The leaf micromorphology and anatomy of gamba grass, Andropogon gayanus Kunth (Poaceae: Panicoideae)
Jeremy Farr A B * , S. Krisantini C D and Melodina Fabillo E *
A B * , S. Krisantini C D and Melodina Fabillo E *
A
B
C
D
E
Handling Editor: Garry Cook
Abstract
Andropogon gayanus, commonly known as gamba grass, is one of the declared weeds of national significance in Australia. Past studies have focused on gross morphology of root structures, biogeochemical behaviour, and ecology, but there has been limited work on comparative descriptions of leaf micromorphology and anatomy.
We investigated and described its leaf micromorphology and anatomy to understand weed biology and ecophysiology.
Optical and scanning electron microscope examination of the adaxial and abaxial leaf surfaces of A. gayanus was carried out. We identified and generated a list of morphological characters that were used to compare several dried herbarium specimens of A. gayanus.
The leaf characters were consistent across all specimens examined, with minor differences in leaf pubescence, indicating this could be a plastic trait.
Andropogon gayanus leaves are well adapted to wet and dry tropical conditions. Plasticity in leaf surface pubescence possibly enhances its adaptability, increasing its success as a weed in Australian ecosystems. The success of A. gayanus in Australia could be because the environment compares favourably with the native environment of the species in Africa, where it has adapted to extremes of wet and dry conditions over a large geographical range.
Plant morphological and taxonomic studies of A. gayanus focused on describing characters of spikelets and caryopses are recommended to understand how reproductive structures aid in its successful proliferation.
Keywords: agrostology, freehand sectioning, gamba grass, leaf characteristics, leaf replica, micromorphology, scanning electron microscope, taxonomy, weeds.
Introduction
Andropogon gayanus Kunth is a grass in the subfamily Panicoideae, tribe Andropogoneae. Grasses belonging to this subfamily are usually two-flowered, have dorsally compressed spikelets and with a staminate or sterile lower floret. Grasses in the subtribe Andropogoneae have a base chromosome number of 10 and exhibit C4 photosynthetic type (Kellogg 2015; Soreng et al. 2017). The native range of A. gayanus is sub-Saharan Africa, but it has garnered notoriety in recent years in parts of South, Central, and North America and northern Australia where it is regarded as a weedy invasive species after its initial introduction to these regions as a valuable fodder crop for ruminants (Javed 1990; Watson and Dallwitz 1992; Ferguson and de Andrade 1997; Rossiter-Rachor et al. 2009, 2017; Setterfield et al. 2010) (Fig. 1a, b). Andropogon gayanus is notable for being a tall grass standing at 2–4 m high, supported by deep roots that improve its resistance to drought, tolerating up to 9 months of water scarcity. The roots propagate vegetatively such that the species is found in thick tussocks or clumps (Fig. 1c, d). As a grass from the humid tropics, the species is drought resistant and tolerant to heavy rains between 750 mm and 1500 mm (Bowden 1964; DAF 2016).
The distribution of Andropogon gayanus (a) globally and (b) in Australia. The plant appears in (c) tall tussocks or clumps reaching 2–4 m high and (d) with inflorescences in paired racemes. Some specimens that were examined at the Queensland Herbarium and Biodiversity Science (BRI) includes accessions coming from (e) north Queensland, Australia, and (f) Africa. Photo source: Global Biodiversity Index Facility (a) (GBIF.org 2024), Australasian Virtual Herbarium (b) (The Australasian Virtual Herbarium (AVH) 2024), Keith R. McDonald (c, d), and BRI (e, f).
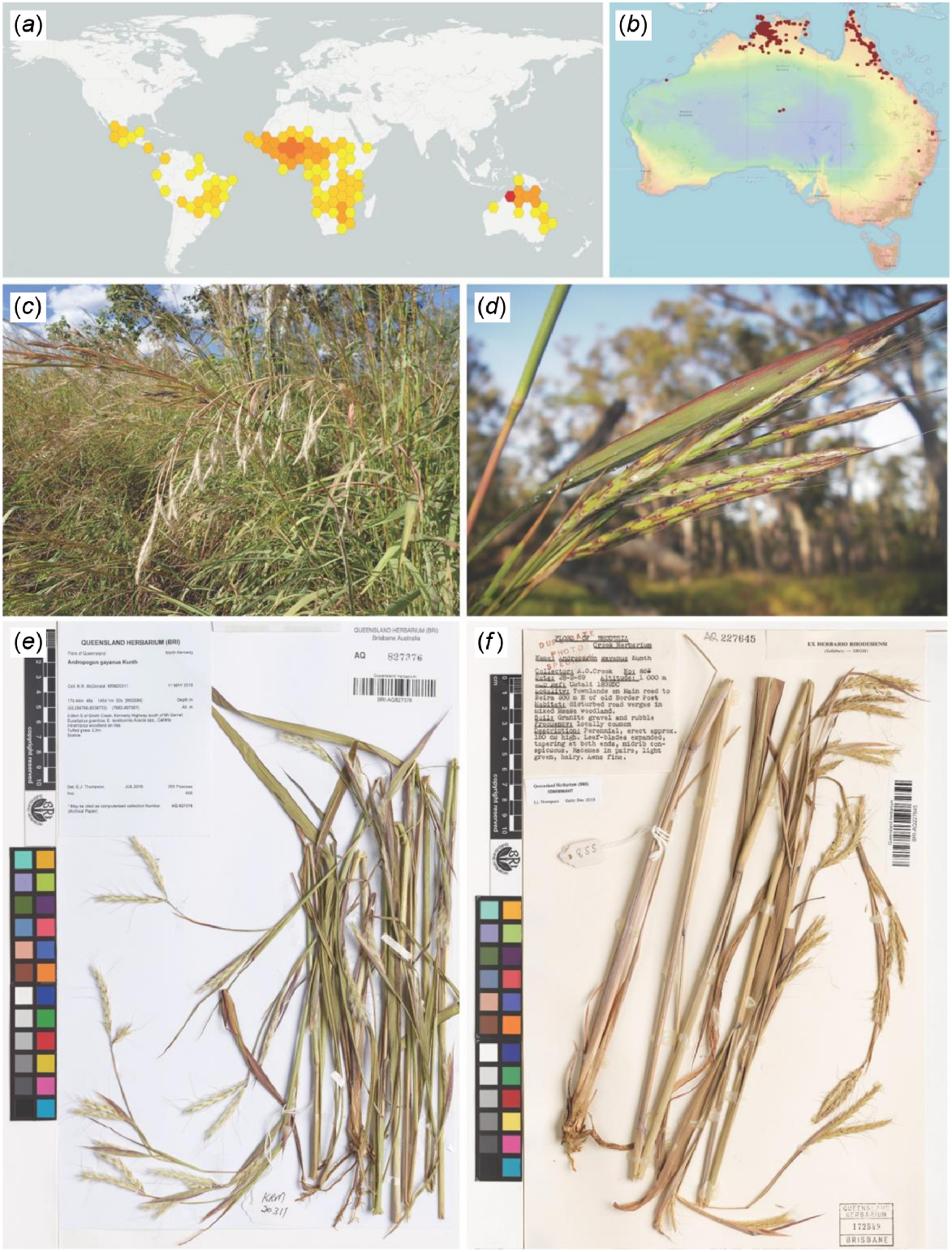
Andropogon gayanus grows in various soil types ranging from light sand to clay loam. It is found across a range of habitats and ecosystems, but it is common predominantly in dry forest–savanna mosaics across the Sahel and Sudanian regions north of the equator, and throughout the Miombo ecoregion south of the equator (Bowden 1964). It can also be found in marshy areas and riparian zones and has tolerance to major seasonal floods. It is notably absent from the central African rainforest (Foster 1962; Bowden 1964; Ojo et al. 2021; Royal Botanic Gardens, Kew 2022). Andropogon gayanus is considered a weed of national significance in Australia, spreading mostly in the northernmost regions (Bebawi et al. 2018) (Fig. 1e, f).
Andropogon gayanus displays variation in reproductive characters, leading some authors to split them into varieties (Stapf 1919; Bor 1960; Foster 1962). In this study we refer to the varieties in the sense of Stapf (1919). There are three varieties of A. gayanus, namely A. gayanus var. gayanus, A. gayanus var. squamulatus and A. gayanus var. bisquamulatus. They differ in the following characters: presence of cilia on one or both margins of the joints and pedicels, presence or absence of hair on the spikelets, degree of hairiness of the base of the awn (beard), and the awn length. A. gayanus var. gayanus possesses joints and pedicels that are ciliate on one margin, glabrous pedicelled spikelets, scanty beard, and 1–2 cm long awns. A. gayanus var. squamulatus has joints and pedicels that are ciliate on both margins, scaberulous and puberulous pedicelled spikelets, dense callus beard on both frontal and lateral sides, and 2–3 cm long awns. A. gayanus var. bisquamulatus has joints and pedicels that are ciliate on both margins, hairy to villous pedicelled spikelets, dense callus beard on the frontal and lateral sides, and 2–3 cm long awns (Bowden 1964).
The value of A. gayanus as a fodder crop has led to its cultivation across Central and South America, parts of south Asia, south-east Asia, and Australia. Andropogon gayanus was introduced into Australia by the Commonwealth Scientific and Industrial Research Organisation (CSIRO) in 1931 in Northern Territory and in 1942 in Queensland. Starting 1946, CSIRO carried out trials at the Katherine Research Station in Northern Territory using cultivars CPI 2312 (supplied in 1931 by C. P. Taylor from Zaria in Nigeria, Africa, var. bisquamulatus) and CPI 9207 (supplied in 1944 by Ramos de Otero, Division of Agrostology, Deodora, Brazil, as var. squamulatus). These varieties were grown by Mr Frank Kent at the Berrimah Experiment Farm from the 1950s until 1981. This led to the creation of cv. ‘Kent’, a cultivar that shares characteristics of A. gayanus var. squamulatus and A. gayanus var. bisquamulatus (Kent 1987; DAF 2016).
As part of a broader program of experimenting and introducing tropical forages, A. gayanus was included in experiments during the 1970s to introduce grasses that would offer cattle improved pastures in the Cape York Peninsula in North Queensland, with results describing the species as an aggressive coloniser (Jones 1979; Anning 1982; Cook and Dias 2006). Grains for A. gayanus were later released to cattle farmers throughout northern Australia in the 1980s and 1990s (Oram 1987; Cameron 2000). The first naturalised specimen in Queensland was found in Cape York in 1992 but is believed to have become naturalised in Cape York earlier, possibly since the early 1980s. The largest populations are associated with original planting sites, with the plant appearing to spread most rapidly along disturbance corridors, often being found on roadsides, but less frequently in undisturbed habitats. The species became a cause of concern as it spread beyond its plantations, eventually covering an estimated 10,000–15,000 km2 of Australian savanna–woodland across Western Australia, Northern Territory, and Queensland (Rossiter-Rachor et al. 2009, 2017; Setterfield et al. 2010; DAF 2016).
The rapid spread of A. gayanus is considered a serious risk to native ecology, property, and human life due to its capacity to withstand long droughts, allowing it to build up large fuel loads during the dry season, substantially increasing the risk of catastrophic wildfires. The fuel load can be attributed to the species growing significantly taller than native grasses, usually between 2 and 4 m high. When wildfires break out, the height of the grass and the density of the fuel load poses serious risk for rural properties and native ecosystems, especially where the species has found footholds within national parks. Unlike native vegetation, which is adapted to wildfires, the fires from A. gayanus are more intense and reach higher, increasing the likelihood of burning the meristems of native trees, prohibiting future growth and reproduction (Setterfield et al. 2010). The Western Australian Government recently announced it was close to eradicating wild forms of A. gayanus. However, it is still a prevalent problem across the northern coasts of Northern Territory and Queensland (Snow 2022).
Several aspects of the weediness of A. gayanus have been addressed in previous studies. It has been shown that the biogeochemical properties, reproduction processes, and the gross morphology of its rhizomes make the plant particularly well adapted to drought (Rossiter-Rachor et al. 2009, 2017; Setterfield et al. 2010). Despite the very focused studies on particular plant characters, information on leaf surface morphology (micromorphology) and anatomy is limited. Studies of leaf epidermal features are of great value in the field of grass systematics and provide a basis for understanding phylogenetic relationships within and between species (Nazir et al. 2013). Leaf morphological studies can also be used in understanding the ecophysiology and weed biology of A. gayanus. The general aim of this study is to describe the leaf surface structure and anatomy of A. gayanus using herbarium specimens collected across its current distribution range. Specifically, selected specimens from the Queensland Herbarium and Biodiversity Science (BRI) are compared based on leaf structure examined using optical and scanning electron microscopes.
Material and methods
All plant specimens examined for this study were from accessions from the Queensland Herbarium and Biodiversity Science (BRI). Specimen slide and scanning electron microscope (SEM) stub preparation and microscopy were undertaken using the microscopy facility at BRI.
Taxon sampling
A total of 100 out of the 147 accessions of A. gayanus found at BRI were examined. These include the known varieties (A. gayanus var. bisquamulatus and A. gayanus var. bisquamulatus) and cultivar (A. gayanus ‘Kent’). Representative accessions from key geographic distribution sites were sampled for leaf morphology and surface structure. Twenty of the accessions were chosen for preparing leaf replicas for optical microscopy and mounting on stubs for scanning electron microscopy (see Appendix 1 in Supplementary material). Sections from the mid-region (halfway between the leaf apex and the ligule) of the leaves were used, as recommended by Ellis (1976, 1979).
Leaf sample preparation and optical microscopy
Leaf epidermal replicas for both abaxial and adaxial surfaces were prepared using nail varnish and adhesive tape (Bianchi and Marchesi 1960; Dean and Ashton 2008). This was done by applying a 50:50 solution of clear nail polish and acetone to the abaxial and adaxial sides of the leaf blade and left to dry. Adhesive tape was then applied over the varnished surfaces and peeled off, leaving a varnish impression of the leaf cell structure on the adhesive tape. The adhesive tape with the leaf replicas were then placed over a glass microscope slide and examined using Leica DMLB compound microscope with camera attachment and ToupView software (ToupTek Photonics) for imaging.
Transverse sections of the leaves were prepared using free-hand sectioning technique, whereby a sharp razor blade was used to cut thinly through the leaves. Sections were mounted on glass slides and protected with glass cover slips. Specimens were viewed using a Nikon eclipse 50i light microscope and images were captured using the NIS-Elements BR digital image analysis software (Nikon Instruments Inc.).
Scanning electron microscopy (SEM)
Abaxial and adaxial leaves approximately 10 mm in length were cut and mounted on stubs covered with carbon tabs. The samples were coated with gold for 30 s using an automatic sputter coater (Agar Scientific). Samples were viewed and imaged using Phenom desktop SEM (ThermoFisher Scientific) at 10 eV, high vacuum, with back scattering.
Selection of characters and terminology used
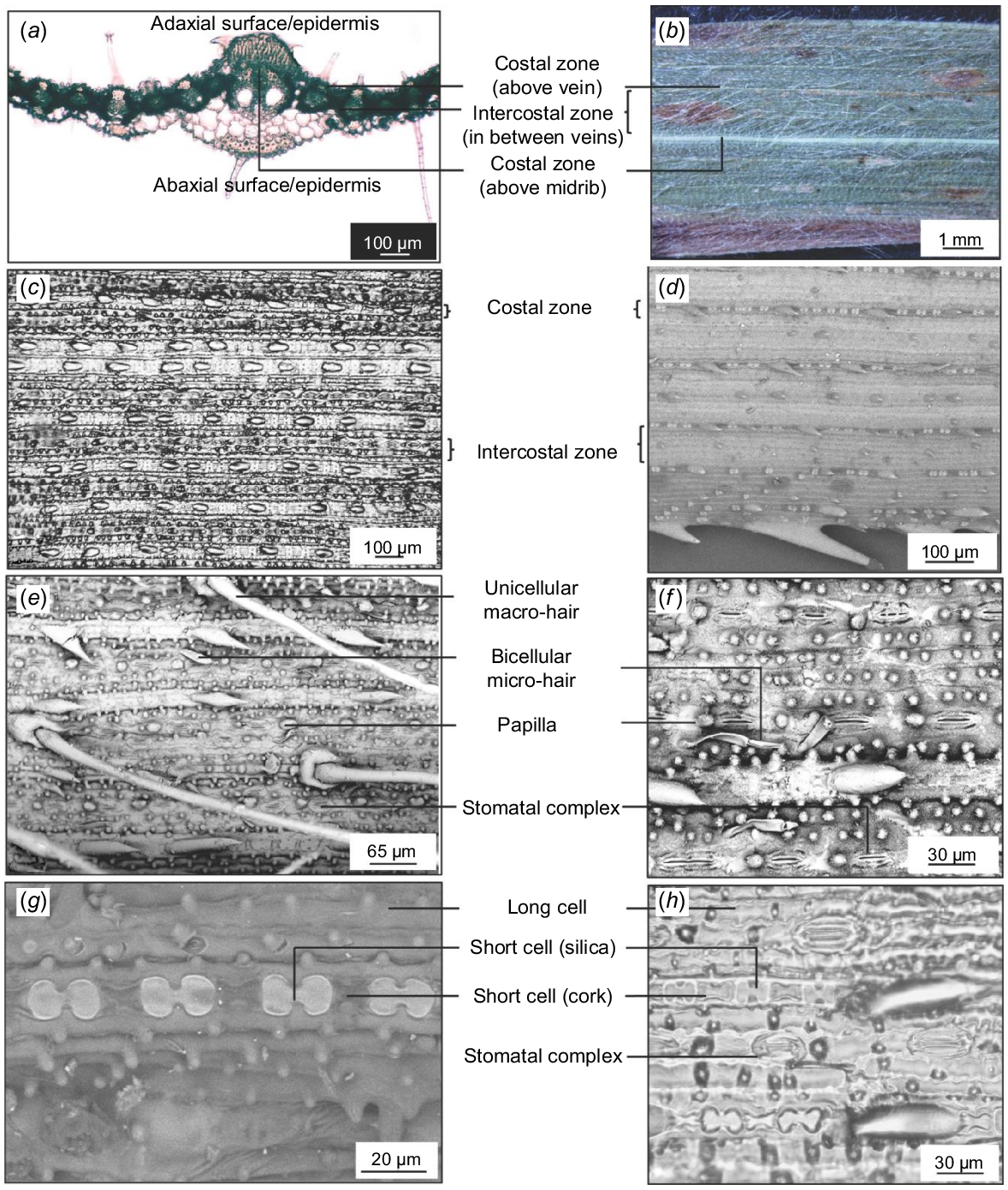
As a starting point, anatomical and micromorphological characters reported for Panicoideae, Andropogoneae, and Andropogon were included (Bowden 1964; Ojo et al. 2021). A total of 34 characters were assessed and variability among accessions was noted. The terminology used was based on Ellis (1976, 1979). Leaf surface morphology and anatomy were assessed, and terminology were used based on the criteria and description provided by Ellis (1976, 1979). The costal (above vein) and intercostal (between veins) zonation and the position and arrangement of cell types in these two zones were identified by examining transverse sections of leaves, along with epidermal replicas and SEM images of the leaf surface.
Results and discussion
A combined description of the characters observed for both abaxial and adaxial surfaces is provided, with variations noted where significant. A summary of the comparison of characters within multiple accessions of A. gayanus is presented in Table 1.
| Character | Description | ||||||
|---|---|---|---|---|---|---|---|
| Andropogon gayanus | A. gayanus var. bisquamulatus | A. gayanus var. squamulatus | A. gayanus ‘Kent’ | ||||
| Accessions from Australia | Accessions from Africa | Accessions from Indonesia | |||||
| Gross morphology | |||||||
| Lamina shape | Linear-lanceolate | Linear-lanceolate | Linear-lanceolate | Linear-lanceolate | Linear-lanceolate | Linear-lanceolate | |
| Tip | Acute | Acute | Acute | Acute | Acute | Acute | |
| Venation | Conspicuous | Conspicuous | Conspicuous | Conspicuous | Conspicuous | Conspicuous | |
| Length (cm) | 30–40 | 30–40 | 30–40 | 30–40 | 30–40 | 30–40 | |
| Width (mm) | To 15 | 10–15 | 10–18 | 10–18 | 7–10 | 10–20 | |
| Abaxial surface presence of hair | Pubescent | Pubescent, glabrous | Pubescent, glabrous | Pubescent | Glabrous | Pubescent | |
| Adaxial surface presence of hair | Pubescent | Glabrous | Pubescent | Pubescent | Glabrous | Pubescent | |
| Midrib conspicuousness (abaxial surface) | Distinctly raised | Distinctly raised | Distinctly raised | Distinctly raised | Distinctly raised | Distinctly raised | |
| Midrib conspicuousness (adaxial surface) | Highly prominent to not prominent | Highly prominent to not prominent | Highly prominent | Highly prominent | Highly prominent | Highly prominent | |
| Leaf margin | Scabrid | Scabrid, smooth | Smooth | Scabrid | Scabrid, smooth | Scabrid | |
| Leaf micromorphology – abaxial | |||||||
| Costal/intercostal zonation | Conspicuous | Conspicuous | Conspicuous | Conspicuous | Conspicuous | Conspicuous | |
| Long cell outline | Slightly undulating | Slightly undulating | Slightly undulating | Slightly undulating | Slightly undulating | Slightly undulating | |
| Long cell – bulliform | Absent | Absent | Absent | Absent | Absent | Absent | |
| Type of short cells present | Cork, silica | Cork, silica | Cork, silica | Cork, silica | Cork, silica | Cork, silica | |
| Silica cell shape | Dumbbell | Dumbbell | Dumbbell | Dumbbell | Dumbbell | Dumbbell | |
| Subsidiary cell shape | Dome | Dome | Dome | Dome | Dome | Dome | |
| Stomatal complex distribution | Common | Common | Common | Common | Common | Common | |
| Micro-hair shape | Panicoid type (two-celled) | Panicoid type (two-celled) | Panicoid type (two-celled) | Panicoid type (two-celled) | Panicoid type (two-celled) | Panicoid type (two-celled) | |
| Macro-hair | Present | Absent | Present, absent | Present | Absent | Present | |
| Prickles | Present | Present | Present | Present | Present | Present | |
| Papillae | Present | Present | Present | Present | Present | Present | |
| Papillae shape | Rounded | Rounded | Rounded | Rounded | Rounded | Rounded | |
| Leaf micromorphology – adaxial | |||||||
| Costal/intercostal zonation | Conspicuous | Conspicuous | Conspicuous | Conspicuous | Conspicuous | Conspicuous | |
| Long cell outline | Deeply undulating | Deeply undulating | Deeply undulating | Deeply undulating | Deeply undulating | Deeply undulating | |
| Long cell – bulliform | Present | Present | Present | Present | Present | Present | |
| Type of short cells present | Cork, silica | Cork, silica | Cork, silica | Cork, silica | Cork, silica | Cork, silica | |
| Silica cell shape | Elliptical to almost square | Elliptical to almost square | Elliptical to almost square | Elliptical to almost square | Elliptical to almost square | Elliptical to almost square | |
| Subsidiary cell shape | Dome | Dome | Dome | Dome | Dome | Dome | |
| Stomatal complex distribution | Not common | Not common | Not common | Not common | Not common | Not common | |
| Micro-hair shape | Panicoid type (two-celled) | Panicoid type (two-celled) | Panicoid type (two-celled) | Panicoid type (two-celled) | Panicoid type (two-celled) | Panicoid type (two-celled) | |
| Macro-hair distribution | Present | Absent | Present, absent | Present | Absent | Present | |
| Prickles | Present | Present | Present | Present | Present | Present | |
| Papillae | Present | Present | Present | Present | Present | Present | |
| Papillae shape | Rounded | Rounded | Rounded | Rounded | Rounded | Rounded | |
| Leaf anatomy | |||||||
| Lamina outline | Expanded, gently undulating | Expanded, gently undulating | Expanded, gently undulating | Expanded, gently undulating | Expanded, gently undulating | Expanded, gently undulating | |
| Size of cells on the abaxial surface | Uniform | Uniform | Uniform | Uniform | Uniform | Uniform | |
| Size of cells on the adaxial surface | Not uniform | Not uniform | Not uniform | Not uniform | Not uniform | Not uniform | |
| Presence of sclerenchyma girder in the midvein | Present | Present | Present | Present | Present | Present | |
Gross morphology of the leaf
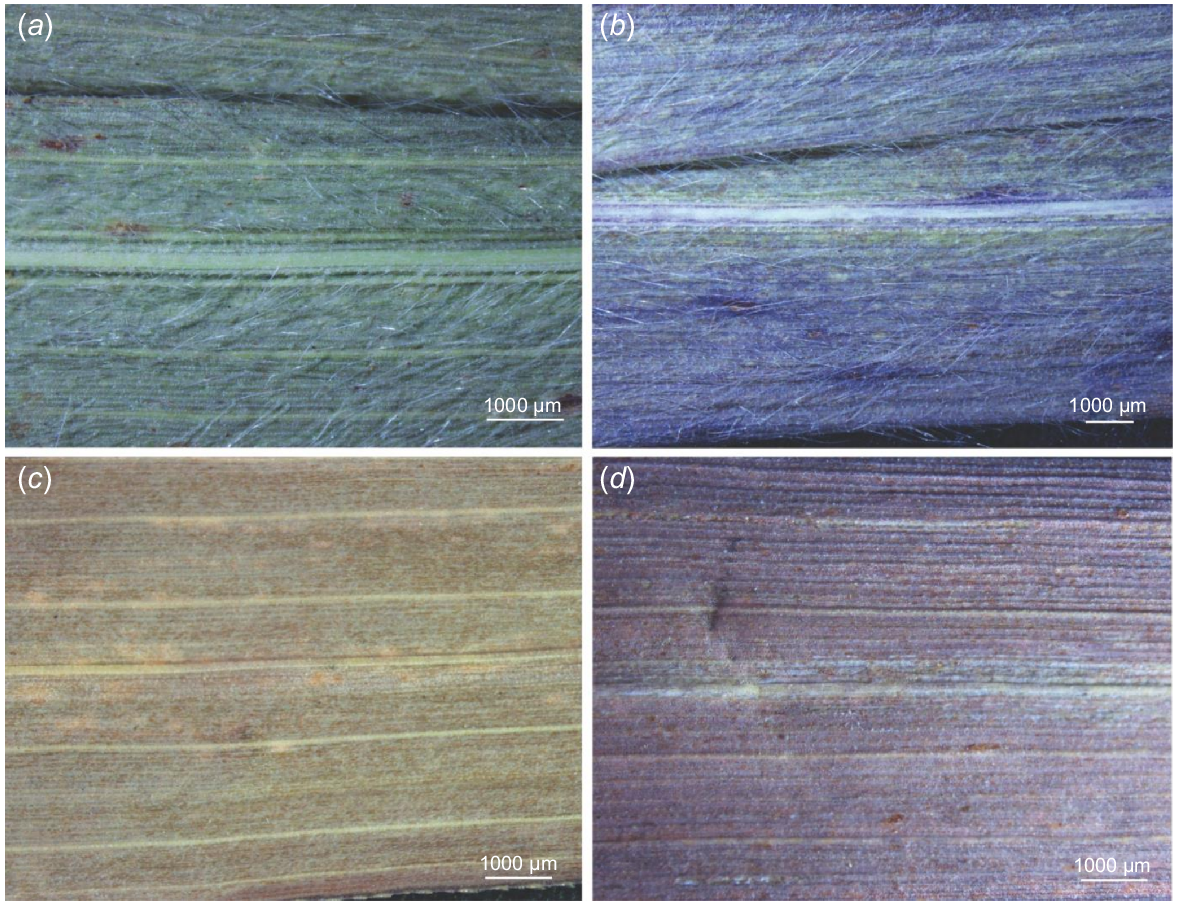
The leaf blades of A. gayanus, the two known varieties (A. gayanus var. bisquamulatus and A. gayanus var. bisquamulatus) and cultivar (A. gayanus ‘Kent’) are linear-lanceolate, with acute tip and conspicuous venation, indicative of prominent costal and intercostal zonation on the abaxial and adaxial sides (Table 1, Fig. 2a–d). The leaves are usually 30–40 cm long and 7–20 mm wide. The leaf margin is usually scabrid due to the presence of prickles. However, some specimens have smooth leaf margins (e.g. accessions from Indonesia, some from Africa, A. gayanus var. squamulatus). The midrib is strong, white, and distinctly raised on the abaxial side and can be highly prominent on the adaxial side but varies and is not prominent in some cases. The variation across specimens examined for the adaxial surface is more pronounced in cases where more specimen accessions were examined (e.g. accessions from Australia and Africa). Both abaxial and adaxial leaf surfaces are generally pubescent for the naturalised specimens (i.e. Australia, Indonesia), the known variety A. gayanus var. bisquamulatus and the cultivar A. gayanus ‘Kent’. Leaves on both surfaces are usually glabrous for the specimens from the native range (i.e. Africa) and the known variety A. gayanus var. squamulatus. Notably, both glabrous and pubescent abaxial surface were observed in specimens from Indonesia (Table 1, Fig. 2a–d).
The two sides of the leaves of Andropogon gayanus collected from different geographic locations. Specimens collected in Australia show prominent venation, dense hair, and conspicuous midvein (a) on the abaxial and (b) and adaxial side. Specimens collected in Africa show prominent venation but are glabrous and have conspicuous midvein (c) on the abaxial and the (d) adaxial side. Note: (a, b) were taken from BRI specimen with accession AQ827376, whereas (c, d) were taken from AQ227645.
Leaf surface micromorphology
Andropogon gayanus has conspicuous costal (above veins)/intercostal (in between veins) zonation on both abaxial and adaxial surfaces of the leaf (Fig. 2a–d).
Structures commonly occurring in the costal zone include long cells, short cells, cork cells, prickles, macro-hairs, and papillae. Those occurring in the intercostal zone include long cells, stomatal complexes, micro-hairs, macro-hairs, and papillae. The distribution of cells in these zones is almost similar on the abaxial and adaxial surfaces, except for the higher density of hairs on the adaxial surface and the absence of papillae on the abaxial surface (Figs 3 and 4).
The abaxial side of Andropogon gayanus collected from different geographic locations has conspicuous costal (above veins)/intercostal (in between veins) zonation. Andropogon gayanus from Australia in (a) transverse section showing abaxial and adaxial surfaces (AQ614816), and (b) showing costal/intercostal zonation (AQ723817). (c) A. gayanus var. squamulatus leaf replica (AQ227650). Scanning electron micrographs showing different cells on costal and intercostal zones of (d) A. gayanus from Australia (AQ591663), (e) A. gayanus from Australia (AQ827376), (f) A. gayanus from Australia (AQ591663), (g) A. gayanus var. bisquamulatus (AQ227643), and leaf replica of (h) A. gayanus from Australia (591663).
The adaxial side of Andropogon gayanus collected from different geographic locations has conspicuous costal (above veins)/intercostal (in between veins) zonation. (a) A. gayanus from Australia (AQ591663). (b) A. gayanus var. squamulatus leaf replica (AQ227650). (c) A. gayanus ‘Kent’ (AQ395415). (d) A. gayanus from Australia (AQ591663). (e) A. gayanus var. bisquamulatus (AQ227643). (f) A. gayanus from Australia (AQ827376).
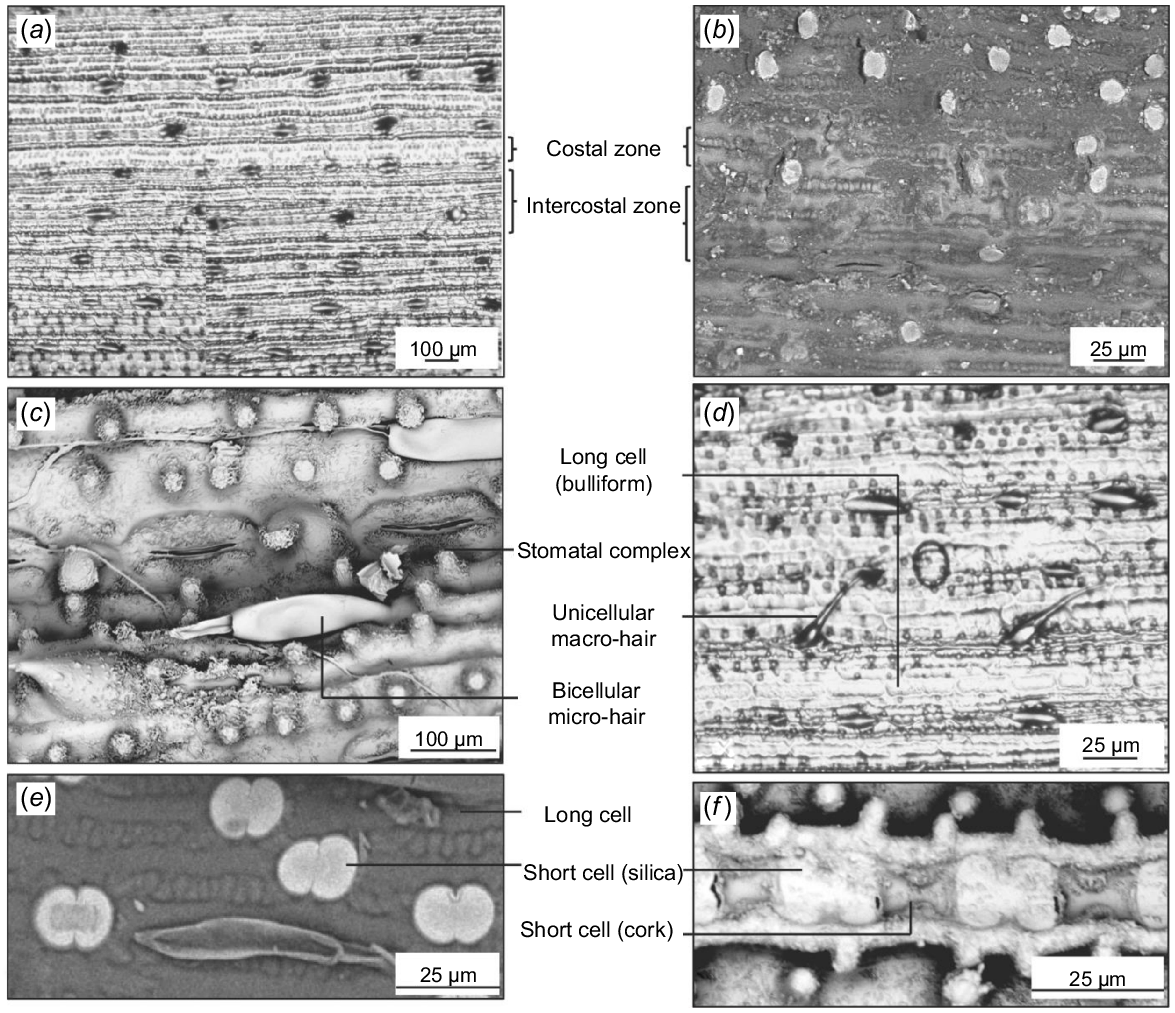
Each costal zone (above vein) of the abaxial surface bears a single file of alternating dumbbell-shaped silica cells and cork cells. The series is interrupted at regular intervals by macro-hairs. At either side of the costal zone is a continuous file of long cells. The intercostal zone of the abaxial surface consists mainly of long cells with papillae. Bicellular micro-hairs are also present. Solitary silica cells or paired silica and cork cells are also found in the intercostal zone. Finally, along the intercostal zone, stomatal complexes alternating with other cells are also observed (Fig. 3a–h).
The adaxial surface is almost similar to the abaxial surface except for the thicker and more sinuous walls the cells create, fewer stomata, micro-hairs and macro-hairs, the presence of shorter, colourless cells called bulliform, and the different shape of the silica cells. Each costal zone has almost square-shaped or elliptical silica cells alternating with cork cells. Macro-hairs with cushion cells at their bases are also found on the costal zone. This is followed by a file of long cells with sinuous walls and bearing circular papillae. The intercostal zone is composed of bulliform cells, which are inflated long cells not interrupted by other cells and with papillae. The bulliform cells look shorter and wider than the rest of the long cells in the files found on the intercostal zone. Fewer short cells occur, including elliptical silica cells and cork cells, which are not always paired with silica cells (Fig. 4a–f).
Leaf anatomy (transverse section)
The leaf vascular bundles of A. gayanus are surrounded by well-developed radiate chlorenchyma except in regions where it is interrupted by girder sclerenchyma. Vascular bundles with bundle sheath, chlorenchyma and sclerenchyma cells constitute the regions between the abaxial and adaxial surfaces. The vascular bundles are separated by vertical plates of parenchyma made of cells without chloroplasts, also known as colourless cells (Fig. 5).
Transverse section of Andropogon gayanus var. bisquamulatus showing (a) primary and secondary vascular bundles and (b) primary vascular bundle (midrib) and tissues.
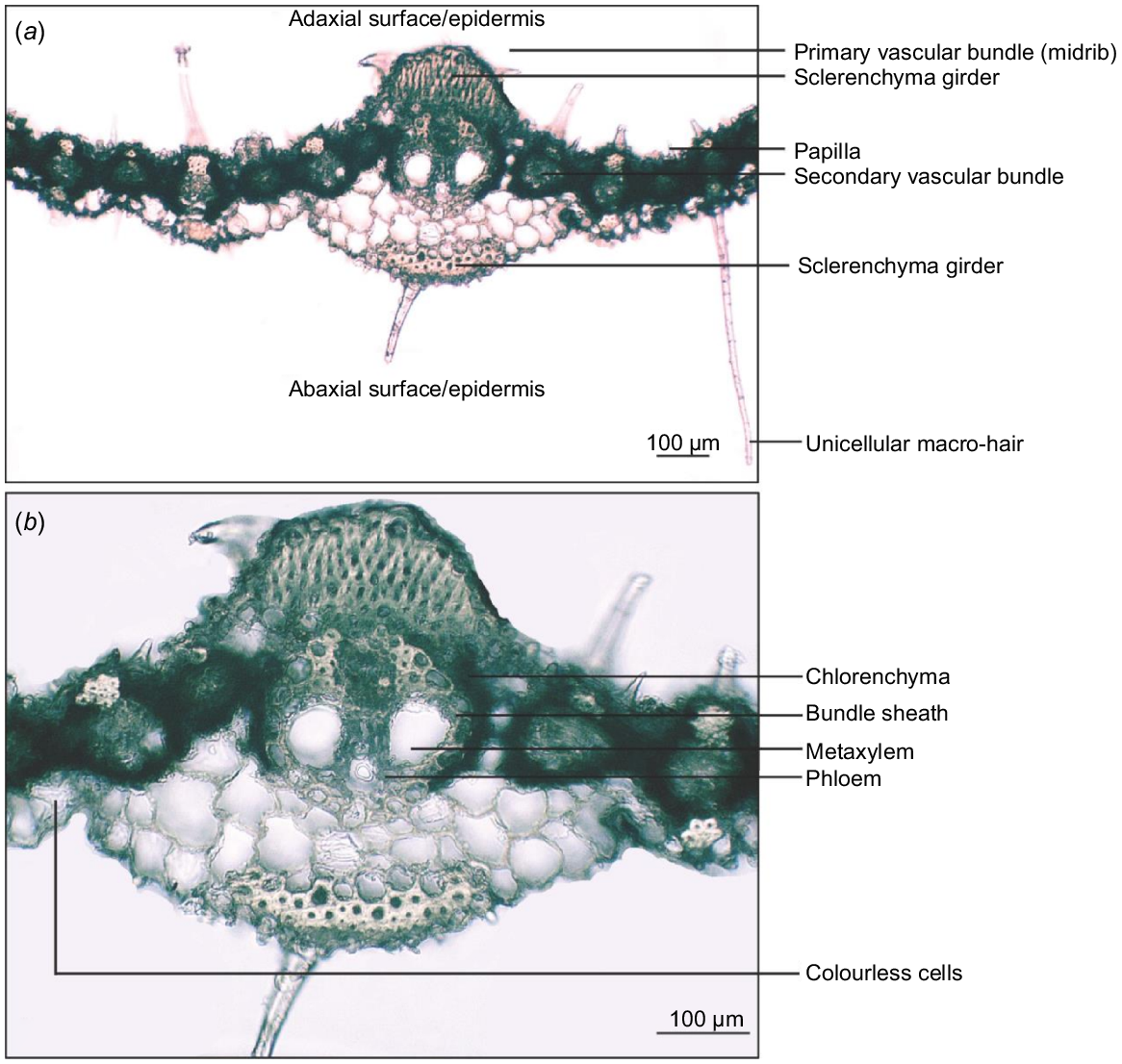
Except for laminar pubescence, the leaf micromorphology and anatomy of A. gayanus, including varieties and cultivars do not vary. The leaf micromorphology and anatomy are typical of the Andropogonae subtribe (as described by Renvoize 1982) with oblong convolute walled (sinuous) long cells that are found in 80% of the genera. The stomata appear in one row per costal zone, where 67% of genera have one or two rows. The adaxial surface is flat (like 77% of the subtribe genera). Like all Andropogonae, the vascular bundles are organised for the C4 pathway for carbon fixation. The primary vascular bundle in the midrib is associated with several layers of parenchyma cells, which is typical within the genus. Most characters of A. gayanus leaf blade are consistent across all specimens examined. The absence or rare presence of stomata on the adaxial surface is an adaptation to semi-arid conditions. Similarly, the abundance of papillae across the abaxial and adaxial surfaces have been shown in other studies to catch and help retain water on the leaf blade. The only notable variation between the accessions sampled was the level of pubescence on the leaf blade. One of the two varieties is consistently glabrous, whereas the other variety and the African and Asian specimens were either glabrous or pubescent. The cultivar and Australian specimens are pubescent. These results could indicate that pubescence is a plastic trait for A. gayanus or that the presence of leaf surface hair is a distinguishing character that could separate the currently known grouping into separate taxa.
Conclusion
Like most of the members of the subfamily Panicoideae, A. gayanus is well adapted for tropical environments. Considering the stability of most characters found in these results it is possible that the success of A. gayanus in Australia is not due to adaptations of the micromorphology and anatomy of the leaf blade. This indicates that the environmental conditions in northern Australia compare favourably with the species’ native range, which is expansive across the African dry tropical savannas. We can look to similar structuring of plant community ecology, habitat types, and adaptation to fire in Australia and Africa, that have allowed for the successful translocation of different species in recent times. The authors recommend taxonomic studies of A. gayanus focused on determining whether the specimens in the native range fall within the same clade as the ones in Australia, the two varieties and the cultivars. Characters of the reproductive structures such as spikelets, caryopses, and pollen should also be investigated to determine how these aid in the successful proliferation of A. gayanus.
Data availability
The data that supports this study will be shared upon reasonable request to the corresponding author.
Author contributions
JF and MF designed the study and conducted the microscopy. JF managed the literature searches, prepared the specimens for microscopy and wrote the initial draft. MF put together the protocols for specimen preparation and microscopy. JF, MF, and SK expanded the ideas from the initial draft and edited the final draft.
Acknowledgements
The authors would like to thank Andrew Franks, the Collections Manager at Queensland Herbarium and Biodiversity Science during the conduct of the study (2022), for allowing the use of the A. gayanus specimen collection and various microscopy equipment to carry out this study. JF acknowledges the University of Queensland for supporting the industry placement that initiated this project.
References
Anning P (1982) Evaluation of introduced grass species for pastures in the dry tropics of north Queensland. Tropical Grasslands 16, 37.
| Google Scholar |
Bebawi FF, Campbell SD, Mayer RJ (2018) Gamba grass (Andropogon gayanus Kunth.) seed persistence and germination temperature tolerance. The Rangeland Journal 40, 463-472.
| Crossref | Google Scholar |
Bianchi A, Marchesi G (1960) The surface of the leaf in normal and glossy maize seedlings. Zeitschrift für Vererbungslehre 91, 214-219.
| Crossref | Google Scholar |
Bowden BN (1964) Studies on Andropogon gayanus Kunth: III. An outline of its biology. The Journal of Ecology 52, 255-271.
| Crossref | Google Scholar |
Cook GD, Dias L (2006) It was no accident: deliberate plant introductions by Australian government agencies during the 20th century. Australian Journal of Botany 54(7), 601-625.
| Crossref | Google Scholar |
Dean M, Ashton PA (2008) Leaf surfaces as a taxonomic tool: the case of Carex section Phacocystis (Cyperaceae) in the British Isles. Plant Systematics and Evolution 273, 97-105.
| Crossref | Google Scholar |
Ellis RP (1976) A procedure for standardizing comparative leaf anatomy in the Poaceae. I. The leaf-blade as viewed in transverse section. Bothalia 12, 65-109.
| Crossref | Google Scholar |
Ellis RP (1979) A procedure for standardizing comparative leaf anatomy in the Poaceae. II. The epidermis as seen in surface view. Bothalia 12, 641-671.
| Crossref | Google Scholar |
Foster WH (1962) Investigations preliminary to the production of cultivars of Andropogon gayanus. Euphytica 11, 47-52.
| Crossref | Google Scholar |
GBIF.org (2024) GBIF occurrence download. Available at https://doi.org/10.15468/dl.7wufz7
Javed A (1990) Andropogon gayanus: a valuable forage grass for tropical and sub-tropical arid rangelands. Rangelands 12, 229-231.
| Google Scholar |
Jones CA (1979) The potential of Andropogon gayanus Kunth in the oxisol and ultisol savannas of tropical America. Herbage Abstracts 49, 1-8.
| Google Scholar |
Kent F (1987) Gamba grass Andropogon gayanus Kunth cv. Kent (Reg. no. A-20a-1). Tropical Grasslands 1, 44-46.
| Google Scholar |
Nazir A, Khan MA, Ahmad F, Ullah K, Shah A (2013) Foliar epidermal studies as an aid to the identification of grasses of tribe Andropogoneae (Poaceae) from Potohar region of Pakistan. Pakistan Journal of Botany 45, 235-241.
| Google Scholar |
Ojo FM, Nwokeocha CC, Faluyi JO (2021) Foliar anatomical studies of Andropogon gayanus Andropogon tectorum complex in Southwestern Nigeria. Notulae Scientia Biologicae 13, 11031.
| Crossref | Google Scholar |
Oram R (1987) Register of Australian herbage plant cultivars. A. Grasses. 20. Andropogon. Andropogon gayanus Kunth (Gamba grass) cv. Kent (Reg. no. A-20a-1). Journal of the Australian Institute of Agricultural Science 2, 123-124.
| Google Scholar |
Renvoize SA (1982) A survey of leaf-blade anatomy in grasses. I. Andropogoneae. Kew Bulletin 37, 315-321.
| Crossref | Google Scholar |
Rossiter-Rachor NA, Setterfield SA, Douglas MM, Hutley LB, Cook GD, Schmidt S (2009) Invasive Andropogon gayanus (Gamba grass) is an ecosystem transformer of nitrogen relations in Australian savanna. Ecological Applications 19, 1546-1560.
| Crossref | Google Scholar |
Rossiter-Rachor NA, Setterfield SA, Hutley LB, McMaster D, Schmidt S, Douglas MM (2017) Invasive Andropogon gayanus (Gamba grass) alters litter decomposition and nitrogen fluxes in an Australian tropical savanna. Scientific Reports 7, 11705-11710.
| Crossref | Google Scholar | PubMed |
Royal Botanic Gardens, Kew (2022) Andropogon gayanus Kunth. Plants of the world online. Available at https://powo.science.kew.org/taxon/387915-1#distribution-map
Setterfield SA, Rossiter-Rachor NA, Hutley LB, Douglas MM, Williams RJ (2010) Turning up the heat: the impacts of Andropogon gayanus (gamba grass) invasion on fire behaviour in northern Australian savannas. Diversity and Distributions 16, 854-861.
| Crossref | Google Scholar |
Snow M (2022) WA on cusp of eradicating gamba grass as Queensland and Northern Territory battle to contain it. Available at https://www.abc.net.au/news/2022-02-21/gamba-grass-eradication-close-in-wa-/100836152
Soreng RJ, Peterson PM, Romaschenko K, Davidse G, Teisher JK, Clark LG, Barberá P, Gillespie LJ, Zuloaga FO (2017) A worldwide phylogenetic classification of the Poaceae (Gramineae) II: an update and a comparison of two 2015 classifications. Journal of Systematics and Evolution 55, 259-290.
| Crossref | Google Scholar |
Stapf O (1919) Gunnera manicata and G. brasiliensis. Bulletin of Miscellaneous Information (Royal Botanic Gardens, Kew) 9, 376-378.
| Crossref | Google Scholar |
The Australasian Virtual Herbarium (AVH) (2024) Council of Heads of Australasian Herbaria. Available at https://avh.chah.org.au [Accessed 3 January 2023]


