Biochar increases soil enzyme activities in two contrasting pastoral soils under different grazing management
Stanislav Garbuz A , Alec Mackay B , Marta Camps-Arbestain A , Brian DeVantier B and Maria Minor A *
A *
A School of Agriculture and Environment, Massey University, PB 11222, Palmerston North, New Zealand.
B AgResearch, Grasslands Research Centre, Palmerston North 4410, New Zealand.
Crop & Pasture Science - https://doi.org/10.1071/CP21790
Submitted: 2 December 2021 Accepted: 18 July 2022 Published online: 12 August 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC)
Abstract
Context: Soil enzyme activities are key regulators of carbon and nutrient cycling in grazed pastures.
Aims: We investigated the effect of biochar addition on the activity of seven enzymes involved in the carbon, nitrogen and phosphorus cycles in a Sil-andic Andosol and a Dystric Cambisol under permanent pastures.
Methods: The study consisted of a one-year field-based mesocosm experiment involving four pastures under different nutrient and livestock practices: with and without effluent under dairy cow grazing on the Andosol, and with either nil or high phosphorus fertiliser input under sheep grazing on the Cambisol. Soil treatments were: (1) willow biochar added at 1% w/w; (2) lime added at the liming equivalence of biochar (positive control); (3) no amendments (negative control).
Key results: Compared with the Cambisol, the Andosol had higher dehydrogenase, urease, alkaline and acid phosphatase and, especially, nitrate-reductase activities, aligning with its higher pH and fertility. In both soils, biochar addition increased the activity of all enzymes, except for acid phosphatase and peroxidase; lime addition increased peroxidase and nitrate-reductase activity.
Conclusions: The increased enzyme activity was strongly positively correlated with soil biological activity following biochar addition. Biochar caused a 40–45% increase in cellulase activity, attributed to increased root biomass following biochar addition. The response in acid and alkaline phosphatase activity can be attributed to the impact of biochar and lime addition on soil pH.
Implications: The results provide more insights in realising the potential benefits of biochar to the provision of ecosystem services for grazed pastures.
Keywords: Andosol, biochar, biological activity, Cambisol, fertility, nutrient cycling, pasture, soil enzymes.
Introduction
A good understanding of how to best manage soils is important in order to maintain or increase the soil capability to meet human needs (Dominati et al. 2010). Soil organic matter (SOM) content and soil fertility play a key role in the ability of soils to be suitable for food production and provision of other ecosystem services (Adhikari and Hartemink 2016). Soil enzymes are an important part of soil processes, providing a link between soil biotic and abiotic components that are integral in nutrient and energy exchange within the soil (Yang and Wang 2002; Sinsabaugh et al. 2008). A measure of soil enzyme presence and activity allows for the indirect quantification of soil processes (flows) that contribute to carbon (C) and nutrient cycling, which in turn contribute to plant growth (Shi 2011; Jog et al. 2012), and soil detoxification and remediation (Rao et al. 2010). Soil enzymes are involved in multiple processes (Table 1), including the C, nitrogen (N), and phosphorus (P) cycles (Sardans et al. 2008; Das and Varma 2011). As a product of biological activity, enzymes are closely linked to abundance, community structure and activity of soil microorganisms, and soil micro- and meso-fauna (Caldwell 2005). Large soil animals, such as earthworms and some arthropods, influence the concentration and activity of soil enzymes in three ways: by releasing their own gut enzymes; by changing the microbial community inside their intestine and in their excreta; and by changing physico-chemical properties of the soil through their burrowing and mixing activities (Moldenke et al. 2000; Kizilkaya et al. 2011). The dynamics of soil nutrient and biological properties and enzymatic activity help to identify the main drivers of the C, N and P biogeochemical cycles (Harrison 2016; Macdonald et al. 2018). Enzymes, therefore, can be seen as indicators that can be used to assess the influence of soil and plant management practices and land use on key soil ecosystem functions (Chang et al. 2007; Garbuz et al. 2016; Holík et al. 2019). The addition of organic amendments influences the physical and chemical environment of the soil, and affects the functional and structural diversity of soil microorganisms, which is the main factor for regulating and maintaining soil enzyme activity (Pérez-Piqueres et al. 2006; Cleveland et al. 2007). A promising organic amendment is biochar, a charcoal produced from biomass pyrolysis (Lorenz and Lal 2014; Lehmann et al. 2021). Biochar affects soil microbial communities through changes in soil bulk density, water retention, soil pH, and soil nutrient content and availability, as well as the provision of some labile C, especially when produced at low temperature of pyrolysis (Masto et al. 2013; De Tender et al. 2016). Generally, biochar application increases the abundance of soil microorganisms (Lehmann et al. 2011; Paz-Ferreiro et al. 2015; Palansooriya et al. 2019), whereas the influence of biochar on soil enzyme activity has been reported to be more variable, being highly dependent on the properties of the biochar and soil characteristics (Ouyang et al. 2014; Paz-Ferreiro et al. 2014; Khadem and Raiesi 2017; Garbuz et al. 2021).
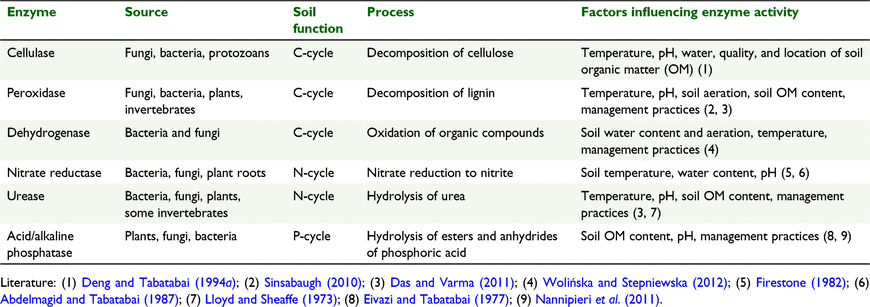
|
Previous studies from our group (Garbuz et al. 2020, 2021) have shown that willow biochar addition (1% w/w ratio), when added to a Sil-andic Andosol and a Dystric Cambisol under grazed pasture, increased the size of both the bacterial and fungal communities, and also plant roots biomass within 12 months of application. In the same study, biochar also reduced soil bulk density (BD) and soil acidity, increased soil organic C and N stocks, and plant-available P (Olsen P). The changes in the stocks and availability of C, N and P indicate that biochar is also likely to be impacting on the activity of soil enzymes involved in the cycles of these three nutrients. However, it is not clear to what degree cellulase, peroxidase and dehydrogenase, which are involved in SOM decomposition, are affected by biochar. In addition, the degree of impact of biochar on nitrate-reductase and urease activity, which are involved in N cycling and used to quantify N transformation rates in soil, is unknown. It is well known that phosphatases, which are involved in mineralisation of organic P and, consequently, play a key role in plant P nutrition (Table 1), are sensitive to soil pH (Juma and Tabatabai 1978) and thus it is likely that their activity will be altered following biochar addition. In view of the above, we hypothesised that the influence of willow biochar produced at low temperature of pyrolysis on microbial biomass and plant root growth (1) should be mirrored in soil enzyme activity, as a part of underlying processes involved in stimulating the biogeochemical cycle regulating C, N and P supply, and (2) is beyond that of just the liming effect of biochar.
Materials and methods
Biochar production and characterisation
Biochar used in this experiment was produced from willow (Salix matsudana L.) chips. Air-dried feedstock (<12% moisture content) was pyrolysed at a maximum heating temperature of 350°C and residence time of 4 h. The characteristics of the biochar were: pH 7.8, organic C (Corg) 703 g kg−1, the ratio of dichromate-oxidisable C out of Corg (Cox/Corg) 51.4%, atomic H/Corg 0.63, liming equivalence 7.3% CaCO3-eq (Garbuz et al. 2021). The biochar used in our study was classified as having a C storage class of 2, a liming class of 1, and a fertiliser class of zero (no nutrient value) (Camps-Arbestain et al. 2015). The choice of willow was based on the fact that it is readily available as it is used extensively in New Zealand in soil conservation and stream bank protection. Willow grows readily from a cutting and has an extensive rooting system.
Study sites
Two soils common under pastures in lower North Island of New Zealand were used in this study: (1) a Dystric Cambisol (IUSS Working Group WRB 2015), Brown soil in the New Zealand soil classification system (Hewitt 2010), from the experimental site of AgResearch Ballantrae Hill Country Research Station, Manawatu (40°18′35″S, 175°49′41″E); (2) a Sil-andic Andosol (IUSS Working Group WRB 2015), Allophanic soil (Hewitt 2010), from Hawera, Taranaki (39°36′28″S, 174°16′30″E). The Andosol used in this study is derived from volcanic ash. Rich in short-range order constituents, it offers high SOM protection, has a high anion retention, good physical properties, and resilience to treading pressure (Molloy 1998). The Cambisol is predominantly derived from loess materials. It has a low anion storage capacity and limited physical resilience to treading pressure. Two pastures grazed by dairy cows throughout the year on the Andosol were selected: one receiving dairy shed effluent (And-EF), and one not receiving effluent (And-NE). Both pastures receive 160 kg of N as fertiliser N ha−1 year−1, 300 kg of 20% potash superphosphate ha−1 year−1, and 1 kg selenium prill ha−1 year−1 (a standard fertiliser regime for NZ pastoral soils under dairy farming). The two selected pastures on the Cambisol were grazed by sheep throughout the year: one (Cam-LF) had received no superphosphate since 1980, and the other (Cam-HF) receives 375 kg superphosphate ha−1 year−1 since 1980 (Mackay et al. 2021).
Field-based mesocosm experiment
The field-based mesocosm experiment was conducted using large soil cores enclosed into PVC cylinders (150 mm Ø, 300 mm length). Four holes (5.1 cm Ø) were made in the wall of each cylinder to allow the free movement of soil organisms (fig. S1 in Garbuz et al. 2021). There were three treatments: (1) no amendments (negative control), (2) 1% of biochar application by weight (equivalent to approximately 10.9 Mg ha−1), and (3) lime (positive control) applied at a rate corresponding to the liming equivalent of biochar (ca. 0.8 Mg ha−1). Each treatment was replicated six times in each of the four pastures. During the southern hemisphere spring of 2017, the PVC cylinders were hammered into the ground and excavated with soil from each of the four pastures. At the laboratory, the turf layer (ca. 20 mm) was split off, and the top 150 mm of soil below the turf layer was removed from all cores. All earthworms from the topsoil were removed, counted, labelled with the core code, and cold-stored. For biochar and lime treatments, the soil was mixed with either biochar or lime, respectively, and put back into cylinders to the depth 20–170 mm; in control mesocosms the soil was also removed, and mixed without amendments. Earthworms and turf layer were placed back, and mesocosms installed in the field. Further details on design, preparation, and installation of mesocosms are provided in Garbuz et al. (2021). The experiment started in late October–November 2017, during the southern hemisphere spring. The sampling (18 cores from each pasture) occurred in November 2018, approximately 12 months after the start of the experiment. Climatological data for the two locations are provided in Supplementary Fig. S1.
Soil physico-chemical and biological properties
Soil samples for chemical analysis and microbial biomass were collected with a corer (30 mm Ø) from five depths: 0–20 mm (the turf), 20–95 mm, 95–170 mm, 170–200 mm, and 200–300 mm, and air-dried. The following variables were measured: soil bulk density (BD), pH, total C, total N (TN), nitrate-N (NO3−–N) and ammonium-N (NH4+–N), Olsen P. Inorganic C was negligible (<0.05%) in the lime-treated soil after 12 months of incubation, and thus total soil C was all organic (Corg). Mesofauna abundance (Collembola, Oribatida and Gamasina) was sampled by taking 50 mm × 50 mm × 50 mm cores from the topsoil (20–95 mm) in each mesocosm cylinder. Fungal and bacterial biomass were measured in mixed topsoil (20–170 mm) using the substrate-induced respiration (SIR) method with selective inhibition (Nakamoto and Wakahara 2004). Fungal (Cf) and bacterial (Cb) biomass C was calculated according to Anderson and Domsch (1978). The sum of Cf and Cb was considered as the microbial biomass. Earthworms from each mesocosm cylinder (full depth) were hand sorted, identified to species when possible, and counted. Plant roots from each cylinder were collected by hand, washed over a 3-mm sieve, oven-dried (40°C) and weighed. Soil properties are summarised in Garbuz et al. (2021) and we relate them to enzyme activities in this study.
Soil enzymes analysis
Soil samples for enzyme analysis were collected with a corer (30 mm Ø) from the same five depths as soil chemistry and microbial biomass samples. All soils were sieved (<2 mm) and air-dried prior to analysis. Alkaline and acid phosphatases, nitrate-reductase, urease, cellulase, peroxidase and dehydrogenase activity were measured in each of the five depths in all three treatments of the two contrasting soils. Details on soil enzyme analysis are provided in Garbuz et al. (2020) and in the supplementary information.
Statistical analysis
Statistical analysis was carried out using SAS 9.4. Normality of data sets was evaluated by the Shapiro–Wilk test. A multicollinearity analysis was done to check simple correlations and variance inflation factors for variables. The data were normalised using z-score prior to analysis. Analysis of variance (ANOVA) with contrast statements and Tukey HSD test were used to investigate the effect of factors: pasture (And-NE, And-EF, Cam-LF, Cam-HF), treatment (control, biochar, and lime) and pasture × treatment interaction on enzyme activities. When the interaction term was not significant, main effects were reported; if the interaction effect was significant, the four pastures were considered separately. Finally, for biochar-treated and control mesocosms we constructed a hypothetical model of causal relationships underlying the observed patterns in soil enzyme activity, biota, and nutrients, and used path analysis (proc CALIS in SAS 9.4) to calculate coefficients associated with each path in the model. Due to sample size, the hypothetical model of causal relationships underlying the observed patterns in soil C-enzymes activity was limited to cellulase and dehydrogenase.
Results
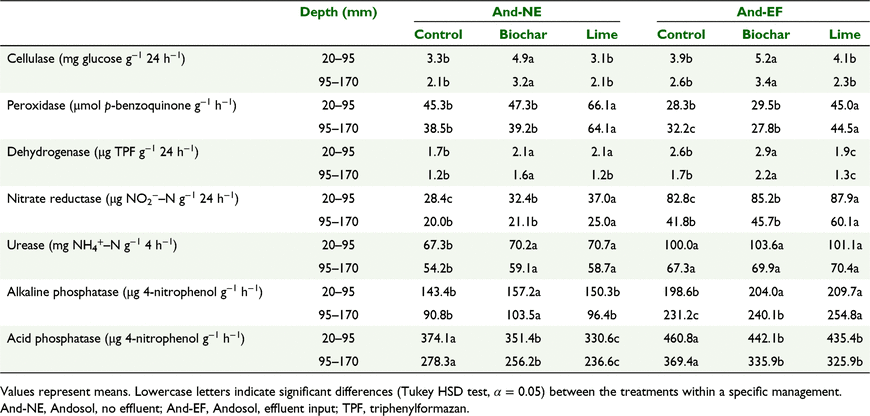
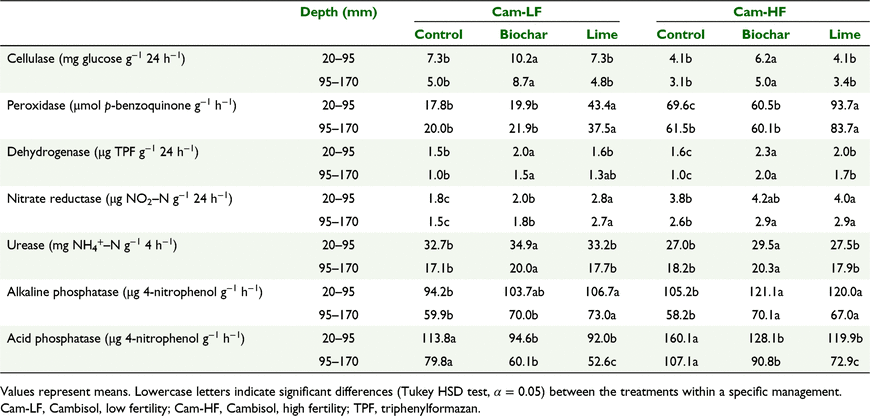
Results for the two soils, the Andosol and the Cambisol, are reported separately (Tables 2 and 3, respectively) unless otherwise indicated, as soil order had a significant effect on the activity of almost all enzymes. Phosphatases, urease, dehydrogenase and, especially, nitrate-reductase activities were higher in the Andosol, whereas cellulase activity was higher in the Cambisol (all P < 0.005). There was no difference in peroxidase activity between the two soil orders. In both soils, with few exceptions (e.g. peroxidase), enzyme activities declined with depth (all P < 0.005); the treatment effects on enzyme activity were observed primarily within the 20–95 mm and 95–170 mm soil depths, where treatments were applied, while in the turf and in layers below 170 mm there was no significant effect of treatments on soil enzyme activities (data not shown).
|
|
There were significant effects of pasture management practices on enzyme activities. Pastures with effluent addition (And-EF) and high P fertiliser input (Cam-HF) had higher phosphatases, nitrate-reductase and dehydrogenase activities (all P < 0.005) than their lower fertility equivalents (Tables 2 and 3, respectively).
Enzymes of the C cycle
Cellulase and dehydrogenase activities were higher in the biochar-treated soil than in the control (all P < 0.001), while the effect on peroxidase was site-dependent (Tables 2 and 3). In all pastures treated with lime, the activity of peroxidase was increased over that of the control and biochar-treated soil (all P < 0.001). Lime had no effect on cellulase activity, while its effect on dehydrogenase was site-dependent (Tables 2 and 3).
Path analysis (biochar-treated and negative control mesocosms only) identified significant pathways for cellulase and dehydrogenase (shown as C-enzymes) that offer a plausible representation of causal relationships (Fig. 1). In both soil types, the activity of cellulase (and C-enzymes for the Cambisol) was closely correlated with microbial biomass, with biochar addition acting as a significant driver for this variable, through changes in soil pH and the SOM pool (Fig. 1). The pathway involving roots in cellulase and dehydrogenase activity (C-enzymes) was important only in Cambisol pastures. In both soil types, the main source of C used by microbes appears to be root-derived, as opposed to the source of N, which appears to be derived from the soil pool, as described below. Pathways involving earthworms and mesofauna were not significant for cellulase and dehydrogenase, suggesting that these enzymes’ activity is largely microbial-driven. On the other hand, pathways for peroxidase indicated the singular importance of pH for activity of this enzyme, with other factors not being significant (data not shown).
Enzymes of the N and P cycle
In both Cambisol pastures urease was higher in the biochar-treated soil than in the control or lime. In the Andosol, the soil receiving effluents (And-EF) had higher urease (all P < 0.005) than the same soil without effluent (And-NE), but no effect of biochar was detected. The activity of nitrate reductase was highest in soil treated with lime in both Andosol pastures, but also higher in biochar-treated soil than in control (Tables 2 and 3). Higher nitrate-reductase activity was correlated with higher earthworm abundances in sites with lower fertility (And-NE and Cam-LF) (both P < 0.001); data on earthworm abundance is provided in Garbuz et al. (2021). Path analysis suggests that the main drivers for nitrate-reductase activity in both the Andosol and Cambisol pastures were available N (NO3−–N) and soil pH (Fig. 2). Pathways involving soil fauna (earthworms and mesofauna) were not significant for nitrate reductase in either soil type. Pathways involving plant roots and microbial biomass in nitrate-reductase activity were important only in Cambisol pastures. Alkaline phosphatase activity was higher (and acid phosphatase lower) in the biochar- and lime-treated soils compared to the control (both P < 0.005).
Discussion
The increase in enzyme activity found in the present study was strongly correlated with greater soil biological activity and plant root biomass in the soils to which biochar had been added. The recurring question for all soil enzyme studies is whether enzyme production is constitutive (linked to the biomass of microbial cells) or inducible (linked to the presence of the substrate for the enzyme) (Moorhead et al. 2012, 2013). The theory of eco-enzymatic stoichiometry suggests that the relationships between microorganisms, enzymes and resources are tightly constrained (Sinsabaugh and Follstad Shah 2012). Further, a review of patterns between microbial biomass and specific enzyme activities reveals that the enzyme production is inducible and responsive to differences in substrate characteristics, and that patterns in C, N, and P acquisition are similar across soil types (Berg 2000; Allison 2005; Moorhead et al. 2013). In our study the enzyme production appears to be inducible, as increased activity of cellulase, peroxidase, dehydrogenase, nitrate reductase, urease, and alkaline phosphatase following biochar addition aligns with increased availability in soil C, N and P measured in the biochar-amended soil (Garbuz et al. 2021).
A number of studies have reported on various effects of biochar on soil biota and soil processes (Jones et al. 2012), and how those then influence the dynamics of soil biogeochemical cycles (Sarathchandra et al. 1988; Teutscherova et al. 2018; Holík et al. 2019). Further, different types of biochar influence bacterial and fungal activities differently, including shifts in the microbial community structure, as for example, changing fungi:bacteria ratio or changing abundance of specific groups of soil bacteria, all of which are responsible for increasing the diversity of the enzymatic pool in soil (Pandian et al. 2016; Gao et al. 2017; Garbuz et al. 2021). Most often, the addition of biochar causes an increase in enzyme activities (Vázquez et al. 2000; Paz-Ferreiro et al. 2014; Mierzwa-Hersztek et al. 2019). Wang et al. (2015a) showed that a small application rate of maize biochar produced at 450°C (0.5% w/w) increased the activity of enzymes involved in the C cycle, while larger application rates (>0.5%) had a negative effect on the activities of these enzymes. These authors also showed that enzymes involved in the N cycle increase with biochar application rate. The effect of biochar on selected enzymes appears to depend on soil chemical properties, available nutrients and SOM, as well as the properties of the biochar (Paz-Ferreiro et al. 2014; Irfan et al. 2019; Oladele 2019), such as its pH (in our biochar 7.8), Cox/Corg (51.4%), and residual nutrient content (negligible).
Enzymes of the C cycle
The positive effect of biochar on dehydrogenase activity was attributed to an increase in microbial biomass C in biochar-treated soils (data for microbial biomass provided by Garbuz et al. 2021). Root C inputs to the rhizosphere, in response to higher root biomass, have been shown to stimulate soil enzyme activity and increase microbial biomass (Brzostek et al. 2013). Dehydrogenase is an important component of microbial metabolic functions (Casida 1977) and is often used as an indicator of soil microbial response to land use practice changes (Watts et al. 2010; Järvan et al. 2014). Biochar application can have a positive effect on dehydrogenase activity and increased C mineralisation in the soil as it has been observed in the short term (positive priming) (Ouyang et al. 2014; Lehmann et al. 2021) which can be related, to some extent, to the presence of labile C supplied in biochar as well as its labile C to labile N ratio if residual N is present (Wang et al. 2012). The high Cox/Corg ratio and the relatively high atomic H/Corg ratio suggests the presence of a considerable fraction of labile C in our biochar. This probably favoured microbial growth and positive priming of native SOM as inferred from a mass balance calculation (Garbuz et al. 2021). However, over one year period, biochar-treated soils in our study showed a 1.2 to 4.0 Mg C ha−1 gain in root C, especially high in Cambisol pastures, which may generate a negative priming over time (Garbuz et al. 2021). Biochar can promote long-term C storage through stabilisation of rhizo-deposits and organic ligands, in general, on biochar surface, as it can act as a new reactive surface (Lehmann et al. 2021).
The increased cellulase activity in biochar-treated soils was correlated with increased root biomass, suggesting that the increased supply of root necromass provided additional substrate for cellulase activity (Sajjad et al. 2002; Sinsabaugh 2010), yet the path analysis suggests that, in the Andosols, this only occurs indirectly through the impact of the enhanced root growth on microbial biomass. The amount of cellulose in our biochar is negligible given that the biochar was produced at 350°C and cellulose is fully carbonised at temperatures above 240°C (Demirbaş 2001). The path analysis indicated both similarities and differences in the drivers of C-enzyme activities in the Andosol and Cambisol pastures. The similarities reflect that, as expected, the most important driver is soil biological activity, while the differences are likely to be a manifestation of different types of SOM, soil aggregation and microbial community structure between the two soils. For example, the fact that in the Cambisol, but not in the Andosol, roots have a direct impact on both measured enzymes involved in the C cycle, could be related to the existence of a more plant-derived OM in the Cambisol, and a more microbial-derived SOM in the Andosol (Herath et al. 2015) due to differences in clay mineralogy and prevalence of microaggregates in the latter (Angst et al. 2021). The increase in root biomass reported by Garbuz et al. (2021) would have caused a direct increase in microbial-derived SOM in both soil types, but this increase would have been diluted in the Andosol where this SOM fraction is already more abundant as suggested by Wang et al. (2015b).
Peroxidase activity generally increases with soil pH (Sinsabaugh 2010). With an increase in pH, the bonds of organic molecules (ligands) with mineral surfaces become weaker, and desorbed SOM becomes more easily degraded and oxidised by peroxidases (Sinsabaugh 2010; Tian and Shi 2014).
Enzymes of the N cycle
Nitrate reductase activity is affected by factors such as nitrate concentration and soil pH, with an optimum at pH 7 (Abdelmagid and Tabatabai 1987). This is consistent with the enzyme activity trends observed in this study: (1) nitrate-reductase activity was highest in the Andosol that received effluents (And-EF) and in the Cambisol with high fertility pasture containing an active legume component (Cam-HF); (2) both lime and biochar increased nitrate-reductase activity, with lime having a more pronounced effect. Andosols are abundant in micropores and microaggregates which can remain saturated with water for long periods under udic moisture conditions (Buurman et al. 2007), as experienced during the study, thus creating favourable anaerobic conditions for nitrate reduction. This, and high substrate availability, could explain the higher nitrate-reductase activity in this soil (Abdelmagid and Tabatabai 1987).
Substrate availability (NO3−–N) in biochar-treated topsoil in our experiment was higher or the same as in control and lime treatments (Garbuz et al. 2021) but, compared to lime, biochar mesocosms had a lower nitrate-reductase activity. In our study, biochar-treated soil had significantly lower bulk density and higher root biomass (Garbuz et al. 2021); we speculate that this would be associated with increased soil aeration, thereby limiting the activity of nitrate reductase (Abdelmagid and Tabatabai 1987; Joseph et al. 2015). Moreover, it is well known that biochar has an effect on redox-regulated N transformations (Chacón et al. 2017). In fact, when produced at low temperature, such as the one used in this study, biochar can act as an electron shuttle (Chacón et al. 2017; Dai et al. 2021), favouring the full reduction of NO3− to N2 (Obia et al. 2015). Yet the impact of biochar electrochemical properties on N-reductase activity is hard to discern with the data available.
There is evidence that biochar and lime affect different groups of soil bacteria responsible for denitrification (Bai et al. 2015; Jha et al. 2016; Harter et al. 2017; Weldon et al. 2019). The positive correlation between earthworm abundance and the activity of nitrate reductase in two of the pastures supports the idea of a link between earthworms and denitrifying bacteria, suggested by some authors (Burtelow et al. 1998; Depkat-Jakob et al. 2010) through increased nitrate input by earthworms. Garbuz et al. (2020), working with the same soils under glasshouse conditions, also showed synergetic interactions between lime, earthworms, and increased nitrate-reductase activity.
The high urease activity in the Andosol might reflect the higher urine input from the lactating dairy cows, up to 55 L urine cow−1 day−1 (Betteridge et al. 1986), compared to 3 L urine sheep−1 day−1 (Ledgard et al. 2008) from sheep grazing in Cambisol. Urease activity has been reported to be strongly correlated with soil bacterial biomass (Amini Kiasari et al. 2018). In our experiment bacterial biomass increased with the addition of biochar (Garbuz et al. 2021), and was correlated with urease activity in all pastures except And-NE. The latter may be explained by the fact that the bacteria–urease correlation relies on a specific group of ureolytic bacteria, but not on the whole bacterial community (Lloyd and Sheaffe 1973). In other studies, urease activity in response to biochar addition has also been unpredictable; for example, rice husk biochar had both negative and positive effects on urease activity in two different acid soils – Ultisol with a pH 5.8, Corg 16 g kg−1 and available N 1.6 g kg−1 and Alfisol with a pH 4.4, Corg 3.7 g kg−1, and available N 0.0156 g kg−1 (Huang et al. 2017; Oladele 2019).
Enzymes of the P cycle
As expected, the addition of alkaline material (lime or biochar) drove an increase in the alkaline phosphotase to acid phosphotase (AlP/AcP) ratio, reflecting the sensitivity of phosphatases to soil pH (Acosta-Martínez and Tabatabai 2000), with activity of acid phosphatase decreasing and alkaline phosphatase increasing. Olsen P values in the soil amended with biochar were higher than in the control in both Andosols and in the Cambisol with high fertility (Garbuz et al. 2021), so the higher alkaline phosphatase activity in the biochar-treated soils compared with the control reflects increased substrate availability. However, this was not the case in the Cambisol with low fertility, indicating that despite an increase in the AlP/AcP ratio, this does not always translate into an increase in plant-available P. In addition to causing an increase in available P through desorption due to increased pH, biochar amendment may also influence P availability because of the increase in SOM. An enrichment in organic ligands would result in chelation of Al3+ and Fe3+ that would otherwise precipitate P (Gao and DeLuca 2016; Gao et al. 2019).
The higher phosphatase activity in the Andosol than Cambisol soil can be explained by the fact that the short-range order inorganic constituents (e.g. allophane) abundant in Andosols have the capacity to immobilise phosphatase (Chatterjee et al. 2014; Jordanova 2017) and protect this enzyme from adverse conditions (Shindo et al. 2002). Phosphatase activities are positively correlated to SOM, which enhances the stability of these enzymes (Bonmati et al. 1991). As root biomass was enhanced in the biochar treatment (Garbuz et al. 2021), we can hypothesise that root-derived SOM increased alkaline phosphatase activity.
Conclusions
Studying the effect that biochar addition has on enzyme activity, a key regulator of C and nutrient cycling of grazed pastures, is important to fully understand the potential benefits of biochar application to the provision of soil ecosystem services. Willow biochar pyrolysed at low temperature and applied at a rate of 10.9 Mg ha−1 had a significant influence on the activities of many of the enzymes involved in C, N and P cycling through a diversity of mechanisms. In our study, the enzyme production appears to be inducible, as increased activity of cellulase, peroxidase, dehydrogenase, nitrate reductase, urease and alkaline phosphatase following biochar addition aligns with increased availability in soil C, N and P measured in the biochar-amended soil. The effects of biochar range from (1) stimulating plant roots and/or the soil microbial community, which resulted in a parallel increase in cellulase and dehydrogenase activity, through to (2) increasing soil alkalinity and/or nutrient stocks, that favours, except for acid phosphatase, the activity of all enzymes. Interestingly, the increase in urease activity may point to the influence of biochar on specific functional groups within the wider soil biological community. Future research is required to better understand the influence biochar addition has on each of the functional groups that make up the soil biological community, and the flow-on effect that has on the enzymes involved in the C, N and P cycles.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
The authors would like to recognise funding from the Massey University PhD Scholarship fund to SG, and the Ministry of Business, Innovation and Employment funded research project (C09X1613) Soil health and resilience – a pathway to prosperity and well-being.
Acknowledgements
We thank the anonymous reviewers for the comments which helped to improve the manuscript. This paper forms part of the PhD Thesis of S. Garbuz.
References
Abdelmagid HM, Tabatabai MA (1987) Nitrate reductase activity of soils. Soil Biology and Biochemistry 19, 421–427.| Nitrate reductase activity of soils.Crossref | GoogleScholarGoogle Scholar |
Acosta-Martínez V, Tabatabai MA (2000) Enzyme activities in a limed agricultural soil. Biology and Fertility of Soils 31, 85–91.
| Enzyme activities in a limed agricultural soil.Crossref | GoogleScholarGoogle Scholar |
Adhikari K, Hartemink AE (2016) Linking soils to ecosystem services—A global review. Geoderma 262, 101–111.
| Linking soils to ecosystem services—A global review.Crossref | GoogleScholarGoogle Scholar |
Allison SD (2005) Cheaters, diffusion and nutrients constrain decomposition by microbial enzymes in spatially structured environments. Ecology Letters 8, 626–635.
| Cheaters, diffusion and nutrients constrain decomposition by microbial enzymes in spatially structured environments.Crossref | GoogleScholarGoogle Scholar |
Amini Kiasari M, Pakbaz MS, Ghezelbash GR (2018) Increasing of soil urease activity by stimulation of indigenous bacteria and investigation of their role on shear strength. Geomicrobiology Journal 35, 821–828.
| Increasing of soil urease activity by stimulation of indigenous bacteria and investigation of their role on shear strength.Crossref | GoogleScholarGoogle Scholar |
Anderson JPE, Domsch KH (1978) A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biology and Biochemistry 10, 215–221.
| A physiological method for the quantitative measurement of microbial biomass in soils.Crossref | GoogleScholarGoogle Scholar |
Angst G, Mueller KE, Nierop KGJ, Simpson MJ (2021) Plant- or microbial-derived? A review on the molecular composition of stabilized soil organic matter. Soil Biology and Biochemistry 156, 108189
| Plant- or microbial-derived? A review on the molecular composition of stabilized soil organic matter.Crossref | GoogleScholarGoogle Scholar |
Bai SH, Reverchon F, Xu C-Y, Xu Z, Blumfield TJ, Zhao H, Van Zwieten L, Wallace HM (2015) Wood biochar increases nitrogen retention in field settings mainly through abiotic processes. Soil Biology and Biochemistry 90, 232–240.
| Wood biochar increases nitrogen retention in field settings mainly through abiotic processes.Crossref | GoogleScholarGoogle Scholar |
Berg B (2000) Litter decomposition and organic matter turnover in northern forest soils. Forest Ecology and Management 133, 13–22.
| Litter decomposition and organic matter turnover in northern forest soils.Crossref | GoogleScholarGoogle Scholar |
Betteridge K, Andrewes WGK, Sedcole JR (1986) Intake and excretion of nitrogen, potassium and phosporus by grazing steers. The Journal of Agricultural Science 106, 393–404.
| Intake and excretion of nitrogen, potassium and phosporus by grazing steers.Crossref | GoogleScholarGoogle Scholar |
Bonmati M, Ceccanti B, Nanniperi P (1991) Spatial variability of phosphatase, urease, protease, organic carbon and total nitrogen in soil. Soil Biology and Biochemistry 23, 391–396.
| Spatial variability of phosphatase, urease, protease, organic carbon and total nitrogen in soil.Crossref | GoogleScholarGoogle Scholar |
Brzostek ER, Greco A, Drake JE, Finzi AC (2013) Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry 115, 65–76.
| Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils.Crossref | GoogleScholarGoogle Scholar |
Burtelow AE, Bohlen PJ, Groffman PM (1998) Influence of exotic earthworm invasion on soil organic matter, microbial biomass and denitrification potential in forest soils of the northeastern United States. Applied Soil Ecology 9, 197–202.
| Influence of exotic earthworm invasion on soil organic matter, microbial biomass and denitrification potential in forest soils of the northeastern United States.Crossref | GoogleScholarGoogle Scholar |
Buurman P, Peterse F, Almendros Martin G (2007) Soil organic matter chemistry in allophanic soils: a pyrolysis-GC/MS study of a Costa Rican Andosol catena. European Journal of Soil Science 58, 1330–1347.
| Soil organic matter chemistry in allophanic soils: a pyrolysis-GC/MS study of a Costa Rican Andosol catena.Crossref | GoogleScholarGoogle Scholar |
Caldwell BA (2005) Enzyme activities as a component of soil biodiversity: a review. Pedobiologia 49, 637–644.
| Enzyme activities as a component of soil biodiversity: a review.Crossref | GoogleScholarGoogle Scholar |
Camps-Arbestain M, Amonette JE, Singh B, Wang T, Schmidt H-P (2015) A biochar classification system and associated test methods. In ‘Biochar for environmental management’. (Eds J Lehmann, S Joseph) pp. 165–194. (Routledge: New York, NY, USA)
Casida LE (1977) Microbial metabolic activity in soil as measured by dehydrogenase determinations. Applied and Environmental Microbiology 34, 630–636.
| Microbial metabolic activity in soil as measured by dehydrogenase determinations.Crossref | GoogleScholarGoogle Scholar |
Chacón FJ, Cayuela ML, Roig A, Sánchez-Monedero MA (2017) The effect of different chemical treatments, pyrolysis conditions and feedstocks on the redox properties of biochar. In ‘EGU general assembly conference abstracts’. p. 9556. (European Geosciences Union General Assembly 2017: Vienna, Austria)
Chang E-H, Chung R-S, Tsai Y-H (2007) Effect of different application rates of organic fertilizer on soil enzyme activity and microbial population. Soil Science and Plant Nutrition 53, 132–140.
| Effect of different application rates of organic fertilizer on soil enzyme activity and microbial population.Crossref | GoogleScholarGoogle Scholar |
Chatterjee D, Datta SC, Manjaiah KM (2014) Fractions, uptake and fixation capacity of phosphorus and potassium in three contrasting soil orders. Journal of Soil Science and Plant Nutrition 14, 640–656.
| Fractions, uptake and fixation capacity of phosphorus and potassium in three contrasting soil orders.Crossref | GoogleScholarGoogle Scholar |
Cleveland CC, Nemergut DR, Schmidt SK, Townsend AR (2007) Increases in soil respiration following labile carbon additions linked to rapid shifts in soil microbial community composition. Biogeochemistry 82, 229–240.
| Increases in soil respiration following labile carbon additions linked to rapid shifts in soil microbial community composition.Crossref | GoogleScholarGoogle Scholar |
Dai Z, Xiong X, Zhu H, et al. (2021) Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 3, 239–254.
| Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes.Crossref | GoogleScholarGoogle Scholar |
Das SK, Varma A (2011) Role of enzymes in maintaining soil health. In ‘Soil enzymology’. (Eds G Shukla, A Varma) pp. 25–42. (Springer: Berlin, Heidelberg)
| Crossref |
De Tender CA, Debode J, Vandecasteele B, D’Hose T, Cremelie P, Haegeman A, Ruttink T, Dawyndt P, Maes M (2016) Biological, physicochemical and plant health responses in lettuce and strawberry in soil or peat amended with biochar. Applied Soil Ecology 107, 1–12.
| Biological, physicochemical and plant health responses in lettuce and strawberry in soil or peat amended with biochar.Crossref | GoogleScholarGoogle Scholar |
Demirbaş A (2001) Biomass to charcoal, liquid, and gaseous products via carbonization process. Energy Sources 23, 579–587.
| Biomass to charcoal, liquid, and gaseous products via carbonization process.Crossref | GoogleScholarGoogle Scholar |
Deng SP, Tabatabai MA (1994a) Cellulase activity of soils. Soil Biology and Biochemistry 26, 1347–1354.
| Cellulase activity of soils.Crossref | GoogleScholarGoogle Scholar |
Depkat-Jakob PS, Hilgarth M, Horn MA, Drake HL (2010) Effect of earthworm feeding guilds on ingested dissimilatory nitrate reducers and denitrifiers in the alimentary canal of the earthworm. Applied and Environmental Microbiology 76, 6205–6214.
| Effect of earthworm feeding guilds on ingested dissimilatory nitrate reducers and denitrifiers in the alimentary canal of the earthworm.Crossref | GoogleScholarGoogle Scholar |
Dominati E, Patterson M, Mackay A (2010) A framework for classifying and quantifying the natural capital and ecosystem services of soils. Ecological Economics 69, 1858–1868.
| A framework for classifying and quantifying the natural capital and ecosystem services of soils.Crossref | GoogleScholarGoogle Scholar |
Eivazi F, Tabatabai MA (1977) Phosphatases in soils. Soil Biology and Biochemistry 9, 167–172.
| Phosphatases in soils.Crossref | GoogleScholarGoogle Scholar |
Firestone MK (1982) Biological denitrification. In ‘Nitrogen in agricultural soils. Vol. 22.’ Agronomy monographs. (Ed. FJ Stevenson) pp. 289–326. (American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America Inc.: Madison, WI, USA)
Gao S, DeLuca TH (2016) Influence of biochar on soil nutrient transformations, nutrient leaching, and crop yield. Advances in Plants & Agriculture Research 4, 348–362.
| Influence of biochar on soil nutrient transformations, nutrient leaching, and crop yield.Crossref | GoogleScholarGoogle Scholar |
Gao L, Wang R, Shen G, Zhang J, Meng G, Zhang J (2017) Effects of biochar on nutrients and the microbial community structure of tobacco-planting soils. Journal of Soil Science and Plant Nutrition 17, 884–896.
| Effects of biochar on nutrients and the microbial community structure of tobacco-planting soils.Crossref | GoogleScholarGoogle Scholar |
Gao S, DeLuca TH, Cleveland CC (2019) Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: a meta-analysis. Science of The Total Environment 654, 463–472.
| Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Garbuz SA, Yaroslavtseva NV, Kholodov VA (2016) Enzymatic activity inside and outside of water-stable aggregates in soils under different land use. Eurasian Soil Science 49, 367–375.
| Enzymatic activity inside and outside of water-stable aggregates in soils under different land use.Crossref | GoogleScholarGoogle Scholar |
Garbuz S, Camps-Arbestain M, MacKay A, DeVantier B, Minor M (2020) The interactions between biochar and earthworms, and their influence on soil properties and clover growth: a 6-month mesocosm experiment. Applied Soil Ecology 147, 103402
| The interactions between biochar and earthworms, and their influence on soil properties and clover growth: a 6-month mesocosm experiment.Crossref | GoogleScholarGoogle Scholar |
Garbuz S, Mackay A, Camps-Arbestain M, DeVantier B, Minor M (2021) Biochar amendment improves soil physico-chemical properties and alters root biomass and the soil food web in grazed pastures. Agriculture, Ecosystems & Environment 319, 107517
| Biochar amendment improves soil physico-chemical properties and alters root biomass and the soil food web in grazed pastures.Crossref | GoogleScholarGoogle Scholar |
Harrison MD (2016) Nutrient dynamics. In ‘Encyclopedia of estuaries’. (Ed. MJ Kennish) pp. 462–463. (Springer: Dordrecht, Netherlands)
| Crossref |
Harter J, El-Hadidi M, Huson DH, Kappler A, Behrens S (2017) Soil biochar amendment affects the diversity of nosZ transcripts: implications for N2O formation. Scientific Reports 7, 3338
| Soil biochar amendment affects the diversity of nosZ transcripts: implications for N2O formation.Crossref | GoogleScholarGoogle Scholar |
Herath HMSK, Camps-Arbestain M, Hedley MJ, Kirschbaum MUF, Wang T, van Hale R (2015) Experimental evidence for sequestering C with biochar by avoidance of CO2 emissions from original feedstock and protection of native soil organic matter. GCB Bioenergy 7, 512–526.
| Experimental evidence for sequestering C with biochar by avoidance of CO2 emissions from original feedstock and protection of native soil organic matter.Crossref | GoogleScholarGoogle Scholar |
Hewitt AE (2010) ‘New Zealand soil classification.’ (Manaaki Whenua Press: New Zealand)
Holík L, Hlisnikovský L, Honzík R, Trögl J, Burdová H, Popelka J (2019) Soil microbial communities and enzyme activities after long-term application of inorganic and organic fertilizers at different depths of the soil profile. Sustainability 11, 3251
| Soil microbial communities and enzyme activities after long-term application of inorganic and organic fertilizers at different depths of the soil profile.Crossref | GoogleScholarGoogle Scholar |
Huang M, Zhou X, Chen J, Cao F, Jiang L, Zou Y (2017) Interaction of changes in pH and urease activity induced by biochar addition affects ammonia volatilization on an acid paddy soil following application of urea. Communications in Soil Science and Plant Analysis 48, 107–112.
| Interaction of changes in pH and urease activity induced by biochar addition affects ammonia volatilization on an acid paddy soil following application of urea.Crossref | GoogleScholarGoogle Scholar |
Irfan M, Hussain Q, Khan KS, Akmal M, Ijaz SS, Hayat R, Khalid A, Azeem M, Rashid M (2019) Response of soil microbial biomass and enzymatic activity to biochar amendment in the organic carbon deficient arid soil: a 2-year field study. Arabian Journal of Geosciences 12, 95
| Response of soil microbial biomass and enzymatic activity to biochar amendment in the organic carbon deficient arid soil: a 2-year field study.Crossref | GoogleScholarGoogle Scholar |
IUSS Working Group WRB (2015) World Reference Base for Soil Resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. FAO, Rome, Italy.
Järvan M, Edesi L, Adamson A, Võsa T (2014) Soil microbial communities and dehydrogenase activity depending on farming systems. Plant, Soil and Environment 60, 459–463.
| Soil microbial communities and dehydrogenase activity depending on farming systems.Crossref | GoogleScholarGoogle Scholar |
Jha N, Palmada T, Berben P, Saggar S, Luo J, McMillan A (2016) Lime enhances denitrification rate and denitrifier gene abundance in pasture soils treated with urine and urine DCD. In ‘Integrated nutrient and water management for sustainable farming’. Occasional Report No. 29. (Eds LD Currie, R Singh) p. 13. (Fertilizer and Lime Research Centre, Massey University: Palmerston North, New Zealand)
Jog R, Nareshkumar G, Rajkumar S (2012) Plant growth promoting potential and soil enzyme production of the most abundant Streptomyces spp. from wheat rhizosphere. Journal of Applied Microbiology 113, 1154–1164.
| Plant growth promoting potential and soil enzyme production of the most abundant Streptomyces spp. from wheat rhizosphere.Crossref | GoogleScholarGoogle Scholar |
Jones DL, Rousk J, Edwards-Jones G, DeLuca TH, Murphy DV (2012) Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biology and Biochemistry 45, 113–124.
| Biochar-mediated changes in soil quality and plant growth in a three year field trial.Crossref | GoogleScholarGoogle Scholar |
Jordanova N (2017) The magnetism of soils distinguished by iron/aluminum chemistry: Planosols, Pozdols, Andosols, Ferralsols, and Gleysols. In ‘Soil magnetism’. (Ed. N Jordanova) pp. 139–220. (Academic Press)
| Crossref |
Joseph S, Husson O, Graber ER, Van Zwieten L, Taherymoosavi S, Thomas T, Nielsen S, Ye J, Pan G, Chia C, Munroe P, Allen J, Lin Y, Fan X, Donne S (2015) The electrochemical properties of biochars and how they affect soil redox properties and processes. Agronomy 5, 322–340.
| The electrochemical properties of biochars and how they affect soil redox properties and processes.Crossref | GoogleScholarGoogle Scholar |
Juma NG, Tabatabai MA (1978) Distribution of phosphomonoesterases in soils. Soil Science 126, 101–108.
| Distribution of phosphomonoesterases in soils.Crossref | GoogleScholarGoogle Scholar |
Khadem A, Raiesi F (2017) Influence of biochar on potential enzyme activities in two calcareous soils of contrasting texture. Geoderma 308, 149–158.
| Influence of biochar on potential enzyme activities in two calcareous soils of contrasting texture.Crossref | GoogleScholarGoogle Scholar |
Kizilkaya R, Karaca A, Turgay OC, Cetin SC (2011) Earthworm interactions with soil enzymes. In ‘Biology of earthworm’. (Ed. A Karaca) pp. 141–158. (Springer: Berlin, Heidelberg)
| Crossref |
Ledgard SF, Menneer JC, Dexter MM, Kear MJ, Lindsey S, Peters JS, Pacheco D (2008) A novel concept to reduce nitrogen losses from grazed pastures by administering soil nitrogen process inhibitors to ruminant animals: a study with sheep. Agriculture, Ecosystems & Environment 125, 148–158.
| A novel concept to reduce nitrogen losses from grazed pastures by administering soil nitrogen process inhibitors to ruminant animals: a study with sheep.Crossref | GoogleScholarGoogle Scholar |
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota — A review. Soil Biology and Biochemistry 43, 1812–1836.
| Biochar effects on soil biota — A review.Crossref | GoogleScholarGoogle Scholar |
Lehmann J, Cowie A, Masiello CA, et al. (2021) Biochar in climate change mitigation. Nature Geoscience 14, 883–892.
| Biochar in climate change mitigation.Crossref | GoogleScholarGoogle Scholar |
Lloyd AB, Sheaffe MJ (1973) Urease activity in soils. Plant and Soil 39, 71–80.
| Urease activity in soils.Crossref | GoogleScholarGoogle Scholar |
Lorenz K, Lal R (2014) Biochar application to soil for climate change mitigation by soil organic carbon sequestration. Journal of Plant Nutrition and Soil Science 177, 651–670.
| Biochar application to soil for climate change mitigation by soil organic carbon sequestration.Crossref | GoogleScholarGoogle Scholar |
Macdonald CA, Delgado-Baquerizo M, Reay DS, Hicks LC, Singh BK (2018) Soil nutrients and soil carbon storage: modulators and mechanisms. In ‘Soil carbon storage’. (Ed. BK Singh) pp. 167–205. (Academic Press)
Mackay AD, Vibart R, McKenzie C, Costall D, Bilotto F, Kelliher FM (2021) Soil organic carbon stocks in hill country pastures under contrasting phosphorus fertiliser and sheep stocking regimes, and topographical features. Agricultural Systems 186, 102980
| Soil organic carbon stocks in hill country pastures under contrasting phosphorus fertiliser and sheep stocking regimes, and topographical features.Crossref | GoogleScholarGoogle Scholar |
Masto RE, Kumar S, Rout TK, Sarkar P, George J, Ram LC (2013) Biochar from water hyacinth (Eichornia crassipes) and its impact on soil biological activity. CATENA 111, 64–71.
| Biochar from water hyacinth (Eichornia crassipes) and its impact on soil biological activity.Crossref | GoogleScholarGoogle Scholar |
Mierzwa-Hersztek M, Gondek K, Klimkowicz-Pawlas A, Chmiel MJ, Dziedzic K, Taras H (2019) Assessment of soil quality after biochar application based on enzymatic activity and microbial composition. International Agrophysics 33, 331–336.
| Assessment of soil quality after biochar application based on enzymatic activity and microbial composition.Crossref | GoogleScholarGoogle Scholar |
Moldenke A, Pajutee M, Ingham E (2000) The functional roles of forest soil arthropods: the soil is a lively place. In ‘Proceedings of the California forest soils council conference on forest soils biology and forest management’. Gen Tech Rep PSW-GTR-178. (USDA Forest Service, Pacific Southwest Research Station: Berkeley, CA, USA)
Molloy L (1998) ‘Soils in the New Zealand landscape: the living mantle.’ 2nd edn. (New Zealand Society of Soil Science: Lincoln, NZ)
Moorhead DL, Lashermes G, Sinsabaugh RL (2012) A theoretical model of C- and N-acquiring exoenzyme activities, which balances microbial demands during decomposition. Soil Biology and Biochemistry 53, 133–141.
| A theoretical model of C- and N-acquiring exoenzyme activities, which balances microbial demands during decomposition.Crossref | GoogleScholarGoogle Scholar |
Moorhead DL, Rinkes ZL, Sinsabaugh RL, Weintraub MN (2013) Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: informing enzyme-based decomposition models. Frontiers in Microbiology 4, 223
| Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: informing enzyme-based decomposition models.Crossref | GoogleScholarGoogle Scholar |
Nakamoto T, Wakahara S (2004) Development of substrate induced respiration (SIR) method combined with selective inhibition for estimating fungal and bacterial biomass in humic andosols. Plant Production Science 7, 70––76.
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. In ‘Phosphorus in action’. Vol. 26. Soil biology. (Eds E Bünemann, A Oberson, E Frossard) pp. 215–243. (Springer)
| Crossref |
Obia A, Cornelissen G, Mulder J, Dörsch P (2015) Effect of soil pH increase by biochar on NO, N2O and N2 production during denitrification in acid soils. PLoS ONE 10, e0138781
| Effect of soil pH increase by biochar on NO, N2O and N2 production during denitrification in acid soils.Crossref | GoogleScholarGoogle Scholar |
Oladele SO (2019) Effect of biochar amendment on soil enzymatic activities, carboxylate secretions and upland rice performance in a sandy clay loam Alfisol of Southwest Nigeria. Scientific African 4, e00107
| Effect of biochar amendment on soil enzymatic activities, carboxylate secretions and upland rice performance in a sandy clay loam Alfisol of Southwest Nigeria.Crossref | GoogleScholarGoogle Scholar |
Ouyang L, Tang Q, Yu L, Zhang R (2014) Effects of amendment of different biochars on soil enzyme activities related to carbon mineralisation. Soil Research 52, 706–716.
| Effects of amendment of different biochars on soil enzyme activities related to carbon mineralisation.Crossref | GoogleScholarGoogle Scholar |
Palansooriya KN, Wong JTF, Hashimoto Y, Huang L, Rinklebe J, Chang SX, Bolan N, Wang H, Ok YS (2019) Response of microbial communities to biochar-amended soils: a critical review. Biochar 1, 3–22.
| Response of microbial communities to biochar-amended soils: a critical review.Crossref | GoogleScholarGoogle Scholar |
Pandian K, Subramaniayan P, Gnasekaran P, Chitraputhirapillai S (2016) Effect of biochar amendment on soil physical, chemical and biological properties and groundnut yield in rainfed Alfisol of semi-arid tropics. Archives of Agronomy and Soil Science 62, 1293–1310.
| Effect of biochar amendment on soil physical, chemical and biological properties and groundnut yield in rainfed Alfisol of semi-arid tropics.Crossref | GoogleScholarGoogle Scholar |
Paz-Ferreiro J, Fu S, Méndez A, Gascó G (2014) Interactive effects of biochar and the earthworm Pontoscolex corethrurus on plant productivity and soil enzyme activities. Journal of Soils and Sediments 14, 483–494.
| Interactive effects of biochar and the earthworm Pontoscolex corethrurus on plant productivity and soil enzyme activities.Crossref | GoogleScholarGoogle Scholar |
Paz-Ferreiro J, Liang C, Fu S, Mendez A, Gasco G (2015) The effect of biochar and its interaction with the earthworm Pontoscolex corethrurus on soil microbial community structure in tropical soils. PLoS ONE 10, e0124891
| The effect of biochar and its interaction with the earthworm Pontoscolex corethrurus on soil microbial community structure in tropical soils.Crossref | GoogleScholarGoogle Scholar |
Pérez-Piqueres A, Edel-Hermann V, Alabouvette C, Steinberg C (2006) Response of soil microbial communities to compost amendments. Soil Biology and Biochemistry 38, 460–470.
| Response of soil microbial communities to compost amendments.Crossref | GoogleScholarGoogle Scholar |
Rao MA, Scelza R, Scotti R, Gianfreda L (2010) Role of enzymes in the remediation of polluted environments. Journal of Soil Science and Plant Nutrition 10, 333–353.
| Role of enzymes in the remediation of polluted environments.Crossref | GoogleScholarGoogle Scholar |
Sajjad MH, Lodhi A, Azam F (2002) Changes in enzyme activity during the decomposition of plant residues in soil. Pakistan Journal of Biological Sciences 5, 952–955.
| Changes in enzyme activity during the decomposition of plant residues in soil.Crossref | GoogleScholarGoogle Scholar |
Sarathchandra SU, Perrott KW, Boase MR, Waller JE (1988) Seasonal changes and the effects of fertiliser on some chemical, biochemical and microbiological characteristics of high-producing pastoral soil. Biology and Fertility of Soils 6, 328–335.
| Seasonal changes and the effects of fertiliser on some chemical, biochemical and microbiological characteristics of high-producing pastoral soil.Crossref | GoogleScholarGoogle Scholar |
Sardans J, Peñuelas J, Estiarte M (2008) Changes in soil enzymes related to C and N cycle and in soil C and N content under prolonged warming and drought in a Mediterranean shrubland. Applied Soil Ecology 39, 223–235.
| Changes in soil enzymes related to C and N cycle and in soil C and N content under prolonged warming and drought in a Mediterranean shrubland.Crossref | GoogleScholarGoogle Scholar |
Shindo H, Watanabe D, Onaga T, Urakawa M, Nakahara O, Huang Q (2002) Adsorption, activity, and kinetics of acid phosphatase as influenced by selected oxides and clay minerals. Soil Science and Plant Nutrition 48, 763–767.
| Adsorption, activity, and kinetics of acid phosphatase as influenced by selected oxides and clay minerals.Crossref | GoogleScholarGoogle Scholar |
Shi W (2011) Agricultural and ecological significance of soil enzymes: soil carbon sequestration and nutrient cycling. In ‘Soil enzymology’. (Eds G Shukla, A Varma) pp. 43–60. (Springer: Berlin, Heidelberg)
| Crossref |
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biology and Biochemistry 42, 391–404.
| Phenol oxidase, peroxidase and organic matter dynamics of soil.Crossref | GoogleScholarGoogle Scholar |
Sinsabaugh RL, Follstad Shah JJ (2012) Ecoenzymatic stoichiometry and ecological theory. Annual Review of Ecology, Evolution, and Systematics 43, 313–343.
| Ecoenzymatic stoichiometry and ecological theory.Crossref | GoogleScholarGoogle Scholar |
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecology Letters 11, 1252–1264.
| Stoichiometry of soil enzyme activity at global scale.Crossref | GoogleScholarGoogle Scholar |
Teutscherova N, Lojka B, Houška J, Masaguer A, Benito M, Vazquez E (2018) Application of holm oak biochar alters dynamics of enzymatic and microbial activity in two contrasting Mediterranean soils. European Journal of Soil Biology 88, 15–26.
| Application of holm oak biochar alters dynamics of enzymatic and microbial activity in two contrasting Mediterranean soils.Crossref | GoogleScholarGoogle Scholar |
Tian L, Shi W (2014) Soil peroxidase regulates organic matter decomposition through improving the accessibility of reducing sugars and amino acids. Biology and Fertility of Soils 50, 785–794.
| Soil peroxidase regulates organic matter decomposition through improving the accessibility of reducing sugars and amino acids.Crossref | GoogleScholarGoogle Scholar |
Vázquez MM, César S, Azcón R, Barea JM (2000) Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants. Applied Soil Ecology 15, 261–272.
| Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants.Crossref | GoogleScholarGoogle Scholar |
Wang C, Anderson C, Suárez-Abelenda M, Wang T, Camps-Arbestain M, Ahmad R, Herath HMSK (2015b) The chemical composition of native organic matter influences the response of bacterial community to input of biochar and fresh plant material. Plant and Soil 395, 87–104.
| The chemical composition of native organic matter influences the response of bacterial community to input of biochar and fresh plant material.Crossref | GoogleScholarGoogle Scholar |
Wang T, Arbestain MC, Hedley M, Bishop P (2012) Chemical and bioassay characterisation of nitrogen availability in biochar produced from dairy manure and biosolids. Organic Geochemistry 51, 45–54.
| Chemical and bioassay characterisation of nitrogen availability in biochar produced from dairy manure and biosolids.Crossref | GoogleScholarGoogle Scholar |
Wang X, Song D, Liang G, Zhang Q, Ai C, Zhou W (2015a) Maize biochar addition rate influences soil enzyme activity and microbial community composition in a fluvo-aquic soil. Applied Soil Ecology 96, 265–272.
| Maize biochar addition rate influences soil enzyme activity and microbial community composition in a fluvo-aquic soil.Crossref | GoogleScholarGoogle Scholar |
Watts DB, Torbert HA, Feng Y, Prior SA (2010) Soil microbial community dynamics as influenced by composted dairy manure, soil properties, and landscape position. Soil Science 175, 474–486.
| Soil microbial community dynamics as influenced by composted dairy manure, soil properties, and landscape position.Crossref | GoogleScholarGoogle Scholar |
Weldon S, Rasse DP, Budai A, Tomic O, Dörsch P (2019) The effect of a biochar temperature series on denitrification: which biochar properties matter? Soil Biology and Biochemistry 135, 173–183.
| The effect of a biochar temperature series on denitrification: which biochar properties matter?Crossref | GoogleScholarGoogle Scholar |
Wolińska A, Stepniewska Z (2012) Dehydrogenase activity in the soil environment. In ‘Dehydrogenases’. (Ed. RA Canuto) pp. 183–210. (InTechOpen: London, UK)
| Crossref |
Yang WQ, Wang KY (2002) Advances on soil enzymology. Chinese Journal of Applied and Environmental Biology 8, 564–570.


