019) also found that the texture and compaction (both factors that affect moisture absorption into soil) of soil inside Triodia rings were not significantly different to that found in bare soil areas outside the rings. Finally, the proposed mechanisms through which water availability might cause ring-formation are not consistent across sites or species (Getzin et al. 2016; Herooty et al. 2020). Experimental work on the role of water availability in ring-formation would be a worthwhile next step, but this is not the goal of the present study.
Previous studies have rejected the idea that ants or termites might be responsible for the die-back in the centre of Triodia hummocks (Getzin et al. 2016, 2019).
Another possibility is that dieback in the centre of ring-forming plants could be caused by depletion of soil nutrients. Rice et al. (1994) found higher levels of soil nitrogen concentrated within T. basedowii, T. pungens and Plectrachne schinzii hummocks compared to outside, whereas available soil phosphorous was lower inside hummocks compared to outside. However, the addition of fertiliser or ash within the boundaries of hummocks did not result in new growth over 4 months following summer rain (Westoby et al. 1988). Thus, the existing evidence suggests that ring formation is not caused by nutrient limitation. We therefore did not consider soil chemistry further in the present study.
Evidence is accumulating that plant–soil feedbacks might contribute to ring-formation in some species. For example, seedlings of Scirpus holoschoenus accumulated twice as much biomass when grown in soil from outside S. holoschoenus tussocks than in soil from inside S. holoschoenus tussocks (Bonanomi et al. 2005). Interestingly, the effects were species specific, with the same soils having much less effect on seedlings of other species (Bonanomi et al. 2005). Models based on negative plant–soil feedbacks have successfully recreated the real-world patterns seen in ring-forming vegetation (Cartenì et al. 2012).
One potential mechanism for ring formation that has not been studied in Triodia is the interaction of the plant with the soil microbial community. Previous studies show that plants can accumulate pathogens in their root zone over time (Bever 1994; Van der Putten et al. 2013; Smith-Ramesh and Reynolds 2017). These pathogens can then weaken plant growth and dominance, facilitating the coexistence of plant species within communities (Klironomos 2002; Bonanomi et al. 2012; Reinhart 2012; Yang et al. 2015; Chung and Rudgers 2016). In addition, soil pathogens can reduce interspecific competitive ability (Petermann et al. 2008; Hendriks et al. 2013) and shape succession of species within a community over time (Oremus and Otten 1981; Van der Putten et al. 1993; Frouz et al. 2016). For example, seedlings grown in soil that has been exposed to the roots of conspecifics have reduced biomass compared to seedlings grown in sterilised conspecific soil or soil exposed to other species (Packer and Clay 2003; Callaway et al. 2004; Gundale et al. 2014). Plants may temporarily escape their pathogens by colonising new soil through clonal growth (van der Stoel et al. 2002; Van der Putten 2003) or by dispersing offspring away from the parent plant (Packer and Clay 2000). In this way, the outward expansion of ring-forming plants may be an escape response from accumulated soil pathogens, with die-back in the centre of the plant being due to older ramets being overwhelmed by soil pathogens. Consistent with this, a study of Bouteloua gracilis in New Mexico, USA showed that roots from the inner edge of grass rings had 1.4 times higher fungal colonisation in the field than did roots from outside the rings (Carlton et al. 2018). While live soil had an overall negative effect on plant growth, glasshouse studies did not show greater negative effects of soil from inside the rings (Carlton et al. 2018). Here, we aimed to test the idea that pathogenic soil microbes might contribute to ring formation in Triodia. Specifically, we tested the hypothesis that microbes in the soil inside naturally formed T. basedowii rings negatively affect the emergence, survival and growth of T. basedowii seedlings.
Materials and methods
Study site
We focused on Triodia basedowii growing naturally on flat, interdune soil in the Northern Territory, Australia. Our study site was located at Deep Well Station, 40 km south of Alice Springs (Fig. 1; details in Table S1 of the Supplementary material). Mean temperatures at this site range from 21.5–36.3°C in January to 4.0–19.8°C in July (Bureau of Meteorology 2017a). The mean annual rainfall, although highly variable year to year (Bureau of Meteorology 2017b), is 282 mm and falls mainly over the warmer months (Bureau of Meteorology 2017a). Vegetation at the site consisted of an open woodland (Specht 1981) of scattered desert oak trees (Allocasuarina decaisneana) up to 15 m tall, with an Acacia shrub layer up to 3 m tall, and a ground layer dominated by ~20% cover of 30 cm high T. basedowii (Table S2 of the Supplementary material). The soil was a red, fine sandy loam with a clay content of 10–20% (Macdonald and Isbell 2009), and dry soil Munsell colour: 2.5YR 4/6. Surface soil samples from other Triodia grasslands in the southern Northern Territory have soil N of 0.015 to 0.022%, soil P of 0.007–0.015%, pH of 5.8–7 and soluble salts of 0.003–0.015% (Winkworth 1967; Rice et al. 1994). Previous significant rainfall occurred during December 2016 (125 mm) and January 2017 (40 mm) (Bureau of Meteorology 2017c). Soil and vegetation data were collected during 27–28 February 2017. Seeds for the glasshouse experiment were collected at Deep Well Station in May 2012 (B. Wright, pers. comm., 9 February 2017).

|
Field sampling
We laid out three 100-m transects with random starting locations and directions. At each 10-m interval along the transects we used a random number generator to select whether we sampled to the left or right of the transect. We then located the nearest T. basedowii that had an internal ring with a diameter greater than 30 cm. We chose a 30-cm diameter to focus our sampling on well-developed rings with substantial central die-back. This size of ring also allowed us to take soil that was unambiguously from the centre of the hummock. The sampled T. basedowii had internal diameters from 0.3 to 1 m, with a mean internal area of 0.265 m2 (n = 30 plants, s.e. = 0.03). If more than one T. basedowii was encountered at the same distance away from the transect, each individual was given a number and then one was chosen using a random number generator. All sampled T. basedowii were at least 2 m (but usually more than 20 m) from established trees.
We then used a random number generator to select a compass direction from the centre of each hummock and collected soil at two points along these radii: inside the hummock, at 10 cm away from the inner edge of the living hummock, and outside the hummock, at 10 cm away from the edge of the plant. Leaf litter and organic matter were removed from the surface of the ground before taking soil samples (following Kardol et al. 2006; Knappová et al. 2016). We used cylindrical cores (10 cm in diameter and 10 cm deep) to extract soil before double-bagging it in polyethylene press-seal bags. Soil was shipped to UNSW Sydney at 4°C to minimise changes in soil biology during transport (Kardol et al. 2006). All soil collection tools were disinfected with 70% ethanol between samples to minimise cross-contamination (Abreu et al. 2013; Cortois et al. 2016).
Soil preparation
We passed soil samples through a 2-mm sieve and removed large roots and stones. Each soil sample was then homogenised (by mixing with a spoon) and divided in half (by weight, visually ensuring equal representation of different soil textures in each fraction) to form live and sterile treatments. Each treatment was then divided into bulk soil (95%) and inoculum (5%) components and bagged in polyethylene press-seal bags.
We used 50 kGy gamma irradiation at the Australian Nuclear Science and Technology Organisation in Sydney to sterilise our soil samples. Gamma irradiation causes fewer changes in soil properties than other methods such as autoclaving (Newman et al. 1977; McNamara et al. 2003); however, all methods of soil sterilisation can cause a flush of nutrients that might affect plant growth (McNamara et al. 2003). We therefore sterilised the bulk soil components for both live and sterile treatments to reduce confounding effects. The inoculum component for the sterile treatment was also sterilised. We then recombined bulk and inoculum soil components to produce the live (95% sterile, 5% live) and sterilised (100% sterile) soil treatments for each hummock position (i.e. inside and outside the T. basedowii rings). The fact that all plants were grown in at least 95% sterilised soil minimises differences between treatments that might have arisen from the effects of sterilisation.
Glasshouse experiment
We placed 290 g of live or sterile soil into new 50 × 50 × 120-mm forestry pots, lined with a square of new paper towel to prevent soil leakage. Pots were saturated with water (~50 mL) shortly before seeds were sown. All equipment that came into contact with soil was disinfected with 70% ethanol between samples to avoid cross-contamination.
We extracted the seeds from the florets by rubbing them against a 1.2-mm sieve and then winnowing them (W. Lewandrowski, pers. comm., 7 March 2017). The seeds weighed an average of 0.92 mg, and were ~1 mm wide and 2 mm long. We then soaked the seeds in smoke-water solution for 24 h to release physiological dormancy before rinsing and drying them (Erickson et al. 2016; W Lewandrowski, pers. comm., 7 March 2017). Twenty-five seeds were sown into each pot (3000 in total), 1–2 mm below the surface of moist soil.
Pots were individually covered with polyethylene wrap to maintain surface soil moisture, and were placed randomly on mesh benches in the UNSW glasshouse with positions randomised weekly to minimise differences between treatments that might be caused by position effects in the glasshouse. Every 3 days, the wrap was lifted, the soil was sprayed with water and the pots were surveyed for the presence of live plants. The amount of water provided was comparable among all pots.
Seedling emergence plateaued after 18 days, after which the polyethylene wrap was removed. One randomly selected seedling from each pot was replanted in the centre of its pot. Plants were then watered at least once daily to maintain soil moisture. The growth phase of the experiment ran for a further 101 days, a duration sufficiently long to develop soil microbe related growth effects (Wagg et al. 2014) while maintaining unique microbe communities (Newman et al. 1977). At the completion of the growth phase, we carefully removed the plants from the soil, separated the roots from the base of the grass and discarded any remaining seed coats. We dried the samples at 70°C for 48 h and then cooled them in bell jars with a desiccant to reduce moisture reabsorption. Root and shoot biomass samples were then weighed using a microbalance (Mettler Toledo XP26, 1-μg precision).
Data analyses
We recorded four traits: seedling emergence (as a percentage of seeds planted); seedling emergence time (days); seedling survival (the number of seedlings harvested at 101 days as a percentage of initial number of seedlings at the start of the growth phase); and total seedling biomass (accumulated at the end of growth phase). Data for seedling emergence, seedling emergence time and total seedling biomass were analysed using generalised linear mixed models (GLMM) with a random effect for hummock and fixed effects for position (inner or outer) and sterilisation (sterile or live). Seedling emergence and survival (both binary) were fitted with binomial models while seedling emergence time was fitted with a negative binomial model. Data for total seedling biomass were log-transformed to improve normality. Data were analysed in R (ver. 3.4.1, R Core Team, R Foundation for Statistical Computing: Vienna, Austria, see https://www.r-project.org/) using the lme4 (ver. 1.1-13, see https://cran.r-project.org/web/packages/lme4/index.html; Bates et al. 2015) and nlme (ver. 3.1-131, J. Pinheiro, D. Bates, S. DebRoy, D. Sarkar and R Core Team, see https://CRAN.R-project.org/package=nlme) packages.
Results
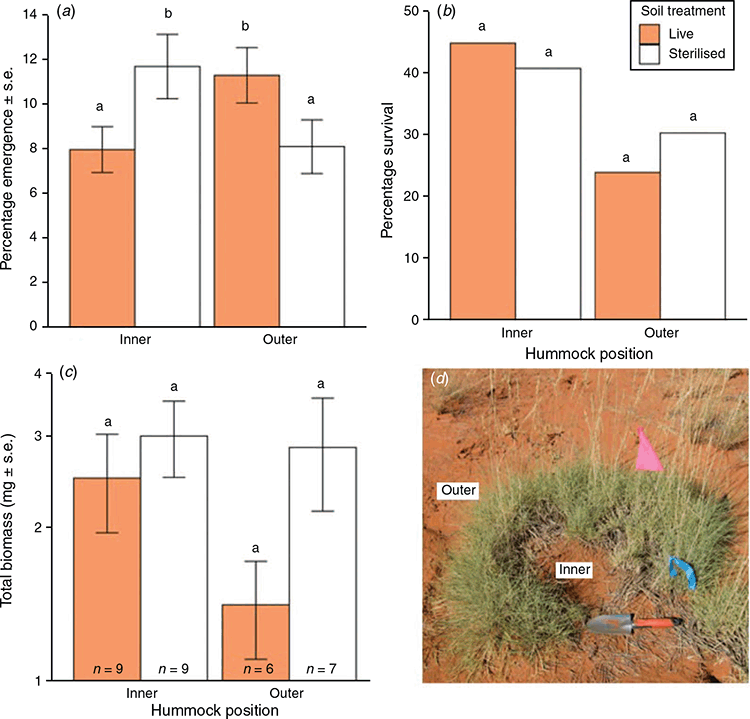
The emergence of T. basedowii seedlings was simultaneously affected by soil source position and sterilisation treatment (P < 0.001, Fig. 2a). Consistent with our hypothesis, we found that seedling emergence was 41% higher in live soil from the outside of hummocks than in live soil from the inside of hummocks (P = 0.037, Fig. 2a), and that seedling emergence inside hummocks was 46% higher for seeds planted in sterilised soil than those planted in live soil (P = 0.015, Fig. 2a). Interestingly, seeds planted in soil from outside hummocks showed 39% higher emergence in live soil compared to sterilised soil (P = 0.034, Fig. 2a). Finally, seedling emergence was 44% higher in sterilised soil from within hummocks than in sterilised soil from outside hummocks, but this difference was marginally non-significant (P = 0.052, Fig. 2a). On average, seedlings emerged 1.9 days earlier in live soil than in sterilised soil (P = 0.007, Fig. S1 of the Supplementary material), with no significant differences in emergence times detected among soil positions (P > 0.3, Fig. S1).
More than half of the seedlings died during the experiment with mortality unrelated to sterilisation (P = 0.730) or soil position (P = 0.219; Fig. 2b). Initial losses of 13 to 27% among treatments were caused by dehydration; however, further steady losses followed during the growth phase of the experiment. Only nine seedlings remained to represent the ‘inner-live’ and ‘inner-sterilised’ treatments, six seedlings to represent the ‘outer-live’ treatment and seven to represent the ‘outer-sterilised’ treatment. This low sample size decreased our ability to detect differences between treatments in subsequent measures. No significant differences were found in the total biomass of T. basedowii seedlings grown in soil from inside or outside hummocks (P = 0.178, Fig. 2c). There were also no significant differences in biomass between seedlings grown in live v. sterile soil (P = 0.461, Fig. 2c).
Discussion
Our study showed that the presence of soil microbes might contribute to the distinctive ring-shaped growth form of the common Australian spinifex grass, T. basedowii. This idea is supported by two key findings. First, consistent with our hypothesis, we found that unsterilised soil from inside hummocks negatively affects seedling recruitment. This was evident from the significantly lower emergence of T. basedowii seedlings in live soil from inside the rings compared to live soil from outside the rings. Second, and also consistent with our hypothesis, we found significantly greater seedling emergence for seeds planted in sterilised soil from inside hummocks than for seeds planted in live soil from inside hummocks (Fig. 2a), suggesting that the presence of soil microbes may restrict T. basedowii recruitment inside hummocks. Our findings are similar to those of Bonanomi et al. (2005), who found that Scirpus holoshoenus from an alluvial grassland in Italy showed substantially less growth in soil from inside tussocks than in soil from outside tussocks.
Most current work on ring-formation in plants in arid ecosystem tends to favour water-limitation as a mechanism (Getzin et al. 2016; Yizhaq et al. 2019; Herooty et al. 2020), whereas evidence for the importance of soil microbes tends to come from more mesic environments (e.g. Bonanomi et al. 2005, 2012). Our findings, and the findings of Carlton et al. (2018) suggest that soil microbes may also have a role in shaping community dynamics and vegetation structure in arid ecosystems. This contributes to a growing body of evidence suggesting that plant–soil feedbacks are important in a wide range of ecosystems worldwide (Piercey et al. 2021).
Soil microbes are often cited as beneficial for plant performance and essential to plant survival on land (e.g. mycorrhizal fungi and rhizobia; Cain et al. 2008; Reece et al. 2012; Evert and Eichhorn 2013). Indeed, recent work on dryland ecosystem restoration has found that in some species, including Triodia, seedling growth and establishment is enhanced with the addition of specific strains of cyanobacteria applied to the seed coats (Muñoz-Rojas et al. 2018; Chua et al. 2020). Consistent with this idea, we saw significantly higher emergence of T. basedowii seeds in live than sterile soil from outside the rings (Fig. 2a). Thus, the expansion of rings outward may allow plants to reach areas with more beneficial microbiota. Conversely, in soil from inside T. basedowii rings, the effects of pathogenic soil microbes seem to outweigh the effects of beneficial soil microbes. This is consistent with findings from other studies, which show that sterilisation often increases the germination success of seeds grown in soil previously occupied by other species (Mohler et al. 2012; Lou et al. 2016), and that seedlings of plants grown in soil exposed to the roots of their own species perform better in sterilised soil samples than in soil samples that contain live microbes (Kardol et al. 2007; Gundale et al. 2014; Carlton et al. 2018).
Our results suggest that die-back in the centre of T. basedowii hummocks might be explained by older culms succumbing to a build-up of pathogenic soil microbes through time. This has been demonstrated, for example, in the European coastal pioneer Ammophila arenaria (also a grass species adapted for growth in sand-dunes), where ramets establishing in new wind-deposited sand initially grow strongly before degenerating over time in the presence of root parasitic nematodes (Van der Putten et al. 1988; van der Stoel et al. 2002). In addition, the outward expansion of T. basedowii hummocks could be explained as an escape response from accumulated soil pathogens with clonal growth away from the pathogenic centre or dispersal of offspring into soil with fewer microbes. This idea is supported by other studies, for example, seedlings of the North American black cherry, Prunus serotina, are far less affected by damping-off fungus when grown in soil away from parent trees compared to soil from within the root zone of parents (Packer and Clay 2000). This combination of die-back and escape as a result of pathogenic soil microbes is consistent with the ring-shaped growth form observed in T. basedowii.
Our study points to three worthwhile directions for future research. First, now that we know that soil microbes can have substantial effects on the establishment of arid-zone plants, it would be well worth using molecular methods to identify the types of microbes involved. Second, our finding that seedlings in live soil treatments emerged 1.9 days earlier than seedlings in sterile soil treatments suggests that perhaps different types of microbes affect seedlings at different times or under different conditions of germination (Funk et al. 2014). Identifying the microbes that are most important at different times would help us to understand the forces shaping plant recruitment, growth, and survival. Third, our non-significant results for seedling survival and biomass are likely related to a small sample size following the loss of many seedling replicates due to non-treatment effects. It would be interesting to determine whether the non-significant trend for seedlings to perform better in sterilised soil than in live soil (Fig. 2c) is upheld in a larger experiment.
Considering the extensive distribution and ecological importance of Triodia species, we know remarkably little about this iconic group of plants. Our data suggest that soil microbes may play a role in the ring formation of the widespread T. basedowii. This new information helps us further understand the unique ecology of Australia’s arid grasslands, and adds to our growing recognition of the crucial function that soil microbes play in terrestrial ecosystem processes worldwide (van der Heijden et al. 2008).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This study was partially funded by the UNSW School of BEES Student Research Project Fund.
Acknowledgements
This study was inspired by Dr Barbara Rice (1944–2009), who is greatly missed. Thanks to Jan Hayes for allowing access to the study site on Deep Well Station; Sharon Ryall for assisting with field equipment; Boyd Wright for supplying the seeds and advice about the study site; Wolfgang Lewandrowski for his tips and enthusiasm on growing Triodia; Peter Jobson for supplying plant species information and access to the NT herbarium, Alice Springs and Frank Hemmings for assistance in identifying specimens; Riin Tamme, Peter Geelan-Small for assistance with statistical analysis and Claire Brandenburger for helpful comments on the manuscript.
References
Abreu AC, Tavares RR, Borges A, Mergulhão F, Simões M (2013) Current and emergent strategies for disinfection of hospital environments. The Journal of Antimicrobial Chemotherapy 68, 2718–2732.| Current and emergent strategies for disinfection of hospital environments.Crossref | GoogleScholarGoogle Scholar | 23869049PubMed |
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Beadle N (1981) ‘The Vegetation of Australia.’ (The Press Syndicate of the University of Cambridge: Cambridge, UK)
Beard JS (1984) The vegetation of the Australian arid zone. In ‘Arid Australia’. (Eds HG Cogger, EE Cameron) pp. 113–119. (Australia Museum: Sydney, NSW, Australia)
Bever JD (1994) Feedback between plants and their soil communities in an old field community. Ecology 75, 1965–1977.
| Feedback between plants and their soil communities in an old field community.Crossref | GoogleScholarGoogle Scholar |
Bonanomi G, Rietkerk M, Dekker SC, Mazzoleni S (2005) Negative plant–soil feedback and positive species interaction in a herbaceous plant community. Plant Ecology 181, 269–278.
| Negative plant–soil feedback and positive species interaction in a herbaceous plant community.Crossref | GoogleScholarGoogle Scholar |
Bonanomi G, Mingo A, Incerti G, Mazzoleni S, Allegrezza M (2012) Fairy rings caused by a killer fungus foster plant diversity in species‐rich grassland. Journal of Vegetation Science 23, 236–248.
| Fairy rings caused by a killer fungus foster plant diversity in species‐rich grassland.Crossref | GoogleScholarGoogle Scholar |
Bonanomi G, Incerti G, Stinca A, Carteni F, Giannino F, Mazzoleni S (2014) Ring formation in clonal plants. Community Ecology 15, 77–86.
| Ring formation in clonal plants.Crossref | GoogleScholarGoogle Scholar |
Brown S, Clarke M, Clarke R (2009) Fire is a key element in the landscape-scale habitat requirements and global population status of a threatened bird: the Mallee Emu-wren (Stipiturus mallee). Biological Conservation 142, 432–445.
| Fire is a key element in the landscape-scale habitat requirements and global population status of a threatened bird: the Mallee Emu-wren (Stipiturus mallee).Crossref | GoogleScholarGoogle Scholar |
Burbidge NT (1945) Morphology and anatomy of the Western Australian species of Triodia R.Br. Transactions of the Royal Society of South Australia 69, 303–308.
Burbidge N (1946) A revision of the Western Australian Species Triodia R.Br. Journal of the Royal Society of Western Australia 30, 15–33.
Bureau of Meteorology (2017a) Climate statistics for Australian locations – Monthly climate statistics: Alice Springs Airport. (Bureau of Meteorology, Commonwealth of Australia: Canberra, ACT, Australia) Available at http://www.bom.gov.au/climate/averages/tables/cw_015590_All.shtml [Verified 21 August 2017]
Bureau of Meteorology (2017b) Rainfall variability. (Bureau of Meteorology, Commonwealth of Australia: Canberra, ACT, Australia) Available at http://www.bom.gov.au/jsp/ncc/climate_averages/rainfall-variability/index.jsp [Verified 26 February 2021]
Bureau of Meteorology (2017c) Latest weather observations for Alice Springs Airport. (Bureau of Meteorology, Commonwealth of Australia: Canberra, ACT, Australia) Available at http://www.bom.gov.au/jsp/ncc/climate_averages/rainfall-variability/index.jsp [Verified 22 August 2017]
Cain ML, Bowman WD, Hacker SD (2008) ‘Ecology.’ (Sinauer Associates Inc.: Sunderland, MA, USA)
Callaway RM, Thelen GC, Rodriguez A, Holben WE (2004) Soil biota and exotic plant invasion. Nature 427, 731–733.
| Soil biota and exotic plant invasion.Crossref | GoogleScholarGoogle Scholar | 14973484PubMed |
Carlton L, Duncritts NC, Chung YA, Rudgers JA (2018) Plant–microbe interactions as a cause of ring formation in Bouteloua gracilis. Journal of Arid Environments 152, 1–5.
| Plant–microbe interactions as a cause of ring formation in Bouteloua gracilis.Crossref | GoogleScholarGoogle Scholar |
Cartenì F, Marasco A, Bonanomi G, Mazzoleni S, Rietkerk M, Giannino F (2012) Negative plant soil feedback explaining ring formation in clonal plants. Journal of Theoretical Biology 313, 153–161.
| Negative plant soil feedback explaining ring formation in clonal plants.Crossref | GoogleScholarGoogle Scholar | 22974971PubMed |
Casson NE, Fox JED (1987) The post-fire regeneration responses of Triodia wiseana and T. basedowii. Australian Rangeland Journal 9, 53–55.
| The post-fire regeneration responses of Triodia wiseana and T. basedowii.Crossref | GoogleScholarGoogle Scholar |
Chua M, Erickson TE, Merritt DJ, Chilton AM, Ooi MK, Muñoz‐Rojas M (2020) Bio‐priming seeds with cyanobacteria: effects on native plant growth and soil properties. Restoration Ecology 28, S168–S176.
| Bio‐priming seeds with cyanobacteria: effects on native plant growth and soil properties.Crossref | GoogleScholarGoogle Scholar |
Chung YA, Rudgers JA (2016) Plant–soil feedbacks promote negative frequency dependence in the coexistence of two aridland grasses. Proceedings of the Royal Society of London – B. Biological Sciences 283, 20160608
| Plant–soil feedbacks promote negative frequency dependence in the coexistence of two aridland grasses.Crossref | GoogleScholarGoogle Scholar |
Cortois R, Schroder-Georgi T, Weigelt A, Van der Putten WH, De Deyn GB (2016) Plant–soil feedbacks: role of plant functional group and plant traits. Journal of Ecology 104, 1608–1617.
| Plant–soil feedbacks: role of plant functional group and plant traits.Crossref | GoogleScholarGoogle Scholar |
Daly BG, Dickman CR, Crowther MS (2007) Selection of habitat components by two species of agamid lizards in sandridge desert, central Australia. Austral Ecology 32, 825–833.
| Selection of habitat components by two species of agamid lizards in sandridge desert, central Australia.Crossref | GoogleScholarGoogle Scholar |
Erickson TE, Shackelford N, Dixon KW, Turner SR, Merritt DJ (2016) Overcoming physiological dormancy in seeds of Triodia (Poaceae) to improve restoration in the arid zone. Restoration Ecology 24, S64–S76.
| Overcoming physiological dormancy in seeds of Triodia (Poaceae) to improve restoration in the arid zone.Crossref | GoogleScholarGoogle Scholar |
Evert RF, Eichhorn SE (2013) ‘Raven Biology of Plants’, 8th edn. (W. H. Freeman and Company: New York, NY, USA)
Frouz J, Toyota A, Mudrak O, Jilkova V, Filipova A, Cajthaml T (2016) Effects of soil substrate quality, microbial diversity and community composition on the plant community during primary succession. Soil Biology & Biochemistry 99, 75–84.
| Effects of soil substrate quality, microbial diversity and community composition on the plant community during primary succession.Crossref | GoogleScholarGoogle Scholar |
Funk FA, Loydi A, Peter G (2014) Effects of biological soil crusts and drought on emergence and survival of a Patagonian perennial grass in the Monte of Argentina. Journal of Arid Land 6, 735–741.
| Effects of biological soil crusts and drought on emergence and survival of a Patagonian perennial grass in the Monte of Argentina.Crossref | GoogleScholarGoogle Scholar |
Gamage HK, Mondal S, Wallis LA, Memmott P, Martin D, Wright BR, Schmidt S (2012) Indigenous and modern biomaterials derived from Triodia (‘spinifex’) grasslands in Australia. Australian Journal of Botany 60, 114–127.
| Indigenous and modern biomaterials derived from Triodia (‘spinifex’) grasslands in Australia.Crossref | GoogleScholarGoogle Scholar |
Getzin S, Yizhaq H, Bell B, Erickson TE, Postle AC, Katra I, Tzuk O, Zelnik YR, Wiegand K, Wiegand T (2016) Discovery of fairy circles in Australia supports self-organization theory. Proceedings of the National Academy of Sciences of the United States of America 113, 3551–3556.
| Discovery of fairy circles in Australia supports self-organization theory.Crossref | GoogleScholarGoogle Scholar | 26976567PubMed |
Getzin S, Yizhaq H, Muñoz‐Rojas M, Wiegand K, Erickson TE (2019) A multi‐scale study of Australian fairy circles using soil excavations and drone‐based image analysis. Ecosphere 10, e02620
| A multi‐scale study of Australian fairy circles using soil excavations and drone‐based image analysis.Crossref | GoogleScholarGoogle Scholar |
Griffin GF (1984) Hummock grasslands. In ‘Management of Australia’s Rangelands’. (Eds GN Harrington, AD Wilson, MD Young) pp. 271–284. (CSIRO Publishing: Melbourne, Vic., Australia)
Grigg AM, Veneklaas EJ, Lambers H (2008) Water relations and mineral nutrition of Triodia grasses on desert dunes and interdunes. Australian Journal of Botany 56, 408–421.
| Water relations and mineral nutrition of Triodia grasses on desert dunes and interdunes.Crossref | GoogleScholarGoogle Scholar |
Gundale MJ, Kardol P, Nilsson MC, Nilsson U, Lucas RW, Wardle DA (2014) Interactions with soil biota shift from negative to positive when a tree species is moved outside its native range. New Phytologist 202, 415–421.
| Interactions with soil biota shift from negative to positive when a tree species is moved outside its native range.Crossref | GoogleScholarGoogle Scholar |
Hendriks M, Mommer L, de Caluwe H, Smit-Tiekstra AE, Van der Putten WH, de Kroon H (2013) Independent variations of plant and soil mixtures reveal soil feedback effects on plant community overyielding. Journal of Ecology 101, 287–297.
| Independent variations of plant and soil mixtures reveal soil feedback effects on plant community overyielding.Crossref | GoogleScholarGoogle Scholar |
Herooty Y, Bar P, Yizhaq H, Katz O (2020) Soil hydraulic properties and water source-sink relations affect plant rings’ formation and sizes under arid conditions. Flora 270, 151664
| Soil hydraulic properties and water source-sink relations affect plant rings’ formation and sizes under arid conditions.Crossref | GoogleScholarGoogle Scholar |
Kardol P, Bezemer TM, Van der Putten WH (2006) Temporal variation in plant–soil feedback controls succession. Ecology Letters 9, 1080–1088.
| Temporal variation in plant–soil feedback controls succession.Crossref | GoogleScholarGoogle Scholar | 16925657PubMed |
Kardol P, Cornips NJ, van Kempen MML, Bakx-Schotman JMT, Van der Putten WH (2007) Microbe‐mediated plant–soil feedback causes historical contingency effects in plant community assembly. Ecological Monographs 77, 147–162.
| Microbe‐mediated plant–soil feedback causes historical contingency effects in plant community assembly.Crossref | GoogleScholarGoogle Scholar |
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417, 67–70.
| Feedback with soil biota contributes to plant rarity and invasiveness in communities.Crossref | GoogleScholarGoogle Scholar | 11986666PubMed |
Knappová J, Pankova H, Munzbergov Z (2016) Roles of arbuscular mycorrhizal fungi and soil abiotic conditions in the establishment of a dry grassland community. PLoS One 11, e0158925
| Roles of arbuscular mycorrhizal fungi and soil abiotic conditions in the establishment of a dry grassland community.Crossref | GoogleScholarGoogle Scholar | 27391899PubMed |
Lazarides M (1997) A revision of Triodia including Plectrachne (Poaceae, Eragrostideae, Triodiinae). Australian Systematic Botany 10, 381–489.
| A revision of Triodia including Plectrachne (Poaceae, Eragrostideae, Triodiinae).Crossref | GoogleScholarGoogle Scholar |
Lou Y, Davis AS, Yannarell AC (2016) Interactions between allelochemicals and the microbial community affect weed suppression following cover crop residue incorporation into soil. Plant and Soil 399, 357–371.
| Interactions between allelochemicals and the microbial community affect weed suppression following cover crop residue incorporation into soil.Crossref | GoogleScholarGoogle Scholar |
Macdonald R, Isbell R (2009) Soil profile. In ‘Australian Soil and Land Survey Field Handbook’, 3rd edn. (Ed. The National Committee on Soil and Terrain) pp. 147–204. (CSIRO Publishing: Melbourne, Vic., Australia)
McNamara NP, Black HIJ, Beresford NA, Parekh NR (2003) Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Applied Soil Ecology 24, 117–132.
| Effects of acute gamma irradiation on chemical, physical and biological properties of soils.Crossref | GoogleScholarGoogle Scholar |
Mohler CL, Dykeman C, Nelson EB, Ditommaso A (2012) Reduction in weed seedling emergence by pathogens following the incorporation of green crop residue. Weed Research 52, 467–477.
| Reduction in weed seedling emergence by pathogens following the incorporation of green crop residue.Crossref | GoogleScholarGoogle Scholar |
Muñoz-Rojas M, Chilton A, Liyanage G, Erickson T, Merritt D, Neilan B, Ooi M (2018) Effects of indigenous soil cyanobacteria on seed germination and seedling growth of arid species used in restoration. Plant and Soil 429, 91–100.
| Effects of indigenous soil cyanobacteria on seed germination and seedling growth of arid species used in restoration.Crossref | GoogleScholarGoogle Scholar |
Murray BR, Dickman CR (1994) Granivory and microhabitat use in Australian desert rodents: are seeds important? Oecologia 99, 216–225.
| Granivory and microhabitat use in Australian desert rodents: are seeds important?Crossref | GoogleScholarGoogle Scholar | 28313875PubMed |
National Vegetation Information System (2007) Australian Natural Resources Atlas: vegetation profiles: fact sheets of the major vegetation groups: MVG 20 – hummock grasslands. (Department of Environment and Energy, Australian Government: Canberra, ACT, Australia) Available at https://www.environment.gov.au/land/publications/factsheets-vegetation-profiles-major-vegetation-groups [Verified 22 September 2017]
Newman EI, Campbell R, Rovira AD (1977) Experimental alteration of soil microbial populations for studying effects on higher plant interactions. New Phytologist 79, 107–118.
| Experimental alteration of soil microbial populations for studying effects on higher plant interactions.Crossref | GoogleScholarGoogle Scholar |
Nicholas AMM, Franklin DC, Bowman D (2009) Coexistence of shrubs and grass in a semi-arid landscape: a case study of mulga (Acacia aneura, Mimosaceae) shrublands embedded in fire-prone spinifex (Triodia pungens, Poaceae) hummock grasslands. Australian Journal of Botany 57, 396–405.
| Coexistence of shrubs and grass in a semi-arid landscape: a case study of mulga (Acacia aneura, Mimosaceae) shrublands embedded in fire-prone spinifex (Triodia pungens, Poaceae) hummock grasslands.Crossref | GoogleScholarGoogle Scholar |
Nicholas AMM, Franklin DC, Bowman DMJS (2011) Floristic uniformity across abrupt boundaries between Triodia hummock grassland and Acacia shrubland on an Australian desert sandplain. Journal of Arid Environments 75, 1090–1096.
| Floristic uniformity across abrupt boundaries between Triodia hummock grassland and Acacia shrubland on an Australian desert sandplain.Crossref | GoogleScholarGoogle Scholar |
Oremus PAI, Otten H (1981) Factors affecting growth and nodulation of Hippophaë rhamnoides l. ssp. rhamnoides in soils from two successional stages of dune formation. Plant and Soil 63, 317–331.
| Factors affecting growth and nodulation of Hippophaë rhamnoides l. ssp. rhamnoides in soils from two successional stages of dune formation.Crossref | GoogleScholarGoogle Scholar |
Packer A, Clay K (2000) Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature 404, 278–281.
| Soil pathogens and spatial patterns of seedling mortality in a temperate tree.Crossref | GoogleScholarGoogle Scholar | 10749209PubMed |
Packer A, Clay K (2003) Soil pathogens and Prunus serotina seedling and sapling growth near conspecific trees. Ecology 84, 108–119.
| Soil pathogens and Prunus serotina seedling and sapling growth near conspecific trees.Crossref | GoogleScholarGoogle Scholar |
Petermann JS, Fergus AJF, Turnbull LA, Schmid B (2008) Janzen–Connell effects are widespread and strong enough to maintain diversity in grasslands. Ecology 89, 2399–2406.
| Janzen–Connell effects are widespread and strong enough to maintain diversity in grasslands.Crossref | GoogleScholarGoogle Scholar | 18831160PubMed |
Piercey RS, Gribben PE, Hanley TC, Moles AT, Hughes AR (2021) Incorporating marine macrophytes in plant–soil feedbacks: emerging evidence and opportunities to advance the field. Journal of Ecology 109, 614–625.
| Incorporating marine macrophytes in plant–soil feedbacks: emerging evidence and opportunities to advance the field.Crossref | GoogleScholarGoogle Scholar |
Ravi S, D’Odorico P, Wang L, Collins S (2008) Form and function of grass ring patterns in arid grasslands: the role of abiotic controls. Oecologia 158, 545–555.
| Form and function of grass ring patterns in arid grasslands: the role of abiotic controls.Crossref | GoogleScholarGoogle Scholar | 18855020PubMed |
Reece JB, Meyers N, Urry LA, Cain ML, Wasserman SA, Minorsky PV, Jackson RB, Cooke BN (2012) ‘Campbell Biology’, 9th edn. (Pearson Education Inc.)
Reinhart KO (2012) The organization of plant communities: negative plant–soil feedbacks and semiarid grasslands. Ecology 93, 2377–2385.
| The organization of plant communities: negative plant–soil feedbacks and semiarid grasslands.Crossref | GoogleScholarGoogle Scholar | 23236909PubMed |
Rice B, Westoby M (1999) Regeneration after fire in Triodia R.Br. Australian Journal of Ecology 24, 563–572.
| Regeneration after fire in Triodia R.Br.Crossref | GoogleScholarGoogle Scholar |
Rice BL, Westoby M, Griffin GF, Friedel MH (1994) Effects of supplementary soil nutrients on hummock grasses. Australian Journal of Botany 42, 687–703.
| Effects of supplementary soil nutrients on hummock grasses.Crossref | GoogleScholarGoogle Scholar |
Sheffer E, Yizhaq H, Gilad E, Shachak M, Meron E (2007) Why do plants in resource-deprived environments form rings? Ecological Complexity 4, 192–200.
| Why do plants in resource-deprived environments form rings?Crossref | GoogleScholarGoogle Scholar |
Smith-Ramesh LM, Reynolds HL (2017) The next frontier of plant–soil feedback research: unraveling context dependence across biotic and abiotic gradients. Journal of Vegetation Science 28, 484–494.
| The next frontier of plant–soil feedback research: unraveling context dependence across biotic and abiotic gradients.Crossref | GoogleScholarGoogle Scholar |
Specht RL (1981) Major Vegetation Formations in Australia. In ‘Ecological Biogeography of Australia’. (Ed. A Keast) pp. 165–293. (Dr W. Junk B.V. Publishers)
van der Heijden MG, Bardgett RD, van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology Letters 11, 296–310.
| The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems.Crossref | GoogleScholarGoogle Scholar | 18047587PubMed |
Van der Putten WH (2003) Plant defense belowground and spatiotemporal processes in natural vegetation. Ecology 84, 2269–2280.
| Plant defense belowground and spatiotemporal processes in natural vegetation.Crossref | GoogleScholarGoogle Scholar |
Van der Putten WH, van Dijk C, Troelstra SR (1988) Biotic soil factors affecting the growth and development of Ammophila arenaria. Oecologia 76, 313–320.
| Biotic soil factors affecting the growth and development of Ammophila arenaria.Crossref | GoogleScholarGoogle Scholar | 28312214PubMed |
Van der Putten WH, van Dijk C, Peters BAM (1993) Plant-specific soil-borne diseases contribute to succession in foredune vegetation. Nature 362, 53–56.
| Plant-specific soil-borne diseases contribute to succession in foredune vegetation.Crossref | GoogleScholarGoogle Scholar |
Van der Putten WH, Bardgett RD, Bever JD, Bezemer TM, Casper BB, Fukami T, Kardol P, Klironomos JN, Kulmatiski A, Schweitzer JA, Suding KN, Voorde TFJ, Wardle DA, Hutchings M (2013) Plant–soil feedbacks: the past, the present and future challenges. Journal of Ecology 101, 265–276.
| Plant–soil feedbacks: the past, the present and future challenges.Crossref | GoogleScholarGoogle Scholar |
van der Stoel CD, Van der Putten WH, Duyts H (2002) Development of a negative plant–soil feedback in the expansion zone of the clonal grass Ammophila arenaria following root formation and nematode colonization. Journal of Ecology 90, 978–988.
| Development of a negative plant–soil feedback in the expansion zone of the clonal grass Ammophila arenaria following root formation and nematode colonization.Crossref | GoogleScholarGoogle Scholar |
Wagg C, Bender SF, Widmer F, van der Heijden MGA (2014) Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proceedings of the National Academy of Sciences of the United States of America 111, 5266–5270.
| Soil biodiversity and soil community composition determine ecosystem multifunctionality.Crossref | GoogleScholarGoogle Scholar | 24639507PubMed |
Watt AS (1947) Pattern and process in the plant community. Journal of Ecology 35, 1–22.
| Pattern and process in the plant community.Crossref | GoogleScholarGoogle Scholar |
Westoby M, Rice B, Griffin G, Friedel M (1988) The soil seed bank of Triodia basedowii in relation to time since fire. Australian Journal of Ecology 13, 161–169.
| The soil seed bank of Triodia basedowii in relation to time since fire.Crossref | GoogleScholarGoogle Scholar |
Winkworth R (1967) The composition of several arid spinifex grasslands of central Australia in relation to rainfall, soil water relations, and nutrients. Australian Journal of Botany 15, 107–130.
| The composition of several arid spinifex grasslands of central Australia in relation to rainfall, soil water relations, and nutrients.Crossref | GoogleScholarGoogle Scholar |
Yang L, Maron JL, Callaway RM (2015) Inhibitory effects of soil biota are ameliorated by high plant diversity. Oecologia 179, 519–525.
| Inhibitory effects of soil biota are ameliorated by high plant diversity.Crossref | GoogleScholarGoogle Scholar | 26001607PubMed |
Yizhaq H, Stavi I, Swet N, Zaady E, Katra I (2019) Vegetation ring formation by water overland flow in water‐limited environments: field measurements and mathematical modelling. Ecohydrology 12, e2135
| Vegetation ring formation by water overland flow in water‐limited environments: field measurements and mathematical modelling.Crossref | GoogleScholarGoogle Scholar |