Softening of temperate annual pasture legume hard seeds on the soil surface and with shallow burial at three contrasting sites in southern Australia
P. G. H. Nichols A B * , D. M. Peck
A B * , D. M. Peck  C , A. Stefanski D , B. J. Wintle A B and R. J. Simpson
C , A. Stefanski D , B. J. Wintle A B and R. J. Simpson  D
D
A
B
C
D
Abstract
Self-regenerating annual pasture legumes generally have hard seeds (impermeable to water) at maturity. The extent and timing of hard-seed softening over summer–autumn is crucial for seedling regeneration and long-term persistence.
This study examined diversity for the magnitude and timing of seed softening among annual pasture legumes.
In total, 42 cultivars in 15 species were grown at Perth, Adelaide and Canberra (with 20 common entries). Freshly ripened seeds or pods were either placed in nylon mesh pockets on the soil surface or buried at 2 cm depth and sampled for germination testing every 28 days until early winter.
The rate and extent of seed softening was greatest in Perth and least in Canberra, with Adelaide being intermediate. Considerable diversity was evident among and within species. Subterranean clover (Trifolium subterraneum) had the greatest and most rapid seed softening, whereas annual medic (Medicago spp.) and most yellow serradella (O. compressus) cultivars had the least, with a range of responses in other species. Burial reduced seed softening in subterranean clover, balansa clover (T. michelianum), biserrula (Biserrula pelecinus), arrowleaf clover (T. vesiculosum), eastern star clover (T. dasyurum) and purple clover (T. purpureum), but increased softening in yellow serradella and had little effect on French serradella (O. sativus) and the annual medics. An important finding was significant cultivar × site and cultivar × site × burial treatment interactions.
Residual hardseededness, seed-softening rates and response to shallow burial differ among and within species and are dependent on environment.
There is sufficient diversity to select annual legumes with appropriate hard seed traits for different regions and farming systems. However, cultivar evaluations need to be conducted under local conditions to ensure that they have the intended adaptation.
Keywords: annual legumes, annual pasture legumes, false break tolerance, hard seeds, pastures, seed burial, seed dormancy, seed softening, seeding regeneration, southern Australia.
Introduction
Self-regenerating, winter-growing annual pasture legumes, in symbiosis with appropriate Rhizobium leguminosarum root nodule bacteria, are highly valued in the farming systems of southern Australia, both as a nutritious feed source for livestock and for their ability to fix atmospheric nitrogen (N) (Nichols et al. 2012). This benefits growth of both non-leguminous species in the pasture and of subsequent crops grown in rotation and is particularly important in southern Australia, where the majority of soils have an inherently low N status (Angus 2001; Peoples and Baldock 2001). The climate of southern Australia varies from a strongly Mediterranean climate in south-western Australia, with up to 65% of rain falling in the winter months, to a more temperate climate in south-eastern Australia, with rainfall being distributed more evenly throughout the year (Gentilli 1971). Annual average rainfall (AAR) across the farming zones of southern Australia varies from about 250 to 1200 mm, with the autumn to spring period being suitable for annual legume growth.
The annual pasture legumes used in southern Australia are native to the Mediterranean basin and surrounding areas and are derived from either naturalised populations that have been accidentally introduced, deliberate introduction of germplasm collected from their native habitats, or breeding programs (Nichols et al. 2012; Smith et al. 2021). Different species are used across the diversity of climates, farming systems and soil types of southern Australia. The most widely sown species are subterranean clover (Trifolium subterraneum L.) and annual medics (Medicago species), estimated to have been sown over 29.3 and 24.6 million hectares respectively (Nichols et al. 2012). Subterranean clover consists of three subspecies: (i) ssp. subterraneum, adapted to well-drained soils of pH (CaCl2) 4.5–6.5; (ii) ssp. yanninicum, adapted to poorly drained soils of pH (CaCl2) 4.5–6.5; and (iii) ssp. brachycalycinum, best adapted to cracking or stony soils of pH (CaCl2) 6.0–8.0 (Katznelson and Morley 1965; Nichols et al. 2013). Two distinctive features of subterranean clover that contribute to its success as a pasture plant are (i) its high tolerance of regular, close grazing, attributable to its prostrate growth habit, and (ii) its ability to bury its burrs (particularly ssp. subterraneum and ssp. yanninicum), which protects seeds from predation by stock. Annual medics tend to be better suited to more alkaline soils and have been widely sown in low–medium rainfall areas (250–500 mm annual average rainfall). Nine species have been commercialised, but the most widely sown are barrel medic (Medicago truncatula Gaertn.), strand medic (M. littoralis Rhode ex Loisel) and burr medic (M. polymorpha L. var. brevispina) (Nichols et al. 2012).
Since the 1990s, a wider range of annual pasture legume species have been commercialised (Loi et al. 2005; Nichols et al. 2007, 2012; Howieson et al. 2021). Serradella (Ornithopus species) cultivars have been developed for their particular adaptation to deep, acidic sands, to which subterranean clover and annual medics are poorly adapted, and for their superior tolerance to alumunium (Kidd et al. 2021). The two main species are yellow serradella (O. compressus L.) and French serradella (O. sativus Brot.), with slender serradella (O. pinnatus (Mill.) Druce) being less widely used. The serradellas have deep roots and other morphological features that enable the efficient capture of water and nutrients (Haling et al. 2016; Kidd et al. 2021), allowing biomass accumulation with up to 30% lower phosphorus (P) fertiliser input than for other pasture legumes (Simpson et al. 2014; Sandral et al. 2019).
Cultivars of several alternative clover (Trifolium) species have also been developed for different soil types and farming systems (Loi et al. 2005; Nichols et al. 2007, 2012; Howieson et al. 2021). Dual-purpose grazing and fodder species include balansa clover (T. michelianum Savi.), Persian clover (T. resupinatum L.), arrowleaf clover (T. vesiculosum Savi.), purple clover (T. purpureum Loisel.) (Nichols et al. 2010) and eastern star clover (T. dasyurum C.Presl.) (Loi et al. 2007). Bladder clover (T. spumosum L.) has been developed as a grazing-tolerant species for soils where subterranean clovers are unable to bury their burrs (Loi et al. 2012), whereas gland clover (T. glanduliferum Boiss.) was developed for its resistance to redlegged earth mites (Halotydeus destructor) and aphids. Biserrula (Biserrula pelecinus L.) has also been released as a deep-rooted pasture legume with greater persistence than subterranean clover in ley farming systems on acid soils (Loi et al. 1999).
Temperate annual pasture legume seeds can have two types of dormancy, namely (i) hardseededness, in which an impermeable seed coat prevents imbibition by water (Aitken 1939; Taylor 2005), and (ii) embryo dormancy, which is the failure of the naked embryo to germinate under conditions normally favourable for germination (Baskin and Baskin 2004). Both types of dormancy may occur in the same seed, although the effects of embryo dormancy are apparent only in seeds with permeable seed coats. Of the two forms of dormancy, hardseededness is considered the most important for regulating germination and is essential to long-term persistence in Mediterranean-type climates (Quinlivan 1971; Taylor 2005). Newly ripened seeds of most annual legumes contain a high proportion of hard seeds. Some of these hard seeds soften (become permeable to water) over the summer–autumn period under the influence of high and fluctuating soil-surface temperatures, the proportion being dependent on both genotype and environmental conditions during both seed set and post-ripening (Quinlivan 1961). These soft seeds are available for germination to re-establish the pasture, whereas the residual hard seeds form a seed bank and soften over subsequent summers.
Hardseededness has two ecologically significant roles (Russi et al. 1992; Taylor 2005). First, it spreads germination of the seed pool over several seasons, allowing the species to persist in risky environments for growth and seed production. In natural grasslands and permanent or semi-permanent pastures, it ensures survival of the species when seed production is severely curtailed, owing to factors such as drought, flooding, insect attack or disease infection (Russi et al. 1992). In ley farming systems, hardseededness also ensures self-regeneration of the pasture after a cropping phase (Taylor 2005). Second, the timing of seed softening acts to prevent germination outside the autumn–winter growing season.
Seed coat impermeability develops in the latter stages of seed maturation, caused by the laying down of palisade cells impregnated with layers of phenolics and suberin in the testa (Smỳkal et al. 2014). This is accompanied by the hilum acting as a one-way hygroscopic valve to reduce seed moisture content (Hyde 1954). Seed softening undergoes two stages (Taylor 1981). The first stage relies on high temperatures to degrade the hilum and lens, which pre-conditions the seed for softening and predisposes it to subsequent breakdown. The second stage relies on fluctuating temperatures to render the seed permeable to water, with different species responding to different temperature amplitudes. The physical changes after the pre-conditioning stage are poorly understood, but Zeng et al. (2005) demonstrated that water enters soft seeds either through the lens or through fractures caused by physicochemical changes in the seed coat.
Several studies, including Norman et al. (2002, 2006), Loi et al. (2005), Taylor (2005), Howieson et al. (2021) and Harrison et al. (2024), have shown that hardseededness varies considerably between and within pasture legume species. Subterranean clover and French serradella are among the least hardseeded species of the annual legumes, whereas annual medic species (Crawford et al. 1989) and yellow serradella (Revell et al. 1998, 1999, 2012) are among the most hardseeded. Ecological studies have also shown that subterranean clover genotypes adapted to low and unreliable rainfall environments tend to have higher residual hard seed levels than do those adapted to more favourable rainfall environments (Piano et al. 1996; Nichols et al. 2009a). High levels of hardseededness are also required for adaptation to ley farming systems to ensure pasture regeneration after the cropping phase (Crawford et al. 1989; Taylor et al. 1991; Loi et al. 2005; Taylor 2005; Nichols et al. 2012). Selection for higher hard seed levels has been an important breeding objective for both subterranean clovers (Nichols et al. 2013) and French serradellas (Harrison et al. 2024) aimed at crop rotations, whereas several alternative annual legumes with inherently high hardseededness have been domesticated (Loi et al. 2005; Nichols et al. 2007, 2012; Howieson et al. 2021). In contrast, selection for lower hard seed levels has been conducted in annual medics for denser seedling regeneration in non-crop years (Crawford et al. 1989; Peck and Howie 2012).
The timing of hard seed softening within the summer–autumn period is crucial in determining the chances of successful seedling regeneration. A major weakness of many self-regenerating annual legumes is their susceptibility for germination following false breaks to the season, defined as germination-inducing rainfall events in summer or autumn, followed by seedling death from subsequent drought (Chapman and Asseng 2001). This can result in major seedling losses, with the consequence being weedy, unproductive pastures when the true break of season occurs. Chapman and Asseng (2001) showed that seed softening in subterranean clover in south-western Australia proceeds at a rapid rate over summer until early March, with little further seed softening beyond March. However, some other species, including burr medic (Taylor and Ewing 1996), yellow serradella (Revell et al. 1998), eastern star clover (Loi et al. 2007) and messina (Melilotus siculus ((Turra) Vitman ex. B.D. Jacks)) (Nichols et al. 2009b), typically soften later in the autumn, when follow-up rainfall is more likely, and are consequently likely to suffer fewer seedling losses following false breaks. Genotypic differences for the timing of hard seed softening have been recognised in subterranean clover (Norman et al. 2006; Newell et al. 2023), yellow serradella (Revell et al. 1998; Newell et al. 2023) and several other species (Revell et al. 2012; Howieson et al. 2021; Jeffery et al. 2021; Nutt et al. 2021a: Harrison et al. 2024).
Although subterranean clover is generally well adapted to grazing systems in the high-rainfall permanent pasture regions of south-eastern Australia, it has high phosphorus (P) fertiliser requirements for productivity and persistence (Simpson et al. 2014; Sandral et al. 2019), is sensitive to high soil aluminium (Al) concentrations and low soil pH (Richardson and Simpson 1989) and is susceptible to premature senescence during spring drought (Hayes et al. 2023). Consequently, there is recent interest in alternative annual pasture legumes able to overcome such limitations in these environments (Kidd et al. 2020; Hayes et al. 2023; Newell et al. 2023). Hayes et al. (2023) showed that both yellow and French serradellas were promising alternatives to subterranean clover on shallow, acidic, infertile soils in the high-rainfall Northern, Central and Southern Tableland and alpine Monaro regions of New South Wales (NSW), but persistence differed among cultivars. Newell et al. (2023) showed that French, yellow and slender serradella cultivars had seed softening patterns substantially different from that of subterranean clover at two contrasting sites in southern NSW. Both cultivar and site × cultivar differences were also evident among serradella cultivars.
Shallow seed burial has been found to affect the breakdown of hard seeds. In previous studies, the rate of seed softening was reduced by burial in subterranean clover (Taylor 1984; Taylor and Ewing 1996), was little affected in annual medics (Taylor and Ewing 1996) and was more rapid in some genotypes of yellow serradella (Revell et al. 1998, 1999; Howieson et al. 2021) and biserrula (Loi et al. 1999). This has led to the concept of ‘summer sowing’, whereby unprocessed (predominantly hard) seeds of particular pasture legume cultivars with deferred rapid seed softening are sown in late summer and early autumn (Nutt et al. 2021a). It also has implications for the fate of freshly ripened seeds following trampling by stock.
This paper examines phenotypic diversity for the magnitude and timing of hard seed softening during the summer–autumn period after seed set of 42 commercial annual legume cultivars in 15 species at three contrasting climatic environments across southern Australia. This included a core group of 20 cultivars of varying flowering times at each site to examine environmental effects. The effect of a shallow burial treatment (simulating a shallow cultivation or trampling event) was also examined. There were several reasons for undertaking this study. Patterns of seed softening in subterranean clover have mainly been conducted on early flowering genotypes in the Mediterranean climate of Western Australia (WA) (Norman et al. 2006), along with limited cultivar comparisons in NSW (Howieson et al. 2021; Newell et al. 2023). There is consequently a need to better understand hard seed dynamics of subterranean clover in mid-season and later-flowering cultivars suited to higher-rainfall areas across southern Australia. Because serradellas are being investigated for use in high-rainfall pastures of eastern Australia, their seed softening behaviour in these environments needs to be understood before sowing recommendations can be made to farmers. Little is known about the seed softening behaviour of key annual medic species, particularly newly released cultivars, in different environments. Finally, most studies on summer–autumn seed softening of alternative annual legume species have focussed on cropping systems in the mixed farming areas of WA and NSW (Loi et al. 2005; Howieson et al. 2021; Nutt et al. 2021a; Harrison et al. 2024) and little information is available on their softening behaviour in other environments and their suitability for other farming systems. The following three hypotheses were tested:
Hypothesis 1: the magnitude and timing of seed softening differs among species and genotypes within species;
Hypothesis 2: the rate of seed softening varies with environment; and
Hypothesis 3: cultivars vary in their seed softening response following shallow burial.
Materials and methods
Experimental sites
This experiment was conducted at the following three sites across southern Australia:
Perth, WA, at the University of Western Australia Field Station in Shenton Park, (31.9508°S, 115.7931°E, elevation 20 m ASL);
Adelaide, South Australia (SA) at the University of Adelaide Waite Campus in Urrbrae (34.9652°S, 138.6322°E, elevation 115 m ASL); and
Canberra, Australian Capital Territory (ACT) at the CSIRO Experiment Station in Ginninderra (35.2030°S, 149.0823°E, elevation 600 m ASL).
Shenton Park and Urrbrae are located close to the coast (5 km and 10 km inland respectively), whereas Ginninderra is situated 150 km inland in the Southern Tablelands of NSW/ACT. The soil in Perth was a deep sand (Kandosol), into which loam had been incorporated, the soil in Canberra was a Yellow Chromosol sandy loam, whereas the Adelaide site was a Red Chromosol with a sandy loam topsoil. Soil pH (in CaCl2) of the top 20 cm was 6.0 in Perth, 6.2 in Adelaide and 5.5 in Canberra.
Air temperatures and rainfall records from the nearest Australian Bureau of Meteorology recording station were used to compare site differences. These corresponded to Perth metropolitan (Station 009225) for Perth, Kent Town (Station 023090) for Adelaide and Ginninderra (Station 070350) for Canberra. At each site, a temperature button, with hourly temperature recordings, was placed on the soil surface from the time of seed placement to record soil surface temperatures, whereas a second button was placed 2 cm below the surface from March to measure soil temperatures of buried seed samples. However, technical difficulties meant that only data from Perth were obtained.
Production of seeds for hard seed tests
In total, 42 annual legume cultivars were evaluated. These consisted of 15 subterranean clovers (Trifolium subterraneum), nine other clovers (Trifolium species), 11 serradellas (Ornithopus species), six annual medics (Medicago species) and biserrula (Biserrula pelecinus) (Table 1). Twenty cultivars, referred to in this study as ‘core cultivars’, were gown in common at all three sites, six were grown in both Perth and Canberra and three were grown in both Perth and Adelaide, whereas eight were grown only in Perth, four only in Canberra and one only in Adelaide (Table 1). Seeds for hard seed studies were obtained from plants grown at their respective sites during the 2016 winter–spring growing season. Common seed lots were sown at the three sites. Clover, serradella and biserrula seeds were obtained from the Department of Primary Industries and Regional Development, Western Australia, whereas annual medic seeds were obtained from the South Australian Research and Development Institute.
| Common name | Species | Cultivar | Perth | Adelaide | Canberra | |
|---|---|---|---|---|---|---|
| Subterranean clover | Trifolium subterraneum L. ssp. subterraneum (Katzn. & Morley) Katzn. | Denmark | ✓ | ✓ | ✓ | |
| Goulburn | ✓ | ✓ | ||||
| Losa | ✓ | ✓ | ||||
| Leura | ✓ | ✓ | ✓ | |||
| Narrikup | ✓ | ✓ | ✓ | |||
| Rosabrook | ✓ | ✓ | ||||
| Seaton Park | ✓ | |||||
| Tammin | ✓ | ✓ | ✓ | |||
| Woogenellup | ✓ | ✓ | ✓ | |||
| Subterranean clover | T. subterraneum L. ssp. yanninicum (Katzn. & Morley) Katzn. | Monti | ✓ | ✓ | ✓ | |
| Gosse | ✓ | |||||
| Larisa | ✓ | |||||
| Napier | ✓ | ✓ | ✓ | |||
| Subterranean clover | T. subterraneum L. ssp. brachycalycinum (Katzn. & Morley) Katzn. | Antas | ✓ | ✓ | ✓ | |
| Mintaro | ✓ | ✓ | ✓ | |||
| Arrowleaf clover | T. vesiculosum Savi. | Arrotas A | ✓ | |||
| Cefalu | ✓ | |||||
| Balansa clover | T. michelianum Savi. | Frontier | ✓ | ✓ | ||
| Paradana | ✓ | ✓ | ✓ | |||
| Bladder clover | T. spumosum L. | AGWEST Bartolo B | ✓ | ✓ | ✓ | |
| Eastern star clover | T. dasyurum C.Presl | AGWEST Sothis C | ✓ | |||
| Gland clover | T. glanduliferum Boiss. | Prima | ✓ | |||
| Persian clover | T. resupinatum L. var. resupinatum | Nitro Plus | ✓ | |||
| Purple clover | T. purpureum Loisel. | Electra | ✓ | |||
| Barrel medic | Medicago truncatula Gaertn. | Jester | ✓ | ✓ | ||
| Paraggio | ✓ | ✓ | ✓ | |||
| Burr medic | M. polymorpha L. | Santiago | ✓ | ✓ | ||
| Scimitar | ✓ | ✓ | ✓ | |||
| Strand medic | M. littoralis Rhode ex Loisel. | Herald | ✓ | ✓ | ✓ | |
| Seraph | ✓ | |||||
| French serradella | Onithopus sativus Brot. | Cadiz | ✓ | ✓ | ✓ | |
| Erica | ✓ | |||||
| Margurita | ✓ | ✓ | ✓ | |||
| Serratas | ✓ | ✓ | ||||
| Yellow serradella | O. compressus L. | Avila | ✓ | ✓ | ||
| Charano | ✓ | |||||
| Santorini | ✓ | ✓ | ✓ | |||
| SerraMax D | ✓ | ✓ | ✓ | |||
| Yelbini | ✓ | |||||
| Yellotas | ✓ | ✓ | ✓ | |||
| Slender serradella | O. pinnatus (Mill.) Druce | Jebala | ✓ | ✓ | ||
| Biserrula | Biserrula pelecinus L. | Casbah | ✓ | ✓ | ✓ |
Scarified seeds were sown into Petri dishes moistened with filter paper and placed in a 15°C temperature cabinet for 3 days. Sowing dates in 2016 were 27 June at Perth and 29 June at Adelaide. Seeds were initially sown on 30 June at Canberra, but poor germination of some cultivars resulted in a subsequent sowing on 22 July, with a further sowing of O. compressus cv. Avila on 8 August. The late sowing time was aimed at compressing flowering time differences to allow sufficient time for late-flowering varieties to set seed before the early flowering varieties senesced. Germinants with visible radicles were transplanted into peat Jiffy pots in a glasshouse and the appropriate Rhizobium strains for each species were applied by watering on a slurry of peat inoculum (Group C for Trifolium species, Group AM for Medicago polymorpha and M. truncatula, Group AL for M. littoralis, Group S for Ornithopus species and Group Bis for B. pelecinus). Seedlings were watered daily and transplanted to the field 5 weeks after germination. In Canberra, all available seedlings from the 30 June sowing were transplanted with additional seedlings, where needed, from the 28 July sowing and from the 8 August sowing for cv. Avila.
Trial design and management
Weed-free seed beds were prepared by cultivation and application of glyphosate herbicide at label rates. Each trial had a randomised block design with three replicates. To minimise weed growth, seedlings were transplanted into 10 cm diameter holes cut into 1200 mm wide × 30 μm thick strips of white plastic mulch film (Integrated Packaging, Resevoir, Victoria, Australia) in Perth and into black woven polypropylene matting (Permathene Noweed Premium Weed Mat, Castle Hill, New South Wales, Australia) in Adelaide and Canberra. Plots consisted of 20 plants. In Perth and Canberra, they consisted of two rows of 10 plants, whereas plots in Adelaide consisted of four rows of five plants. Plant spacing in each direction within plots was 20 cm, with plots separated by 1 m. In Perth, single superphosphate with potash (6.8% P, 12.4% K and 8.3% S) was drilled in just prior to transplanting at a rate of 180 kg/ha and was also applied by hand at the same rate on 15 September. However, the sites at Canberra and Adelaide were not fertilised, because of high-fertility conditions following crop experiments. No symptoms of any nutrient deficiencies were observed at any of the sites. The sites were hand-weeded throughout the experiment, and Bifenthrin (100 mL/ha) was applied on 9 September to control redlegged earth mites (Halotydeus destructor) at Canberra.
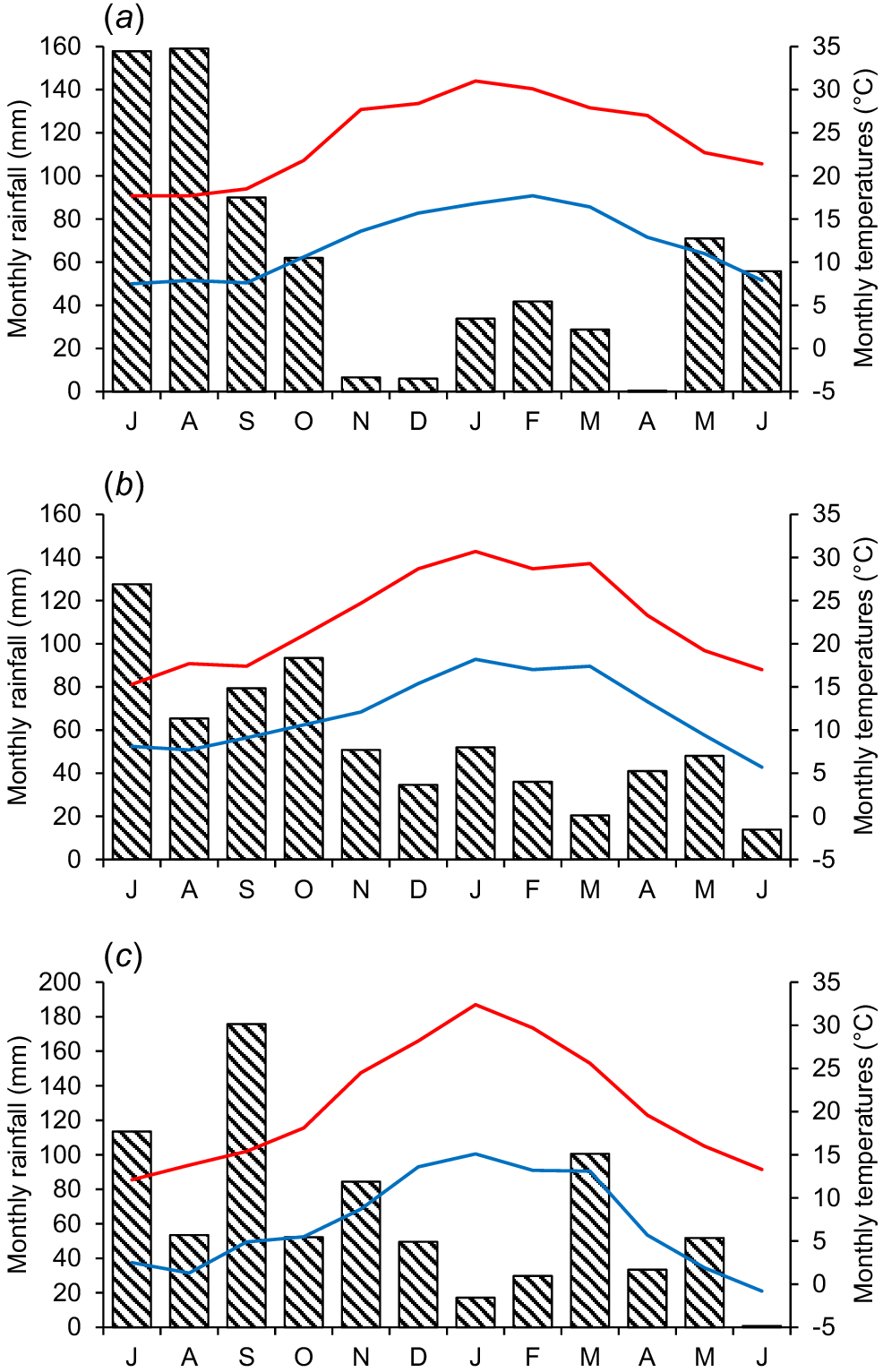
Sites were rainfed, but the Perth trial had supplementary irrigation from 10 October until 23 November, to enable later-flowering cultivars to set seed. Monthly rainfall and temperature data for the three sites from 1 July 2016 to 30 June 2017 are shown in Fig. 1.
Monthly rainfall (mm) and temperature (°C) data for (a) Perth, (b) Adelaide, and (c) Canberra. Rainfall for the experimental period of 1 July 2016–30 June 2017 is shown as bars. Mean monthly maximum and minimum air temperatures during the same period are shown as red and blue lines, respectively. Data from the closest Australian Bureau of Meteorology stations: Perth metropolitan (Station 009225) for Perth; Kent Town (Station 023090) for Adelaide and Ginninderra (Station 070350) for Canberra.
To enable subterranean clovers to bury their burrs for normal seed development (Collins et al. 1976), the plastic was cut away from subterranean clover plots prior to flowering in Perth, whereas a 2 cm layer of weed-free sand was applied on top of the polypropylene matting in Adelaide and Canberra. The plastic strips or polypropylene matting remained in place for other species.
Flowering and senescence
Days to first flowering (DFF) was recorded as the number of days from germination to when 50% of plants in each plot had at least one open flower. Plants were checked every 3–4 days. Time to senescence was also recorded at Perth, determined as the date when no green leaves, petioles or stems remained in the plot. This allowed the reproductive period to be calculated as the difference between the number of days from first flowering to senescence.
Seed harvesting and processing
Fruits (burrs, pods or seed heads) were harvested when plants were fully senesced. In Perth, plants were harvested on 3 January, except for Cefalu arrowleaf clover and Electra purple clover, which did not fully senesce until 12 January, and Arrotas arrowleaf clover, which was too late flowering to produce seeds. Plants in Adelaide were also harvested on 3 January, whereas plants were harvested in Canberra on 31 January. Sampling was focussed on fruits closest to the crowns, because these were considered likely to be the most mature. Sufficient fruits were harvested from each plot to provide 700 seeds for testing.
Prior to seed processing, fruits were placed in an oven at 35°C overnight for easier seed extraction. Soil particles were first gently removed by sieving. The seeds of most species were readily extracted from fruits by gentle rubbing onto ribbed rubber matting. Debris was then removed by sieving to give a clean seed sample. However, whole pods of serradellas and barrel medics were used for hard seed testing, because seeds were too difficult to extract from their pods without causing significant scarification or damage. Samples of harvested material were stored at ambient room temperatures before and after seed processing.
Seed samples for hard seed testing
Seed samples from each plot were divided into seven lots of 100 seeds to allow the following seven sampling times: (i) a sample to determine initial hardseededness at field ripeness; (ii) five sampling times every 28 days over the summer–autumn period (from February to June); and (iii) a sample buried 2 cm below the surface from February to June. Serradella pod segments (each containing a single seed) were counted and allocated similarly to each sampling time. For barrel medics, 15 pods were allocated to each treatment; this was shown to be sufficient for at least 100 seeds.
Seed and pod samples for hard seed testing were placed inside 60 mm × 60 mm pockets of nylon insect screen (0.88 mm mesh) formed using a laboratory heat sealer, using the procedure of Revell et al. (1998). Pockets were arranged in strips in the same order as the seed production plots and the edges were heat sealed to prevent seed escape. The strips of pockets were then pinned flat to the soil surface in full sunlight on a patch of cleared soil. In this way seeds and pods formed a single layer with good soil contact. For the buried treatment, one seed lot was buried to 2 cm at the same time the February sample was measured, following prior exposure on the soil surface. This was aimed at simulating shallow cultivation or trampling by stock.
Germination tests
An initial germination test was conducted at the time when samples were placed in the field. Remaining samples were removed from the field for germination testing every 28 days over the summer–autumn period. Sampling dates at each site were 23 February, 23 March, 20 April, 18 May and 15 June. Particular focus was given to two sampling times. From the Chapman and Asseng (2001) analyses of false break occurrences in south-western Australia, 23 March was used as the date when seeds were considered to be most prone to germination from a false break. The final sampling date of 15 June was used to determine the proportion of residual hard seeds that remained in the seed bank as dormant seeds for germination in future years.
Germination tests were conducted according to standards of the International Seed Testing Association (ISTA 2025). Seed and pod samples were placed in Petri dishes (10 cm diameter) containing a sheet of Whatman No. 1 filter paper moistened with 3.5 mL of filtered water. Petri dishes were placed inside sealed ziplock plastic bags (325 mm × 230 mm) in the dark at a constant temperature of 15°C for 14 days. Preliminary counts were made 7 days after wetting up, with a second count after 14 days.
For the initial (freshly harvested) sample, seeds were classified into the following categories: (i) germinated (seed imbibed with an emerged radical at least 2 mm long); (ii) hard (seed not imbibed); (iii) dormant (seed imbibed with no emerged radical but firm when prodded with forceps); or (iv) dead (seed imbibed with no emerged radical but soft when prodded with forceps, often accompanied by fungal attack). However, the procedure differed for barrel medics and serradellas, whereby germinated seeds erupting from pods were first counted. Barrel medic pods were then manually opened and the remaining seeds inside were counted and classified as above. For serradellas, hard seeds were distinguished from soft seeds by prodding pod segments with forceps. It was assumed that all soft, ungerminated serradella seeds were dead, because they could not be distinguished from dormant seeds. Only hard seeds were counted for subsequent samples removed from the field, because some germination of softened seeds occurred following rainfall events over the summer–autumn period. Hard seeds remaining after 14 days of imbibition were taken as the measure of hardseededness for all genotype, time and site comparisons.
Statistical analyses
Two-way ANOVAs for percentage hard seed were conducted for each site, with cultivar and sampling time as main effects. Two-way ANOVAs were also conducted to compare days to first flowering and percentage of hard seed remaining on 23 March (to simulate the effect of a false break) and on 15 June (to estimate residual hard seeds remaining in the seed bank) for the 20 core cultivars common to the three sites, using cultivar and site as main effects. Additional entries common to Perth and Adelaide and to Perth and Canberra were also compared in this way. To compare the effects of burr burial on residual hard seeds remaining on 15 June, a three-way ANOVA across the 20 core cultivars common to the three sites was conducted, with site, burial treatment and cultivar as factors. Differences among means and the interactions among variables were assessed using least significant differences (P = 0.05). All analyses were conducted using Genstat (ver. 23.1, VSN International Ltd).
Results
Climatic conditions
Rainfall received during 1 July−20 November, corresponding to the period of growth following transplantation, flowering and seed set, was similar at the three sites, with 475 mm at Perth, 417 mm at Adelaide and 455 mm at Canberra (Fig. 1). This was non-limiting for growth and seed set, particularly with supplementary November irrigation in Perth. Rainfall post-seed set from 1 December to 30 April differed markedly among the sites, being 111 mm at Perth, 184 mm at Adelaide and 265 mm at Canberra (Fig. 1).
Canberra was considerably cooler than Perth and Adelaide during the 2016 winter and spring growing season (July–November), reflecting its higher altitude (Fig. 1). This was most pronounced for mean minimum winter and spring temperatures at Canberra, which were 1.9°C and 6.4°C respectively, compared with 7.7°C and 10.6°C respectively, at Perth and 7.9°C and 10.6°C respectively, at Adelaide. Mean maximum temperatures at Canberra during these periods were also 3–4°C cooler than those at the other two sites (Fig. 1).
Mean summer (December 2016–February 2017) maximum temperatures following seed ripening were similar at the three sites, but the mean summer minimum temperature at Canberra (14.0°C) was ~3°C cooler than both Perth and Adelaide. Mean autumn (March–May) maximum temperatures in 2017 varied across the sites, being 25.9°C at Perth, 23.9°C at Adelaide and 20.4°C at Canberra (Fig. 1). The mean autumn minimum temperature at Canberra of 6.9°C was also considerably lower than that in Perth and Adelaide (both 13.4°C). The soil surface at Perth had a 1.6°C higher mean temperature than at 2 cm depth from 1 March to 13 June 2017 (Table 2). The surface temperature also had a higher amplitude, with a 20.1°C difference between the mean maximum and mean minimum temperatures, compared with 16.3°C at 2 cm depth (Table 2).
| Location | Overall maximum (°C) | Overall minimum (°C) | Mean maximum (°C) | Mean minimum (°C) | Overall mean (°C) | |
|---|---|---|---|---|---|---|
| Surface | 51.0 | 5.0 | 33.3 | 13.2 | 24.5 | |
| 2 cm depth | 45.5 | 5.5 | 30.9 | 14.6 | 22.9 |
Flowering and seed set
Days from sowing to first flowering at Perth, Adelaide and Canberra are shown in Table 3. There was a highly significant (P < 0.001) cultivar effect for flowering times at each site, with a large range among and within species. The annual medics had the earliest flowering cultivars at each site, whereas Tammin and Leura were the earliest and latest flowering subterranean clovers respectively. Cadiz and Margurita were the earliest flowering French serradellas, whereas Serratas was the latest. Of the yellow serradellas, Yelbini was the earliest flowering cultivar at Perth and SerraMax the earliest flowering at Canberra and Adelaide, whereas Avila was the latest flowering at Perth and Canberra. Of the other species, gland clover at Perth and Frontier balansa clover at Perth and Adelaide were the earliest flowering. However, Arrotas arrowleaf clover did not flower at Perth until 7 December (37 days later than did Leura subterranean clover) and failed to set seed. It was consequently excluded from hard seed screening. Senescence dates and calculated reproductive periods at Perth are shown in Supplementary Table S1.
| Species | Cultivar | Perth | Adelaide | Canberra | |
|---|---|---|---|---|---|
| Subterranean clover (ssp. subterraneum) | Denmark | 123.0 (0.0) | 124.3 (0.6) | 131.7 (3.1) | |
| Goulburn | 124.3 (1.5) | A | 137.0 (5.3) | ||
| Leura | 126.3 (1.5) | 130.0 (1.0) | 143.0 (0.0) | ||
| Losa | 95.3 (0.6) | A | 119.0 (7.2) | ||
| Narrikup | 113.0 (2.6) | 114.0 (1.0) | 126.3 (5.5) | ||
| Rosabrook | 120.7 (0.6) | A | 133.0 (8.7) | ||
| Seaton Park | A | A | 110.0 (2.0) | ||
| Tammin | 87.7 (0.6) | 89.0 (0.0) | 113.0 (5.7) | ||
| Woogenellup | 113.7 (3.1) | 120.3 (0.6) | 119.7 (5.0) | ||
| Subterranean clover (ssp. yanninicum) | Gosse | A | A | 120.3 (2.3) | |
| Larisa | A | A | 130.0 (3.5) | ||
| Monti | 100.7 (1.5) | 100.7 (1.2) | 120.7 (4.2) | ||
| Napier | 119.7 (0.6) | 122.7 (1.5) | 128.0 (1.0) | ||
| Subterranean clover (ssp. brachycalycinum) | Antas | 121.3 (2.3) | 122.3 (0.6) | 128.7 (0.6) | |
| Mintaro | 101.7 (2.3) | 99.3 (0.6) | 118.3 (4.9) | ||
| Arrowleaf clover | Arrotas | 163.3 (4.7) | A | A | |
| Cefalu | 124.7 (2.1) | A | A | ||
| Balansa clover | Frontier | 95.7 (3.8) | 97.0 (3.5) | A | |
| Paradana | 107.7 (2.5) | 110.7 (1.5) | 111.0 (2.0) | ||
| Bladder clover | Bartolo | 104.3 (2.1) | 108.3 (1.5) | 118.0 (6.1) | |
| Eastern star clover | Sothis | 104.7 (1.5) | A | A | |
| Gland clover | Prima | 97.7 (2.5) | A | A | |
| Persian clover | Nitro Plus | 112.0 (2.6) | A | A | |
| Purple clover | Electra | 131.7 (1.5) | A | A | |
| Barrel medic | Jester | 88.3 (2.3) | 87.0 (0.0) | A | |
| Paraggio | 93.0 (1.0) | 88.0 (0.0) | 88.3 (2.9) | ||
| Burr medic | Santiago | 74.0 (1.0) | 77.3 (1.5) | A | |
| Scimitar | 77.0 (2.6) | 79.3 (0.6) | 84.0 (0.0) | ||
| Strand medic | Herald | 79.7 (0.6) | 77.3 (1.5) | 77.0 (0.0) | |
| Seraph | A | 93.7 (2.3) | A | ||
| French serradella | Cadiz | 103.3 (1.2) | 104.7 (0.6) | 123.7 (1.5) | |
| Erica | A | A | 127.0 (1.7) | ||
| Margurita | 103.7 (2.5) | 102.3 (2.5) | 120.7 (2.5) | ||
| Serratas | 123.0 (1.0) | A | 136.0 (2.6) | ||
| Yellow serradella | Avila | 132.3 (3.5) | A | 136.7 (4.9) | |
| Charano | 92.0 (0.0) | A | A | ||
| Santorini | 99.7 (2.1) | 99.3 (0.6) | 113.7 (4.0) | ||
| SerraMax | 91.7 (0.6) | 95.3 (2.1) | 114.0 (15.6) | ||
| Yelbini | 79.7 (1.5) | A | A | ||
| Yellotas | 118.3 (2.1) | 117.7 (1.5) | 119.0 (12.3) | ||
| Slender serradella | Jebala | 107.5 (0.7) | A | 126.0 (7.0) | |
| Biserrula | Casbah | 105.7 (0.6) | 100.3 (1.5) | 113.7 (3.8) | |
| Cultivar differences within sites (all cultivars sown) | P < 0.001 | P < 0.001 | P < 0.001 | ||
| l.s.d. values (5%) within sites (all cultivars sown) | 3.35 | 2.30 | 8.71 | ||
| Site differences (20 common cultivars) | P < 0.001; l.s.d. (5%) 1.32 | ||||
| Site × cultivar interactions (20 common cultivars) | P < 0.001; l.s.d. (5%) 5.73 | ||||
A two-way ANOVA for flowering time of the 20 core cultivars common to each site showed a highly significant (P < 0.001) site effect, with a mean flowering time of 105 days from sowing for both Perth and Adelaide and 116 days for Canberra. Cultivar and site × cultivar interactions were both highly significant (P < 0.001) (Table 3).
Initial hard seed levels
The hard seed level at field ripening of Cadiz French serradella was ≤0.3% at all three sites and for Serratas French serradella it was ≤6.5% at both Perth and Canberra. At Perth, all other varieties had >85% initial hard seed levels, apart from Avila yellow serradella (73.8%), whereas at Adelaide, all other varieties had >80% initial hard seed levels, apart from Margurita French serradella (33.0%) and Yellotas yellow serradella (37.7%). At Canberra, initial hard seed levels (measured in February) of all other varieties were >80%, apart from Erica French serradella (32.0%), subterranean clovers Seaton Park (66.7%), Monti (71.7%), Gosse (72.7%) and Larisa (75.3%), and Herald strand medic (78.7%).
Hard seed softening over summer–autumn on the soil surface
Graphs of hard seed softening on the soil surface over the summer–autumn period in Perth, Adelaide and Canberra are shown in Figs 2, 3, and 4 respectively.
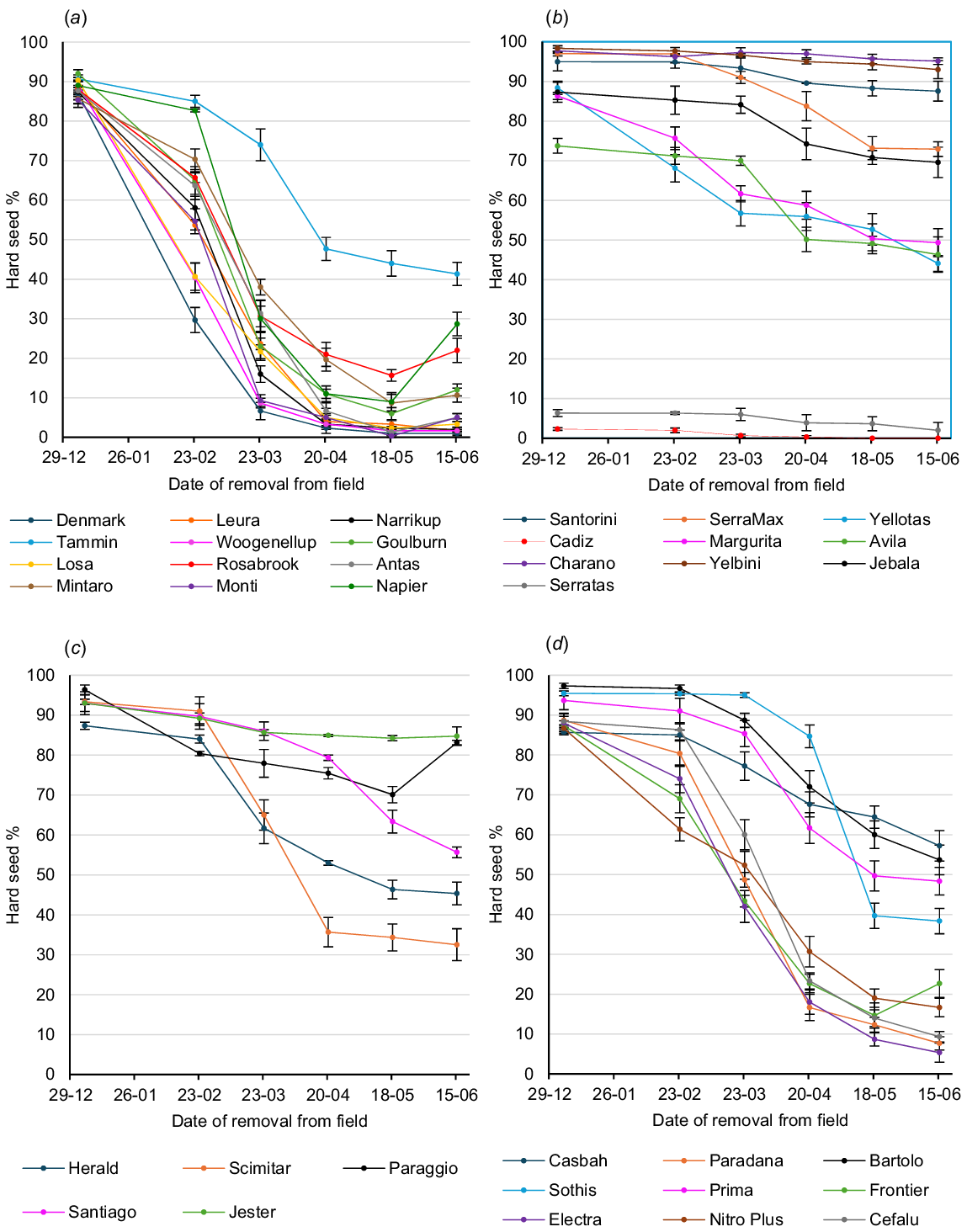
Hard seed softening of annual legume cultivars on the soil surface over the summer–autumn period at Perth: (a) subterranean clovers (b) serradellas; (c) annual medics; and (d) other species. Values are means of three replicates. Error bars represent standard errors. Cultivar effect across all sampling times: P < 0.001; l.s.d. = 2.7; sample time effect across all cultivars: P < 0.001; l.s.d. = 1.1; cultivar × sample time interaction: P < 0.001; l.s.d. = 6.5.
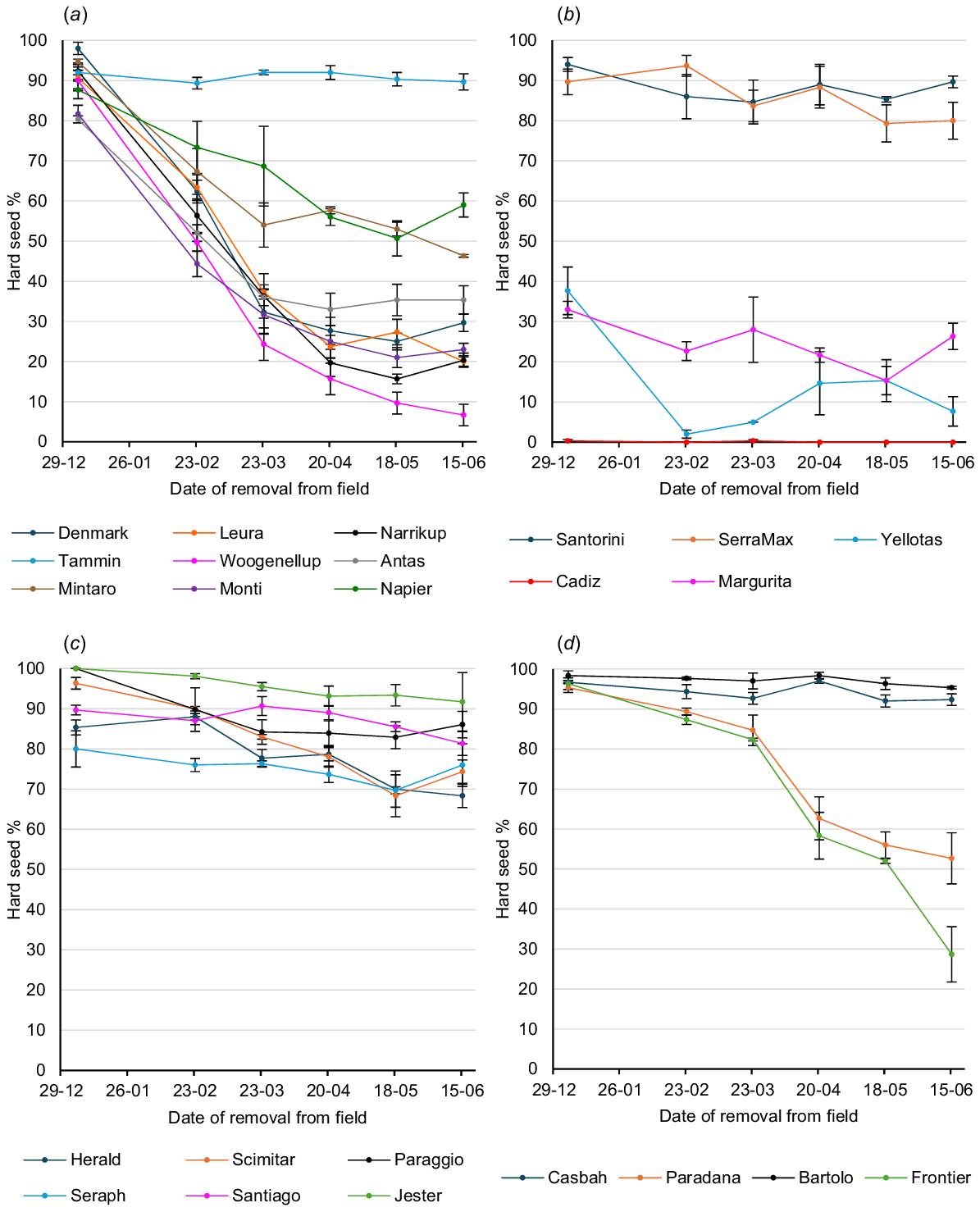
Hard seed softening of annual legume cultivars on the soil surface over the summer–autumn period at Adelaide: (a) subterranean clovers (b) serradellas; (c) annual medics; and (d) other species. Values are means of three replicates. Error bars represent standard errors. Cultivar effect across all sampling times: P < 0.001; l.s.d. = 4.5; sample time effect across all cultivars: P < 0.001; l.s.d. = 2.3; cultivar × sample time interaction: P < 0.001; l.s.d. = 11.0.
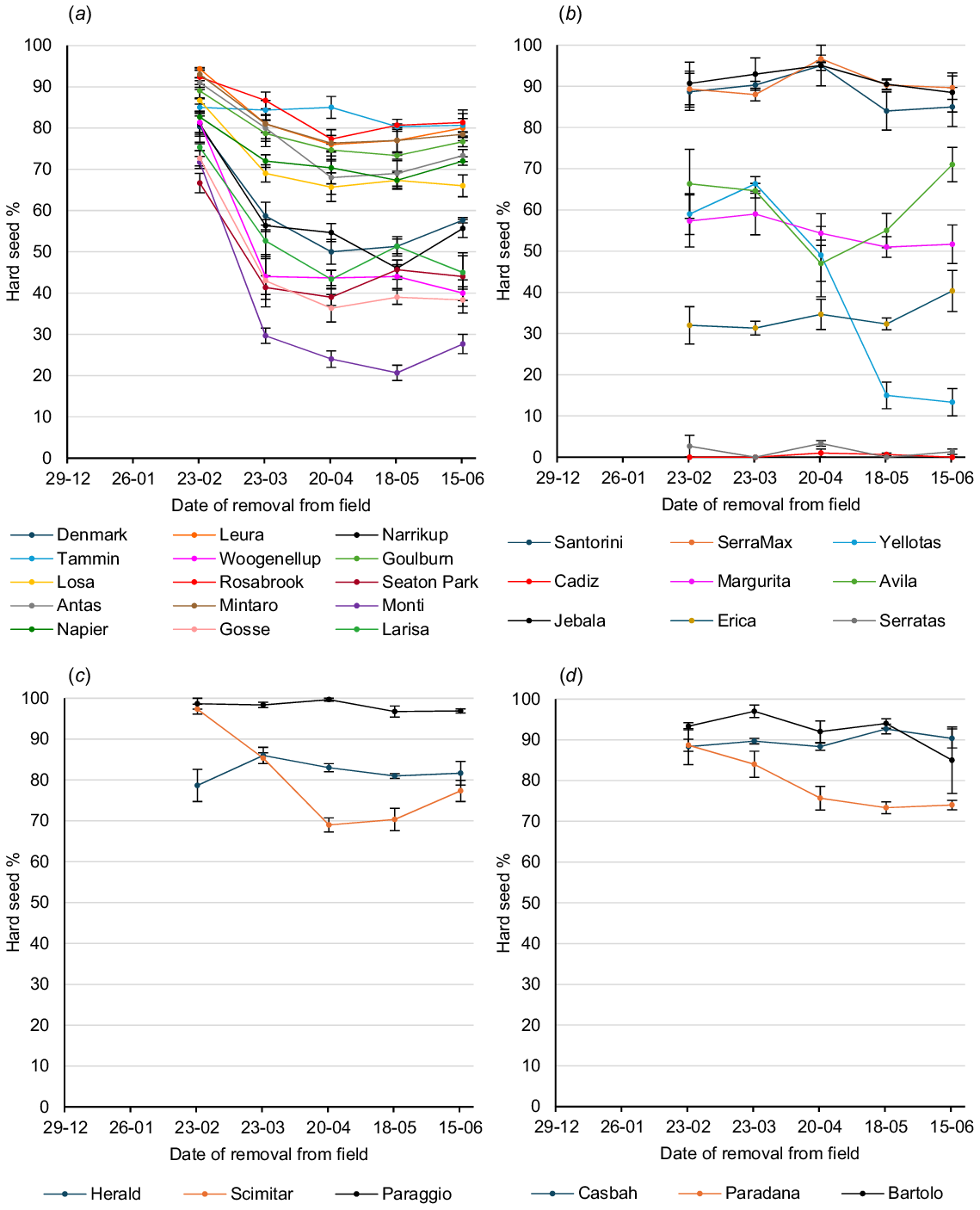
Hard seed softening of annual legume cultivars on the soil surface over the summer–autumn period at Canberra: (a) subterranean clovers (b) serradellas; (c) annual medics; and (d) other species. Values are means of three replicates. Error bars represent standard errors. Cultivar effect across all sampling times: P < 0.001; l.s.d. = 4.3; sample time effect across all cultivars: P < 0.001; l.s.d. = 1.8; cultivar × sample time interaction: P < 0.001; l.s.d. = 9.7.
Highly significant (P < 0.001) site differences were found for hard seed level (as a percentage of the original seed set) of the 20 cultivars common to all sites at each sampling period in February, March, April, May and June. Overall seed softening over summer was most rapid at Perth and least rapid at Canberra, with Adelaide being intermediate. On 23 March, mean hard seed level of the 20 core cultivars was 48.0% at Perth, 56.7% at Adelaide and 71.6% at Canberra. Further seed softening in the following 28 days at Perth reduced the mean hard seed level on 20 April to 35.6%, whereas further seed softening at Adelaide and Canberra reduced hard seed levels only to 53.1% and 67.6% respectively. Very little softening occurred subsequently at each site, so that on 15 June, mean hard seed levels were 31.5% at Perth, 50.1% at Adelaide and 64.0% at Canberra.
Highly significant (P < 0.001) cultivar differences for hard seed content across the three sites were found at each sampling period in February, March, April, May and June among the 20 core cultivars.
On 23 March, Bartolo bladder clover, Santorini and SerraMax yellow serradellas, Paraggio barrel medic and Casbah biserrula had mean hard seed levels across the three sites greater than 85%, whereas Monti and Woogenellup subterranean clovers had less than 30% and Cadiz French serradella had a mean of just 0.3%. At the winter sampling of 15 June, the most hardseeded across sites were Paraggio barrel medic (88.7%), Santorini (87.4%) and SerraMax (80.9%) yellow serradellas, and Casbah biserrula (80.0%). Conversely, Yellotas yellow serradella (21.7%) and Woogenellup (16.1%), Monti (18.6%) and Narrikup (26.0%) subterranean clovers were the least hardseeded, apart from Cadiz French serradella (0.0%).
Among the species groups, subterranean clover had the greatest extent of seed softening over the summer–autumn period. By 23 March, mean hard seed level across the nine core cultivars was 45.8%. Softening continued over the next 4 weeks to give a mean hard seed level of 37.1% on 20 April, and negligible softening occurred thereafter; so, that the mean hard seed level on 15 June was 36.3%. Tammin softened least of the subterranean clover cultivars, with hard seed levels of 83.4% and 70.6% on 23 March and 15 June respectively. Core cultivars of the other species, apart from Cadiz French serradella, were all more hardseeded than the core subterranean clovers on both 23 March and 15 June. Each species group showed cultivar variation. Paraggio barrel medic had a higher hard seed level than did Herald strand medic and Scimitar burr medic on both 23 March and 15 June. Yellow serradella Yellotas and French serradella Margurita had lower hard seed levels than did yellow serradella cultivars Santorini and SerraMax on both 23 March and 15 June, although this result was affected by the very low initial hard seed levels of Yellotas and Margurita at Adelaide. Among the alternative species, Paradana balansa clover had much lower hard seed levels than did Bartolo bladder clover and Casbah biserrula on both 23 March and 15 June.
Highly significant (P < 0.001) site × cultivar interactions were also found on each of the February–June sampling times for the 20 core cultivars and three sites. Examples of this can be shown on 23 March, where Paradana balansa clover was ranked fourth for hard seed level at Adelaide, but 9th and 11th at Canberra and Perth respectively, whereas Margurita French serradella was ranked ninth at Perth, but 15th and 17th respectively, at Canberra and Adelaide. Similar examples were found on 15 June, where Bartolo bladder clover was ranked first for hard seed level at Adelaide, but 5th and 12th at Perth and Canberra respectively, Margurita French serradella was ranked sixth at Perth, but 14th and 16th at Adelaide and Canberra respectively, Yellotas yellow serradella was ranked eighth at Perth, but 18th and 19th at Adelaide and Canberra respectively, and Leura subterranean clover was ranked seventh at Canberra, but 17th at both Adelaide and Perth.
Highly significant (P < 0.001) cultivar effects for hard seed levels of the additional six cultivars sown jointly at Perth and Canberra (Avila yellow serradella, Jebala slender serradella, Serratas French serradella and Goulburn, Losa and Rosabrook subterranean clovers) were found for the February–June sampling periods. Highly significant (P < 0.001) site effects and cultivar × site interactions were also found at each sampling time. Seed softening over summer for the six cultivars was again more rapid at Perth than Canberra, with mean hard seed levels on 23 March of 39.2% at Perth and 65.3% at Canberra. As with the 20 core cultivars, further seed softening occurred at Perth in the following 4 weeks (mean of 27.6%), with little subsequent softening thereafter, while little seed softening occurred at Canberra after 23 March. This resulted in mean residual hard seed levels on 15 June for the six cultivars of 25.9% at Perth and 64.1% at Canberra.
Cultivar Losa was the least hardseeded of the three subterranean clovers at both Perth and Canberra. At Perth, hard seed levels declined rapidly from an initial 90.3% on 4 January to 40.7% by 23 February. By 23 March, the hard seed levels of Losa, Goulburn and Rosabrook had declined to 21.7%, 23.0% and 30.7% respectively, whereas further softening resulted in residual hard seed levels on 15 June of 3.3% for Losa, 12.0% for Goulburn and 22.0% for Rosabrook. In Canberra, the ranking of subterranean clover hard seed levels was the same as for Perth on both 23 March and 15 June. However, mean hard seed levels at Canberra were much higher, being 78.1% on 23 March and 74.7% on 23 June. Among the serradellas, Serratas French serradella had only 6.4% of its initial sample as hard seed when measured in Perth on 4 January and 6.3% in Canberra on 23 February and less than 2% remained as residual hard seeds at both sites on 15 June. This contrasted with Jebala slender serradella and Avila yellow serradella, which still had 88.5% and 71.0% respectively, of hard seeds on 15 June at Canberra and 69.6% and 46.3% respectively, at Perth. Most seed softening of Avila occurred in the 28 days after 23 March at both sites. This was also apparent for Jebala in Perth, but not in Canberra.
Highly significant (P < 0.001) cultivar effects for hard seed levels of the additional three cultivars sown jointly at Perth and Adelaide (Jester barrel medic, Santiago burr medic and Frontier balansa clover) were found at each sampling period from February to June. Site effects were highly significant (P < 0.001) for samples from February to May, but not significant on 15 June. Cultivar × site interactions were highly significant (P < 0.001) in the February–April sampling times, weakly significant (P < 0.05) on 18 May and not significant on 15 June. As for the 20 core cultivars, seed softening over summer was less rapid at Adelaide than at Perth, with mean hardseededness for the three cultivars of 89.5% at Adelaide and 71.7% at Perth on 23 March and 67.2% at Adelaide and 54.4% at Perth on 15 June. The two annual medics were markedly more hardseeded than was cv. Frontier on each sampling date. On 15 June, hard seed levels of Jester and Santiago at Adelaide were 91.7% and 81.3% respectively, whereas at Perth they were 84.7% and 55.7% respectively. This contrasts with Frontier balansa clover with 28.7% at Adelaide and 22.7% at Perth. However, of note was the rapid hard seed decline of cv. Frontier to 43.3% by 23 March in Perth, whereas its hard seed level at Adelaide was 82.3%.
Seed softening of buried seeds
Comparisons of the residual hard seeds on 15 June of all cultivars at each site, following placement either on the surface or buried at 2 cm depth, are shown in Fig. 5.
Hard seed levels of annual legume seeds on 15 June following seed softening over the summer–autumn period either placed on the soil surface (blue bars) or buried at 2 cm depth (orange bars) at (a) Perth, (b) Adelaide, or (c) Canberra. Values are means of three replicates. Error bars represent standard errors. Unsown cultivars are designated by x.
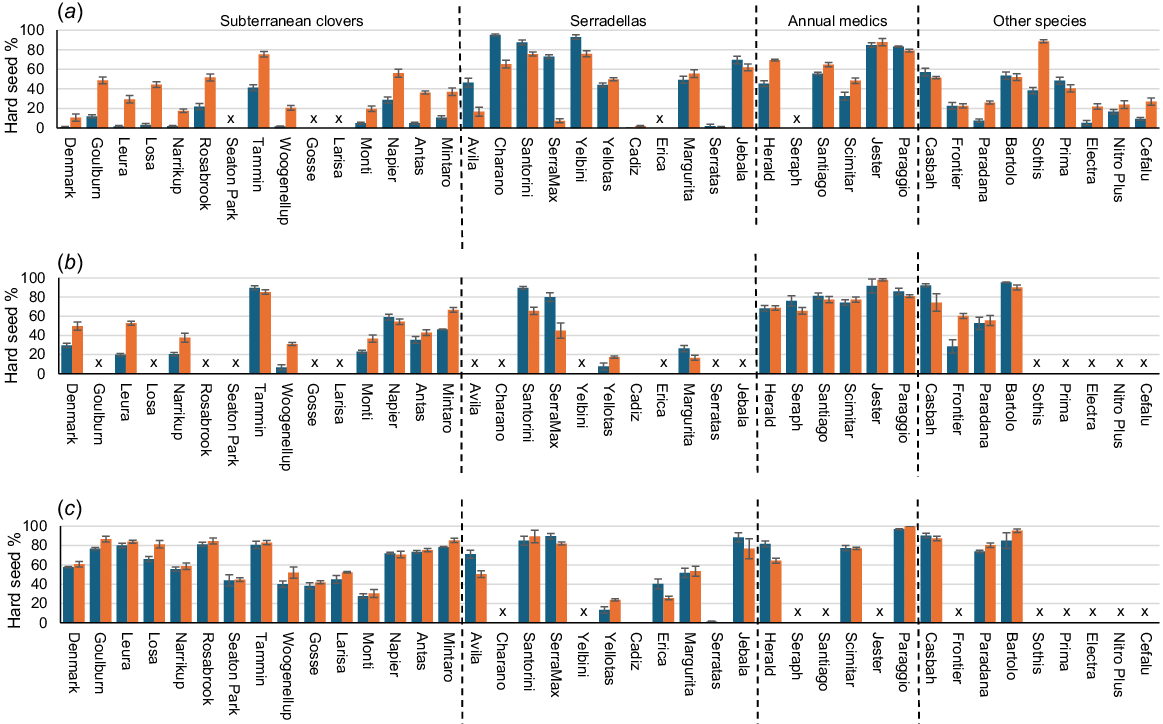
The 20 core cultivars common to the three sites were analysed in more detail to determine the effects of seed burial at different sites. A preliminary ANOVA for the nine common subterranean clover cultivars showed a highly significant (P < 0.001) cultivar effect, but neither subspecies nor subspecies by burial treatment effects were significant. The subspecies were therefore grouped together in subsequent analyses. However, there was a highly significant difference between the two serradella species (P < 0.001), so consequently French and yellow serradellas were treated as separate groups in subsequent analyses. There were insufficient representatives of the three annual medic and three alternative legume species to subdivide into species groups, so single groups were used for comparisons, even though it was recognised that balansa clover, bladder clover and biserrula showed different seed-softening dynamics. Results from a three-way ANOVA (with site, burial treatment and species group as factors) across the 20 core cultivars is shown in Table 4, with species groups of subterranean clover, annual medics, French serradella, yellow serradella and other species.
| Species group | Perth | Adelaide | Canberra | Site mean | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surface | Buried | Mean | Surface | Buried | Mean | Surface | Buried | Mean | Surface | Buried | Mean | ||
| Subterranean clover | 10.8 | 33.6 | 22.2 | 36.7 | 50.8 | 43.7 | 62.8 | 66.7 | 64.8 | 36.8 | 50.4 | 43.6 | |
| Yellow serradella | 58.5 | 28.6 | 43.6 | 43.8 | 27.4 | 35.6 | 51.5 | 52.8 | 52.2 | 51.3 | 36.3 | 43.8 | |
| French serradella | 24.7 | 28.8 | 26.8 | 13.2 | 8.3 | 10.8 | 25.8 | 26.7 | 26.3 | 21.2 | 21.3 | 21.3 | |
| Annual medics | 62.1 | 68.1 | 65.1 | 79.6 | 73.2 | 76.4 | 85.2 | 82.7 | 83.9 | 75.7 | 74.6 | 75.1 | |
| Other species | 39.5 | 43.2 | 41.3 | 80.1 | 76.2 | 78.2 | 83.1 | 87.7 | 85.4 | 67.6 | 69.0 | 68.3 | |
| Species mean | 31.5 | 41.0 | 36.2 | 50.1 | 52.5 | 51.3 | 65.5 | 67.6 | 66.6 | 49.1 | 53.7 | 51.4 | |
Site effect: P < 0.001; l.s.d. = 5.1. Species group effect: P < 0.001; l.s.d. = 7.3. Burial treatment effect: P < 0.05; l.s.d = 4.2. Site × species group interaction: P < 0.001; l.s.d. = 12.6. Species group × burial treatment interaction: P < 0.001; l.s.d = 10.3. Site × burial treatment interaction: P = not significant. Site × species group × burial treatment interaction: P = not significant.
Across all species groups, burial at 2 cm depth significantly (P < 0.05) reduced the mean amount of seed softening over the three sites. The site effect was highly significant (P < 0.001), with Perth showing the greatest difference between burial treatments, but neither the site × burial treatment nor the three-way site × species group × burial treatment interactions were significant (Table 4). However, there was a highly significant (P < 0.001) species group × burial treatment interaction. The overall reduction in seed softening among species groups can be attributed to the high number of subterranean clover entries (9), which had a 37.0% reduction in seed softening following shallow burial. This was particularly noticeable at Perth, with a three-fold reduction, and at Adelaide, with a 38.4% reduction. Reductions in seed softening following burial can generally be seen among the other non-core subterranean clover cultivars, particularly at Perth (Fig. 5).
However, pod burial had the opposite effect on the two core yellow serradellas, which showed a 29.2% mean increase in seed softening with shallow burial. This was most noticeable at Perth (51.1% increase) and at Adelaide (37.4% increase), whereas burial at Canberra had little effect. This difference in seed softening following pod burial was largely attributable to a 71.7% mean reduction in hardseededness ofcv. SerraMax (Fig. 5), which was particularly pronounced in Perth (89.9% reduction) and Adelaide (53.1% reduction), compared with Canberra (9.6% reduction). Significantly increased seed softening following pod burial was also observed in the other non-core yellow serradellas at Perth and Adelaide, and with cv. Avila at Canberra, apart from cv. Yellotas at each site and cv. Santorini at Canberra (Fig. 5).
There were no significant differences in the mean response to shallow burial in the core cultivar groups of French serradella, annual medics or other species across the three sites (Table 4). However, there were significant reductions in seed softening following burial at an individual cultivar level in the balansa clovers Paradana at Perth (but not Adelaide and Canberra) and Frontier at Adelaide (but not Perth), Casbah biserrula at Adelaide (but not the other two sites) and in Electra purple clover, Sothis eastern star clover and Cefalu arrowleaf clover at Perth (Fig. 5). Scimitar burr medic also had significantly less softening following burial at Perth (but not Adelaide and Canberra), whereas Herald strand medic softened more with burial at Perth, but less at Canberra (Fig. 5). Also of note was the inconsistent reaction to burial of Bartolo bladder clover across the three sites, with greater seed softening than surface-placed seeds at Adelaide, less seed softening at Canberra and negligible differences at Perth.
Discussion
This study evaluated the hard seed softening of a wide range of pasture legumes, many of which have not been evaluated previously or in contrasting environments. The results showed that the extent and pattern of seed softening of annual pasture legumes over the summer–autumn period after seed maturity differed among the three contrasting test sites and among cultivars within sites. Thus, Hypotheses 1 and 2 were shown to hold true. The extent of seed softening from shallow burial was reduced in some cultivars and increased in some, whereas other cultivars showed no effect. Thus, Hypothesis 3 only partially held true.
Cultivar differences for hardseededness
Considerable genetic variation was demonstrated among the annual legumes for both the extent of residual hard seeds carried over in the seed bank for germination in subsequent seasons and for tolerance to false breaks, owing to differences in the timing of hard seed softening over the summer–autumn period. This diversity conforms with previous studies, including Norman et al. (2002, 2006), Loi et al. (1999, 2005), Jeffery et al. (2021), Howieson et al. (2021), Nutt et al. (2021a) and Harrison et al. (2024) and indicates that considerable gains can be made by selection of annual pasture legumes for both traits to match the requirements needed for different target environments and farming systems. Across the sites, the soft seeded Cadiz and Serratas French serradellas had negligible hard seeds at seed maturity, whereas the French serradellas bred for hardseededness (Margurita and Erica) demonstrated higher initial and residual hard seed contents, similarly to the experiments of Howieson et al. (2021), Newell et al. (2023) and Harrison et al. (2024). Of the other annual legumes, subterranean clovers had the greatest amount of seed softening over the summer–autumn period, in accordance with the studies of Evans and Smith (1999), Loi et al. (2005), Norman et al. (2006), Nichols et al. (2013), Howieson et al. (2021) and Newell et al. (2023). Of note was the greater hardseededness of cv. Tammin. The annual medics, yellow serradellas (apart from Yellotas) and Casbah biserrula were the most hardseeded annual legumes, which is in general agreement with the studies of Crawford et al. (1989), Loi et al. (2005), Revell et al. (1998, 1999) and Nichols et al. (2012).
Patterns of seed softening
The timing of seed softening over the summer–autumn period for seeds and pods placed on the soil surface was highly species-specific. The subterranean clovers softened rapidly, with the majority of seed softening occurring by late March, with little further softening beyond this date. This is in accordance with previous studies by Norman et al. (2006), Loi et al. (1999) and Newell et al. (2023). Chapman and Asseng (2001) showed that subterranean clover in the low- and medium-rainfall environments of WA was particularly vulnerable to germination from false breaks in March and April, because reliable winter rains do not generally commence until May. The similar patterns of Paradana and Frontier balansa clovers, Nitro Plus Persian clover, Cefalu arrowleaf clover and Electra purple clovers suggests that these cultivars are similarly vulnerable to false breaks. In contrast, several cultivars, including Scimitar and Santiago burr medics, SerraMax and Avila yellow serradellas, Casbah biserrula, Prima gland clover, Bartolo bladder clover and Sothis eastern star clover, demonstrated a more sigmoid pattern of seed softening, with a delay in the timing of major seed softening, making them less vulnerable to false breaks. This was particularly noticeable at Perth and accords with previous studies of Taylor (1996), Revell et al. (1998), Loi et al. (1999), Howieson et al. (2021) and Nutt et al. (2021a). The timing of the season break varies across southern Australia and is changing as a result of climate change (Flohr et al. 2021). Potential exists to match hard seed breakdown patterns with the season break at different locations.
The situation is different for pasture legumes used in permanent pastures in more reliable, higher-rainfall regions. Here, subterranean clover that germinates following favourable early seasonal rainfall can compete with other pasture components and weeds and deliver a long, productive growing season. However, subterranean clover seed softening patterns must still provide some protection against seedling loss after a false break and the ability to regenerate after a delayed break to the growing season. Although there is a diversity of seed softening patterns available, it is seemingly difficult to select pasture legumes that will have an optimal seed softening and germination strategy for the majority of seasons in these higher-rainfall environments. An alternative option may be to sow a mixture of legumes with differing seed softening attributes that are able to respond to different seasonal conditions.
Effects of shallow seed or pod burial
A reduction in subterranean clover seed softening from shallow burial was previously observed by Taylor and Ewing (1996) among early flowering ssp. subterraneum cultivars at a low-rainfall site in WA. This study showed a similar reduction in a wider range of cultivars at higher-rainfall sites that was consistent among the three subspecies. The contrasting increase in seed softening from pod burial of the yellow serradellas confirmed the results of Revell et al. (1998, 1999), Taylor and Revell (2002) and Howieson et al. (2021). This was particularly pronounced for SerraMax in Perth and Adelaide, which had rapid seed softening following pod burial, compared with little softening when pods remained on the surface. Seed burial of Prima gland clover also increased seed softening at Perth, being similar to the findings of Howieson et al. (2021). The annual medics were little affected by burr burial, conforming with the results of Taylor and Ewing (1996). Loi et al. (1999) found that shallow burial of Casbah biserrula seeds increased rates of seed softening. This effect was observed at Adelaide, but not at Perth or Canberra. Howieson et al. (2021) found that softening of Margurita French serradella increased with shallow burial, but this was not supported in our study. New findings in this study were reductions in seed softening at Perth following burial for Electra purple clover, Sothis eastern star clover, Cefalu arrowleaf clover and Nitro Plus Persian clover. The effects of burr burial among sites for Casbah biserrula, Bartolo bladder clover and the balansa clovers Paradana and Frontier were inconsistent and are discussed below.
Site effects on hard seed softening
This study confirmed the rate and extent of hard seed softening in annual pasture legumes is site dependent. Seed softening in general was greatest at Perth and least at Canberra, with softening being intermediate in Adelaide. Site differences have previously been shown for subterranean clover (Norman et al. 2006), balansa clover (Nair et al. 2004) and several other annual legumes (Howieson et al. 2021). Differences in the extent of seed softening among the three sites can largely be explained by differences in mean summer–autumn temperatures, which were greatest in Perth and lowest in Canberra, with Adelaide being intermediate. The warmer autumn temperatures in Perth also meant that seed softening continued later into autumn than at the other two sites. These results conform with the two-stage seed softening process described by Taylor (1981, 2005), in which the first stage relies on high temperatures to degrade the hilum and lens, pre-conditioning the seed for softening, and the second stage relies on fluctuating temperatures to render the seed permeable to water. The modifying effect of shallow burial on the extent of seed softening in some cultivars was also presumably because of changes in the temperature regimes compared with those on the soil surface. This was apparent in the soil temperatures at Perth, in which mean autumn temperatures 2 cm below the soil surface were 1.6°C lower than on the surface, with a 3.8°C reduction in temperature amplitude. It is likely that similar temperature modifications from burial also occurred at Adelaide and Canberra. Further studies are needed to determine whether differences in the mean or amplitude of temperatures had the greatest effect on seed softening.
Cultivar × site interactions
An important finding from this study was significant cultivar × site interactions for seed softening of some key cultivars. This included differences in relative rankings among sites for residual hardseededness of Bartolo bladder clover, Margurita French serradella, Yellotas yellow serradella and Leura subterranean clover, and for each of the February–June sampling times of some cultivars, including Margurita and Paradana balansa clover. Although the relative responses to shallow burial were similar across sites for subterranean clover, French serradella and annual medics, there were relative differences for some cultivars among sites. In particular, Casbah biserrula, Bartolo bladder clover, Paradana and Frontier balansa clovers, Scimitar burr medic and Herald strand medic demonstrated inconsistent burr burial effects among sites. Howieson et al. (2021) also noted site differences for seed softening of buried seeds of Casbah, with much greater softening at three sites in NSW than in WA, and Newell et al. (2023) found site × cultivar differences among serradella cultivars at two contrasting sites in NSW.
Implications for selection of annual pasture legumes for local farming systems
Pasture legume cultivars in Australia have typically been developed regionally, often with little evaluation across Australia. The differences among sites in this study highlighted the risks of taking a cultivar and agronomic package from one region, with the expectation of similar performance in another region. This is also of importance in other regions of the globe where annual legumes are used in sown pastures, including the Mediterranean basin, Chile, South Africa, the United States of America and New Zealand (Porqueddu et al. 2016). This issue is exacerbated with some of the more recently commercialised species, which in many cases have had little evaluation in regional farming systems beyond their initial location of development. Thus, there is a need for regional seed softening studies to go along with other agronomic characterisation for local cultivar recommendations.
This study has shown that the effects of seed burial on seed softening rates is not always applicable for cultivars across site and climatic differences. This may mean that revolutionary new practices, such as summer sowing in the low and medium cropping districts of WA and NSW (Howieson et al. 2021; Nutt et al. 2021a), may need to be tested in new environments to be sure the expected outcome eventuates. Indeed, Howieson et al. (2021) and Nutt et al. (2021a) suggested that Casbah biserrula is better suited to summer sowing in NSW than WA, because of a better matching of its seed softening pattern.
The fitting of suitable cultivars adapted to permanent or semi-permanent pastures in cooler, temperate regions, such as the Northern, Central and Southern Tableland and alpine Monaro regions of NSW, requires attention, particularly because many of the newly commercialised species were developed for lower-rainfall, pasture–crop rotation systems. Late-flowering subterranean clovers are well adapted to this zone but the search for more P-efficient, acid-tolerant species with greater tolerance of false breaks (Kidd et al. 2020; Hayes et al. 2023) has shown both yellow and French serradellas as promising alternatives. However, Newell et al. (2023) showed French, yellow and slender serradella cultivars have substantially different seed softening patterns from that of subterranean clover in southern NSW, raising concerns about their adaptation to these environments. The diversity observed among those species in this study suggests that there is an opportunity to select serradella cultivars for these environments with appropriate seed softening patterns and residual hard seed levels. The effect of burr burial to reduce hardseededness presents new opportunities for the use of yellow serradella in permanent pastures, where high hardseededness is less important, although its implementation is problematic in such environments.
This study showed sufficient diversity to breed and select other pasture legumes with improved seed softening dynamics for different environments and farming systems. Diversity for seed softening patterns also suggests the selection for greater resistance to germination from false breaks. Examples include the higher residual hardseededness of Tammin and Margurita than other subterranean clovers and French serradellas respectively, and the lower hardseededness of Yellotas than other yellow serradellas. Heritability for hardseededness has been shown to be high for subterranean clover (Nichols et al. 2013), French serradella (Nutt et al. 2021b) and balansa clover (Nair et al. 2004), indicating the potential to breed cultivars of these species with differing degrees of hardseededness. Although Peck and Howie (2012) described selection to reduce hardseededness in annual medics, all annual medics cultivars in this study were hardseeded, reflecting their development for ley pasture systems (Nichols et al. 2012). However, Taylor (1996) found considerable diversity for hardseededness among 34 burr medic accessions, with soft seed in June ranging from 6.8% to 69.6%, with a range in the timing of summer–autumn seed softening. This indicates the potential to breed annual medics with different levels of residual hardseededness and the timing of softening for different uses (e.g. ley or permanent pasture) and locations.
Study limitations and further research
This study produced empirical results on differences in hardseededness among genotypes and environments, rather than mechanistic ones. Soil temperature measurements on the surface and 2 cm below the surface at each site would have provided stronger distinctions among the sites and allowed temperature effects at the two depths to be examined in more detail. Seed moisture content has been shown to be important for development and softening of hard seeds (Taylor 2005; Harrison et al. 2021); however, it was not measured in this experiment. Measurement of seed moisture contents during seed softening should be examined in further studies to help explain genotype, site and soil depth effects. Furthermore, this experiment was conducted only over a single summer–autumn period after seed maturation and studies over subsequent summer–autumn periods are needed to measure longer-term seed softening.
Conclusions
The general conclusion from one summer–autumn period of seed softening is that there is wide diversity between and within annual pasture legume species for both residual hardseededness and the pattern of hard seed softening over the summer–autumn following seed maturity and that the extent and timing is dependent on environment, primarily summer–autumn temperatures. Shallow burial of seeds or pods has a modifying effect on seed softening, but its effects are species and/or cultivar specific and often differ among growing environments. Collectively, the results indicate a need to better tailor annual legumes for specific environmental conditions and farming systems and demonstrate substantive diversity in patterns of seed softening among legume cultivars and species that can be utilised for this purpose. However, it is also clear that a greater understanding of the mechanisms and genetics of hardseededness and seed softening is also needed before these traits can be fully exploited. From a practical perspective, farmers can sow a mixture of broadly adapted annual legume species, or cultivars within species, with different hard seed dynamics to help counter seasonal differences in seed softening and germination conditions and provide greater stability to pasture legume content.
Data availability
The data that supports this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
The research in Perth and Adelaide was conducted as part of the project ‘Pre-breeding in annual legumes’ funded by Meat and Livestock Australia (project no. P.PBE.0037). P. Nichols and B. Wintle conducted this study as employees of the Department of Primary Industries and Regional Development, Western Australia.
Acknowledgements
The support of Michael Blair and Rohan Hungerfield in assisting with the management of the UWA Shenton Park Field Station plots and Jeff Hill for the Waite Institute plots is greatly appreciated.
References
Aitken Y (1939) The problem of hard seeds in subterranean clover. Proceedings of the Royal Society of Victoria 51, 187-213.
| Google Scholar |
Angus JF (2001) Nitrogen supply and demand in Australian agriculture. Australian Journal of Experimental Agriculture 41, 277-288.
| Crossref | Google Scholar |
Baskin JM, Baskin CC (2004) A classification system for seed dormancy. Seed Science Research 14, 1-16.
| Crossref | Google Scholar |
Chapman R, Asseng S (2001) An analysis of the frequency and timing of false break events in the Mediterranean region of Western Australia. Australian Journal of Agricultural Research 52, 367-376.
| Crossref | Google Scholar |
Collins WJ, Francis CM, Quinlivan BJ (1976) The interrelation of burr burial, seed yield and dormancy in strains of subterranean clover. Australian Journal of Agricultural Research 27, 787-792.
| Crossref | Google Scholar |
Crawford EJ, Lake AWH, Boyce KG (1989) Breeding annual Medicago species for semiarid conditions in southern Australia. Advances in Agronomy 42, 399-437.
| Crossref | Google Scholar |
Evans PM, Smith FA (1999) Patterns of seed softening in subterranean clover in a cool, temperate environment. Agronomy Journal 91, 122-127.
| Crossref | Google Scholar |
Flohr BM, Ouzman J, McBeath TM, Rebetzke GJ, Kirkegaard JA, Llewellyn RS (2021) Redefining the link between rainfall and crop establishment in dryland cropping systems. Agricultural Systems 190, 103105.
| Crossref | Google Scholar |
Haling RE, Yang Z, Shadwell N, Culvenor RA, Stefanski A, Ryan MH, Sandral GA, Kidd DR, Lambers H, Simpson RJ (2016) Root morphological traits that determine phosphorus-acquisition efficiency and critical external phosphorus requirement in pasture species. Functional Plant Biology 43, 815-826.
| Crossref | Google Scholar |
Harrison RJ, Howieson JG, Yates RJ, Nutt BJ (2021) Long-term storage of forage legumes greatly alters the hard seed breakdown pattern in situ. Grass and Forage Science 76, 72-81.
| Crossref | Google Scholar |
Harrison RJ, Nutt BJ, Yates RJ, Hackney BF, Howieson JG (2024) The de-domestication of Ornithopus sativus Brot. to develop cultivars with physical dormancy (hardseed). Grass and Forage Science 79, 366-380.
| Crossref | Google Scholar |
Hayes RC, Newell MT, Li GD, Haling RE, Harris CA, Culvenor RA, Badgery WB, Munday N, Price A, Stutz RS, Simpson RJ (2023) Legume persistence for grasslands in tableland environments of south-eastern Australia. Crop & Pasture Science 74, 712-738.
| Crossref | Google Scholar |
Howieson JG, Harrison RJ, Yates RJ, Hackney B, Loi A, Nutt BJ (2021) Hard seed breakdown patterns of unprocessed forage legume seed sown into dry soil in summer in southern Australia. Grass and Forage Science 76, 82-92.
| Crossref | Google Scholar |
Hyde EOC (1954) The function of the hilum in some Papilionaceae in relation to the ripening of the seed and the permeability of the testa. Annals of Botany 18, 241-256.
| Crossref | Google Scholar |
ISTA (2025) International rules for seed testing. International Seed Testing Association. Available at https://www.seedtest.org/en/publications/international-rules-seed-testing.html
Jeffery RP, Ryan MH, Ayers NL, Nichols PGH (2021) Salinity tolerance and avoidance mechanisms at germination among messina (Melilotus siculus) accessions. Crop & Pasture Science 72, 641-651.
| Crossref | Google Scholar |
Katznelson J, Morley FHW (1965) A taxonomic revison of sect. Calycomorphum of the genus Trifolium. I. The geocarpic species. Israel Journal of Botany 14, 112-134.
| Google Scholar |
Kidd DR, Di Bella CE, Kotula L, Colmer TD, Ryan MH, Striker GG (2020) Defining the waterlogging tolerance of Ornithopus spp. for the temperate pasture zone of southern Australia. Crop & Pasture Science 71, 506-516.
| Crossref | Google Scholar |
Kidd DR, Ryan MH, Colmer TD, Simpson RJ (2021) Root growth response of serradella species to aluminium in solution culture and soil. Grass and Forage Science 76, 57-71.
| Crossref | Google Scholar |
Loi A, Cocks PS, Howieson JG, Carr SJ (1999) Hardseededness and the pattern of softening in Biserrula pelecinus L., Ornithopus compressus L., and Trifolium subterraneum L. seeds. Australian Journal of Agricultural Research 50, 1073-1082.
| Crossref | Google Scholar |
Loi A, Howieson JG, Nutt BJ, Carr SJ (2005) A second generation of annual pasture legumes and their potential for inclusion in Mediterranean-type farming systems. Australian Journal of Experimental Agriculture 45, 289-299.
| Crossref | Google Scholar |
Loi A, Nutt BJ, Revell CK, Snowball R (2007) AGWEST Sothis: Trifolium dasyurum (eastern star clover). Australian Journal of Experimental Agriculture 47, 1512-1515.
| Crossref | Google Scholar |
Loi A, Nutt BJ, Howieson JG, Yates RJ, Norman HC (2012) Preliminary assessment of bladder clover (Trifolium spumosum L.) as an annual legume for ley farming systems in southern Australia. Crop & Pasture Science 63, 582-591.
| Crossref | Google Scholar |
Nair RM, Craig AD, Rowe TD, Biggins SR, Hunt CH (2004) Genetic variability and heritability estimates for hardseededness and flowering in balansa clover (Trifolium michelianum Savi) populations. Euphytica 138, 197-203.
| Crossref | Google Scholar |
Newell MT, Haling RE, Hayes RC, Stefanski A, Li GD, Simpson RJ (2023) Hard seed breakdown patterns of serradella (Ornithopus spp.) in two contrasting environments of south-eastern Australia. Crop & Pasture Science 74, 700-711.
| Crossref | Google Scholar |
Nichols PGH, Loi A, Nutt BJ, Evans PM, Craig AD, Pengelly BC, Dear BS, Lloyd DL, Revell CK, Nair RM, Ewing MA, Howieson JG, Auricht GA, Howie JH, Sandral GA, Carr SJ, de Koning CT, Hackney BF, Crocker GJ, Snowball R, Hughes SJ, Hall EJ, Foster KJ, Skinner PW, Barbetti MJ, You MP (2007) New annual and short-lived perennial pasture legumes for Australian agriculture – 15 years of revolution. Field Crops Research 104, 10-23.
| Crossref | Google Scholar |
Nichols PGH, Cocks PS, Francis CM (2009a) Evolution over 16 years in a bulk-hybrid population of subterranean clover (Trifolium subterraneum L.) at two contrasting sites in south-western Australia. Euphytica 169, 31-48.
| Crossref | Google Scholar |
Nichols PGH, Malik AI, Stockdale M, Colmer TD (2009b) Salt tolerance and avoidance mechanisms at germination of annual pasture legumes: importance for adaptation to saline environments. Plant and Soil 315, 241-255.
| Crossref | Google Scholar |
Nichols PGH, Snowball R, D’Antuono MF, Barbetti MJ (2010) Resistance to clover scorch disease (Kabatiella caulivora) among accessions of purple clover (Trifolium purpureum) and its relationship to the eco-geography of collection sites. Crop & Pasture Science 61, 44-49.
| Crossref | Google Scholar |
Nichols PGH, Revell CK, Humphries AW, Howie JH, Hall EJ, Sandral GA, Ghamkhar K, Harris CA (2012) Temperate pasture legumes in Australia – their history, current use, and future prospects. Crop & Pasture Science 63, 691-725.
| Crossref | Google Scholar |
Nichols PGH, Foster KJ, Piano E, Pecetti L, Kaur P, Ghamkhar K, Collins WJ (2013) Genetic improvement of subterranean clover (Trifolium subterraneum L.). 1. Germplasm, traits and future prospects. Crop & Pasture Science 64, 312-346.
| Crossref | Google Scholar |
Norman HC, Cocks PS, Galwey NW (2002) Hardseededness in annual clovers: variation between populations from wet and dry environments. Australian Journal of Agricultural Research 53, 821-829.
| Crossref | Google Scholar |
Norman HC, Smith FP, Nichols PGH, Si P, Galwey NW (2006) Variation in seed softening patterns and impact of seed production environment on hardseededness in early-maturing genotypes of subterranean clover. Australian Journal of Agricultural Research 57, 65-74.
| Crossref | Google Scholar |
Nutt BJ, Loi A, Hackney B, Yates RJ, D’Antuono M, Harrison RJ, Howieson JG (2021a) ‘Summer sowing’: a successful innovation to increase the adoption of key species of annual forage legumes for agriculture in Mediterranean and temperate environments. Grass and Forage Science 76, 93-104.
| Crossref | Google Scholar |
Nutt BJ, Harrison RJ, Mccomb JA, Howieson JG (2021b) The breeding system of Ornithopus sativus Brot. subsp. sativus. Grass and Forage Science 76, 3-9.
| Crossref | Google Scholar |
Peck DM, Howie JH (2012) Development of an early season barrel medic (Medicago truncatula Gaertn.) with tolerance to sulfonylurea herbicide residues. Crop & Pasture Science 63, 866-874.
| Crossref | Google Scholar |
Peoples MB, Baldock JA (2001) Nitrogen dynamics of pastures: nitrogen fixation inputs, the impact of legumes on soil nitrogen fertility, and the contributions of fixed nitrogen to Australian farming systems. Australian Journal of Experimental Agriculture 41, 327-346.
| Crossref | Google Scholar |
Piano E, Pecetti L, Carroni AM (1996) Climatic adaptation in subterranean clover populations. Euphytica 92, 39-44.
| Crossref | Google Scholar |
Porqueddu C, Ates S, Louhaichi M, Kyriazopoulos AP, Moreno G, del Pozo A, Ovalle C, Ewing MA, Nichols PGH (2016) Grasslands in ‘Old World’ and ‘New World’ Mediterranean-climate zones: past trends, current status and future research priorities. Grass and Forage Science 71, 1-35.
| Crossref | Google Scholar |
Quinlivan BJ (1961) The effect of constant and fluctuating temperatures on the permeability of the hard seeds of some legume species. Australian Journal of Agricultural Research 12, 1009-1022.
| Crossref | Google Scholar |
Quinlivan BJ (1971) Embryo dormancy in subterranean clover seeds. II. Its value relative to impermeability in field germination regulation. Australian Journal of Agricultural Research 22, 607-614.
| Crossref | Google Scholar |
Revell CK, Taylor GB, Cocks PS (1998) Long-term softening of surface and buried hard seeds of yellow serradella grown in a range of environments. Australian Journal of Agricultural Research 49, 673-685.
| Crossref | Google Scholar |
Revell CK, Taylor GB, Cocks PS (1999) Effect of length of growing season on development of hard seeds in yellow serradella and their subsequent softening at various depths of burial. Australian Journal of Agricultural Research 50, 1211-1223.
| Crossref | Google Scholar |
Revell CK, Ewing MA, Nutt BJ (2012) Breeding and farming system opportunities for pasture legumes facing increasing climate variability in the south-west of Western Australia. Crop & Pasture Science 63, 840-847.
| Crossref | Google Scholar |
Richardson AE, Simpson RJ (1989) Acid-tolerance and symbiotic effectiveness of Rhizobium trifolii associated with a Trifolium subterraneum L.-based pasture growing in an acid soil. Soil Biology and Biochemistry 21, 87-96.
| Crossref | Google Scholar |
Russi L, Cocks PS, Roberts EH (1992) Seed bank dynamics in a Mediterranean grassland. Journal of Applied Ecology 29, 763-771.
| Crossref | Google Scholar |
Sandral GA, Price A, Hildebrand SM, Fuller CG, Haling RE, Stefanski A, Yang Z, Culvenor RA, Ryan MH, Kidd DR, Diffey S, Lambers H, Simpson RJ (2019) Field benchmarking of the critical external phosphorus requirements of pasture legumes for southern Australia. Crop & Pasture Science 70, 1080-1096.
| Crossref | Google Scholar |
Simpson RJ, Richardson AE, Nichols SN, Crush JR (2014) Pasture plants and soil fertility management to improve the efficiency of phosphorus fertiliser use in temperate grassland systems. Crop & Pasture Science 65, 556-575.
| Crossref | Google Scholar |
Smith RW, Harris CA, Cox K, McClements D, Clark SG, Hossain Z, Humphries AW (2021) A history of Australian pasture genetic resource collections. Crop & Pasture Science 72, 591-612.
| Crossref | Google Scholar |
Smỳkal P, Vernoud V, Blair MW, Soukup A, Thompson RD (2014) The role of the testa during development and in establishment of dormancy of the legume seed. Frontiers in Plant Science 5, 351.
| Crossref | Google Scholar |
Taylor GB (1981) Effect of constant temperature treatments followed by fluctuating temperatures on the softening of hard seeds of Trifolium subterraneum L. Australian Journal of Plant Physiology 8, 547-558.
| Crossref | Google Scholar |
Taylor GB (1984) Effect of burial on the softening of hard seeds of subterranean clover. Australian Journal of Agricultural Research 35, 201-210.
| Crossref | Google Scholar |
Taylor GB (1996) Effect of the environment in which seeds are grown and softened on the incidence of autumn seed softening in two species of annual medics. Australian Journal of Agricultural Research 47, 141-159.
| Crossref | Google Scholar |
Taylor GB (2005) Hardseededness in Mediterranean annual pasture legumes in Australia: a review. Australian Journal of Agricultural Research 56, 645-661.
| Crossref | Google Scholar |
Taylor GB, Ewing MA (1996) Effects of extended (4–12 years) burial on seed softening in subterranean clover and annual medics. Australian Journal of Experimental Agriculture 36, 145-150.
| Crossref | Google Scholar |
Taylor GB, Revell CK (2002) Seed softening, imbibition time, and seedling establishment in yellow serradella. Australian Journal of Agricultural Research 53, 1011-1018.
| Crossref | Google Scholar |
Taylor GB, Maller RA, Rossiter RC (1991) A model describing the influence of hard seededness on the persistance of an annual forage legume, in a ley farming system, in a mediterranean-type environment. Agriculture, Ecosystems & Environment 37, 275-301.
| Crossref | Google Scholar |
Zeng LW, Cocks PS, Kailis SG, Kuo J (2005) The role of fractures and lipids in the seed coat in the loss of hardseededness of six Mediterranean legume species. The Journal of Agricultural Science 143, 43-55.
| Crossref | Google Scholar |


