Factors influencing the germination, establishment and distribution of Micromyrtus minutiflora (Myrtaceae), in western Sydney, New South Wales
Tanya Bangel A B , Alison Hewitt C * , E. Charles Morris
C * , E. Charles Morris  A and Anthony M. Haigh
A and Anthony M. Haigh  A
A
A School of Science, Western Sydney University, Locked Bag 1797, Penrith, NSW 2751, Australia.
B WSP Australia Pty Limited, 51-55 Bolton Street, Newcastle, NSW 2300, Australia.
C Hawkesbury Institute for the Environment, Western Sydney University, Locked Bag 1797, Penrith, NSW 2751, Australia.
Australian Journal of Botany 71(1) 12-27 https://doi.org/10.1071/BT22058
Submitted: 28 May 2022 Accepted: 20 January 2023 Published: 15 February 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Little is known of the ecology of Micromyrtus minutiflora, a threatened plant species endemic to the Cumberland subregion, New South Wales, Australia.
Aims: To fill ecological knowledge gaps of M. minutiflora, including habitat preferences, population size and structure, survivorship; and responses to fire and disturbance to inform appropriate management practices.
Methods: Surveys of distribution and abundance, regeneration mode and response to fire, survivorship, size-class analysis, greenhouse germination experiments using soil blocks; and plant tissue and soil analyses.
Key results: Micromyrtus minutiflora was estimated to have a population of ~4.3 × 106, an extent of occurrence of ~13 000 ha and ~1.5% area of occupancy within study areas. It is strongly associated with low tree canopy cover (few trees), dispersive clay soils and Castlereagh Scribbly Gum Woodland. In the one area studied, survivorship decreased by ~15% per annum across 14 years. However, total population numbers were stable across 3 years, indicating continuous recruitment. Size distributions were unimodal and continuous from small to large plants, consistent with recruitment matching mortality. Plants lack a lignotuber and have a high shoot:root ratio. Seedling emergence was unaffected by mechanical disturbance, but heat and smoke significantly increased germination.
Conclusions: This species is abundant on dispersive clays but restricted to the northern Cumberland Plain in open woodland areas. It is an obligate seeder; plants are killed by fire and can flower within 2 years of germination.
Implications: Understanding its soil seedbank dynamics is pertinent in determining an appropriate fire regime to maintain habitat while not directly threatening populations. Populations require regular monitoring.
Keywords: conservation biology, Cumberland Plain, ecology, fire ecology, germination, Myrtaceae, population dynamics, rare species, survivorship.
Introduction
The Cumberland Plain vegetation communities of greater Sydney and the habitats they provide for threatened flora species are at a great threat of being lost as a result of the increasing demands and pressures placed on them by society. It was estimated in 2003 that only 13% of their pre-settlement distribution remained (Tozer 2003). Since this time, the size and quality of remnants has been in continual decline because of activities such as clearing, development, recreation, weed encroachment, fire, pathogens and illegal rubbish dumping. These pressures have resulted in further loss, degradation and fragmentation of threatened flora habitats, thereby increasing the importance of conserving the habitats and populations that remain.
Accurate and reliable information is critical to the success of threatened flora conservation efforts. Information such as the species distribution, abundance, population change, its specific ecological requirements, responses to stochastic events and issues threatening the species survival all assist in determining the most appropriate management actions for a species. Unfortunately, for many threatened flora species this information is missing or may be based on ‘expert guesses’ (Keith 2000), which can lead to unsuitable, inadequate, or poorly designed restoration efforts. These have potential to significantly affect the long-term survival and future conservation of threatened species (NSW Department of Environment and Conservation 2005), especially where there are limited funding and resources available.
Micromyrtus minutiflora (F.Muell.) Benth. (Myrtaceae) is endemic to western Sydney, New South Wales (NSW), Australia, specifically to an area of the north-western Cumberland Plain. Prior to this study, estimates of the species total abundance and the largest remaining population differed among sources. In 2002, the NSW Threatened Species Scientific Committee Final Determination reported an estimate of its total population as ∼1800 individuals, with the largest population reported to be in the Castlereagh Nature Reserve (NSW Department of Environment Climate Change and Water 2002). The Commonwealth Approved Conservation Advice for the species (Australian Department of the Environment, Water, Heritage and the Arts 2008) similarly reported an estimated total population of 1800 individuals, but noted that the largest population was held within the Australian Defence Industries site (ADI), now forming part of Wianamatta Regional Park (James et al. 1999). However, a 2007 BioNet Atlas record reported 36 000 individuals as occurring at Wiannamatta Nature Reserve (see http://www.bionet.nsw.gov.au/). M. minutiflora is listed as ‘Endangered’ under the NSW Biodiversity Conservation Act 2016 and ‘Vulnerable’ under the Commonwealth Environmental Protection and Biodiversity Conservation Act 1999. A fundamental requirement for the assessment of extinction risk is knowledge of the abundance and distribution of a species based on reliable data (Keith 2000). Therefore, we aimed to collect this information through targeted field surveys based on available data.
Little is known about M. minutiflora apart from its botanical description (Bentham 1866; Wilson and Harden 2002), and its recorded occurrences within government databases such as Atlas of Living Australia (https://www.ala.org.au), The Australasian Virtual Herbarium (https://www.avh.chah.org.au) and NSW BioNet (see https://www.environment.nsw.gov.au/topics/animals-and-plants/biodiversity/nsw-bionet). A slender spreading shrub up to 2 m, which was first described from a specimen collected at Richmond (see the Australian Plant Name Index at https://id.biodiversity.org.au/name/apni/73020), as its name suggests, M. minutiflora produces miniature, white flowers that result in tiny indehiscent nuts (Wilson and Harden 2002). Some scleromorphic species of Myrtaceae retain their seeds within woody fruit that are shed after fire, whereas others shed their seeds immediately when mature or gradually over time (Benson and McDougall 1998). Nothing is known about the dispersal of seeds or fruits of M. minutiflora, but Thomas et al. (2007) reported that its more widespread sister species M. ciliata that also occurs on the Cumberland Plain produces a soil seedbank. A narrow geographical range and disjunct occurrences within fragmented remnants heightens the risk of extinction because of stochastic events, reduced genetic variability, reproductive failure and dispersal boundaries (Hewitt et al. 2019). In addition, a lack of knowledge of its basic ecology may lead to unsuitable conservation or restoration efforts that could affect its long-term survival.
Effective management plans for threatened species require information about the species habitat constraints. Benson and McDougall (1998) reported that M. minutifora is found in shrubby woodland containing Eucalytpus sclerophylla, Angophora bakeri, Melaleuca decora and Eucalyptus fibrosa (Castlereagh Scribbly Gum Woodland) on low-nutrient sandy clay or gravelly clay soils of Tertiary alluvium. The online Threatened Biodiversity Profile Data Collection maintained within the NSW BioNet Atlas identifies five vegetation communities as being associated with and providing potential habitat for M. minutiflora. These five communities include Agnes Banks Woodland, Cooks River–Castlereagh Ironbark Forest, Castlereagh Scribbly Gum Woodland, Cumberland Shale Plains Woodland and Cumberland Shale–Sandstone Ironbark Forest (NSW Department of Planning and Environment 2022). Benson and McDougall (1998) reported that nothing was known about dispersal or establishment of M. minutiflora, nor of its response to fire.
Field observations suggested that M. minutiflora is more abundant in open areas of low tree canopy cover (few trees) or past disturbance, such as occurs following vegetation removal, fire or soil mechanical disturbance. Tree canopy-cover measurements for areas of occurrence and non-occurrence were therefore made to test whether the abundance of M. minutiflora was consistently associated with high or low tree canopy cover (many or few trees). A comparison was made between occurrence within Castlereagh Scribbly Gum Woodland (found on sandier clay soils with a more open canopy) and Cooks River–Castlereagh Ironbark Forest (found on more clayey soils with a more closed forest structure); the two communities are often adjacent to each other in the region (Pendall et al. 2022). The initial population survey (N. Bennett, unpubl. data, 2008) showed that the species occurred in a revegetating clearing between these two communities. The germination response of M. minutiflora to soil mechanical disturbance was also tested in a controlled experiment with the hypothesis that this disturbance would increase the rate of germination.
For conservation of threatened plant species, important questions revolve around population dynamics, namely, whether numbers are stable through time, are increasing, or are in decline. Population dynamics can be studied by demographic methods using time-series sampling of individual plants (Harper 1977). An associated approach is to look at age or size-class distributions at a point in time, which may give indications of population dynamics from the distribution of individuals over size classes (Krebs 1985). Because M. minutiflora is a spreading (not erect) shrub with few branches that trail over other plants, the length of the longest stem of individual M. minutiflora was measured as a proxy for plant size, and data were examined to determine the range of size classes and numbers of individual plants within each size class within populations. The aim was to establish the general shape of the size-class distribution, and whether there were features of the size-class distribution that might indicate low recruitment, or bottlenecks in progression through size classes.
Although population structure can be evaluated through the proxy of plant size-class analysis, these data do not provide information about population decline owing to aging. Species with limited distributions or restricted habitat preferences or specific establishment cues may be susceptible to population decline as individuals age. Population decline was monitored for a single population through records of survivorship of tagged plants.
Populations of M. minutiflora are restricted to the soil landscape types of ‘Agnes Banks’, ‘Berkshire Park’ and ‘Blacktown’ (Bannerman and Hazelton 2011). These comprise clay and clayey sand soils derived from Tertiary alluvial sediments, with ‘Agnes Banks’ having deep acid soils overlying the clayey subsoils of the ‘Berkshire Park’ type (Bannerman and Hazelton 2011). These soils occur wholly within Mitchell Landscape ‘Hawkesbury–Nepean Terrace Gravels’ comprising poorly drained texture contrast (sandy A horizon overlying clayey B horizon) Tertiary river sediments of heavy clays and gravels, with areas of deeper fluvial deposited sands and an overlying Pliocene–Pleistocene sand body occurring at Agnes Banks (NSW Department of Environment Climate Change and Water 2016). The boundary of vegetation communities in which the species was frequently recorded, predominantly Castlereagh Scribbly Gum Woodland and Cooks River–Castlereagh Ironbark Forest, have been reported to be a function of the interaction of localised drainage conditions and the thickness of the Tertiary alluvium mantle (Tozer 2003). This indicates that there are several interconnected soil-related factors such as nutrient and water availability that may determine the limit of distribution of plants that occur in these communities. This study examined the nutrient composition and physical properties of soils from areas where M. minutiflora is present and adjacent areas where it is absent, with the aim of determining soil factors that may explain its restricted distribution.
Fire is a major disturbance factor that influences the timing and magnitude of recruitment in fire-prone vegetation communities in the Sydney basin (Auld and Ooi 2009). Many species germinate in response to cues from fire, such as heat, smoke or their combination (Auld and O’Connell 1991; Thomas et al. 2007, 2010). Thomas et al. (2007) reported that several species of Myrtaceae that form soil seedbanks in the Sydney region, including Micromyrtus ciliata, responded to fire cues; however, not all such Myrtaceae tested responded to these cues. The passage of fire also provides a post-fire habitat with higher light and soil nutrient availability and lower competition from other plants (Thomas et al. 2007), thereby increasing opportunities for many species to regenerate (Chick et al. 2019). It was therefore deemed ecologically important to test the emergence of M. minutiflora seedlings in response to fire; it is also important to understand the response of adult plants.
The response of adult plants of M. minutiflora to fire is currently stated as uncertain (NSW Department of Planning and Environment 2022) and it is absent from the NSW Flora Fire Response database (V2.1; NSW Office of Environment and Heritage 2014a). The two main fire-response strategies of Australian native plant species are to resprout or to be killed and re-establish from seed, with a smaller number of taxa being reliant on dispersal back into burnt areas following fire (Clarke et al. 2015). Resprouting species survive fire events and re-establish by growth from protected buds beneath the surface of the soil or from meristematic tissue protected by bark. Resprouters usually have a low seed output (Lamont and Barrett 1988; Zammitt 1988) but higher starch content within root structures than in shoots, and a resultant higher root:shoot ratio (Zammit and Westoby 1987; Pate et al. 1990; Bell et al. 1996). In contrast, re-seeders are killed by the passage of fire and re-establish through seed germination, displaying lower root:shoot ratios and higher seed production (Bell 2001). In addition, it is thought that plants with stem diameters less than 20 mm (like M. minutiflora) will succumb to fire (Bradstock and Myerscough 1988) without epicormic buds from which they can resprout (Burrows 2002). It was hypothesised that M. minutiflora is an obligate seeder and, as such, does not contain any root or belowground stem structure for resprouting, will have a low root:shoot ratio and will not resprout following fire. The investigation therefore examined M. minutiflora root structure for the presence or absence of lignotubers, measured root:shoot ratios and recorded the species life history and fire response at sites across a range of times following fire.
This study sought to increase the knowledge of M. minutiflora, with aims of assisting future conservation and management actions for the species. In particular, the study aimed to fill knowledge gaps regarding the abundance and distribution, correlates of occurrence and vegetation structure, survivorship, edaphic properties of soil nutrient composition and structure, responses to soil mechanical disturbance and fire, root:shoot ratios, and time to first flowering. Gaining a greater understanding of the species ecology will enable more targeted management strategies to be implemented and assist in planning and delivering successful conservation outcomes for the species.
Materials and methods
Study area
Micromyrtus minutiflora grows within Cumberland Plain vegetation communities of western Sydney. Specifically, all of its known records are restricted to the floodplains between Richmond, Cranebrook, Penrith, Ropes Crossing, St Marys, Marsden Park and Mulgoa of NSW, Australia (see http://www.bionet.nsw.gov.au/ and NSW Office of Environment and Heritage 2022) (Fig. 1). Climatically, this region has a mean annual maximum temperature of 23.9°C, a mean annual minimum temperature of 10.5°C and a mean annual rainfall of 797 mm (Bureau of Meteorology 2022).
This work was conducted under NSW National Parks and Wildlife Act 1974 Scientific Licences SL100052 (A. M. Haigh) and SL102496 (A. Hewitt). Access to Londonderry Drop Zone (LDZ) was granted by the Australian Department of Defence. Access to areas in Mulgoa was granted by Greater Sydney Parklands Trust and the two adjoining private landowners.
Distribution and abundance
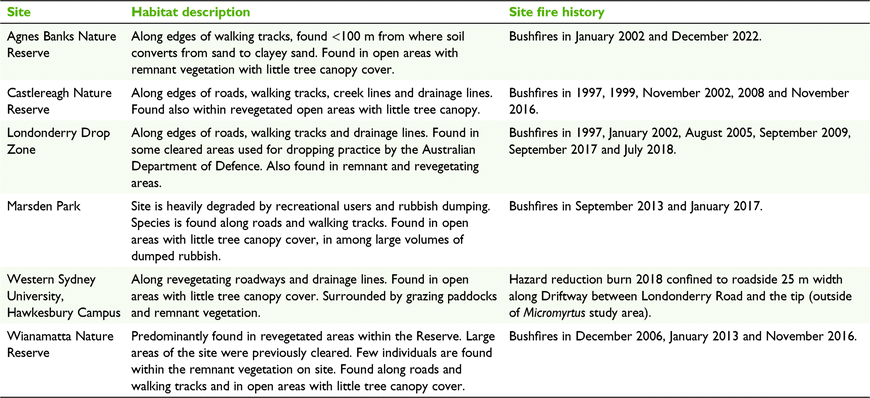
BioNet records informed targeted distribution and abundance surveys within the Richmond, Penrith, St Marys and Marsden Park region in summer and autumn of 2010–11 (see http://www.bionet.nsw.gov.au/). When the species was found at a location, the distribution and abundance of the plants at the location were recorded. Access was granted to study the species in detail at the following six study areas, following confirmation of its presence: Agnes Banks Nature Reserve (ABNR); Castlereagh Nature Reserve (CNR), Londonderry Drop Zone (LDZ); Marsden Park (MP); Wianamatta Nature Reserve (WNR) and the Hawkesbury Campus of Western Sydney University (WSU) (Fig. 1, Table 1).
|
Five locations in addition to the six main study areas were surveyed to confirm previous BioNet records and refine the known distribution limits of M. minutiflora. In 2011, locations in St Marys, Ropes Crossing and Vineyard at the eastern and northern edges of the species distribution were inspected. Windsor Downs Nature Reserve was also surveyed, despite an absence of previous records, because it was suspected to contain potential habitat, and, if present, would have formed a limit to the known distribution of the species. In 2022, two new records were noted in BioNet from the Fernhill Estate, in Mulgoa. If present, the disjunct records at Mulgoa would form a considerable extension of the species narrow distribution. A summary of the additional sites and the surveys completed is presented in Supplementary Table S1.
The presence, distribution of the population and preferred habitat attributes were determined by the completion of random meander surveys in 2010, 2011 and 2022 (Cropper 1993). Where M. minutiflora was observed, the boundaries of distinct patches of plants within each population were mapped on foot by using a hand-held GPS (Garmin 60CSx) and uploaded to Google Earth (ver. 6.0.3.2197). These boundaries were used to calculate the area of occupancy, or the smallest area occupied within this broader extent of occurrence, within the six main study areas.
Micromyrtus minutiflora extent of occurrence, or the area encompassing all known sites of presence, was estimated by applying the convex-polygon method (International Union for Conservation of Nature and Natural Resources 1994)) (Fig. 1). Preparation of the extent of occurrence involved the removal of BioNet records (see http://www.bionet.nsw.gov.au/ and NSW Office of Environment and Heritage 2022) from evidently cleared areas suspected to no longer support native vegetation (on the basis of aerial photographic interpretation of Google Earth satellite imagery, taking into consideration date of record and accuracy of coordinates), those records considered to be potential misidentifications, and records where no individuals had been recorded during targeted surveys completed as part of this study in 2010–11 or 2022 (Table S1). All remaining confirmed and non-field verified BioNet records were included within the extent of occurrence shown in Fig. 1.
Population size in 2010–11 at each of the six study areas was extrapolated using a combination of three sampling techniques adapted from the sampling design, systematic field methodologies and analytical technique principles to estimate abundance described by Keith (2000) and the concept of multi-staged stratified sampling as described by Cochran (1977). The distribution pattern, and the size and shape of occupancy at each study area determined which of the three techniques would be implemented in 2010–11. At all study areas, a total census was taken of small patches, defined as discrete areas of <20 m2, because it was possible to count all individuals. Random sampling was adopted at all intermediate-sized patches, defined as discrete areas between >20 m2 and <20 ha. All plants were counted within at least one, most commonly five and occasionally up to twenty 4- × 4-m (16-m2) quadrats that were randomly selected within patches that were randomly selected from the area that each population occupied (Tables S2–S7). Large patches, defined as areas of >20 ha, were divided into 20-ha independent strata. Within each of these strata, two 40- × 40-m (1600-m2) plots were randomly selected and all plants were counted within five quadrats (16 m2) from within each of 1600-m2 plots. Within each of these plots, 4- × 4-m quadrats were randomly selected from the 100 possible such quadrats. At WSU, in 2008, a half census was conducted on the basis of 4- × 4-m plots, followed by random sampling in subsequent years.
The total abundance of M. minutiflora within each patch and at each study area was estimated using the total-census and density-plot data collected in 2010–11, as described above. The mean density of plants per square metre was calculated by dividing the number of plants by the area they occupied. The total number of plants was calculated by multiplying the total area of occupancy in square metres by the mean plant density within all study areas. Confidence limits of the estimated total number of plants were calculated by inserting the upper and lower 95% confidence limits of mean density. Because population estimates, except for very small patches, were extrapolated from small samples, these values may under- or over-estimate the true value. Estimates of percentage relative precision (PRP) based on the formula (below) are included in Tables 2 and S8.
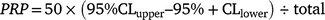
It is noted that M. minutiflora is, in appearance, similar to a non-threatened congener M. ciliata that occurs in the area. This species tends to have a more prostrate, thicker woody growth form than does the taller, more slender M. minutiflora. However, to make a positive identification distinguishing the two species, flowers are required. It is acknowledged that flowers were not always present on all plants during the surveys and, as such, misidentification at some locations may have been possible. Whereas the two species have both been recorded at LDZ and WNR, during field surveys, midsummer to late autumn, M. minutiflora was recorded as flowering at these sites, outside the known flowering time of M. ciliata, spring to early summer. Further, only a single record of M. ciliata has been recorded at each of these sites. Neither of these have been verified by herbarium specimens (see Micromyrtus minutiflora data at http://ava.ala.org.au/occurrences/search?taxa=Micromyrtus+minutiflora).
Flora surveys
As part of a separate project, 148 quadrat surveys were conducted across the species extent of occurrence between January and April 2021, by using the NSW Government’s Biodiversity Assessment Method (20- × 20-m plots) recording GPS coordinates, flora species present and their cover scores, with cover scores for each growth form (tree, shrub, grass, forb, other) being later derived from the plot data (NSW Department of Planning Industry and Environment 2020). Sites were chosen with a range of times since fire from 6 months to 25 years and frequency of fire from zero to four burns in the past 25 years. Seventy-eight plots were within Castlereagh Scribbly Gum Woodland, 52 within Cooks River–Castlereagh Ironbark Forest and 18 within Agnes Banks Woodland. Detailed records of the fire regeneration mode (seedling or resprout), life stage (juvenile or adult) and reproductive status (flowering, fruiting or vegetative) of any M. minutiflora in or adjacent to these plots were made in the field.
Tree canopy and understorey cover
Tree canopy cover, total understorey cover of all plants and M. minutiflora cover were estimated at five study areas (CNR, LDZ, MP, WNR and WSU) by line-intercept method (Elzinga et al. 2019). A hand-held GRS Densitometer with a mirror at 45° (Geographic Resource Solutions, Arcata, CA, USA) was used to visualise the presence or absence of each cover type at 50 points (one recording every 2 m) along a 100-m transect. The proportion of presence scores for each cover type along each transect was then converted to a percentage cover score. Tree canopy cover was defined as any plant >1.6 m and understorey (shrub or forb) cover as any plant <1.6 m. M. minutiflora cover was recorded as a subset of understorey cover. For example, a value of 20% cover for M. minutiflora indicates that, at 20% of measurement points, M. minutiflora was found.
In each area, an equal number of randomly placed transects was set up within the M. minutiflora populations and within adjacent vegetation where M. minutiflora was absent. The size of the areas determined the number of transects. Small study areas (MP and WSU) had 10 transects and larger study areas (CNR, LDZ and WNR) had between 30 and 40 transects established within and outside of the M. minutiflora populations. ABNR was not measured because the area was too small to sample with replicate 100-m line-point transects. Transects within the M. minutiflora populations were contained within the boundaries of the population.
Stem length (length of longest stem)
Because the ages of plants were unknown, the length of the longest stem was used to determine the size structure of populations and to determine whether population structure indicated a pulse of recruitment following stochastic events such as soil mechanical disturbance or fire. The length of the longest stem was measured on up to 10 plants per quadrat in each patch. At three study areas with very large populations (CNR, LDZ and WNR), a random sample of patches was selected from the total population, to give n = 500 plants per area. At the three remaining study areas, data from all plants were used (range n = 89–236 plants). Data for stem length were used to construct size-class distributions for the raw data and log-transformed data. Individuals were assigned to the following growth stages on the basis of stem length: seedling (<5 cm), juvenile (5–15 cm) or mature (>15 cm). A detailed description of the methodology is provided in the Supplementary material.
Survivorship
Survivorship of M. minutiflora was recorded at the WSU population. In March 2008, Nicholas Bennett tagged 196 adult specimens (N. Bennett, unpubl. data). The survivorship of these tagged plants was recorded again in March 2010 (J. Kremer, unpubl. data), February 2011, September 2015 and April 2022. Survivorship (lχ) values were calculated by dividing nx or the number of plants alive at Time x by ni or the initial number of plants. Survivorship values were plotted on a log scale against time to show trends.
Plant tissue and soil analysis
Plant tissue samples were collected from WNR (n = 5), WSU (n = 5), CNR (n = 5), LDZ (n = 4) and ABNR (n = 1). Available resources limited total tissue sample size to 20. Tissue samples comprised fully expanded young leaves and shoots. Two soil samples were collected at each of four study areas (ANBR, CNR, WNR and WSU), one from where M. minutiflora was present and one from where it was absent. Samples collected were between 800 and 1000 g of soil collected from a single location. Soil was sampled to a depth of 10 cm from within which the root system commonly occurred. Litter, when present, was removed. There were no rocks or gravel present in the soils.
Plant tissue was oven-dried for 48 h at 80°C. Samples were then ground for 2 min in a Retsch MM 301 ball mill, transferred to specimen jars and sent to Waite Analytical Services in Adelaide. Plant tissue was analysed using radial view inductively coupled plasma–optical emission spectrometry (ICP-OES) to identify the target elements of aluminium (Al), iron (Fe), manganese (Mn), boron (B), copper (Cu), zinc (Zn), sulfur (S), calcium (Ca), magnesium (Mg), phosphorus (P), potassium (K), sodium (Na), molybdenum (Mo), cobalt (Co), nickel (Ni), cadmium (Cd), lead (Pb), selenium (Se), titanium (Ti) and chromium (Cr). Plant tissue samples were also analysed for nitrogen (N) at the Hawkesbury Institute for the Environment (WSU laboratories) by using a TruSpec Micro CHN Determinator (Leco Corporation, Saint Joseph, MI, USA; Dumas method). Soil samples were commercially analysed (Sydney Environmental and Soil Laboratory) for the plant-available nutrients of phosphate (PO43−), K, sulfate (SO42−), Ca, Mg, Fe, Mn, Zn, Cu and B with Mehlich 3 (1984) extract, by using ICP. Electrical conductivity, pH, chloride (Cl−) and nitrate (NO3−) were analysed using the methods of Rayment and Higginson (1992). The physical characteristics of texture, structure and permeability class of the soils were also determined using the methods of Rayment and Higginson (1992).
Soil seedbank emergence experiment
Intact soil blocks (50 mm deep) were collected from within the dripline of existing mature M. minutiflora at WSU. With the least possible disturbance, 64 circular soil blocks were removed and transferred into plastic pots (220-mm diameter), covered and transported to the laboratory.
Mechanical disturbance was applied to half of the soil blocks by hand-tilling with a sterile fork to a depth of 10 mm, the remaining undisturbed blocks acted as the control. Soil blocks from each of the two disturbance treatments were then assigned to a fire treatment of heat, smoke or a combination of the two. There was one mechanical-disturbance treatment (32 blocks), and one no-disturbance treatment (32 blocks); then, heat, smoke or their combination was applied to the blocks in each. Heat was applied prior to smoke for soil blocks subjected to the combined heat and smoke treatment. Heat treatments were applied by placing the soil blocks in an 80°C fan-forced oven for 30 min. Each block was checked to ensure that the centre reached 60°C by using a Grant 2020 Series Squirrel Data Logger (Keison, Chelmsford, Essex, UK). Thomas et al. (2007) identified that heat shock of 50–75°C yielded the highest germination response in Micromyrtus ciliata. After 30 min, blocks were removed from the oven and allowed to cool to room temperature. Aerosol smoking was achieved by placing soil blocks in a wooden smoker similar to the glass chamber used by Thomas et al. (2007). The smoke was generated by burning dry leaf and stick litter in a beekeeper’s burner. An electric pump directed the smoke into a condensing tube (to cool and dry the smoke) and into the wooden chamber. Smoke was applied to each soil block for a 10-min period. Each treatment was replicated eight times and each soil block was independently subjected to treatments (Morrison and Morris 2000). The experiment consisted of 64 blocks allocated to the following: no-treatment control (8 blocks), heat and no tilling (8), smoke and no tilling (8), heat and smoke and no tilling (8); tilling and no other treatments (8), heat and tilling (8), smoke and tilling (8), and heat and smoke and tilling (8). These treatments were also applied to known quantities of seed collected from the WSU population; however, none germinated after a period of 6 months and the experiment had to be abandoned.
Following treatment, all soil blocks were maintained at 22°C in a greenhouse located at the WSU Hawkesbury Campus. Soil blocks were watered regularly to ensure that the soil did not dry out. Pots were arranged randomly and re-randomised every 2 weeks. After 10 weeks, all emerged grasses and dicots were recorded, except those within an exclusion zone (10-mm annulus around the outside of the soil block). This exclusion zone was intended to eliminate the disturbance effects caused by digging and transferring the soil blocks into pots. Seedlings appearing to be M. minutiflora were recorded. There were no other seedlings nor adult plants of similar appearance at WSU. In accordance with the requirements of the scientific licence, at the completion of this experiment, each soil block was returned to its collection site.
Root architecture and root:shoot ratio
Ten whole M. minutiflora plants were carefully excavated in situ from each of the five study areas (ABNR, CNR, LDZ, WNR and WSU) by using a spade, hand-weeder and brush. The tools were used to loosen the soil and to carefully extract the roots before the plants were transferred to the laboratory where they were washed to remove remaining soil particles. These plants ranged in size to give a representation of the population found at each site. The roots of all plants were examined and the presence or absence of underground reshooting structures such as lignotubers and vegetative spread was recorded. Photographs were taken of the root structure before root and shoot components were separated. All specimens were dried for a period of 48 h in an 80°C fan-forced oven and the root and shoot dry weights were determined.
Flower production
In 2008 at WSU, data were recorded on the number of flowering stems and the total number of flowers per plant for 50 plants at one time during late summer.
Data analysis
Comparison of M. minutiflora variables across study areas was made using one-way ANOVA (cation balance ratios, density, cover, root:shoot ratio, and total population size at WSU). Data for density and cover were patch means or densities per quadrat (total population size at WSU). Data for root:shoot ratio were from the 10 plants sampled and cation balance ratios were based on the soil samples. Comparison of tree canopy cover and understorey cover at study areas where M. minutiflora was present or absent was made using two-way ANOVA with presence or absence of M. minutiflora as a fixed factor, location as a random factor, with n = 9 transects nested within each vegetation by site combination as the error term. Where the vegetation × location interaction was not significant at P > 0.25, the interaction term was pooled with the error term and used to test main effects. Comparison of seedling emergence in the soil-block experiment was made using three-way ANOVA with heat, smoke and disturbance as fixed factors. Data were number of emergents per soil block. Homogeneity of variances was assessed by Cochran’s test, and appropriate transformations (square root or natural logarithm) were used where significant heterogeneity was detected. Comparison of means after ANOVA in SPSS (ver. 19, IBM) were made using Tukey’s HSD test for the appropriate interaction means, or main effect means if no interactions or main effects were significant.
Flora survey data were examined for associated species, correlations in cover scores against richness and cover of each growth form; and an indicator species analysis was conducted using plant community type in PC-Ord (ver. 7, MjM Software Design, Gleneden Beach, OR, USA). Life-history data were tabled against time since fire and fire frequency.
Results
Distribution and abundance
The presence of M. minutiflora was confirmed at each of the six study areas surveyed in detail as part of this study (including ABNR, CNR, LDZ, MP, WSU and WNR). The species was not confirmed present at the additional survey locations of Mulgoa, St Marys, Ropes Crossing and Vineyard as previously indicated by BioNet searches in 2010 and 2022, thereby reducing the species known distribution range (Fig. 1, Table S1).
The extent of occurrence of M. minutiflora, on the basis of the results of this study, is now considered to be restricted to the floodplains between Richmond, Cranebrook, Ropes Crossing and Marsden Park of NSW, Australia. The total extent of occurrence of M. minutiflora in 2022 is estimated to be ∼13 000 ha and the area of occupancy within this is ~1.5% (Fig. 1, Table S9). Quantification of the area of occupancy is based on the M. minutiflora distribution polygons within the six study areas surveyed and does not include areas where the species occurs elsewhere. On the basis of BioNet records, it is suspected that the entire area of occupancy would not increase dramatically if all populations were surveyed because large areas of the extent of occurrence are cleared, microhabitats required by the species are largely absent, and the remaining BioNet records are scattered and low in abundance.
The total population of plants across the six study areas in 2011 was estimated to be ∼4.3 × 106 individuals, with a 95% confidence interval of ∼1.3 × 106 (Table S8). Abundance estimates for each area are tabulated in the Tables S2–S7. WNR contained the largest population (∼1.1 × 106) and a significantly higher density of plants than in the other study areas (F5,78 = 4.446, P = 0.035; Fig. 2). CNR had the largest area (99.8 ha) occupied by M. minutiflora.
Flora surveys
Micromyrtus minutiflora was recorded in 25 of 148 survey plots located across 38 sites. Of these, 24 of 78 plots were in Castlereagh Scribbly Gum Woodland, 1 of 52 plots was within Cooks River–Castlereagh Ironbark Forest and none of 18 plots was within Agnes Banks Woodland plant community types. Cover scores of M. minutiflora per plot were significantly positively correlated with higher per plot shrub-cover scores (r2 = 0.0449, P < 0.01), numbers of listed rare species (r2 = 0.0685, P < 0.01) and higher forb richness (r2 = 0.0695, P < 0.01). There was no significant correlation with fire frequency or time since fire. The species was not recorded resprouting following fire, but only ever as seedlings, juveniles or mature plants (Table S10), confirming its status as an obligate seeder. It was found flowering at sites 18 months, also at 2, 4, 7, 10, 14 and 19 years post-fire and absent from plots that had not burnt in the previous 20 years (Table S10).
Other species occurring more than 80% of the time within plots where M. minutiflora occurred were the tree species Eucalyptus sclerophylla; shrubs Dillwynia tenuifolia, Hakea sericea and Melaleuca nodosa; and groundcovers Entolasia stricta, Lomandra multiflora and Cyathochaeta diandra.
Tree-canopy and understorey cover
Vegetation with M. minutiflora present had significantly lower (28%) tree canopy cover (fewer trees) than did vegetation where M. minutiflora was absent (64%, vegetation main effect: F1,4 = 34.56; P < 0.005; Fig. 3). Understorey cover within areas containing M. minutiflora (80%) did not differ significantly from that in areas where the species was absent (75%). The M. minutiflora cover was significantly different across the five study areas surveyed (F4,5 = 2.907; P = 0.032); however, the Tukey HSD post hoc test was unable to detect significant differences among study areas (Fig. 4).
Stem length (length of longest stem)
Stem length ranged from 2 to 232 cm over the six populations. Examination of population structure using size-class analysis of raw and log-transformed size data showed that low numbers of plants were present over the 1–15 cm size class (seedlings and juveniles) in all populations (Supplementary Fig. S1). Numbers of plants rose steeply in successively larger size classes, corresponding to stem lengths in the range 16–30 cm across the six populations, to peak at or near the median value for both raw and log-transformed data (Fig. S1, S2). Numbers then declined over even larger size classes, to give an approximately unimodal and continuous distribution over the full range of stem lengths. A more detailed analysis of size class distributions is given in the Supplementary material.
Survivorship
The number of tagged plants in the WSU population decreased at each survey time between 2008 and 2022. Only 120 of the original 196 individual plants tagged in February 2008 survived in 2011, 13 were alive in 2015, and only two survived to 2022. Although these are limited data points based on one population at a single location, the adult population appears to be decreasing approximately linearly by 15% each year (Fig. 5). Despite mortality of adult plants, the total population density at WSU remained the same over 2008–2011 (Table 2). Changes in the total plant numbers between 2008 and 2011 were insufficient to be detected as significant (F2,147 = 0.356, P = 0.701); the total population appears to have remained stable. The population size was not estimated in 2015 or 2022.
Plant tissue and soil analyses
There were no major differences in soil chemical properties between where M. minutiflora was present and absent at the study areas or among the study areas (Tables S11–S14). The Ca:Mg ratio was the only exception, being significantly lower at locations where M. minutiflora were present (F1,6 = 6.063, P < 0.05). Soils where M. minutiflora was present also showed higher Mg concentrations; however, the difference was not statistically significant. There were similar Ca concentrations between the two soil locations (Table S11). Soils across most locations had predominantly loam and clay textures (Table S12). The average permeability was between 60 and 120 mm h−1. However, at ABNR, the soil where M. minutiflora was absent had a sandy texture, showing a very rapid infiltration rate, and a higher permeability than at any of the other locations. At the WNR, the soil where M. minutiflora was present showed a higher clay content and a lower permeability than did the soils across all other locations.
Root architecture and root:shoot ratios
Micromyrtus minutiflora has a small root system that contains no visible root nodules or large storage structures such as a lignotuber (Fig. 6). All plants across the five study areas had a main central root (that reached ∼50–100 mm in depth) from which branched a series of lateral roots. These lateral roots spread approximately horizontally in all directions extending beyond the shoot canopy (Fig. 6). The root system of M. minutiflora appears to be one with a small root:shoot ratio in the range of 0.109–0.163. The root:shoot ratios were not significantly different across the five study areas (F4,45 = 0.384, P = 0.812) averaging 0.146. The mean shoot:root ratio was 7.22.
Soil seedbank emergence experiment
The emergence of M. minutiflora from soil blocks was very strongly increased by the fire-related cues of heat and smoke (main effects significant; heat, F1,56 = 11.1, P = 0.002; smoke, F1,56 = 12.05, P = 0.001) (Fig. 7). Smoke and heat interacted significantly (F1,56 = 4.24; P = 0.044). However, emergence after the combination of smoke and heat was less than that expected if they combined to produce an additive effect (Fig. 7). Some limited emergence also occurred in the control treatment, indicating that heat and smoke are not essential requirements for emergence (Fig. 7). Disturbance, by itself or combined with other treatments, had no significant effect on the emergence of seedlings.
Flower production
On the basis of the single time observation of flowering in late summer, M. minutiflora produces large numbers of flowers. On average, plants had seven stems (range 2–24), with the longest stem, on average, being 78 cm long (range 22–156 cm) and with an average of 2456 (range 30–7967) flowers per plant.
Discussion
This study has increased the knowledge of M. minutiflora’s ecology considerably. The knowledge gained will enable more targeted conservation and management strategies to be developed for the species. This study has identified that M. minutiflora has a narrow geographical distribution and a high local abundance. Within the areas where it occurs, it can be locally abundant, such as, for example, up to nearly a third of the shrub layer (CNR) within some transects or dominant within some patches at WNR, >500 individuals per 16-m2 quadrat in some patches. Its presence is associated with low tree canopy cover (few trees), high understorey canopy cover, high forb richness and the presence of other threatened species of the area. There were no major differences in soil chemical properties between where M. minutiflora was present and absent at the study areas; however, the species predominantly grows in Castlereagh Scribbly Gum Woodland, which typically occurs on clay and clayey sand Tertiary alluvium sediments. M. minutiflora showed a positive germination response to fire cues but was unaffected by disturbance. It was found to be an obligate seeder, but can flower within 18 months to 2 years of germination.
Distribution and abundance
This study has provided updated estimates of the abundance, extent of occurrence and area of occupancy of M. minutiflora. The largest population reported prior to this study was at WNR, with 36 000 individuals in 2007 (see http://www.bionet.nsw.gov.au/). However, all other sites were reported to hold fewer than 300 individuals.
This study has quantified an extent of occurrence of ~13 000 ha, which is considerably less than what was estimated previously on the basis of all BioNet records. Disjunct records at Mulgoa some 16 km to the south were confirmed to not be M. minutiflora by this study, and therefore were not included in the extent of occurrence. Some other sites with earlier BioNet records (St Marys, Ropes Crossing and Vineyard) were also found to lack M. minutiflora (Table S1) and, so, were excluded from the extent of occurrence. It is further estimated that M. minutiflora’s area of occupancy within this larger total extent of occurrence is ~200 ha on the basis of the distribution polygons of this study, representing about 1.5% of the species extent of occurrence.
The surveys conducted in 2010–11 across the six major populations provided an abundance estimate of 4.3 × 106, with a 95% confidence limit of ~1.3 × 106, i.e. between 3 × 106 and 5.6 × 106. The estimates of population size for each patch within an area vary greatly in precision. The most precise estimate (within 10%) of a population was for WSU in 2008, based on a systematic survey of 454 of 851 quadrats. To illustrate the effect of sampling effort, we took random samples of different sizes. Precision of the estimate of population size increased with sample size (Table 2). Surveying the same area with 50 random quadrats in 2010 and 2011 resulted in estimates that were similar but less precise (Table 2). The precision of the total population estimates for the other areas ranged from 33 to 61%. Obviously, greater sampling effort increases precision, but at the cost of considerable effort for a result that would differ by less than an order of magnitude. Although population estimates based on abundance surveys are essential for the determination of a threatened species status, these should include reports of confidence bounds (Keith 2000). The total population of M. minutiflora may be higher than we have estimated, with inclusion of plants on unsurveyed privately owned land. In several locations where M. minutiflora was found, there were simply too many individuals to count and previous abundance counts have likely been underestimates. Large, localised populations are not unusual for some rare plants (Lesica et al. 2006), with endemism being largely due to habitat specificity (Rabinowitz 1981).
Small populations generally have smaller genetic pools and are considered to be more vulnerable to extinction when changes in the environment occur (Young and Brown 1996; Reed 2005; Hewitt et al. 2019). Analysis of population size and fitness has concluded that a population size of 5000 is necessary to maintain 95% of original fitness of a population (Reed 2005). Most populations of M. minutiflora are above the figure. Despite this, the species has a restricted distribution and its habitat is at risk of further decline and fragmentation from anthropogenic disturbances given its location in the western Sydney.
This study has shown that large populations of M. minutiflora are held within two nature reserves of north-western Sydney, namely, WNR and CNR. Following receipt of ‘The ecology of Micromyrtus minutiflora’ (T. Bangel, unpubl. data), NSW Office of Environment and Heritage (now Department of Planning and Environment) classified WNR as a priority management site for the species under the NSW Government’s Saving our Species program. This contrasts with the previously identified locations of ADI and CNR in the approved conservation advice (NSW Department of Environment Climate Change and Water 2002; Australian Department of the Environment, Water, Heritage and the Arts 2008). Adopted management activities at WNR include the regular monitoring of species abundance as well as the monitoring of encroachment of woody species and weed infestation and their removal to ensure the maintenance of 20 ha of open habitat for M. minutiflora. The remaining populations found at ABNR, CNR, LDZ, MP and WSU are not included in these management practices. With only 8% of the remnant Cumberland Plain vegetation being protected within national parks and conservation reserves, it is predicted that increasing relevant areas under protection in western Sydney and managing habitat within populations to ensure the persistence the species will aid in conserving M. minutiflora.
Occurrence of M. minutiflora across the six study areas was consistently associated with low (28%) tree canopy cover (few trees), thereby confirming field observations that M. minutiflora was found in open sites. At WNR, M. minutiflora appears to be regenerating well over bare ground and areas of low tree canopy caused by past land-use practices. This reserve is presently regenerating from previous management as a radio transmitter site where it was frequently cleared (NSW Office of Environment and Heritage 2014b). These findings are also consistent with the flora survey finding that M. minutiflora was most often recorded within Castlereagh Scribbly Gum Woodland community with its lower canopy cover, as opposed to Cooks River–Castlereagh Ironbark Forest with its more closely spaced trees and more dense forest canopy (Pendall et al. 2022).
Soil mechanical disturbance has been shown to increase seedling emergence in some Australian species (Spooner et al. 2004). Soil mechanical disturbance by itself did not affect the emergence of M. minutiflora from experimental soil blocks. Perhaps it is the open space generated by disturbance, rather than soil disturbance itself, that accounts for the species common occurrence in disturbed areas. Disturbances such as tree-fall gaps and clearing have been shown to increase the supply of water, light and nutrients within an area, thus reducing the effects of competition from other species (Chapin et al. 1987).
Soil analysis indicated that the species occurs on soils with a clay content of between 10 and 30% and a significantly lower Ca:Mg ratio in areas where M. minutiflora was present versus absent (higher Mg content). Emerson (1983) reported that when Ca:Mg ratios were low in clay soils, the structure tended to be dispersive, being sticky when wet and hard when dry. In dispersive soils, clay particles coalesce on wetting and develop into dense, structureless hardsetting soils with low porosity and high soil strength that restricts root growth (Daniells 2012). The generally horizontal distribution of roots within the top 5–10 cm of soil indicates that M. micromyrtus can tolerate these soil conditions. Tozer (2003) stated that Castlereagh Scribbly Gum Woodland commonly occurred on sandy loam soils, but that underlying soils with a high clay content are exposed because of erosion. This is consistent with our finding of patches of M. minutiflora in mechanically disturbed areas. The low tree canopy cover consistently found where M. micromyrtus occurred may indicate the inability of the tree species in these communities to tolerate these dispersive clay soils.
Five vegetation communities are currently identified as providing potential habitat for M. minutiflora (NSW Department of Planning and Environment 2022). These include one Cumberland Plain Woodland (Cumberland Shale Plains Woodland) and four Castlereagh Woodland (Agnes Banks Woodland, Cooks River–Castlereagh Ironbark Forest, Castlereagh Scribbly Gum Woodland and Cumberland Shale–Sandstone Ironbark Forest) vegetation communities that have been previously described in detail (Benson and Howell 1990; Benson et al. 1996; James et al. 1999; Tozer 2003; NSW Department of Planning and Environment 2022). The factors influencing the distribution, characteristics and composition of these communities include soils, geology, landscape features and climate. The Castlereagh Woodlands differ from the Cumberland Plain Woodlands mostly in that they occur predominantly on low-nutrient soils derived from Tertiary alluvium rather than fertile soils on Wianamatta shale (Benson et al. 1996). This study identified that M. minutiflora was most prevalent in the Castlereagh Woodlands, specifically Castlereagh Scribbly Gum Woodland, indicating that the factors determining the boundaries of these communities may also be determining the distribution of this and other rare species it was found associated with. Future investigations to identify additional populations of the species should focus on areas of Castlereagh Woodlands within the region, including transitional boundaries with Swamp Woodland not identified as potential habitat for the species (NSW Department of Planning and Environment 2022), within and adjacent the extent of occurrence illustrated in Fig. 1.
Micromyrtus minutiflora was frequently found in association with other rare species of the area, including Dillwynia tenuifolia, Grevillea juniperina subsp. juniperina, Persoonia nutans, Pultenaea parviflora, Acacia bynoeana and Allocasuarina glareicola. It was also observed opportunistically occurring alongside Hibbertia puberula subsp. puberula at CNR within Castlereagh Scribbly Gum Woodland (T. Bangel, unpubl. data). Rare species have been reported to co-occur in a range of plant communities including arable lands (Bellanger et al. 2012; Fanfarillo et al. 2020), heaths, woodlands, and forests (Sritharan et al. 2021). In a study of more than 300 biological assemblages, Calatayud et al. (2020) reported that rare species mostly occurred together. Although co-occurrence is not evidence of an ecological interaction (Blanchet et al. 2020), it may result from species requirements for similar micro-environmental factors or past environmental history. The finding of M. minutiflora predominance in threatened Castlereagh Scribbly Gum Woodland and its association with other threatened species of the area highlight the importance of conserving this community as a whole in the region.
Population structure and persistence
Stem length of M. minutiflora spanned at least one and up to two orders of magnitude and included growth stages from seedlings to large mature plants. The observations of mortality from the tagged plants and of stable population numbers in WSU population suggest that demographic gains were approximately equal with population losses. The continuous distribution of size classes indicates that after seedling recruitment, the processes of movement among size classes by growth or loss by mortality are occurring across the population. There are no breaks or dips in the distribution suggesting a bottleneck, nor are there populations that lack small plants, indicating a failure of recruitment. The low numbers of individuals (<5% of total population in all study areas) in the 1–15-cm size class might suggest that seedling recruitment is low, and insufficient to match the estimated average mortality of 15%. An alternative explanation is that although numbers of seedlings are low, they are able to pass through this size class quickly. In short, the first size class of M. minutiflora may have low numbers in it, but if there is a high flux of individuals through it, and slower rates of progression through successive size classes, there will be an increasing buildup of individuals in higher size classes until loss by mortality begins to exceed gains from growth.
The linear decrease in adult survival indicates that the risk of mortality of adult M. minutiflora plants is constant (Krebs 1985). A constant risk of mortality is fairly common in shrubs and trees and is known as Deevey Type II survivorship (Hutchings 1997). Although an annual mortality rate of 15% was observed within the WSU population, this was not evident in the total population estimates between 2008 and 2011 that showed the population to be stable. This implies that the recruitment of seedlings is occurring concurrently and is matching the death of adult plants. Although population size was not estimated in 2015 nor 2022, no obvious decrease in size was apparent in 2022, despite 99% of tagged plants having died, thus confirming that recruitment had occurred frequently.
Micromyrtus minutiflora flowers throughout much of the year (Benson and McDougall 1998; Wilson and Harden 2002), possibly producing hundreds to thousands of fruit per plant annually; however, nothing is known about the persistence of the seeds that these fruit contain. Thomas et al. (2007) reported that the congener M. ciliata produced a soil seedbank. We showed that seedlings emerged from soil collected from under mature M. minutiflora plants when subjected to fire cues, confirming the presence of a soil seedbank, but its size remains unknown. Baumann (2008) reported that for Melaleuca quinquenervia that despite producing hundreds of thousands of seeds per plant per year, it was the transition from the canopy seedbank to seedling that was the key vulnerability in the lifecycle of this plant. The woody nuts of M. minutiflora would be expected to persist longer than the non-dormant seeds of M. quinquenervia.
There has been no research conducted into the breeding system, pollination or seed fertility of M. minutiflora. The apparent large reproductive output and current survivorship within the WSU population indicate that the species should be able to maintain a stable population. However, the narrow distribution of the species suggests that soil characteristics, dispersal agents, barriers or viability of seed produced may be limiting factors restricting its distribution. Further research would be required to confirm these hypotheses.
Response to fire
Past studies suggest that plants with the same post-fire regenerative strategies of reseeding, resprouting or seeder-resprouters typically also have the same or similar root morphology and anatomical characteristics (Pate et al. 1990; Bell et al. 1996; Bell 2001; Saura-Mas and Lloret 2013). The shallow, fibrous root morphology of M. minutiflora is consistent with reseeding species, compared with resprouters, which have large deep penetrating roots (Pate et al. 1990; Bell 2001). There were no visible vegetative or resprouting structures, such as lignotubers, present on any of the field specimens collected, indicating that M. minutiflora does not reproduce asexually. The root biomass allocation was small, only 12% of total plant biomass. Reseeding species in past studies have had larger shoot:root ratios than have resprouters, because they tend to invest in aboveground organs that can reproduce large volumes of seeds rather than in belowground root structures that can resprout (Zammit and Westoby 1987; Pate et al. 1990; Hansen et al. 1991; Bowen and Pate 1993; Bell et al. 1996). The shoot:root ratio of M. minutiflora is consistent with it being a reseeder. Bradstock and Myerscough (1988) found that Banksia plants with stem diameters less than 20 mm invariably succumb to fire, regardless of the species post-fire regenerative strategy. The stem diameters of M. minutiflora were consistently much less than 20 mm (T. Bangel and A. M. Haigh, unpubl. data). The overall root morphology, lack of belowground organs capable of resprouting, high shoot:root ratio, stem diameters consistency being less than 20 mm and post-fire observations from the flora surveys of plants only present as seedlings post-fire all support the species fire response as an obligate reseeder.
Heat and smoke in the laboratory had a positive effect on the emergence of M. minutiflora seedlings from soil blocks collected in the field. However, there was still emergence recorded in the control blocks, similarly to the results of some Sydney region shrub species that form soil seedbanks from the Ericaceae and Myrtaceae families studied by Thomas et al. (2007). This indicated that, although M. minutiflora is an obligate seeder, its recruitment is not constrained to the post-fire environment. This supports the interpretation of the stem-length size-class data indicating continuous recruitment. However, the emergence of seedlings may peak after fire, and fire, in conjunction with seasonal temperatures (Mackenzie et al. 2021), could promote emergence in areas containing a soil seedbank (Read et al. 2000).
The life-history data collected in plots of varied times since fire indicated that M. minutiflora does not resprout following fire, rather it emerges as a seedling and can reach reproductive maturity as early as 18 months post-fire. Watson (2005) also recorded M. minutiflora flowering at two sites as early as 2 years after fire. The species was not recorded at any site subject to fire within 18 months prior to the surveys conducted in 2021 or 2010–11. Further, Pendall et al. (2022) observed shifts in the composition of resprouters and reseeders in response to higher fire frequencies in Castlereagh Scribbly Gum Woodland and Cooks River–Castlereagh Ironbark Forest where M. minutiflora occurs. Therefore, the species is likely to be sensitive to short-return fire intervals, especially given its post-fire regenerative strategy as an obligate seeder. Fire intervals as are currently recommended at a minimum of 8 and a maximum of 30 years (NSW Department of Environment Climate Change and Water 2011; NSW National Parks and Wildlife Service 2016) appear to be adequate for the species. However, maintenance of an open tree-canopy structure above existing populations of M. minutiflora may be a more important management strategy than any particular fire regime because the species will recruit without fire and reach reproductive maturity within a short period.
In summary, this study has provided an understanding of the distribution, abundance, population dynamics, fire response, reproductive strategy and habitat characteristics of M. minutiflora to inform its conservation management. Key threats to the species conservation include habitat removal for urban development, short-return fire intervals and habitat degradation, especially given the species narrow distribution within western Sydney and its specific habitat requirements, which are also major determinants that influence human impacts in the locality. Therefore, of utmost importance is the conservation of its habitat and remaining populations throughout its distribution, especially within areas that are not already protected. This will assist in linking and enhancing its habitat while also providing opportunities to identify new populations. However, simply conserving habitat is not enough, management of this habitat is also important in conserving the species because it has specific habitat preferences and is susceptible to habitat degradation. Key actions for managing the species habitat include prevention of invasive weed encroachment, grazing, arson, destructive recreational use and ensuring appropriate fire regimes are implemented. Fire management should specifically focus on preventing short-return fire intervals and raising awareness of the species requirements and occurrence to the appropriate stakeholders. Finally, regular monitoring of abundances, extents and health of known populations is required. As the species is myrtaceous, monitoring of the species should also include regular checks for myrtle rust (Austropuccinia psidii). These management actions are likely to assist in the management of other rare and threatened flora species and threatened ecological communities in the region that M. minutiflora was frequently found to be associated with.
Supplementary material
Supplementary material is available online.
Data availability
Data are available from the authors on request.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
T. Bangel received a Valette Williams Scholarship in Botany from the Australian Plants Society North Shore Group and a summer scholarship from Western Sydney University, Centre for Plants and the Environment. A. Hewitt received funding from the Australian Department of Agriculture, Water and the Environment (grant number GA-2000691).
Acknowledgements
The contributions of undergraduate students Nicholas Bennett in 2008 and Jenny Kremer in 2010, who completed initial work on this project, are gratefully acknowledged. The authors thank Mark Emmanuel (Western Sydney University) for measuring N in plant tissues. The authors thank the Australian Department of Defence for access to LDZ. The authors thank the Greater Sydney Parklands Trust and the two adjoining private landowners for access to areas in Mulgoa.
References
Auld TD, O’Connell MA (1991) Predicting patterns of post-fire germination in 35 eastern Australian Fabaceae. Australian Journal of Ecology 16, 53–70.| Predicting patterns of post-fire germination in 35 eastern Australian Fabaceae.Crossref | GoogleScholarGoogle Scholar |
Auld TD, Ooi MKJ (2009) Heat increases germination of water-permeable seeds of obligate-seeding Darwinia species (Myrtaceae). Plant Ecology 200, 117–127.
| Heat increases germination of water-permeable seeds of obligate-seeding Darwinia species (Myrtaceae).Crossref | GoogleScholarGoogle Scholar |
Australian Department of the Environment, Water, Heritage and the Arts (2008) Approved conservation advice for Micromyrtus minutiflora. In effect under the EPBC Act from 3 July 2008. (DEWHA: Canberra, ACT, Australia) Available at http://www.environment.gov.au/biodiversity/threatened/species/pubs/11485-conservation-advice.pdf
Bannerman SM, Hazelton PA (2011) ‘Soil landscapes of the Penrith 1:100 000 sheet interactive CD-ROM.’ (NSW Office of Environment and Heritage: Sydney, NSW, Australia)
Baumann A (2008) Regeneration of broad-leaved paperbark trees in the fringing forest of the Myall Lakes. PhD thesis, University of Sydney, NSW, Australia.
Bell DT (2001) Ecological response syndromes in the flora of southwestern Western Australia: fire resprouters versus reseeders. The Botanical Review 67, 417–440.
| Ecological response syndromes in the flora of southwestern Western Australia: fire resprouters versus reseeders.Crossref | GoogleScholarGoogle Scholar |
Bell TL, Pate JS, Dixon KW (1996) Relationships between fire response, morphology, root anatomy and starch distribution in south-west Australian Epacridaceae. Annals of Botany 77, 357–364.
| Relationships between fire response, morphology, root anatomy and starch distribution in south-west Australian Epacridaceae.Crossref | GoogleScholarGoogle Scholar |
Bellanger S, Guillemin J-P, Bretagnolle V, Darmency H (2012) Centaurea cyanus as a biological indicator of segetal species richness in arable fields. Weed Research 52, 551–563.
| Centaurea cyanus as a biological indicator of segetal species richness in arable fields.Crossref | GoogleScholarGoogle Scholar |
Benson DH, Howell J (1990) ‘Taken for granted: the bushland of Sydney and its suburbs.’ (Kangaroo Press: Sydney, NSW, Australia)
Benson D, McDougall L (1998) Ecology of Sydney plant species Part 6: dicotyledon family Myrtaceae. Cunninghamia 5, 808–983.
Benson DH, Howell J, McDougall L, Hawkesbury–Nepean Catchment Management Trust (1996) ‘Mountain devil to mangrove: a guide to natural vegetation in the Hawkesbury–Nepean catchment. The lands managed by the Hawkesbury–Nepean Catchment Management Trust.’ (Royal Botanic Gardens: Sydney, NSW, Australia)
Bentham G (1866) Myrtaceae to Compositae. In ‘Flora Australiensis: a description of the plants of the Australian territory’. Vol. 3, pp. 63–66. (Lovell Reeve and Co: London, UK)
Blanchet FG, Cazelles K, Gravel D (2020) Co-occurrence is not evidence of ecological interactions. Ecology Letters 23, 1050–1063.
| Co-occurrence is not evidence of ecological interactions.Crossref | GoogleScholarGoogle Scholar |
Bowen BJ, Pate JS (1993) The significance of root starch in post-fire shoot recovery of the resprouter Stirlingia latifolia R. Br.(Proteaceae). Annals of Botany 72, 7–16.
| The significance of root starch in post-fire shoot recovery of the resprouter Stirlingia latifolia R. Br.(Proteaceae).Crossref | GoogleScholarGoogle Scholar |
Bradstock RA, Myerscough PJ (1988) The survival and population response to frequent fires of two woody resprouters Banksia serrata and Isopogon anemonifolius. Australian Journal of Botany 36, 415–431.
| The survival and population response to frequent fires of two woody resprouters Banksia serrata and Isopogon anemonifolius.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2022) Climate statistics for Australian locations: Summary statistics RICHMOND – UWS HAWKESBURY. Available at http://www.bom.gov.au/climate/averages/tables/cw_067021.shtml [Verified 30 November 2021]
Burrows GE (2002) Epicormic strand structure in Angophora, Eucalyptus and Lophostemon (Myrtaceae) – implications for fire resistance and recovery. New Phytologist 111–131.
| Epicormic strand structure in Angophora, Eucalyptus and Lophostemon (Myrtaceae) – implications for fire resistance and recovery.Crossref | GoogleScholarGoogle Scholar |
Calatayud J, Andivia E, Escudero A, Melián CJ, Bernardo-Madrid R, Stoffel M, Aponte C, Medina NG, Molina-Venegas R, Arnan X, Rosvall M, Neuman M, Noriega JA, Alves-Martins F, Draper I, Luzuriaga A, Ballesteros-Cánovas JA, Morales-Molino C, Ferrandis P, Herrero A, Pataro L, Juen L, Cea A, Madrigal-González (2020) Positive associations among rare species and their persistence in ecological assemblages. Nature Ecology & Evolution 4, 40–45.
| Positive associations among rare species and their persistence in ecological assemblages.Crossref | GoogleScholarGoogle Scholar |
Chapin FS, Bloom AJ, Field CB, Waring RH (1987) Plant responses to multiple environmental factors. BioScience 37, 49–57.
| Plant responses to multiple environmental factors.Crossref | GoogleScholarGoogle Scholar |
Chick MP, York A, Sitters H, Stefano JD, Nitschke CR (2019) Combining optimization and simulation modelling to measure the cumulative impacts of prescribed fire and wildfire on vegetation species diversity. Journal of Applied Ecology 56, 722–732.
| Combining optimization and simulation modelling to measure the cumulative impacts of prescribed fire and wildfire on vegetation species diversity.Crossref | GoogleScholarGoogle Scholar |
Clarke PJ, Lawes MJ, Murphy BP, Russell-Smith M (2015) A synthesis of postfire recovery traits of woody plants in Australian ecosystems. Science of The Total Environment 534, 31–42.
| A synthesis of postfire recovery traits of woody plants in Australian ecosystems.Crossref | GoogleScholarGoogle Scholar |
Cochran WG (1977) ‘Sampling techniques’, 3rd edn. (Wiley: New York, NY, USA)
Cropper S (1993) ‘Management of endangered plants.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Daniells IG (2012) Hardsetting soils: a review. Soil Research 50, 349–359.
| Hardsetting soils: a review.Crossref | GoogleScholarGoogle Scholar |
Elzinga CL, Salzer DW, Willoughby JW (2019) ‘Measuring and monitoring plant populations.’ (US Bureau of Land Management Papers)
Emerson WW (1983) Inter-particle bonding. In ‘Soils: an Australian viewpoint’. pp. 477–497. (CSIRO Division of Soils: Melbourne, Vic., Australia; and Academic Press: London, UK)
Fanfarillo E, Latini M, Abbate G (2020) Patterns of co-occurrence of rare and threatened species in winter arable plant communities of Italy. Diversity 12, 195–206.
| Patterns of co-occurrence of rare and threatened species in winter arable plant communities of Italy.Crossref | GoogleScholarGoogle Scholar |
Hansen A, Pate JS, Hansen AP (1991) Growth and reproductive performance of a seeder and a resprouter species of Bossiaea as a function of plant age after fire. Annals of Botany 67, 497–509.
| Growth and reproductive performance of a seeder and a resprouter species of Bossiaea as a function of plant age after fire.Crossref | GoogleScholarGoogle Scholar |
Harper JL (1977) ‘Population biology of plants.’ (Academic Press: London, UK)
Hewitt A, Rymer P, Holford P, Morris EC, Renshaw A (2019) Evidence for clonality, breeding system, genetic diversity and genetic structure in large and small populations of Melaleuca deanei (Myrtaceae). Australian Journal of Botany 67, 36–45. 10.1071/BT18148
Hutchings MJ (1997) The structure of plant populations. In ‘Plant ecology’, 2nd edn. (Ed. MJ Crawley) pp. 325–358. (Blackwell Science)
International Union for Conservation of Nature and Natural Resources (1994) ‘IUCN Red List Categories.’ (IUCN Species Survival Commission: Kew, London, UK)
James T, McDougall L, Benson D (1999) ‘Urban bushland biodiversity survey. Stage 1: Western Sydney: native flora.’ (NSW National Parks and Wildlife Service: Sydney, NSW, Australia)
Keith DA (2000) Sampling designs, field techniques and analytical methods for systematic plant population surveys. Ecological Management & Restoration 1, 125–139.
| Sampling designs, field techniques and analytical methods for systematic plant population surveys.Crossref | GoogleScholarGoogle Scholar |
Krebs CJ (1985) ‘Ecology: the experimental analysis of distribution and abundance’, 3rd edn. (Harper and Row: New York, NY, USA)
Lamont BB, Barrett GJ (1988) Constraints on seed production and storage in a root-suckering Banksia. The Journal of Ecology 76, 1069–1082.
| Constraints on seed production and storage in a root-suckering Banksia.Crossref | GoogleScholarGoogle Scholar |
Lesica P, Yurkewycz R, Crone EE (2006) Rare plants are common where you find them. American Journal of Botany 93, 454–459.
| Rare plants are common where you find them.Crossref | GoogleScholarGoogle Scholar |
Mackenzie BDE, Auld TD, Keith DA, Ooi MKJ (2021) Fire seasonality, seasonal temperature cues, dormancy cycling, and moisture availability mediate post-fire germination of species with physiological dormancy. Frontiers in Plant Science 12, 795711
| Fire seasonality, seasonal temperature cues, dormancy cycling, and moisture availability mediate post-fire germination of species with physiological dormancy.Crossref | GoogleScholarGoogle Scholar |
Morrison DA, Morris EC (2000) Pseudoreplication in experimental designs for the manipulation of seed germination treatments. Austral Ecology 25, 292–296.
| Pseudoreplication in experimental designs for the manipulation of seed germination treatments.Crossref | GoogleScholarGoogle Scholar |
NSW Department of Environment and Conservation (2005) Recovering bushland on the Cumberland Plain: best practice guidelines for the management and retsoration of bushland. DEC 2005/177. (NSW DEC: Sydney, NSW, Australia) Available at https://www.environment.nsw.gov.au/resources/nature/RecoveringCumberlandPlain.pdf
NSW Department of Environment Climate Change and Water (2002) Micromyrtus minutiflora (a shrub) – endangered species listing. NSW Scientific Committee final determination. Available at https://www.environment.nsw.gov.au/topics/animals-and-plants/threatened-species/nsw-threatened-species-scientific-committee/determinations/final-determinations/2000-2003/micromyrtus-minutiflora-a-shrub-endangered-species-listing
NSW Department of Environment Climate Change and Water (2011) Cumberland Plain Recovery Plan. DECCW 2010/501. (NSW DECCW: Sydney, NSW, Australia) Available at https://www.environment.nsw.gov.au/-/media/OEH/Corporate-Site/Documents/Animals-and-plants/Recovery-plans/cumberland-plain-recovery-plan-100501.pdf
NSW Department of Environment Climate Change and Water (2016) The native vegetation of the Sydney metropolitan area - version 3.1 (OEH, 2016) VIS_ID 4489. (NSW DECCW) Available at https://datasets.seed.nsw.gov.au/dataset/the-native-vegetation-of-the-sydney-metropolitan-area-oeh-2016-vis-id-4489
NSW Department of Planning and Environment (2022) Micromyrtus minutiflora – profile. (NSW OEH: Sydney, NSW, Australia) Available at https://www.environment.nsw.gov.au/threatenedspeciesapp/profile.aspx?id=10529
NSW Department of Planning Industry and Environment (2020) Biodiversity assessment method. NSW Department of Planning Industry and Environment, Sydney, NSW, Australia.
NSW National Parks and Wildlife Service (2016) Castlereagh nature reserve fire management strategy. Available at https://www.environment.nsw.gov.au/-/media/OEH/Corporate-Site/Documents/Parks-reserves-and-protected-areas/Fire-management-strategies/castlereagh-nature-reserve-fire-management-strategy-160688.pdf
NSW Office of Environment and Heritage (2014a) NSW flora fire response database (Version 2.1). NSW OEH, Sydney, NSW, Australia.
NSW Office of Environment and Heritage (2014b) Wianamatta Nature Reserve statement of management intent. DEC20140250. (NSW OEH: Sydney, NSW, Australia) Available at https://www.environment.nsw.gov.au/-/media/OEH/Corporate-Site/Documents/Parks-reserves-and-protected-areas/Parks-statement-of-management-intent/wianamatta-nature-reserve-statement-of-management-intent-140250.pdf
NSW Office of Environment and Heritage (2022) Micromyrtus minutiflora management strategy. (NSW OEH: Sydney, NSW, Australia) Available at https://www.environment.nsw.gov.au/savingourspeciesapp/project.aspx?ProfileID=10529
Pate JS, Froend RH, Bowen BJ, Hansen A, Kuo J (1990) Seedling growth and storage characteristics of seeder and resprouter species of Mediterranean-type ecosystems of SW Australia. Annals of Botany 65, 585–601.
| Seedling growth and storage characteristics of seeder and resprouter species of Mediterranean-type ecosystems of SW Australia.Crossref | GoogleScholarGoogle Scholar |
Pendall E, Hewitt A, Boer MM, Carrillo Y, Glenn NF, Griebel A, Middleton JH, Mumford PJ, Ridgeway P, Rymer PD, Steenbeeke GL (2022) Remarkable resilience of forest structure and biodiversity following fire in the peri-urban bushland of Sydney, Australia. Climate 10, 86
| Remarkable resilience of forest structure and biodiversity following fire in the peri-urban bushland of Sydney, Australia.Crossref | GoogleScholarGoogle Scholar |
Rabinowitz D (1981) Seven forms of rarity. In ‘The biological aspects of rare plant conservation’. (Ed. H Synge) pp. 205–217. (Wiley: New York, NY, USA)
Rayment GE, Higginson FR (1992) ‘Australian laboratory handbook of soil and water chemical methods.’ (Inkata Press: Melbourne, Vic., Australia)
Read TR, Bellairs SM, Mulligan DR, Lamb D (2000) Smoke and heat effects on soil seed bank germination for the re-establishment of a native forest community in New South Wales. Austral Ecology 25, 48–57.
| Smoke and heat effects on soil seed bank germination for the re-establishment of a native forest community in New South Wales.Crossref | GoogleScholarGoogle Scholar |
Reed DH (2005) Relationship between population size and fitness. Conservation Biology 19, 563–568.
| Relationship between population size and fitness.Crossref | GoogleScholarGoogle Scholar |
Saura-Mas S, Lloret F (2013) Adult root structure of mediterranean shrubs: relationship with post-fire regenerative syndrome. Plant Biology 16, 147–154.
| Adult root structure of mediterranean shrubs: relationship with post-fire regenerative syndrome.Crossref | GoogleScholarGoogle Scholar |
Spooner PG, Lunt ID, Briggs SV, Freundenberger D (2004) Effects of soil disturbance from roadworks on roadside shrubs in a fragmented agricultural landscape. Biological Conservation 117, 393–406.
| Effects of soil disturbance from roadworks on roadside shrubs in a fragmented agricultural landscape.Crossref | GoogleScholarGoogle Scholar |
Sritharan MS, Scheele BC, Blanchard W, Lindenmayer DB (2021) Spatial associations between plants and vegetation community characteristics provide insights into the processes influencing plant rarity. PLoS ONE 16, e0260215
| Spatial associations between plants and vegetation community characteristics provide insights into the processes influencing plant rarity.Crossref | GoogleScholarGoogle Scholar |
Thomas PB, Morris EC, Auld TD (2007) Response surfaces for the combined effects of heat shock and smoke on germination of 16 species forming soil seed banks in south-east Australia. Austral Ecology 32, 605–616.
| Response surfaces for the combined effects of heat shock and smoke on germination of 16 species forming soil seed banks in south-east Australia.Crossref | GoogleScholarGoogle Scholar |
Thomas PB, Morris EC, Auld TD, Haigh AM (2010) The interaction of temperature, water availability and fire cues regulates seed germination in a fire-prone landscape. Oecologia 162, 293–302.
| The interaction of temperature, water availability and fire cues regulates seed germination in a fire-prone landscape.Crossref | GoogleScholarGoogle Scholar |
Tozer K (2003) The native vegetation of the Cumberland Plain, western Sydney: systematic classification and field identification of communities. Cunninghamia 8, 1–75.
Watson PJ (2005) Fire frequencies for Western Sydney’s woodlands: indications from vegetation dynamics. PhD thesis. Western Sydney University, Penrith, NSW, Australia. Available at http://handle.uws.edu.au:8081/1959.7/24673
Wilson P (2002) Micromyrtus. In ‘Flora of New South Wales’. (Ed. GJ Harden) Vol. 2, pp. 215–217. (UNSW Press: Sydney, NSW, Australia)
Young AG, Brown AHD (1996) Comparative population genetic structure of the rare woodland shrub Daviesia suaveolens and its common congener D. mimosoides. Conservation Biology 10, 1220–1228.
| Comparative population genetic structure of the rare woodland shrub Daviesia suaveolens and its common congener D. mimosoides.Crossref | GoogleScholarGoogle Scholar |
Zammit C, Westoby M (1987) Seedling recruitment strategies in obligate-seeding and resprouting Banksia shrubs. Ecology 68, 1984–1992.
| Seedling recruitment strategies in obligate-seeding and resprouting Banksia shrubs.Crossref | GoogleScholarGoogle Scholar |
Zammitt C (1988) Dynamics of resprouting in the lignotuberous shrub Banksia oblongifolia. Australian Journal of Ecology 13, 311–320.
| Dynamics of resprouting in the lignotuberous shrub Banksia oblongifolia.Crossref | GoogleScholarGoogle Scholar |



