Fire responses of flora in a sclerophyll–rainforest vegetation complex in the Nightcap Range, North Coast, New South Wales
Andrew Benwell A *
A *
A
Abstract
Species fire responses were investigated in a mixed sclerophyll–rainforest ecosystem in the Nightcap Range, North Coast, New South Wales.
To examine rates of seedling recruitment and resprouting in functional and phytogeographic components of wet sclerophyll forest (WSRf), and adjacent open forest (OF) and rock outcrop shrubland (RO).
Species resprouting and seedling recruitment traits (fire responses) were recorded in 45 stem plots and 225 seedling subplots in WSRf, OF and RO. Species fire responses were classified, community fire-response spectra compiled and rates of seedling recruitment and resprouting in WSRf examined in relation to primary fire response, growth-form, habitat and broad functional and phytogeographic species groupings. Species size-regenerative class distribution was used to analyse population structure, fire impact, regeneration and recruitment in resprouter species that comprised most of the mesic-Gondwanan element of the WSRf flora.
WSRf, OF and RO habitats had distinctively different fire-response spectra. In WSRf, there was a high proportion of mesophyll resprouter species of Gondwanan origin with nil or very low seedling recruitment, a distinct component of mesophyll seeders of Indo-Malayan origin, as well as sclerophyll seeders and resprouters that also comprised most of the OF and RO floras. Resprouters comprised 75% of the WSRf flora, 50% OF and 10% pavement shrubland. Continuous size-class distributions indicated recruitment between fire events in the majority of mesophyll resprouters in WSRf. Lower total seedling density appeared to reflect inherent species traits and less canopy disturbance by fire. Large sclerophyll species forming the unburnt canopy of WSRf had very low seedling recruitment.
Different habitats (WSRf, OF and RO) and functional and phytogeographic clades in WSRf display distinctive patterns of resprouting and seedling-recruitment fire response. Fire responses of species that maintain species population and community composition are governed by fire regime, habitat variables and inherent species traits.
The distinctive fire-response spectrum of WSRf appears to be a direct consequence of the overlap of ‘new’ and ‘old’ floras in this broad vegetation type.
Keywords: fire, Gondwanan, phytogeography, rainforest, recruitment, resprouting, sclerophyll, seedling.
Introduction
The Nightcap Range on the North Coast of New South Wales (NSW) supports a diverse vegetation of wet and dry sclerophyll forest and rainforest (FCNSW 1989; DPE 2023), which typifies much of the narrow zone of high rainfall on the eastern margin of mainland Australia, between the Great Escarpment and the coast, and in Tasmania. This is a distinctive phytogeographic region where the sclerophyll flora that dominates most of Australia meets and overlaps with the remnants of Australia’s Tertiary rainforests that once covered most of the continent. The rainforest flora includes Gondwanan and recent immigrant elements from Southeast Asia, whereas the sclerophyll flora contains Gondwanan and autochthonous elements, the latter having evolved within Australia during its northward drift into drier subtropical latitudes in the Tertiary and Quaternary (Barlow 1981; Byrne et al. 2011; Weston and Jordan 2017). The recently evolved flora in forest is mostly sclerophyll and pyrogenic, whereas much of the older flora is mesophytic, fire-retardant and dominates the mesic end of the vegetation spectrum. At a regional scale, the two floras are clearly separated in dry sclerophyll forest and rainforest, whereas overlap is most evident in wet sclerophyll forest (WSF), where elements of the two floras often occur in the same plant community.
Research on the fire ecology of WSF has been conducted mainly in the temperate zone in Victoria (Ashton 1981) and Tasmania (Jackson 1968; Hill and Read 1984; Prior et al. 2022; Bowman et al. 2023) and high-elevation, north-eastern NSW (Smith and Guyer 1983; Campbell and Clarke 2006; Knox and Clarke 2012). Little fire ecology research has been conducted in WSF and rainforest–open forest ecotones in subtropical eastern Australia, examples including Floyd (1976) and Baker et al. (2022) on the NSW North Coast, and Fensham et al. (2003), Williams et al. (2012) and Tng et al. (2012) in eastern Queensland, whereas other research had an Australia-wide perspective (Tng et al. 2013; Wardell-Johnson et al. 2017). Some of these studies noted divergence in the fire-response traits of mesic and sclerophyll components of wet sclerophyll, but there is generally little research on how species and communities respond to fire in terms of mortality, vegetative regeneration and seed recruitment, species-level demographic traits that can underlie changes in plant community composition and type.
Vegetation dynamics in sclerophyll forest consist predominantly of cycles of fire disturbance and successional regrowth, whereas in mature rainforest, gap formation by falling, senescent trees and cyclone damage is usually the dominant disturbance regime and driver of vegetation dynamics (White 1979; Huston 1994; Murphy et al. 2014). However, during drought years, fires in dry sclerophyll (open) forest often burn into wet sclerophyll and the margins of rainforest, causing damage to rainforest species equivalent to that occurring to sclerophyll flora in open forest, although usually being less due to lower fire intensity. Rainforest has a marked dampening effect on fire intensity because of mesophytic flora, microclimate, low litter accumulation and topography. Records indicate that historical bushfires on the NSW North Coast generally penetrated rainforest for only 10–50 m, rarely more than 100 m (Floyd 1990). It has long been thought that the greater flammability of sclerophyll forest limits the distribution of rainforest by killing and stunting juvenile rainforest plants establishing in open forest between fires, thereby inhibiting rainforest expansion. However, recent studies have found that many rainforest species exhibit regenerative traits after fire (Pausas et al. 2004; Clarke et al. 2015). Baker et al. (2022) found that most late successional rainforest species in littoral, dry, temperate and subtropical rainforest resprouted after fire, larger stems were not significantly reduced in rainforest and many species exhibited ‘fire-cued’ seedling recruitment, findings that appear to contradict the assumption of the sensitivity of rainforest to fire and are further examined in this paper.
Plant responses to fire involve two basic population-maintaining processes, resprouting (R) and seedling recruitment (S), which can exhibit as three primary fire responses, namely, obligate seed regeneration (R−S+), facultative resprouting (R+S+) and obligate resprouting (S−R+), or OSR, FR and OR for short, or four if no regeneration (R−S−) is included (Naveh 1975; Gill 1981; Pausas et al. 2004). Diverse species fire responses are a striking feature of plant communities, and all three primary fire responses will generally be present in a fire-prone plant community. Most species that resprout ultimately depend on S to maintain population, as individuals eventually die as a result of senescence, competition, or environmental stress. Some species can reproduce vegetatively, which is generally included as a subcategory of resprouting. The two basic regenerative modes (R and S) involve persistence by asexual and sexual reproduction respectively (Bond and Midgley 2003; Verdú et al. 2007). As well as primary fire responses, various subcategories of associated traits are recognised, where species utilise different seed sources and modes of resprouting to re-establish population (Gill 1981). The species forming a plant community also vary in other aspects of fire response, including levels of fire-induced mortality and resprouting (Vesk et al. 2004), post-fire seedling density and survivorship (Benwell 1998) and primary and secondary juvenile periods.
In this study, seedling recruitment and resprouting fire responses were examined in a mixed rainforest–sclerophyll ecosystem in the Nightcap Range after a major fire in 2019. The main interest was in WSF to better understand how the mesophyll and sclerophyll components of this broad vegetation are apparently both able to maintain population and co-exist in the same plant community. The rainforest flora occurring in this broad vegetation type, usually shielded from fire within rainforest, is relatively exposed to fire in WSF, so its fire response is readily examined. With the present risk of significant climatic change, fire-response studies have the potential to serve as the ‘canary in the coal-mine’ in detecting early stages of change in plant community as reflected in species responses to fire. The principal aims of this study were therefore to determine
variation among species in post-fire rates of vegetative recovery and seedling recruitment;
whether those rates differed between WSRf and more fire-prone ecosystems adjacent to them;
whether rates varied between broad functional and phytogeographic components of the WSRf flora (e.g. mesophyll/sclerophyll; Gondwanan/autochthonous).
Materials and methods
Study site and vegetation
The Nightcap Range (28.58S, 153.37E; Fig. 1) has an elevation of 200–900 m and a mean annual rainfall of 1800–2500 mm. Soils are brown podzols of low to medium fertility formed on rhyolite, with smaller areas of higher fertility, red–brown krasnozem on intermediate to basic volcanics (Stevens 1976; Australian Government 1992). The major vegetation formations in the order of extent are wet sclerophyll forest, dry sclerophyll forest, rainforest and rock outcrop shrubland (FCNSW 1989). High rainfall and a warm subtropical climate promote rainforest development, whereas low-nutrient substrates favour sclerophyll vegetation (Beadle 1954; Adam 1992). In the Nightcap Range, the rainforest flora occurs both in rainforest, a closed canopy formation and extensively in wet sclerophyll–rainforest (WSRf) as an understorey below a very tall, open forest canopy of myrtaceous brush box (Lophostemon confertus), turpentine (Syncarpia glomulifera), blackbutt (Eucalyptus pilularis), flooded gum (Eucalyptus grandis), tallowwood (Eucalyptus microcorys), pink bloodwood (Corymbia intermedia) and at high-elevation New England blackbutt (Eucalyptus campanulata). Accumulation of sclerophyll litter produced by the upper stratum of WSRf may increase bushfire penetration, but this is counteracted by the mesophytic understorey flora and microclimate, which dampen fire spread and intensity. Drier sclerophyll forest dominated mainly by blackbutt, and patches of rock outcrop shrubland cover the ridge lines and most of the northern flanks of the range.
2019 bushfire and fire history
Extremely dry conditions developed in eastern NSW and south-eastern Queensland in 2019 because of a positive Indian Ocean Dipole and Sudden Stratospheric Warming above Antarctica (BOM 2019). The forest fire-danger index in the Richmond–Tweed region was the highest on record and spring rainfall one of the lowest. Lightening ignited the Nightcap Range fire in November 2019, which was fanned by moderate, 40–50 km/h NW winds and 5% relative humidity (M. Wiseman, NPWS, pers. comm.).
Major fires in the Nightcap Range before 2019 occurred in 1968 and 1926, a fire interval of 40–50 years. The drier northern slopes of the range were also burnt in the 1990s (M. Wiseman, NPWS, pers. comm.). Rainfall data for Mullumbimby near the base of the range show that annual rainfall fell well below average and vegetation was dry enough for bushfire at intervals of 4–25 years between 1898 and 1988, although fire frequency at a specific location will be lower because of several factors. By carbon dating soil charcoal layers in the Nightcap Range, Turner (1984) estimated the frequency of intense fire in brush box forest at 325–380 years and in blackbutt at 280 years. Of eight major wildfires in 3000 years, only one fire approximately 1100 years ago penetrated rainforest. Wildfire appears to be part of the Nightcap Range environment, although fires recur under a low-frequency fire regime.
Survey design
Species fire responses after the 2019 fire were sampled in 45 plots, 36 in WSRf, four in drier open forest and five in rock outcrop shrubland, which included rock pavement and fringing woodland (Benwell 2007). The sampling focused on WSRf, because primary interest was in how rainforest and sclerophyll floristic components in this vegetation type responded to fire. To compare fire impact and species responses in different habitats, samples were also taken from adjacent drier OF and RO. The higher plot number in WSRf aimed to maximise the number of species captured and reduce the standard error of sampled resprouting and seedling recruitment traits as far as practicable, given limited time and resources.
Sites carrying a well-developed rainforest understorey were selected in WSF (i.e. WSRf), where the 6–15+ m high mid-stratum of rainforest trees, shrubs, palms and vines had been burnt, scorched or defoliated. Sites were stratified to include lower to mid-slopes of varying aspect and most were on rhyolite geology and brown podzolic soil, with a few on small areas of red–brown soil on intermediate volcanics. Plots were at elevations of 250 m to 600 m asl (Fig. 1). More lightly burnt WSF and true rainforest with a closed canopy and without sclerophyll emergents were not sampled because fire damage was either very light or absent, the fire extinguishing on the rainforest edge or a short distance inside.
Data collection
Stem mortality and resprouting were recorded in 20 m × 6 m plots and seedling recruitment in five 1 m × 1 m subplots. The height, diameter at breast height and species of all live and dead stems in plots were recorded and each stem was assigned to one of the following three regenerative classes: (1) stem 100% defoliated, plant resprouting, (2) stem dead (defoliated, plant not resprouting) and (3) foliage crown unburnt and intact, lower trunk with or without visible scorching. Presence of minor basal resprouts on individuals in (3) was also recorded. ‘Defoliated’ meant all foliage was dead, either scorched and still attached to the stem, shed as litter, or combusted by the fire. The mode of resprouting of live stems was recorded as basal, epicormic or root sucker. Because the fire reached only moderate intensity in WSRf, dead stems assumed to be alive and ‘green’ when burnt were left standing in a mummified state, and, combined with live stems, provided a record of approximate forest structure at the time of fire. Dead stems were identified to species from dead leaves still attached to stems, bark texture, stem blaze and smell, stem form and branching architecture. Unidentified stems were recorded as unknowns. Stem data were recorded by plot quarter to facilitate relocating individuals 2 years after fire. Vines and herbaceous perennials resprouting in plots were recorded as present and notes were made on the mode of resprouting and relative abundance.
Seedling recruitment was recorded in five 1 m × 1 m subplots placed 4 m apart along the centre line of each plot. Seedlings were identified from their cotyledons and first true leaves and counts of each species recorded along with identification features. Seedlings of unidentified species were given a temporary name, collected outside plots and grown-on in a nursery for identification. Monocots without obvious cotyledons and several dicots were excavated to confirm that they were seedlings and not small resprouts from roots, rhizomes, or corms. Marker stakes were installed at the corners of each subplot. Plots and seedling subplots were recorded 6–12 months and 18–24 months after fire. In Year 2, stems recorded in Year 1 were identified using species, height, diameter, regeneration mode and plot quarter data and re-recorded. Species identification was checked and any additional mortalities, late resprouts (usually small plants), number of root suckers and condition of epicormic resprouts were noted. Counts of seedlings and the maximum seedling height of each species were recorded in each sub-plot.
Classifying fire response
Species fire responses were classified in terms of primary fire response, and more detailed resprouting and seedling recruitment traits incorporating mode of resprouting and seed source (Table 1) based on Gill (1981). Additional fire-response categories were included for ‘live escapes’ where the foliage crown of a stem remained intact and functioning (Table 1, ‘E’); a seed source category for seedlings that appeared to be from unburnt tree crowns (Table 1, ‘SU’); and a category for stems apparently defoliated by drought, not fire (Table 1, ‘DR’).
| Abbreviation | Species fire response | |
|---|---|---|
| R | Resprouting after 100% defoliation by fire, pre-fire stem dead or alive (R3) | |
| R1 | Basal (collar or lignotuber) resprouts only | |
| R2 | Root suckering with/without basal resprouts | |
| R3 | Epicormic resprouts with/without basal resprouts | |
| E | Crown intact, foliage above scorch height; ‘live escape’ | |
| E1 | Trunk base lightly scorched, canopy intact, minor basal or epicormic resprouting | |
| E2 | Trunk base lightly scorched, canopy intact, no minor basal or epicormic resprouting | |
| DR | Large tree apparently defoliated by drought, not fireA | |
| DR1 | Stem and root system dead | |
| DR2 | Stem and root system alive, reshooting in canopy | |
| S | Seedling regeneration (obligate seeders and facultative resprouters); seed source | |
| S1 | Soil seedbank: perennials and fire ephemerals | |
| S2 | Bradysporous seed release | |
| S3 | Post-fire flowering | |
| SU | Unburnt sources, e.g. seed fall from intact crowns or dispersed by birds and mammals; wind-dispersed (mostly exotics) |
Species size–regenerative class distributions
Size-class histograms of resprouter tree and shrub species with >10 stems were prepared from stem height data and used to interpret species population dynamics (particularly recruitment) under recent ecological conditions (Daubenmire 1968; Lykke 1998; Vlam et al. 2017). Heights of pre-fire live and standing dead stems were allocated to height classes (0–1, 1–2, 2–5, 5–10, 10–15, 15–20, 20–30 and 30–40 m) to reconstruct approximate species population and forest structure at the time of fire, and combined with regenerative class to show how each size class was affected by fire. Species histograms were described in terms of shape (right-skewed, left-skewed, bell, flat), type of distribution (continuous or truncated) and whether small size classes were present. If a population is recruiting new individuals, a distribution including large and small size class individuals (continuous) is expected. If recruitment has not been occurring for some time, a truncated distribution lacking small size-class individuals is usually recorded. A truncated distribution may indicate unsuitable conditions for recruitment or intermittent recruitment. The size-class distributions were used as an indicator of population recruitment in resprouter species, which comprised most of the mesic component of WSRf, and overlaid with regenerative class data, how each size class was affected by fire.
Data analysis
The five-point vegetal-damage scale of Chafer et al. (2004) was used to describe the degree of fire damage to vegetation (Table 2). To quantify fire damage in relation to forest structure in WSRf, pre-fire heights of stems (dead and alive) were assigned to six vertical strata (0–1 m, >1–5 m, >5–10 m, >10–15 m, >15–25 m and >25 m) and the number of individuals in the three regenerative classes (i.e. dead; 100% defoliated and resprouting; and foliage crown intact, trunk burnt) was summed for each strata. Number and percentage of stems in the three regenerative classes were compared in WSRf and OF and tested for significant differences by using a standard Student’s t-tests if normally distributed or a non-parametric Mann–Whitney (Wilcoxon rank-sum) tests when skewed.
| Damage score | Damage-level description | Field indicators | |
|---|---|---|---|
| 1 | Low | Ground fuel and low shrubs burnt. | |
| 2 | Moderate | Ground fuel and shrubs up to 4 m burnt. | |
| 3 | High | Ground fuel, shrubs incinerated, canopy scorched. | |
| 4 | Very high | Ground fuel, shrubs incinerated and canopy completely burnt. | |
| 5 | Extreme | All green vegetation burnt and stems <10 mm thick incinerated. |
Histograms showing the frequency of three primary fire responses (OSR, FR and OR) in each of the three habitats (WSRf, OF and RO) were compared in terms of (1) mean number of OSR, FR and OR species per plot, and (2) total number and percentage of OSR, FR and OR species per habitat.
The seedling recruitment patterns of species and communities were compared in terms of the demographic trait, mean number of seedlings per square metre, or seedling density. This metric represented net seedling density (i.e. total germinations less total mortalities) at a given time after disturbance. Following Benwell (1998), mean seedling density for comparison of species and communities was recorded 6–12 months after fire, when seedling numbers approximated peak density and most species had their first adult leaves and cotyledons to confirm that they were seedlings and to assist identification.
Fire responses in WSRf described in terms of seedling recruitment and resprouting traits were examined in broad functional-phytogeographical components of the WSRf flora, including primary fire response (OSR, FR, OR), growth-form (herbaceous, woody), preferred habitat (mesophytic/rainforest vs sclerophytic/open forest) and successional niche (early successional vs late successional, on the basis of information on species ecology in PlantNet (Royal Botanic Gardens, Sydney), Harden et al. 2006, 2007 and Floyd 2008). A functional group identifies a group of species that share an aspect of species ecology that usually has some adaptive significance (Miller and Murphy 2017; Kearney et al. 2021). A species assigned to rainforest habitat shows best development in rainforest habitat. It may also be found in sclerophyll habitat, but is generally smaller in number and stature. Early successional species are those that are more prominent in the initial stages of wet sclerophyll–rainforest succession and included some long-lived species. Late successional or mature phase species are those prominent in later stages of this succession. Nearly all sclerophyll species were classed early successional in the context of rainforest succession (see Supplementary data S1).
The representation of primary fire response and functional components in plant community fire response was examined in terms of number of species and seedling density per square metre. Statistical comparisons were again performed using non-parametric Mann–Whitney (Wilcoxon rank-sum) tests for skewed data, or paired Student’s t-tests for data approximating a normal distribution. For frequency data, chi-squared tests were used.
Pre-fire heights of stems of WSRf woody tree and shrub species in the ‘100% defoliated and basal resprouting’ regenerative class (mostly mesic-Gondwanan taxa) were allocated to the five height strata used to describe fire impact (0–1 m, >1–2 m, >2–5 m, >5–10 m and >10–15 m) and height of resprouting regrowth of each stem measured 2 years later to make a preliminary estimate of rate of regrowth and how fast burnt WSRf would regenerate pre-fire understorey structure.
Species seedling growth rates in WSRf were compared in terms of the maximum height of seedlings recorded in subplots. Mean maximum seedling height was compared in OSR and FR primary fire-response categories using Mann–Whitney tests and by comparing the number of FR and OSR species above and below median maximum seedling height by using chi-squared tests.
Results
Species composition
In total, 264 vascular species were recorded in the three habitats sampled. Species richness (mean ± s.e.) per plot was highest in WSRf (36.3 ± 1.4), followed by OF (30.0 ± 4.5) and RO (18.2 ± 3.4). Of the 214 species recorded in WSRf, 86.5% were woody species (including vines) and 13.5% herbaceous; 76.1% of species were classed as mesophytic or rainforest species and 23.9% as sclerophyll or open forest species.
Intensity and impact of 2019 fire
The fire in WSRf survey plots was a moderately intense ground and mid-stratum fire, under extremely dry conditions and build-up of woody fuel, not having been burnt for 40–50 years (M. Wiseman, NPWS, pers. comm.). Fire intensity was dampened by mesic standing fuels, microclimate and wind strength up to a moderate 40 km/h (M. Wiseman, NPWS, pers. comm.). By contrast, the fire often crowned upslope in OF, and in RO the shallow, humic soil as well as standing vegetation were incinerated, removing soil and roots down to bedrock.
Rainforest tree and shrub species in WSRf were damaged or killed by burning and scorching of the stem system and sometimes roots where soil erosion on steep slopes had exposed large roots or the whole root crown at the base of trunks. Approximately 50% of taller (>10 m) rainforest trees in WSRf had intact, unburnt canopies, although trunks often showed scorching up to 4–5 m above ground, but with bark being apparently intact. These trees were referred to as ‘live escapes’ in Year 1, although later examination showed that partial damage to the lower trunk of many had in fact occurred, because dead bark later fell away, showing new bark regrowing over fire scars. A sign of this damage in ‘live escapes’ in Year 1 was minor basal stem shoots, even though bark at the tree base appeared undamaged (Table 1, E1). The 40–60 m high sclerophyll canopy of WSRf escaped fire damage, except for occasional, old brush box (L. confertus) and blackbutt (E. pilularis) felled by the fire burning into tree butts hollowed out by previous fires.
The proportion of resprouting stems and dead stems increased as size class decreased, except in the smallest (0–1 m) size class (Fig. 2), which may be due to complete incineration of some stems. Nearly all stems in the two smallest size classes were either resprouting or dead, decreasing to approximately 50% in the 10–15 m stratum and 20% in the 25+ m stratum.
Fire impact on rainforest understorey in WSRf, showing the total number stems/individuals in six size classes, either resprouting after 100% defoliation, dead or with canopy intact. n = 2522.
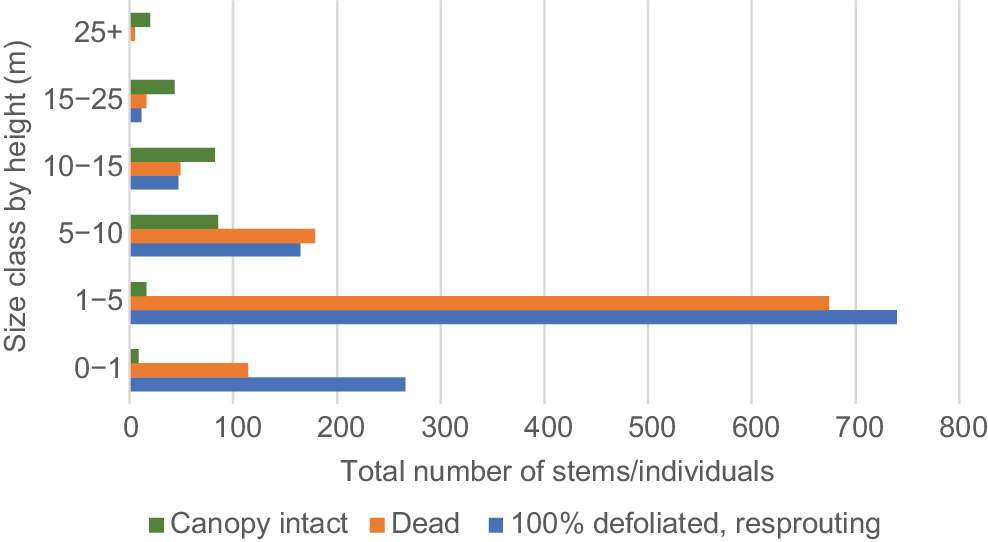
A smaller proportion of mortality and resprouting in the three largest size classes appeared to be due to drought stress rather than fire, because there was no evidence of burn damage on these individuals. Percentages of dead and resprouting, drought-affected individuals by size class were as follows: 10–15 m, 5.5%; 15–25 m, 18.5%; and 25+ m, 5%.
Community fire-response spectra
There were marked differences between adjacent vegetation formations in their fire-response spectra. WSRf had a much higher proportion of resprouter species, the great majority with nil or very low seedling recruitment. Open forest had roughly equal proportions of resprouters and seeders, and rock outcrop was dominated by seeders (Fig. 3).
Percentage of FR, OR and OSR species in WSRf, OF, RO and Pavement habitats (RO including pavement and fringing open woodland). As vegetation structural complexity increases, the percentage of resprouter species increases and that of obligate seeders decreases.
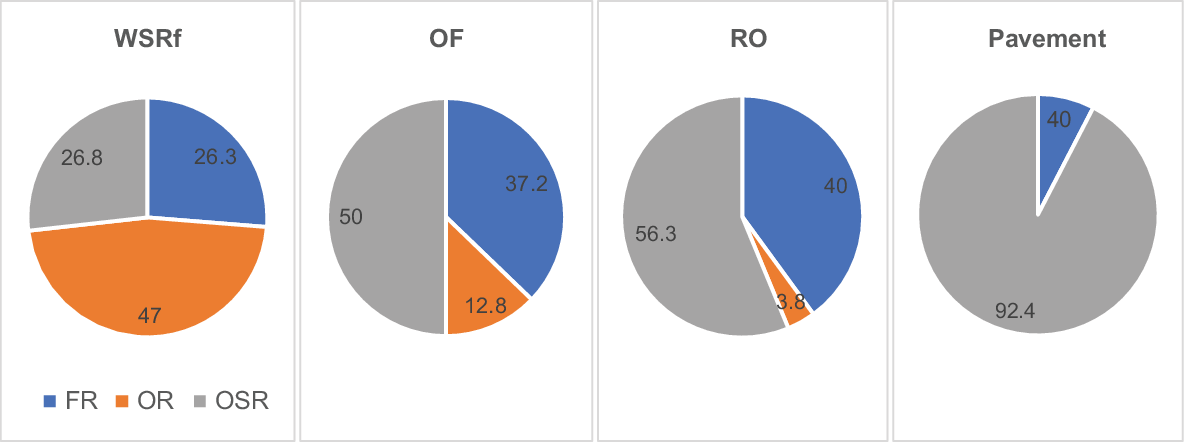
A continuum of primary fire response appeared to parallel gradients in vegetation structure and habitat. As structural complexity of vegetation increased from RO to WSRf, the proportion of R species (FR and OR) increased; as vegetation biomass and structural complexity decreased from WSRf to RO, the proportion of OSR species increased.
The proportion of facultative resprouter species in habitats followed a trend similar to that for obligate seeders, decreasing in WSRf and increasing in OF and RO, but differed in pavement habitat, where resprouting is apparently not a viable regeneration strategy, because underground parts are killed during extreme drought–fire conditions owing to a lack of insulating soil depth.
Seedling recruitment
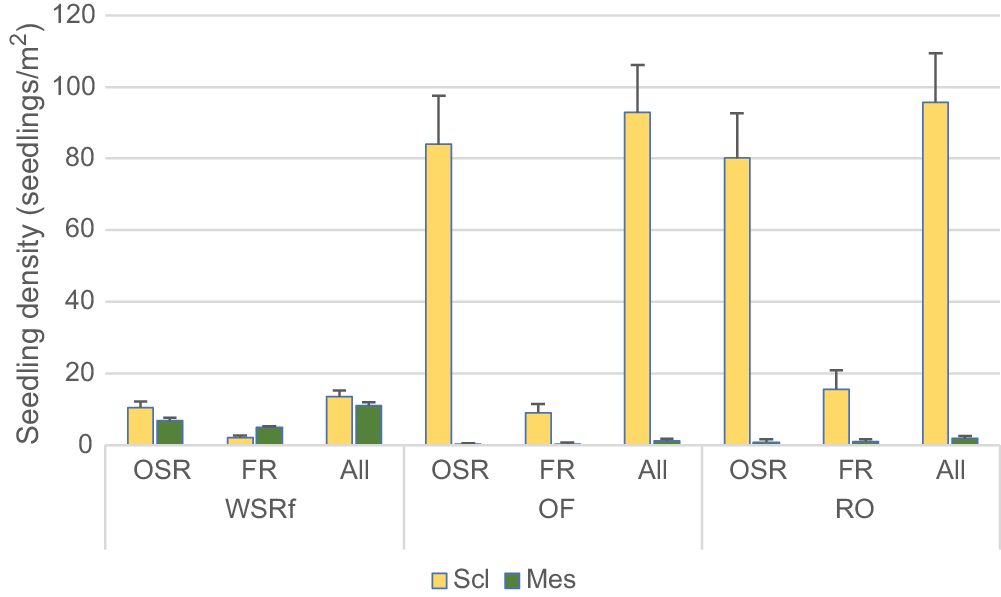
Mean total seedling density 6–12 months post-fire was used as a demographic index of seedling recruitment in different habitats (WSRf, OF and RO) and functional components of the same plant community. A summary of seedling recruitment in relation to habitat, primary fire response, growth-form, early versus late successional flora, and sclerophyll (open forest) versus mesophyll (rainforest) flora is provided below.
Mean total seedling density (woody and herbaceous species combined) was much lower in WSRf than in adjoining OF and RO, namely 25.3 ± 2.1, 94.1 ± 13.5 and 97.8 ± 13.6 seedlings/m2 respectively (WSRf/OF; Z = −5.937; P < 0.001). Of the total seedling population in WSRf, 43.4% were rainforest species and 58.9% sclerophyll species. Sclerophyll species occurring in both WSRf and OF had a much lower seedling density in WSRf, including Dodonaea triquetra, Lepidosperma laterale, Billardiera rubens, Lomandra longifolia and Lepidosperma clipeicola (e.g. D. triquetra WSRf, 0.6 ± 0.2 seedlings/m2, OF, 10.3 ± 4.0 seedlings/m2; Z = −6.524; P < 0.01). Overall, the floristic and demographic pattern of seedling recruitment appeared to be well differentiated between habitats, even though they directly adjoin each other.
Obligate seeders comprised the majority of the seedling population in terms of seedling density and number of species recruiting seedlings (Fig. 4). Total seedling density of OSRs in WSRf was significantly higher (more than double) than than that of FRs (OSR 18.17 ± 1.95; FR 7.13 ± 0.54; Z = −5.884; P < 0.001). Of 214 species recorded in WSRf plots, seedlings were recorded for 116 species (54.2%), which comprised 74 obligate seeders and 42 facultative resprouters. The remaining 98 species were resprouters without recorded seedlings.
In WSRf, there was no significant difference in the total seedling density of mesophyll and sclerophyll components (mesophyll, 11.86 ± 0.89; sclerophyll, 13.44 ± 1.85), whereas in OF and RO, there were many more seedlings of sclerophyll than mesophyll species and nearly all were OSRs (Fig. 4).
The great majority of seedlings in WSRf were early successional species (91.2%), with the remainder being late successional (8.7%). Mean seedling densities of early and late successional species were 23.09 ± 2.09/m2 and 2.21 ± 0.27/m2 respectively. Approximately half of the early successional species in WSRf were mesophyll species (e.g. Homolanthus, Trema, Polyscias) belonging to the recent immigrant flora rather than the old Gondwanan rainforest flora. The other half of the species were early successional sclerophyll flora. Few of the sclerophyll flora were classed as late successional (e.g. Eucalyptus, Casuarina). The proportion of FR species was about equal in early versus late successional flora, whereas the proportion of OSR species was very uneven. In summary, WSRf was characterised by much lower seedling density than were OF and RO, having roughly equal recruitment by OSRs and FRs in terms of seedling density and number of species with seedlings (unlike for OF), and weak penetration of WSRf by seedlings of sclerophyll flora. Penetration of OF by mesophyll flora was very weak.
Seedling population in Year 2
Mean seedling density increased in WSRf in Year 2 and fell in OF and RO, falling faster in RO. The increase in WSRf was largely due to one species, coachwood (Ceratopetalum apetalum), which recorded seedling densities up to 440/m2 in Year 2, from <10/m2 in Year 1. Other rainforest (mesophyll) species also recorded an increase in seedlings in Year 2, but at very low densities (Fig. 5).
Seedling density in selected common mesophyll FR species in WSRF in Years 1 and 2 divided into species that decreased in density in Year 2 (>Year1) and increased in Year 2 (>Year2). Common OSR species showing a typical decrease in seedling density in Year 2 are included. Standard errors are shown.
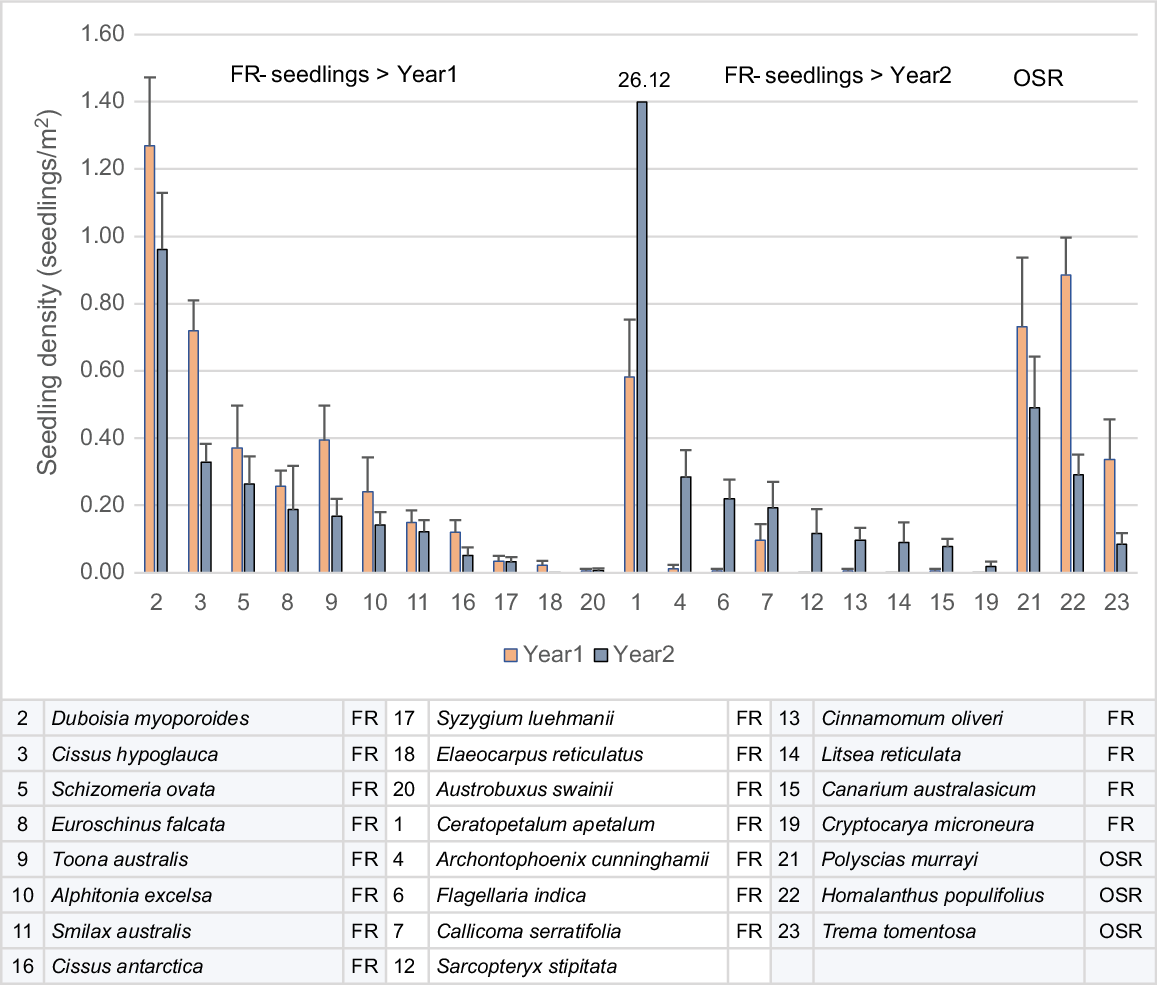
FRs with seedling densities that decreased in Year 2 were moderately long-lived, hardy rainforest pioneers and generally in the category of early successional species. FRs that increased in Year 2 were mature phase species. OSRs were early successional but faster-growing and shorter-lived than were early successional FRs. Early successional OSR and FR mesophylls exhibited fire-cued recruitment, because seedling density peaked in the first year after fire. OSR mesophylls such as Homolanthus populifolius, Trema tomentosa and Polyscias murrayi appear to produce long-lived soil seedbanks, but the extent of seedbanks in FR early successional species is unclear, although they may also be present in species such as Cissus hypoglauca and Alphitonia excelsa. Red cedar (Toona ciliata), an early successional but also long-lived species, releases seed in December–January, so seed dispersed from unburnt crowns may have been the source of recorded seedlings, rather than fire-cued soil seedbank. Bangalow palm was classified as an FR (Fig. 5), because, although adults and juveniles were killed when 100% defoliated by fire, some seedlings continued to grow from the burnt coleoptile (seedling shoot) and woody seed, exhibiting fire adaptation. Cryptocarya microneura was unique among resprouting mesophylls in occasional individuals flowering prolifically from basal resprouts in the first year after fire.
Seedling recruitment of upper-stratum species in WSRf
The seedling density response of sclerophyll canopy species in WSRf was very low to low, despite the understorey being burnt to a height of approximately 10 m, and deposition of ash. Seedlings of sclerophyll dominants were rare in WSRf and limited to occasional bare patches and charcoal beds left by incinerated stumps and fallen tree trunks already dead at the time of the fire. Eucalyptus, Corymbia and Syncarpia had low seedling density in OF (e.g. Eucalyptus spp.: WSRf, 0.13 ± 0.04; OF, 0.95 ± 0.50; RO, 0.20 ± 0.07), whereas L. confertus was highest on rock outcrop fringes (WSRf, 0.36 ± 0.14; OF, 0.50 ± 0.40; RO, 4.30 ± 2.45), an unexpected result that suggested that this species requires highly disturbed, open conditions for optimum seedling recruitment.
Resprouting
Of 214 species in WSRf plots, 152 were resprouters and the remainding species were obligate seeders, except for a small number with no regeneration. The striking feature of fire response in WSRf was the high percentage of resprouters without recorded seedlings (47%), therefore being classed in this study as obligate resprouters with respect to fire, although rare seedlings may not have been detected by sampling. Year 2 seedling-density data and size-class distributions indicated that these species recruit new individuals from seed in unburnt conditions between fires but this needs to be confirmed by further research. Facultative and obligate resprouters were present in both WSRf and OF, but obligate resprouters were absent from pavement habitat. Resprouter and seeder clades in WSRf had roughly equal proportions of woody and herbaceous species.
The dominant mode of resprouting in WSRf was basal resprouting from stem base or root crown. Coppicing (i.e. multiple basal resprouts) was common, but pre-fire structure indicated that most coppice had thinned to a single dominant stem. Several rainforest species exhibited epicormic resprouting from trunk and large branches (e.g. Austrobuxus swainiii), but overall this mode of resprouting was uncommon in mesophyll resprouters. By contrast, most tree species in OF were prominent epicormic resprouters, notably Eucalyptus and Allocasuarina torulosa. In WSRf, the percentage of stems resprouting was highest in small size classes and decreased as size class increased. Mortality and resprouting were roughly equal in size classes from 1–5 m to 15–25 m, suggesting stability in total stems (Fig. 2). The percentage of resprouting stems in tree and shrub species in WSRf varied from high to low, which was consistent with the pattern of resprouting among species being a continuum of response (Vesk et al. 2004). Percentage of stems resprouting appears to be species- as well as size class-specific, although stem frequency was too low to confirm this for many species. Linear regression found no relationship between percentage stems resprouting and seedling density in 11 resprouting rainforest species also recruiting seedlings.
Root suckering was recorded in 24 tree and shrub species in WSRf, usually in combination with basal resprouting. These were all mesophyll (rainforest) species. Some species such as Flindersia bennettiana, A. excelsa and Triunea youngiae only suckered close (<1 m) to the parent stem and others such as Litsea australis, Archidendron muellerianum and Croton verrauxii up to 1 m or more from dead parent stems (adventitious suckering). Root suckers were common in some vines (e.g. Palmeria scandens and Rubus nebulosus), but rare overall. Vines generally resprouted near ground level, and epicormically as well in a few species (e.g. C. hypoglauca and Flagellaria indica). Root-suckering species were rare in OF (e.g. Dampieria stricta, Tetrarrhena juncea) and absent in rock pavement habitat (Table 3).
| Item | Mean number dead stems/plot (± s.e.) | Mean number resprouting stems | Mean number crown-intact stems | |
|---|---|---|---|---|
| WSRf | 30.2 ± 3.3 (40.0%) | 35.0 ± 2.4 (49.3%) | 7.0 ± 0.8 (9.6%) | |
| OF | 18.3 ± 3.7 (38.6%) | 29.3 ± 5.8 (57.6%) | 1.8 ± 1.0 (2.9%) | |
| RO | 18.0 ± 3.5 (89.1%) | 1.8 ± 1.1 (11.0%) | 0 (0%) | |
| WSRf cf. OF | Z = 1.17; P = 0.241 | Z = 0.76; P = 0.443 | Z = 2.43; P = 0.01 |
Mann–Whitney (Z) tests compare the three regenerative categories in WSRf and OF.
Resprouter rate of growth
Rates of basal resprouter regrowth relative to pre-fire height (2019) in five vertical height strata after 2 years were as follows: 0–1 m, 60%; 1–2 m, 34%; 2–5 m, 25%; 5–10 m, 15%; 10–15 m, 9% (Table 4). The taller an individual is pre-fire in the 100% defoliated class, the lower is its percentage regrowth and, presumably, the longer it will take to reach pre-fire height. Assuming a constant rate of regrowth, which may be an under-estimation (e.g. Falster and Westoby 2005), resprouting stems in the 2–5 m stratum would take roughly 6–8 years to reach 2019 pre-fire height and those in 10–15 m stratum 15–20 years.
| Item | Height class (m) | |||||
|---|---|---|---|---|---|---|
| 0–1 | 1–2 | 2–5 | 5–10 | 10–15 | ||
| 2021 height | 0.35 ± 0.02 | 0.54 ± 0.03 | 0.82 ± 0.03 | 1.09 ± 0.07 | 1.11 ± 0.07 | |
| 2019 height | 0.57 ± 0.02 | 1.58 ± 0.03 | 3.34 ± 0.05 | 7.07 ± 0.13 | 12.26 ± 0.20 | |
| %Regrowth at 2 years | 61.4 | 34.2 | 24.6 | 15.4 | 9.1 | |
Seedling growth rate
Comparing the mean maximum height (cm) of seedlings, OSR seedlings in WSRf were 2.5 times taller than were FR seedlings after 2 years (FR, 37.39 ± 7.37; OSR, 97.09 ± 9.32; Z = −5.394, P < 0.001). Similarly, comparing the number of FR and OSR species above and below median maximum seedling height, chi-squared was highly significant (χ2 = 28.98; P < 0.0001) (above median: FR, 10, OSR, 39; below median: FR, 36, OSR, 12). If the three tallest FRs and OSRs are considered, the seedling height difference is not great, but as more species are added to the mean, the difference becomes obvious owing to the tail of FR species with slow seedling height growth. Comparing means of species with more than 10 seedlings, the same trends were evident. Ranking species by seedling height, of the 15 tallest, three were FR and 12 OSR.
Stem size–regenerative-class distribution
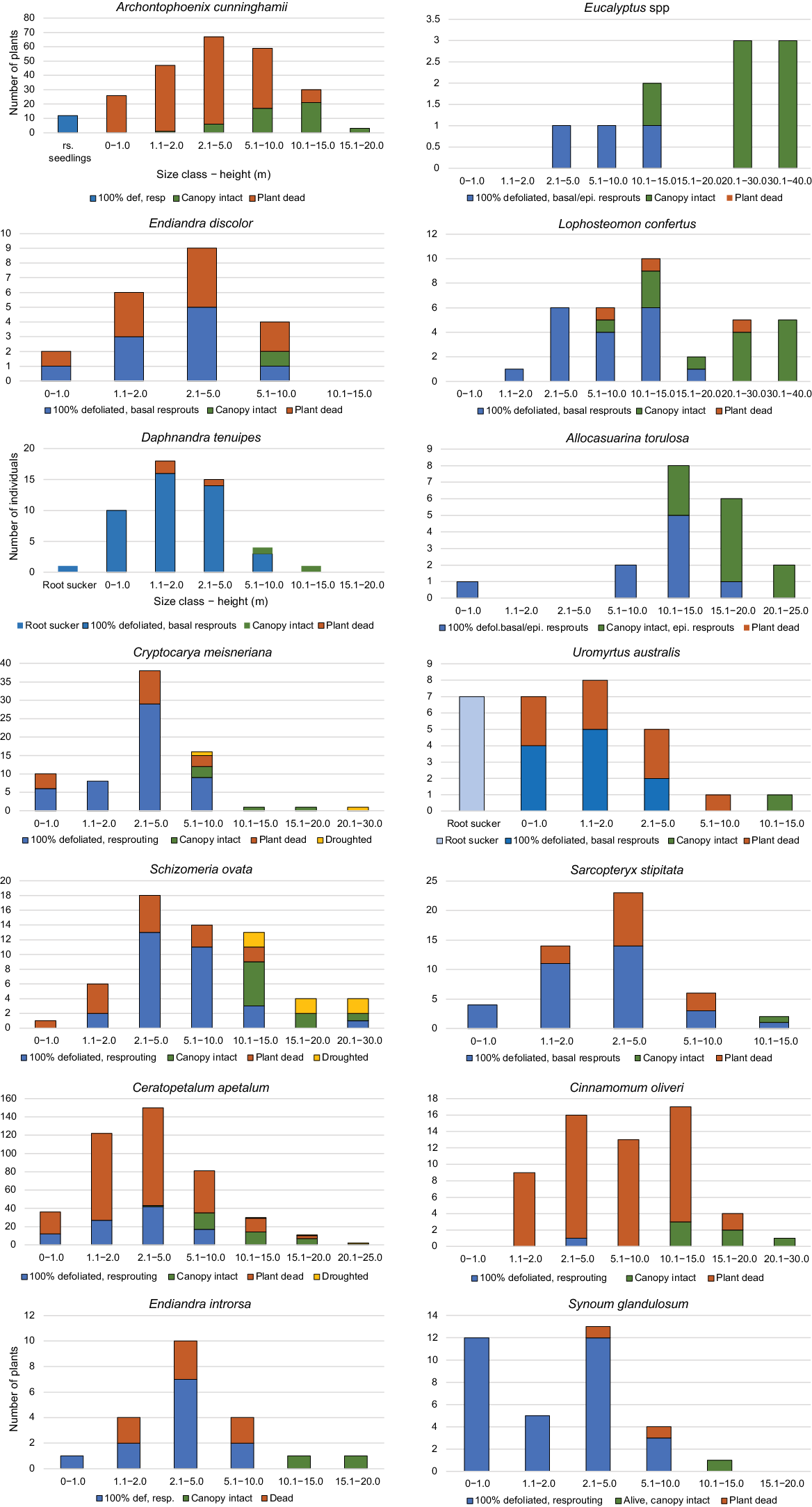
Percentage of species with continuous and truncated size-class distributions varied significantly with number of stems, with species with <20 stems being more likely to have a truncated distribution (χ2 = 8.344; P = 0.004). Even though the total sample was more than 2500 stems (Fig. 2), results need to be interpreted cautiously. Considering 25 resprouting species with >20 stems, 82.6% had a continuous distribution, indicating input of new individuals to populations from seed (or roots suckers). Approximately 17.4% had a truncated size-class distribution, with small resprouting plants being absent or few. Approximately 75% of the resprouter species with a continuous size-class distribution recorded no seedlings or evidence of root sucking. These species appeared to be obligate resprouters post-fire, so that the continuous size-class distribution may result from recruitment between fires (i.e. after Year 1). Taxa with a truncated distribution such as large sclerophyll trees may require more intense disturbance, and mesophyll rainforest species different conditions for recruitment than prevailing at present (Table 5, Fig. 6).
| Species | Number of stems (n) | SCD type | SCD shape | Small size class (y/n) | Resprouting vigour | Seedlings/m2 Year 1 | Root suckers (n) | Mesophyll/sclerophyll | %Resprouting | %Intact | %Dead | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ackama paniculata | 13 | Continuous | Flat | Yes | Low | No | Mes | 15.4 | 30.8 | 53.8 | ||
| Acmena smithii | 57 | Continuous | Bell | Yes | Strong | A | Mes | 82.5 | 0 | 17.5 | ||
| Acronychia bauerlenii | 17 | Continuous | Bell | Yes | Strong | 0.02 | 26 | Mes | 58.8 | 5 | 35.3 | |
| Allocasuarina torulosa | 19 | Truncated | Bell | No | Strong | 0.03 | Scl | 47.4 | 52.6 | 0 | ||
| Archontophoenix cunninghamii | 244 | Continuous | Bell | Yes | Very weak | 0.01 | Mes | 4.9 | 24.5 | 75.4 | ||
| Austrobuxus swainii | 16 | Truncated | Bell | No | Low | 0.01 | Mes | 12.5 | 50 | 31.2 | ||
| Callicoma serratifolia | 25 | Truncated | Left skew | No | Low | 0.1 | Mes | 25 | 41.7 | 33.3 | ||
| Ceratopetalum apetalum | 432 | Continuous | Bell | Yes | Low | 0.58 | Mes | 22.7 | 9.5 | 67.1 | ||
| Cinnamomum oliveri | 60 | Continuous | Bell | No | Weak | 0.01 | Mes | 1.7 | 10 | 88.3 | ||
| Cryptocarya glaucescens | 90 | Continuous | Bell | Yes | Strong | No | Mes | 84.4 | 2.2 | 12.2 | ||
| Cryptocarya meisneriana | 75 | Continuous | Bell | Yes | Strong | No | Mes | 69.3 | 6.7 | 21.3 | ||
| Cryptocarya microneura | 32 | Continuous | Bell | Yes | Strong | A | Mes | 71.9 | 18.8 | 9.4 | ||
| Cryptocarya rigida | 92 | Continuous | Bell | Yes | Strong | No | Mes | 89.3 | 0.8 | 9.8 | ||
| Daphnandra tenuipes | 48 | Continuous | Left skew | Yes | Strong | No | 1 | Mes | 89.6 | 4.2 | 6.3 | |
| Duboisia myoporoides | 12 | Continuous | Flat | Yes | Strong | 1.27 | 26 | Mes | 75 | 0 | 25 | |
| Elaeocarpus reticulatus | 58 | Continuous | Bell | Yes | Strong | 0.02 | Mes | 91.4 | 0 | 8.2 | ||
| Endiandra discolor | 21 | Continuous | Bell | Yes | Strong | No | Mes | 47.6 | 4.8 | 47.6 | ||
| Endiandra introrsa | 21 | Continuous | Bell | Yes | Strong | No | Mes | 57.1 | 9.5 | 33.3 | ||
| Eucalyptus spp. | 10 | Truncated | Left skew | No | Low | 0.05 | Scl | 30 | 70 | 0 | ||
| Guioa semiglauca | 18 | Continuous | Bell | Yes | Strong | A | 2 | Mes | 94.4 | 0 | 5.6 | |
| Helicia ferruginea | 10 | Truncated | Flat | No | Low | No | Mes | 10 | 0 | 90 | ||
| Hickesbeachia pinnatifolia | 11 | Truncated | Left skew | No | Strong | No | 1 | Mes | 81.8 | 9.1 | 9.1 | |
| Jagera pseudorhus | 11 | Truncated | Bell | No | Strong | No | 23 | Mes | 81.8 | 0 | 18.2 | |
| Linospadix monostachys | 35 | Continuous | Flat | Nil | No | Mes | 0 | 0 | 100 | |||
| Litsea australis | 26 | Continuous | Flat | Yes | Strong | No | 101 | Mes | 53.8 | 3.8 | 34.6 | |
| Lophostemon confertus | 27 | Truncated | Flat | No | Strong | 0.35 | Scl | 40.7 | 51.9 | 7.4 | ||
| Melicope hayseii | 11 | Continuous | Right skew | Yes | Strong | 0.02 | 3 | Mes | 81.8 | 0 | 9.1 | |
| Neolitsea dealbata | 57 | Continuous | Bell | Yes | Strong | No | Mes | 56.1 | 0 | 43.9 | ||
| Pilidiostigma glabrum | 18 | Continuous | Bell | No | Strong | No | 3 | Mes | 93.3 | 0 | 6.7 | |
| Polyscias elegans | 15 | Truncated | Left skew | No | Weak | 0.01 | 16 | Mes | 13.3 | 0 | 86.7 | |
| Quintinia verdonii | 10 | Truncated | Bell | No | Medium | 0.02 | Mes | 40 | 20 | 40 | ||
| Sarcopteryx stipitata | 49 | Continuous | Bell | Yes | Strong | No | Mes | 67.3 | 2 | 30.6 | ||
| Schizomeria ovata | 60 | Continuous | Bell | Yes | Strong | 0.37 | Mes | 50 | 15 | 25 | ||
| Syncarpia glomulifera | 26 | Truncated | Bell | No | Strong | 0.05 | Scl | 46.2 | 19.2 | 26.9 | ||
| Synoum glandulosum | 35 | Continuous | Right skew | Yes | Strong | No | Mes | 91.4 | 2.9 | 5.7 | ||
| Syzygium oleosum | 51 | Continuous | Bell | Low | Strong | No | Mes | 60.8 | 11.8 | 27.5 | ||
| Triunea youngiae | 18 | Truncated | Left skew | No | Weak | No | 3 | Mes | 22.2 | 0 | 77.8 | |
| Trochocarpa laurina | 37 | Continuous | Bell | Yes | Strong | A | Mes | 45.9 | 5.4 | 48.6 | ||
| Uromyrtus australis | 22 | Continuous | Right skew | Yes | Strong | No | 7 | Mes | 50 | 4.5 | 45.5 | |
| Wilkea huegeliana | 46 | Continuous | Right skew | Yes | Strong | No | Mes | 69.6 | 0 | 30.4 |
For SCD type, a continuous distribution is one with stems (alive and dead) in all size classes, indicating on-going recruitment (although possible high mortality during fire); a truncated distribution is missing small size classes (alive or dead), indicating interrupted recruitment under current ecological conditions.
Size–regenerative-class distributions of selected species showing percentage of stems in size classes in three regenerative classes (resprouting, canopy intact and dead). Continuous size-class distribution with some stems in small size classes predominated in mesophyll/rainforest species. Eucalyptus spp. and Allocasuarina torulosa had truncated distributions lacking in small size-class individuals as did some rainforest species, indicating very low recruitment.
Discussion
Parameters of the 2019 fire
The wildfire in WSRf did not fit readily into the distinction between crown fire and surface fire, because the fire was concentrated in the rainforest understorey up to a height of approximately 10 m and the 30–50 m high sclerophyll canopy was largely unscathed. Paradoxically, the fire could be described as a ‘WSF mid-stratum rainforest fire’. This is consistent with the findings of Clarke et al. (2014) of equivalent ignitability and combustibility of rainforest and sclerophyll forest leaves and fuels. Even under the extremely dry conditions, the fire was prevented from crowning by the height and openness of the canopy, moderate winds, the dampening effect of mesophyll understorey flora, limited litter build-up and topography. The overall effects of the fire on WSRf were to (1) substantially thin out size classes of rainforest trees lower than scorch height (<10–15 m), (2) cause non-lethal damage to many rainforest tree trunks by partial killing of vascular tissue and burning of exposed roots around the tree base, and (3) largely eliminate a few rainforest species by causing near 100% mortality of individuals (notably Linospadix monostachys).
Generally masked by the passage of fire, the effect of drought alone, one of the severest on record, could be seen in occasional large trees with canopies well above flame scorch height that had been killed or defoliated without any evidence of significant burn damage to trunk or roots, apparently owing to drought stress. Monitoring of unburnt rainforest elsewhere in the district recorded extensive leaf wilting and shedding, particularly in vines, and dieback of mature trees and saplings (Ecos Environmental 2019). Extensive crown-dieback owing to drought alone occurred in dry sclerophyll forest during the same climatic event (Losso et al. 2022). It was assumed that most stems in smaller size classes left standing after the fire were alive and ‘green’ when burnt, but an unknown number may have died and dried out before the fire and burnt leaving no trace. This may have occurred selectively to some species such as the shrubs Tasmannia insipida and Psychotria simmondsiana, which are common in the study area, but left little evidence post-fire. In the 2019 drought–fire event, the most damaging agent of disturbance appears to have been fire, but fire responses probably represent to some extent the cumulative effect of drought and fire.
In 20–30 m high OF upslope of WSRf, dominated by E. pilularis, the fire often crowned reflecting absence of mesophyll understorey, a lower canopy able to carry fire, higher fuel load and topographic updrafts feeding oxygen to the fire. Fire damage in rock outcrop vegetation was often extreme, with shrubs, roots and organic soil being consumed. Dominant Leptospermum, Callistemon, Allocasuarina, Eucalyptus and Lophostemon regenerated from canopy seedbanks of fire-resistant capsules and other species probably from seed in cracks and fissures in the underlying rock or soil washed downslope from rock outcrop fringes.
Plant fire response in WSRf
The main differences between plant fire response in WSRf and adjoining OF and RO were as follows, in WSRf: (1) much lower total seedling density, (2) many resprouter species with nil or very low seedling recruitment, and (3) higher total seedling density in Year 2 than in Year 1. Because the majority of obligate and near-obligate resprouter species had a continuous size-class distribution indicating on-going recruitment of new individuals from seed (some species produced clonal recruits), it appears this occurs between fires and seedlings can attain fire resistance before the next fire (Fensham et al. 2003). Most were mature phase rainforest/mesophyll species that produce non-persistent, recalcitrant seed (Sommerville et al. 2021) destroyed by fire. Freshly fallen fruit were observed under canopy-intact trees of some of these species in Year 1 (e.g. Schizomeria ovata, Sarcopteryx stipitata), indicating seed input from unburnt crowns in or around plots, or bird dispersal. These results only partly agree with Baker et al. (2022) that post-fire seedling recruitment in rainforest flora is fire-cued, because most of the rainforest flora in WSRf appeared to recruit seed in unburnt conditions between fires, apart from a component of mesophyll OSRs, which have soil seedbanks, fast growth rate and rapid maturation typical of disturbance specialists.
The low total seedling density in WSRf compared with OF and RO reflects species traits, particularly the lack of adaptation to fire reflected by the very weak seedling recruitment response in the first year after fire by most (but not all) mesophyll species, but also probably the fewer ‘gaps’ created by fire disturbance and greater competition from resprouting vegetation (Keeley and Zedler 1978; Sousa 1984). There was no significant difference in the mean number of resprouting stems in WSRf and OF, but the number of crown intact stems was significantly higher in WSRf (Table 3), being consistent with fewer gaps and less seedling recruitment.
Net seedling density in OF and RO followed the normal post-fire pattern of a flush of seed germination and a peak in seedling density in Year 1, followed by a decreasing density in Year 2. In WSRf, there was a similar, smaller surge of recruitment in Year 1, but net seedling density continued to increase in Year 2, apparently owing to non-dormant seed being produced by mature-phase rainforest trees with unburnt crowns, particularly C. apetalum. It is possible that seedling recruitment in this and other mature-phase rainforest species was enhanced by early post-fire habitat conditions, which increased seed production in trees with intact crowns. No difference in seedling recruitment behaviour was evident in a few species from predominantly mesophyll genera that are common in both WSRf and OF, including Elaeocarpus reticulatus, Cryptocarya rigida and Trochocarpa laurina, which resprouted strongly and exhibited very low or nil, post-fire seedling recruitment in both habitats, suggesting an inherently rigid fire response.
Large sclerophyll trees forming the unburnt canopy of WSRf responded with very low seedling recruitment, suggesting that drought–fire events more intense than 2019 may have occurred in the past 500 years to account for present populations and structure of WSRf. Seedling recruitment, which the sclerophyll canopy species ultimately depend on to replace aging individuals, was significantly higher in OF and the fringes of RO where fire intensity was higher. Acacia orites and Callitris macleayana, growing up to 20–35 m high, are killed when completely burnt (OSRs), but tall individuals often survived mid-stratum fire. The myrtaceous canopy dominants in WSRf behaved as facultative resprouters (FRs), with seedling density depending on fire intensity and habitat. Several mesophyll FRs in WSRf had a higher seedling density in Year 1 (e.g. C. hypoglauca and A. excelsa) and recruitment appears to be enhanced by fire. These were usually hardy rainforest pioneer species. Other features of fire response in WSRf were loss of some species by failure to regenerate (e.g. L. monostachys) and a much higher number of root-suckering species than of OF and RO (24 species). Root suckering, a form of clonal reproduction, often characterises relict floras and stressful growing conditions (Rossetto and Kooyman 2005; Weber et al. 2014).
Overall, the following four categories of seedling recruitment could be discerned in the WSRf flora:
Mesophyll OSRs: soil seedbank, high seedling density, rapid growth; SE Asian migrant origin.
Mesophyll FRs: mostly soil seedbank, low to moderate seedling density, slower seedling growth; mixed origin.
Sclerophyll OSRs and FRs: mostly soil seedbank, either specialising in WSF or more common in OF; Australian origin, or mixed.
Mesophyll ORs: seed fall and dispersal from unburnt tree crowns, Gondwanic.
The distinctive fire-response spectrum of WSRf appears to be a direct consequence of the overlap of ‘new’ and ‘old’ floras in this broad vegetation type. Species seedling-recruitment traits in these two components appear to be strongly influenced by biogeographic history and successional niche. Fire response in the mesophyll flora was largely a function of differentially represented plant clades of Gondwanan and Indo-malayan biogeographic origin. Most mesophyll OSRs were of recent immigrant origin, although there were exceptions, including the vine Streptothamnus moorei, a Gondwanan relict and the conifer C. macleayana.
In a more intense drought–fire event, one can envisage higher mortality of rainforest trees and sclerophyll dominants. NPWS reported (unpubl. data) areas of higher-intensity crown fire in WSRf at the western end of the park, outside the survey area. More extreme fire intensity could reduce rainforest trees with intact canopies to zero, so that mortality rates are even higher, and many rainforest species persist solely by basal and root-sucker resprouting. Seedlings of mesophyll OSRs would probably still be present among higher densities of sclerophyll OSRs and high densities of sclerophyll canopy species, assuming seed being available. If a species is subjected to increasing environmental stress, growth is slowed, stature stunted, seed production is reduced, seedling recruitment is less likely and resprouting is relied on as a means of persistence. The rainforest growing as an understorey in WSRf is largely a resprouting rainforest growing under relatively stressful conditions in terms of habitat (low-fertility soil) and disturbance regime. Resprouting rainforest also occurs in South Africa (Bond and Midgley 2003), the Canary Islands (Fernández-Palacios and Arévalo 1998) and probably elsewhere in habitats receiving substantial mean annual rainfall but also exposed to periodic drought and fire.
Plant species fire-response traits such as seedling density may vary with fire intensity and frequency, but at a broader ecological scale, patterns of fire response are correlated with a complex of additional factors. Plant fire responses in the Nightcap Range at species and community levels were influenced by three general groups of factors, namely, fire-regime components, habitat variables and inherent species traits. Habitat was clearly an important factor, as shown by the contrasting fire-response spectra of wet sclerophyll–rainforest, open forest and outcrop shrubland. Specific habitat variables driving variation in fire response appeared to be soil depth, microclimate, topography and vegetation structure, including the extent of structural gaps post-fire. Given habitat and fire parameters, it appears that species fire response was largely determined by inherent species attributes reflecting biogeographic history, successional niche and life history.
Conclusions
The fire-response spectrum of WSRf was distinct from adjoining OF and RO and generally characterised by much lower total seedling recruitment and a high proportion of resprouting species.
Obligate seeders dominated the seedling population in WSRf, OF and RO, and had a higher seedling density than did facultative resprouters.
Obligate seeders were generally early successional, sclerophyll, or, if mesophyll, belonged to the ‘new’ immigrant rainforest flora from Southeast Asia, with exceptions.
The great majority of late successional, mesophyll-Gondwanan flora in WSRf were resprouters with nil or very low seedling recruitment post-fire, and preliminary evidence indicates that seedling recruitment in this floristic element occurs mainly between fires, which needs to be confirmed.
Predominant fire responses (OSR, FR and OR) in phytogeographic clades in WSRf were Gondwanan-rainforest (OR), immigrant-rainforest (OSR) and autochthonous-sclerophyll components (OSR and FR), with exceptions.
The majority of the rainforest flora of WSRf appears to be tolerant of fire because of the capacity of the mesophyll-Gondwanan component to regenerate by basal resprouting (assuming sufficient rainfall), and OSR attributes in most rainforest species of recent immigrant origin.
An increase in fire intensity and frequency may destabilise the present balance between the level of fire damage and resilience capacity of the mesophyll-Gondwanan rainforest element, resulting in a decline and loss of species.
Aspects of plant fire ecology in WSRf needing further research include the following:
Recruitment processes in mesophyll resprouters between fires.
Recolonisation of burnt forest by non-soil seedbank/late successional rainforest species with high fire mortality rates, particularly L. monostachys, Cinnamomum oliveri and probably T. insipida and P. simmondsiana, which may serve as indicators of ecosystem stability and resilience.
Recruitment and population structure of sclerophyll canopy species.
Acknowledgements
Many thanks go to David Keith, David Bowman, Dick Williams and an anonymous reviewer for comments on earlier versions of this paper that greatly improved its contents, and to Matt Wiseman of NPWS for reviewing a draft of the paper, facilitating access to Nightcap National Park and supplying information on fire history.
References
Baker AG, Catterall C, Wiseman M (2022) Rainforest persistence and recruitment after Australia’s 2019–2020 fires in subtropical, temperate, dry and littoral rainforests. Australian Journal of Botany 70(3), 189-203.
| Crossref | Google Scholar |
Beadle NCW (1954) Soil phosphate and the delimitation of plant communities in eastern Australia. Ecology 35(3), 370-375.
| Crossref | Google Scholar |
Benwell AS (1998) Post-fire seedling recruitment in coastal heathland in relation to regeneration strategy and habitat. Australian Journal of Botany 46, 75-101.
| Crossref | Google Scholar |
Benwell A (2007) Response of rock-outcrop and fringing vegetation to disturbance by fire and drought. Australian Journal of Botany 55, 736-748.
| Crossref | Google Scholar |
Bond WJ, Midgley JJ (2003) The evolutionary ecology of sprouting in woody plants. International Journal of Plant Sciences 164(S3), S103-S114.
| Crossref | Google Scholar |
Bowman DMJS, Ondei S, Lucieer A, Foyster S, Prior LD (2023) Forest–sedgeland boundaries are historically stable and resilient to wildfire at Blakes Opening in the Tasmanian Wilderness World Heritage Area, Australia. Landscape Ecology 38(1), 205-222.
| Crossref | Google Scholar |
Byrne M, Steane DA, Joseph L, Yeates DK, Jordan GJ, Crayn D, Aplin K, Cantrill DJ, Cook LG, Crisp MD, Keogh JS, Melville J, Moritz C, Porch N, Sniderman JMK, Sunnucks P, Weston PH (2011) Decline of a biome: evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota. Journal of Biogeography 38, 1635-1656.
| Crossref | Google Scholar |
Campbell ML, Clarke PJ (2006) Response of montane wet sclerophyll forest understorey species to fire: evidence from high and low intensity fires. Proceedings of the Linnean Society of New South Wales 127, 63-73.
| Google Scholar |
Chafer CJ, Noonan M, Macnaught E (2004) The post-fire measurement of fire severity and intensity in the Christmas 2001 Sydney wildfires. International Journal of Wildland Fire 13, 227-240.
| Crossref | Google Scholar |
Clarke PJ, Prior LD, French BJ, Vincent B, Knox KJE, Bowman DMJS (2014) Using a rainforest–flame forest mosaic to test the hypothesis that leaf and litter fuel flammability is under natural selection. Oecologia 176, 1123-1133.
| Crossref | Google Scholar | PubMed |
Clarke PJ, Lawes MJ, Murphy BP, Russell-Smith J, Nano CEM, Bradstock R, Enright NJ, Fontaine JB, Gosper CR, Radford I, Midgley JJ, Gunton RM (2015) A synthesis of postfire recovery traits of woody plants in Australian ecosystems. Science of The Total Environment 534, 31-42.
| Crossref | Google Scholar | PubMed |
DPE (2023) Plant community type classification and map of NSW. Department of Planning and Environment, Environment and Heritage Group. Available at https://www.environment.nsw.gov.au/topics/animals-and-plants/biodiversity/nsw-bionet/state-vegetation-type-map
Ecos Environmental (2019) Woolgoolga to Ballina Pacific highway upgrade threatened rainforest communities and rainforest plants monitoring program annual report 2019. Report to Transport for NSW. Ecos Environmental. Available at https://majorprojects.planningportal.nsw.gov.au/
Falster DS, Westoby M (2005) Tradeoffs between height growth rate, stem persistence and maximum height among plant species in a post-fire succession. Oikos 111(1), 57-66.
| Crossref | Google Scholar |
Fensham RJ, Fairfax RJ, Butler DW, Bowman DMJS (2003) Effects of fire and drought in a tropical eucalypt savanna colonized by rain forest. Journal of Biogeography 30, 1405-1414.
| Crossref | Google Scholar |
Fernández-Palacios JM, Arévalo JR (1998) Regeneration strategies of tree species in the laurel forest of Tenerife (The Canary Islands). Plant Ecology 137, 21-29.
| Crossref | Google Scholar |
Floyd AG (1976) Effect of burning on regeneration from seeds in wet sclerophyll forest. Australian Forestry 39(3), 210-220.
| Crossref | Google Scholar |
Hill RS, Read J (1984) Post-fire regeneration of rainforest and mixed forest in western Tasmania. Australian Journal of Botany 32, 481-493.
| Google Scholar |
Jackson WD (1968) Fire, air, water and earth – an elemental ecology of Tasmania. Proceedings of the Ecological Society of Australia 3, 9-16.
| Google Scholar |
Kearney MR, Jusup M, McGeoch MA, Kooijman SALM, Chown SL (2021) Where do functional traits come from? The role of theory and models. Functional Ecology 35(7), 1385-1396.
| Crossref | Google Scholar |
Keeley JE, Zedler PH (1978) Reproduction of chaparral shrubs after fire: a comparison of sprouting and seeding strategies. American Midland Naturalist 99, 142-161.
| Crossref | Google Scholar |
Knox KJE, Clarke PJ (2012) Fire severity, feedback effects and resilience to alternative community states in forest assemblages. Forest Ecology and Management 265, 47-54.
| Crossref | Google Scholar |
Losso A, Challis A, Gauthey A, Nolan RH, Hislop S, Roff A, Boer MM, Jiang M, Medlyn BE, Choat B (2022) Canopy dieback and recovery in Australian native forests following extreme drought. Scientific Reports 12, 21608.
| Crossref | Google Scholar | PubMed |
Lykke AM (1998) Assessment of species composition change in savanna vegetation by means of woody plants’ size class distributions and local information. Biodiversity and Conservation 7, 1261-1275.
| Crossref | Google Scholar |
Murphy HT, Metcalfe DJ, Bradford MG, Ford AJ (2014) Community divergence in a tropical forest following a severe cyclone. Austral Ecology 39(6), 696-709.
| Crossref | Google Scholar |
Naveh Z (1975) The evolutionary significance of fire in the Mediterranean region. Vegetatio 29, 199-208.
| Crossref | Google Scholar |
Pate JS, Froend RH, Bowen BJ, Hansen A, Kuo J (1990) Seedling growth and storage characteristics of seeder and resprouter species of Mediterranean-type ecosystems of SW Australia. Annals of Botany 65(6), 585–-601.
| Google Scholar |
Pausas JG, Bradstock RA, Keith DA, Keeley JE (2004) Plant functional traits in relation to fire in crown-fire ecosystems. Ecology 85(4), 1085-1100.
| Crossref | Google Scholar |
Prior LD, Foyster SM, Furlaud JM, Williamson GJ, Bowman DMJS (2022) Using permanent forest plots to evaluate the resilience to fire of Tasmania’s tall wet eucalypt forests. Forest Ecology and Management 505, 119922.
| Crossref | Google Scholar |
Rossetto M, Kooyman RM (2005) The tension between dispersal and persistence regulates the current distribution of rare palaeo-endemic rain forest flora: a case study. Journal of Ecology 93, 906-917.
| Crossref | Google Scholar |
Smith JMB, Guyer IJ (1983) Rainforest-eucalypt forest interactions and the relevance of the biological nomad concept. Australian Journal of Ecology 8, 55-60.
| Crossref | Google Scholar |
Sommerville KD, Errington G, Newby Z-J, Liyanage GS, Offord CA (2021) Assessing the storage potential of Australian rainforest seeds: a decision-making key to aid rapid conservation. Biodiversity and Conservation 30, 3185-3218.
| Crossref | Google Scholar |
Sousa WP (1984) The role of disturbance in natural communities. Annual Review of Ecology and Systematics 15, 353-391.
| Crossref | Google Scholar |
Tng DYP, Murphy BP, Weber E, Sanders G, Williamson GJ, Kemp J, Bowman DMJS (2012) Humid tropical rain forest has expanded into eucalypt forest and savanna over the last 50 years. Ecology and Evolution 2(1), 34-45.
| Crossref | Google Scholar | PubMed |
Tng DYP, Jordan GJ, Bowman DMJS (2013) Plant traits demonstrate that temperate and tropical giant eucalypt forests are ecologically convergent with rainforest not savanna. PLoS ONE 8(12), e84378.
| Crossref | Google Scholar | PubMed |
Turner J (1984) Radiocarbon dating of wood and charcoal in an Australian forest ecosystem. Australian Forestry 47, 79-83.
| Crossref | Google Scholar |
Verdú M, Pausas JG, Segarra-Moragues JG, Ojeda F (2007) Burning phylogenies: fire, molecular evolutionary rates, and diversification. Evolution 61(9), 2195-2204.
| Crossref | Google Scholar | PubMed |
Vesk PA, Warton DI, Westoby M (2004) Sprouting by semi-arid plants: testing a dichotomy and predictive traits. Oikos 107(1), 72-89.
| Crossref | Google Scholar |
Vlam M, van der Sleen P, Groenendijk P, Zuidema PA (2017) Tree age distributions reveal large-scale disturbance-recovery cycles in three tropical forests. Frontiers in Plant Science 7, 1984.
| Crossref | Google Scholar |
Weber LC, VanDerWal J, Schmidt S, McDonald WJF, Shoo LP (2014) Patterns of rain forest plant endemism in subtropical Australia relate to stable mesic refugia and species dispersal limitations. Journal of Biogeography 41(2), 222-238.
| Crossref | Google Scholar |
White PS (1979) Pattern, process, and natural disturbance in vegetation. The Botanical Review 45, 229-299.
| Crossref | Google Scholar |
Williams PR, Parsons M, Jensen R, Tran C (2012) Mechanisms of rainforest persistence and recruitment in frequently burnt wet tropical eucalypt forests. Austral Ecology 37(2), 268-275.
| Crossref | Google Scholar |