Resistance to herbicides in the broadleaf weeds Raphanus raphanistrum, Sisymbrium orientale, and Sonchus oleraceus as determined by random weed surveys in south-eastern Australia
John C. Broster A * , Peter Boutsalis B , Gurjeet S. Gill B and Christopher Preston
A * , Peter Boutsalis B , Gurjeet S. Gill B and Christopher Preston  B
B
A
B
Abstract
Broadleaf weeds infest crops and reduce crop yield. The use of herbicides to control these weeds results in the evolution of herbicide resistance.
There is limited information about the extent and distribution of herbicide resistance in broadleaf weeds in south-eastern Australia, so a survey was conducted to provide this information for three key broadleaf weeds of this region.
A survey of three broadleaf weeds of grain crops collected wild radish (Raphanus raphanistrum), Indian hedge mustard (Sisymbrium orientale), and sowthistle (Sonchus oleraceus) across south-eastern Australia between 2013 and 2017. Samples were screened for resistance to commonly used herbicides.
A total of 1760 fields were visited with 74 R. raphanistrum, 95 S. orientale, and 488 S. oleraceus seed samples collected. Resistance to the ALS-inhibiting herbicide chlorsulfuron was most common in these species, present in 66% of S. oleraceus, 34% of S. orientale, and 17% of R. raphanistrum samples tested. R. raphanistrum and S. orientale also had samples resistant to atrazine, diflufenican and 2,4-D. No resistance to glyphosate was found in any species. Multiple resistance to two or more herbicide modes of action was present in all three species.
Herbicide resistance was identified in all three weed species in all the states where they were collected. All species had resistance to more than one herbicide mode of action.
While the frequency of resistance in these broadleaf weeds in south-eastern Australia is low to most herbicides, the accumulation of multiple resistance means that alternative management strategies will be required to effectively manage these weeds.
Keywords: 2,4-D, atrazine, chlorsulfuron, diflufenican, glyphosate, Indian hedge mustard, multiple resistance, sowthistle, wild radish.
Introduction
Weeds are a major constraint to grain production around the world. In Australia, weeds and their control cost the grains industry AU$3.3 billion per annum (Llewellyn et al. 2016). Herbicides are the main weed management tool used in grain production systems in Australia. A consequence of the intensive use of herbicides is the evolution of resistance to herbicides. Management of herbicide resistant weeds in Australia is estimated to cost grain growers AU$187 million per annum (Llewellyn et al. 2016).
While grass weeds remain the most important weeds of grain production systems in Australia, there are a number of important broadleaf weeds of concern. Many of these have also evolved resistance to herbicides (Walsh et al. 2001; Lu et al. 2007; Owen et al. 2015a; Long et al. 2019). Raphanus raphanistrum (wild radish), Sisymbrium orientale (Indian hedge mustard), and Sonchus oleraceus (sowthistle) are the 2nd, 9th, and 11th most costly weeds in Australian grain cropping in terms of revenue loss (Llewellyn et al. 2016).
R. raphanistrum, S. orientale, and S. oleraceus are all weeds of winter crops in south-eastern Australia, emerging in autumn or early winter with the crop. S. oleraceus can also emerge in spring or early summer and can also be a problem weed of summer fallows (Widderick et al. 2010). R. raphanistrum is a highly competitive weed that can reduce wheat (Triticum aestivum) yield by up to 40% (Eslami et al. 2006). It produces seeds enclosed in pod segments that can break off and contaminate grain (Cheam 1986). R. raphanistrum has a long-lived seed bank and can emerge from deep in the soil profile (Reeves et al. 1981; Chauhan et al. 2006a). S. orientale is a small-seeded weed species that germinates close to the soil surface (Boutsalis and Powles 1998; Chauhan et al. 2006b). It produces a large number of seeds that are held in siliques, which break open after maturity (Boutsalis and Powles 1998). S. oleraceus is also a small-seeded weed species, germinating close to the soil surface (Chauhan et al. 2006c; Widderick et al. 2010). S. oleraceus produces a large number of seeds each bearing a pappus that allows them to disperse long distances (Sheldon and Burrows 1973). In addition to being an important weed of summer fallows, S. oleraceus can also cause problems by its presence at harvest resulting in discolouration of harvested grain (Widderick et al. 2010).
Resistance to the ALS-inhibiting (aetolactate synthase inhibiting) herbicides was first reported in Australia in 1990 for both S. orientale and S. oleraceus (Boutsalis and Powles 1995), and in 1997 for R. raphanistrum (Hashem et al. 2001). Since then, S. orientale has evolved resistance to 2,4-D, atrazine and diflufenican (Preston et al. 2013; Dang et al. 2017, 2018), S. oleraceus has evolved resistance to chlorsulfuron, glyphosate, and 2,4-D (Cook et al. 2014; Merriam et al. 2018; Krishnan et al. 2025) and R. raphanistrum has evolved resistance to atrazine, 2,4-D, diflufenican, and glyphosate (Walsh et al. 2004; Ashworth et al. 2014).
Surveys of weed resistance can be used to better understand the extent and distribution of herbicide resistance in farming systems. Surveys of herbicide resistance in grass weeds in south-eastern Australia have identified high frequencies of resistance to herbicides in Lolium rigidum (Broster et al. 2022a) and lower levels of resistance in Avena spp. and other grass weeds (Broster et al. 2023a). A survey of R. raphanistrum from Western Australia (WA) found resistance to chlorsulfuron, 2,4-D and diflufenican was widespread in the state (Owen et al. 2015a). Despite these findings there have been few surveys of broadleaf weed resistance in south-eastern Australia (Merriam et al. 2018). This study was undertaken to determine the extent and distribution of herbicide resistance in R. raphanistrum, S. orientale, and S. oleraceus in south-eastern Australia.
Materials and methods
Crop survey
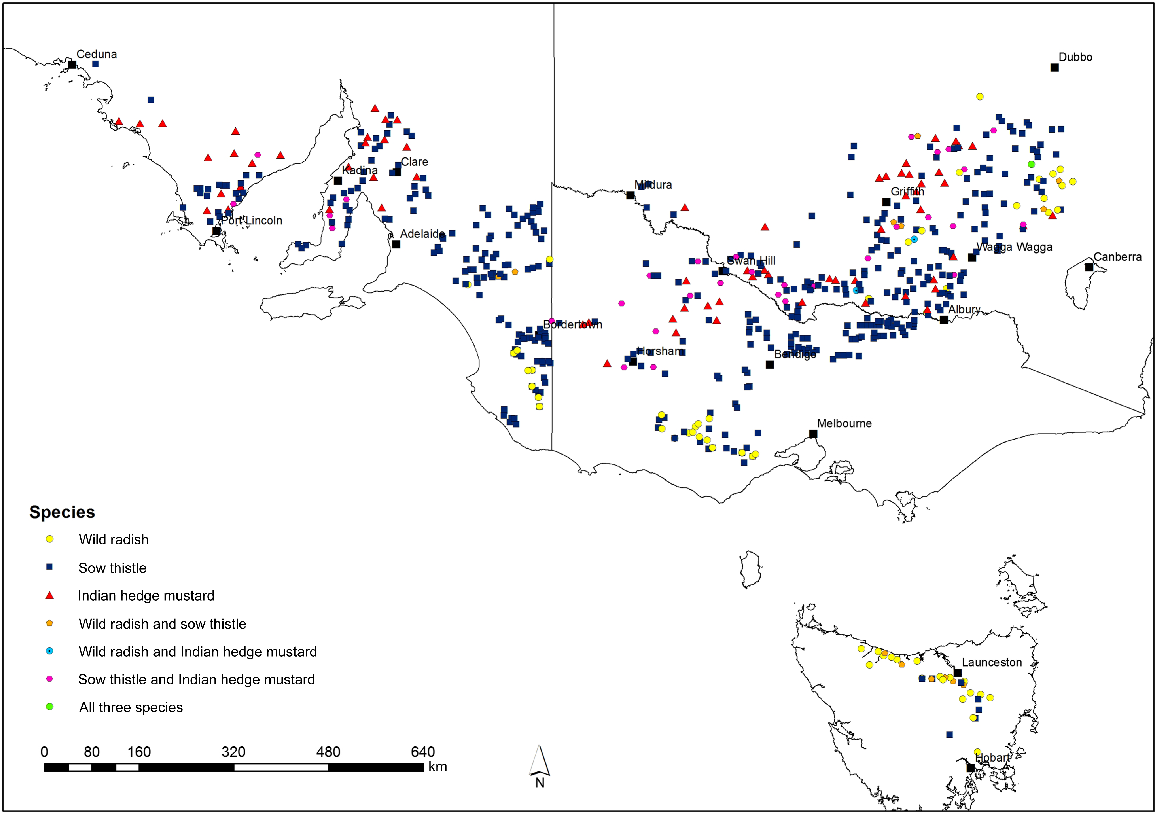
From 2013 to 2017, surveys were conducted in the cropping regions of South Australia (SA), Victoria (VIC), Tasmania (TAS), and southern New South Wales (NSW) (Fig. 1) to collect seeds of residual weeds from crop fields. Fields were visited at random within designated regions (Table 1) by travelling for a predetermined distance (5 or 10 km depending upon the size of surveyed area) with two or three regions visited in any year. At each stop, a single field was surveyed with the aim to visit a minimum of 120 crop fields in each region and collect Raphanus raphanistrum L., Sisymbrium orientale L., and Sonchus oleraceus L. Seeds were collected by removing mature seed pods or heads from the plants. After collection, the samples were stored for after-ripening with all other seed samples collected in the survey. A more detailed description of the sampling methodology is described by Boutsalis et al. (2012) and Broster et al. (2022a). However, the samples collected represent the potential seed bank input and therefore the resistance status of the weed populations present next year.
Survey area across south-eastern Australia. Each point represents a field where one or more of the three broadleaf weeds were collected. The different symbols and colours represent different weed species present: Raphanus raphanistrum (yellow circles); Sisymbrium orientale (red triangles); Sonchus oleraceus (blue squares); R. raphanistrum and S. orientale (blue circles); R. rapahnistrum and S. oleraceus (orange circles); S. orientale and S. oleraceus (pink circles); and all three species (green circles).
| Region | Year surveyed | Fields visited | Number of samples collected | |||
|---|---|---|---|---|---|---|
| R. raphanistrum | S. orientale | S. oleraceus | ||||
| SA | ||||||
| Eyre Peninsula | 2014 | 167 | 0 | 14 | 24 | |
| Mid-north | 2013 | 150 | 0 | 16 | 47 | |
| Mallee | 2017 | 164 | 4 | 0 | 29 | |
| South-east | 2017 | 74 | 9 | 0 | 90 | |
| VIC | ||||||
| Wimmera Mallee | 2015 | 140 | 0 | 19 | 29 | |
| Southern | 2014 | 116 | 14 | 0 | 18 | |
| North-east | 2016 | 122 | 0 | 0 | 72 | |
| NSW | ||||||
| Western | 2015 | 164 | 2 | 23 | 59 | |
| Plains | 2016 | 152 | 6 | 12 | 35 | |
| Southern | 2017 | 162 | 4 | 8 | 32 | |
| Slopes | 2013 | 199 | 3 | 1 | 33 | |
| High Rainfall | 2014 | 75 | 7 | 2 | 8 | |
| TAS | 2014 | 75 | 25 | 0 | 12 | |
| Total | 1760 | 74 | 95 | 488 | ||
Plant growth and herbicide treatment
Seeds collected were allowed to after-ripen over summer and were sown the following autumn. Samples collected from SA and VIC were tested at the Waite Campus, University of Adelaide. Samples collected from TAS and NSW were tested at Charles Sturt University (CSU), Wagga Wagga. At University of Adelaide, R. raphanistrum seed was soaked for 30 min in a sodium hypochlorite solution (21 g L−1) followed by washing for 30 min in running water to break seed dormancy. Seeds of all species were sown into trays containing cocoa peat potting and watered (Boutsalis et al. 2012). R. raphanistrum seeds were covered by 5 mm of potting soil; however, seed of the other two species that require light exposure to stimulate germination were left on the soil surface. Once germinated, seedlings were transplanted into pots containing potting soil with 10 plants per pot. There were three replicate pots per field sample. Methods were similar at CSU except R. raphanistrum seeds were processed using a gristing mill to remove them from their pods and all species were sown into garden loam.
All herbicides were applied post-emergent to plants at the 4−6-leaf stage. The herbicides rates used and species treated with each herbicide are in Table 2. Herbicides were applied as described by Boutsalis et al. (2012) or Broster et al. (2012). A standard susceptible population was included in every test. At CSU the susceptible populations for each species were compilations of five to ten different populations, all with 0% survival, from either previous surveys (Broster et al. 2015, 2023b) or the CSU commercial herbicide resistance testing service. The susceptible populations used at the University of Adelaide had been identified in previous surveys. Due to limited seed availability for some samples not all samples were tested with all herbicides.
| Herbicide | Rate used (g a.i. or a.e. ha−1) | Trade name | Manufacturer | Species screened | |
|---|---|---|---|---|---|
| Chlorsulfuron | 15 | Tackle | Adama Australia | All three | |
| Imazamox + imazapyr | 19.8 + 9 | Intervix | BASF Australia | R. raphanistrum and S. orientale only | |
| Atrazine | 600 | Farmozine | Adama Australia | R. raphanistrum and S. orientale only | |
| Diflufenican | 50 | Brodal options | Bayer CropScience Australia | R. raphanistrum and S. orientale only | |
| 2,4-D | 455 | Amicide Advance | Nufarm Australia | All three | |
| Glyphosate | 540 | Weedmaster Argo | Nufarm Australia | All three (except R. raphanistrum from SA) |
Plants were counted as survivors to the herbicide if they had new shoot growth at 21 days after treatment. Following Boutsalis et al. (2012), samples from each field were considered resistant when 20% or more of the individuals in that accession had survived 21 days after herbicide application.
Statistical analysis
A G test (McDonald 2014) was used to test whether the frequency of resistance was similar between species for each herbicide tested except for glyphosate, where there was no survival. The null hypothesis was that resistance occurred equally across the species for each herbicide. The G test was also used to test whether the frequency of resistance to chlorsulfuron was the same as the frequency of resistance to imazamox + imazapyr for R. raphanistrum and S. orientale. The frequency of resistance between different groups was considered to be different when P < 0.05.
Results
A total of 1760 crop fields were visited; however, broadleaf weeds were identified in only a minority of the fields. R. raphanistrum was collected from only 74 fields and S. orientale from 95 fields (Table 1). S. oleraceus was more common, being collected from 488 crop fields. The distribution of R. raphanistrum and S. orientale were localised, being absent from 4 and 5 of the 13 regions surveyed, respectively. In contrast, S. oleraceus was collected in every region (Table 1).
Due to the low number of weed samples collected from some cropping regions, resistance frequencies were calculated on a state basis (Table 3). Of the herbicides tested, the highest frequency of resistance was to the ALS-inhibiting herbicide chlorsulfuron (Table 3). Resistance was present to this herbicide in all three species in all four states. Overall, the frequency of resistance to chlorsulfuron was higher in S. oleraceus (both P = <0.0001; Table 4) than the other two weed species, and higher in S. orientale than in R. raphanistrum (P = 0.0001; Table 4).
| State | Herbicide | ||||||
|---|---|---|---|---|---|---|---|
| Chlorsulfuron | Imazamox + imazapyr | Atrazine | Diflufenican | 2,4-D | Glyphosate | ||
| Resistance (% samples) | |||||||
| SA | |||||||
| R. raphanistrum | 39 | 23 | 0 | 0 | 39 | – | |
| S. orientale | 43 | 13 | 3 | 20 | 3 | 0 | |
| S. oleraceus | 80 | – | – | – | 6 | 0 | |
| VIC | |||||||
| R. raphanistrum | 29 | 0 | 0 | 0 | 7 | 0 | |
| S. orientale | 42 | 5 | 21 | 47 | 32 | 0 | |
| S. oleraceus | 78 | – | – | – | 3 | 0 | |
| NSW | |||||||
| R. raphanistrum | 5 | 0 | 6 | 17 | 0 | 0 | |
| S. orientale | 24 | 0 | 0 | 0 | 2 | 0 | |
| S. oleraceus | 45 | – | – | – | 0 | 0 | |
| TAS | |||||||
| R. raphanistrum | 8 | 32 | 0 | 0 | 9 | 0 | |
| S. oleraceus | 9 | – | – | – | 0 | 0 | |
| Mean | |||||||
| R. raphanistrum | 17 | 15 | 2 | 5 | 12 | 0 | |
| S. orientale | 34 | 6 | 6 | 16 | 9 | 0 | |
| S. oleraceus | 66 | – | – | – | 3 | 0 | |
Samples were considered to be resistant if >20% of individuals within that population survived application of the herbicide. A dash indicates species was not screened to this herbicide.
| Comparison | G statistic | P-value | |
|---|---|---|---|
| R. raphanistrum vs S. orientale | |||
| Chlorsulfuron | 15.9 | 0.0001 | |
| Imazamox + imazapyr | 7.35 | 0.007 | |
| Diflufenican | 18.6 | <0.0001 | |
| Atrazine | 5.55 | 0.019 | |
| 2,4-D | 0.91 | 0.34 | |
| R. raphanistrum vs S. oleraceus | |||
| Chlorsulfuron | 578 | <0.0001 | |
| 2,4-D | 47.6 | <0.0001 | |
| S. orientale vs S. oleraceus | |||
| Chlorsulfuron | 39.8 | <0.0001 | |
| 2,4-D | 26.2 | <0.0001 | |
| Chlorsulfuron vs Imazamox + imazapyr | |||
| R. raphanistrum | 0.114 | 0.74 | |
| S. orientale | 40.7 | <0.0001 | |
Imazamox + imazapyr is an ALS-inhibiting herbicide combination from a different chemical class to chlorsulfuron. Only R. raphanistrum and S. orientale were tested for resistance to this herbicide. Resistance to imazamox + imazapyr was identified in R. raphanistrum in all states, except NSW. The frequency of resistance to imazamox + imazapyr in R. raphanistrum at 15% was similar to the frequency of resistance to chlorsulfuron (17%) (P = 0.74; Table 4). Resistance to imazamox + imazapyr in S. orientale was identified in SA and VIC only. In contrast to R. raphanistrum, resistance to imazamox + imazapyr in S. orientale occurred at a lower frequency compared to resistance to chlorsulfuron (P = <0.0001; Table 4). Overall, only 6% of S. orientale samples tested had resistance to imazamox + imazapyr, compared to 34% with resistance to chlorsulfuron (Table 3).
Atrazine is a PSII-inhibiting herbicide widely used to control broadleaf weeds in Australia. Resistance to atrazine was identified in S. orientale samples from SA and VIC, but not NSW (Table 3). In contrast, resistance to atrazine was found in R. raphanistrum samples from NSW, but not SA, VIC or TAS. S. oleraceus was not tested to this herbicide. Overall, the frequency of resistance to atrazine was low; 2% of R. raphanistrum samples and 6% of S. orientale samples with S. orientale having more resistance to this herbicide than R. raphanistrum (P = 0.019; Table 4).
Diflufenican is a phytoene-desaturase-inhibiting herbicide used in Australia for the control of Brassicaceous and other broadleaf weeds (Dang et al. 2018). As with resistance to atrazine, resistance to diflufenican was identified in S. orientale samples from SA and VIC, but not NSW (Table 3), and resistance in R. raphanistrum was identified only in NSW. Overall, the frequency of resistance to diflufenican was greater in S. orientale (16%) than R. raphanistrum (5%) (P = <0.0001; Table 4).
2,4-D is an auxin mimic herbicide widely used for broadleaf weed control in cereal crops and chemical fallows. Resistance to 2,4-D was found in all three weed species in SA and Vic (Table 3). Resistance to 2,4-D was present in 39% of R. raphanistrum samples tested from SA and 32% of S. orientale samples tested from VIC. Resistance to 2,4-D was also identified in S. orientale in NSW and R. raphanistrum in TAS (Table 3). Overall, the frequency of resistance to 2,4-D was 12% in R. raphanistrum, 9% in S. orientale, and 3% in S. oleraceus. The frequency of resistance to 2,4-D was greater in both R. raphanistrum and S. orientale than in S. oleraceus (both P = <0.0001; Table 4).
Glyphosate is an EPSPS (enzyme 5-enolpyruvylshikimate-3-phosphate synthase) inhibiting herbicide that is widely used prior to crop seeding and in glyphosate tolerant (RoundupReady) canola (Brassica napus) (Hudson and Richards 2014). No resistance to glyphosate was found in any of these weed species in this survey (Table 3).
Of the samples collected, 66% of R. raphanistrum samples were susceptible to all herbicides tested, compared with 55% of S. orientale samples and 30% of S. oleraceus samples (Table 5). Most resistant populations of all three species were resistant to only one herbicide mode of action. A total of 7% of R. raphanistrum samples, 8% of S. orientale samples, and 2% of S. oleraceus samples were resistant to two herbicide modes of action. Only S. orientale had samples resistant to three herbicide modes of action (5%) and no species had samples with resistance to more than three herbicide modes of action (Table 5).
| Herbicide modes of action with resistance | R. raphanistrum | S. orientale | S. oleraceus | |
|---|---|---|---|---|
| % of samples tested | ||||
| 0 | 66 | 55 | 30 | |
| 1 | 27 | 32 | 68 | |
| 2 | 7 | 8 | 2 | |
| 3 | 0 | 5 | 0 | |
| 4 | 0 | 0 | – | |
| 5 | 0 | 0 | – | |
Samples of R. raphanistrum and S. orientale were tested with herbicides from five modes of action, while samples of S. oleraceus were tested with herbicides from three modes of action.
Discussion
This survey of more than 1700 crop fields in south-eastern Australia identified the broadleaf weeds R. raphanistrum, and S. orientale were not as common as grass weeds in this region and S. oleraceus was less common than ryegrass and wild oats, but more common than brome grass (Bromus spp.) and barley grass (Hordeum spp.) (Broster et al. 2022a, 2023a). Previous herbicide resistance surveys in south-eastern Australia identified L. rigidum in 91% of sample fields in VIC, 86% of fields in SA (Boutsalis et al. 2012), 76% and 65% of fields in southern (Broster et al. 2011a) and south-western (Broster et al. 2013) NSW, respectively, and 90% of fields in TAS (Broster et al. 2012). Avena spp. were found in 43% and 54% of fields in southern (Broster et al. 2011b) and south-western (Broster et al. 2013) NSW, respectively, and 20% of fields in TAS (Broster et al. 2012). Bromus spp. were less common than the other two grass weed species, and were found in 41% of fields in SA, 25% of fields in VIC (Boutsalis et al. 2014), 6% of fields in Vic (Broster et al. 2010) and 8% of fields in TAS. Of the broadleaf weeds, S. oleraceus was most common being present in 28% of fields across the region, followed by S. orientale in 5% of fields, and R. raphanistrum in 4% of fields (Table 1). R. raphanistrum was more common in a survey of WA fields where it was collected from 21% of fields (Owen et al. 2015a) compared with south-eastern Australia.
As in south-eastern Australia, grass weeds were more common in WA crop fields with L. rigidum collected from 78% of fields (Owen et al. 2014), Avena spp. collected from 23% of fields (Owen and Powles 2016) and Bromus spp. collected from 20% of fields (Owen et al. 2015b). Likewise, Beckie et al. (2013) also found grass weeds were more common in crops in the Canadian prairies. In their surveys, the most common weeds were the grass weeds Avena fatua (present in 68% of fields), Lolium persicum (present on 21% of fields), followed by the broadleaf weeds Thapsia arvense and Galium spp. at 11% of fields each, and Polygonum convolvulus and Chenopodium album in 9% of fields each. There are several factors that influence the diversity of weed species in cropping systems including: type of crop, soil type, and environment (Fried et al. 2008, Hawes et al. 2010). Within crop types, management factors can become important (Fried et al. 2008). Cropping systems with high frequency of cereal crops in rotations tend to contain higher frequencies of grass weeds (Blackshaw et al. 1994; Derksen et al. 2002; Murphy and Lemerle 2006). Broster et al. (2022b) reported that broadleaf weeds were less common than expected in cereal crops compared with broadleaf crops in a study that comprised 752 of the NSW fields in this study. In south-eastern Australia, 78% of fields visited were sown to the cereal crops, wheat and barley (Hordeum vulgare) (Broster et al. 2022a). This is similar to the frequency of cereal crops in WA at 81% (Owen et al. 2014), but higher than the 60% of fields sown to cereal crops in the Canadian prairies (Beckie et al. 2013). The high frequency of cereal crops likely explains the dominance of grass weed species in all three systems.
Resistance was present in all three broadleaf weeds collected in south-eastern Australia. The levels of resistance in broadleaf weeds are generally low, except for resistance to chlorsulfuron (Table 3). Resistance to ALS-inhibiting herbicides, like chlorsulfuron, is common in broadleaf weeds. Of the 176 weed species with resistance to ALS-inhibiting herbicides, more than 100 are broadleaf weed species (Heap 2024). Resistance to ALS-inhibiting herbicides occurs rapidly (Boutsalis and Powles 1995; Preston and Powles 2002). The frequency of resistance to sulfonylurea herbicides in R. raphanistrum in south-eastern Australia at 17% of samples was much lower than resistance in WA, where 73% of R. raphanistrum samples tested had >20% survival to chlorsulfuron (Owen et al. 2015a). In the Canadian prairies, all Sonchus asper samples, 40% of Stelleria media samples and 16% of Galium spp. samples tested had resistance to ALS-inhibiting herbicides (Beckie et al. 2013). Resistance to ALS-inhibiting herbicides is often the result of target site mutations in ALS gene; however, non-target site mechanisms can also occur (Yu and Powles 2014). Differences in specific mutations provide differences in resistance to sulfonylurea and imidazolinone herbicides. For example, in R. raphanistrum in WA, there was a much higher frequency of resistance to sulfonylurea herbicides than imidazolinone herbicides (Owen et al. 2015a). In south-eastern Australia, there was no difference in the frequency of resistance between these two groups in R. raphanistrum, but there was in S. orientale (Table 4). These differences suggest differences in the selection intensity between regions of Australia in imidazolinone tolerant crops.
Resistance was also present to other herbicides in all three broadleaf weed species (Table 3). Resistance to atrazine was present in both R. raphanistrum and S. orientale at low frequencies. Atrazine resistance is more common in broadleaf weeds overseas than in Australia. Similar to the results here, atrazine resistance was identified in only one of 96 R. raphanistrum samples collected in a survey in WA (Owen et al. 2015a). Triazine herbicides are mainly used in canola and pulse crops in Australian grain production. As these represent less than 20% of the crops grown in south-eastern Australia (Broster et al. 2022a), the selection pressure in Australian grain production for resistance remains low.
Of greater concern, is resistance to 2,4-D, which was present in all three weed species (Table 3). 2,4-D is a relatively inexpensive herbicide, which is widely used for broadleaf weed control in cereal crops (Preston et al. 2013). The loss of 2,4-D and the ALS-inhibiting herbicides due to resistance reduces broad-spectrum broadleaf herbicide options, making broadleaf weed control in cereals more difficult (Walsh et al. 2004; Preston et al. 2013). Resistance to 2,4-D was not found in a weed survey of the Canadian prairies (Beckie et al. 2013) or a survey of Amaranthus rudis in Missouri, USA (Schultz et al. 2015); however, about a third of R. rapahnistrum samples collected from WA had greater than 20% survival when treated with this herbicide (Owen et al. 2015a). Multiple resistance, defined here as resistance to two or more herbicide modes of action, was relatively rare in this survey, affecting 7% of R. raphanistrum samples, 13% of S. orientale samples, and 2% of S. oleraceus samples (Table 5). This is in contrast to a survey of R. raphanistrum in WA where Owen et al. (2015a) concluded that multiple resistance was widespread and prevalent in this weed species. Likewise, more than half of the A. rudis populations in Missouri had multiple resistance (Schultz et al. 2015). The lower level of multiple resistance in broadleaf weeds in south-eastern Australia provides an opportunity for farmers to use a diverse range of herbicides to control these weeds. Rotation of herbicide modes of action or herbicide mixtures involving different modes of action will be necessary to reduce the selection pressure for resistance.
Conclusion
This research has shown that while broadleaf weeds are less common in crop fields of south-eastern Australia than grass weeds, herbicide resistance has evolved in these weeds. Resistance was present in at least two of the weed species to herbicides in four different modes of action. This highlights the risks of relying on herbicides alone for broadleaf weed control in grain crops. Additional weed control practices will need to be introduced. These could include tillage, crop competition or harvest weed seed control (Cirujeda et al. 2003; Walsh et al. 2013; Van der Meulen and Chauhan 2017). However, tillage is unlikely to be effective on R. raphanistrum, as its seeds can easily emerge from depth (Reeves et al. 1981). Likewise, harvest weed seed control is unlikely to be effective on S. oleraceus, as its seeds are easily dislodged from the plant and dispersed by wind (Widderick et al. 2010), therefore, different management practices will be needed for each of these weed species because of inherent differences in their biology which will affect their suitability for various management practices.
Data availability
The data that support this study are available in the article and upon request to the corresponding author.
Conflicts of interest
Christopher Preston is an Associate Editor of Crop & Pasture Science, but was not involved in the peer review or any decision-making process for this paper. The authors declare no other conflicts of interest.
Declaration of funding
This research was funded by the Grains Research and Development Corporation grant number UCS00020.
Acknowledgements
The authors hank Alicia Merriam, David Brunton, Geetha Velappan, Ruwan Lenorage, and Allison Chambers for technical assistance.
References
Ashworth MB, Walsh MJ, Flower KC, Powles SB (2014) Identification of the first glyphosate-resistant wild radish (Raphanus raphanistrum L.) populations. Pest Management Science 70(9), 1432-1436.
| Crossref | Google Scholar | PubMed |
Beckie HJ, Lozinski C, Sherriff S, Brenzil CA (2013) Herbicide-resistant weeds in the Canadian prairies: 2007 to 2011. Weed Technology 27(1), 171-183.
| Crossref | Google Scholar |
Blackshaw RE, Larney FO, Lindwall CW, Kozub GC (1994) Crop rotation and tillage effects on weed populations on the semi-arid Canadian prairies. Weed Technology 8(2), 231-237.
| Crossref | Google Scholar |
Boutsalis P, Powles SB (1995) Resistance of dicot weeds to acetolactate synthase (ALS)-inhibiting herbicides in Australia. Weed Research 35(3), 149-155.
| Crossref | Google Scholar |
Boutsalis P, Powles SB (1998) Seedbank characteristics of herbicide-resistant and susceptible Sisymbrium orientale. Weed Research 38(5), 389-395.
| Crossref | Google Scholar |
Boutsalis P, Gill GS, Preston C (2012) Incidence of herbicide resistance in rigid ryegrass (Lolium rigidum) across southeastern Australia. Weed Technology 26(3), 391-398.
| Crossref | Google Scholar |
Boutsalis P, Kleemann S, Gill GS, Preston C (2014) A hidden threat: widespread group B herbicide resistance in brome across south-eastern Australia. In ‘Proceedings of the 19th Australasian weeds conference’, 1–4 September 2014, Hobart, Tasmania, Australia. (Ed. M Baker) pp. 202–205. (Tasmanian Weed Society)
Broster JC, Koetz EA, Wu H (2010) A survey of southern New South Wales to determine the level of herbicide resistance in brome grass and barley grass populations. In ‘Proceedings of the 17th Australasian weeds conference’, 26–30 September 2010, Christchurch, New Zealand. (Ed. SM Zydenbos) pp. 274–277. (New Zealand Plant Protection Society)
Broster JC, Koetz EA, Wu H (2011a) Herbicide resistance levels in annual ryegrass (Lolium rigidum Gaud.) in southern New South Wales. Plant Protection Quarterly 26(1), 22-28.
| Google Scholar |
Broster JC, Koetz EA, Wu H (2011b) Herbicide resistance in wild oats (Avena spp.) in southern New South Wales. Plant Protection Quarterly 26(3), 106-110.
| Google Scholar |
Broster JC, Koetz EA, Wu H (2012) Herbicide resistance frequencies in ryegrass (Lolium spp.) and other grass species in Tasmania. Plant Protection Quarterly 27, 36-42.
| Google Scholar |
Broster JC, Koetz EA, Wu H (2013) Herbicide resistance levels in annual ryegrass (Lolium rigidum Gaud.) and wild oats (Avena spp.) in south-western New South Wales. Plant Protection Quarterly 28(4), 126-132.
| Google Scholar |
Broster JC, Chambers AJ, Weston LA (2015) The extent of herbicide resistance in Tasmanian wild radish populations. In ‘Proceedings of the 17th Australian agronomy conference’, 21–24 September 2015, Hobart, Tasmania, Australia. (Eds TB Acuᶐña, C Moeller, D Parsons, M Harrison) pp. 582–585. (Australian Society of Agronomy: Hobart, TAS.)
Broster J, Boutsalis P, Gill GS, Preston C (2022a) The extent of herbicide resistance in Lolium rigidum Gaud. (annual ryegrass) across south-eastern Australia as determined from random surveys. Crop & Pasture Science 73(11), 1308-1317.
| Crossref | Google Scholar |
Broster JC, Chambers AJ, Weston LA, Walsh MJ (2022b) Annual ryegrass (Lolium rigidum), wild oats (Avena spp.) and sowthistle (Sonchus oleraceus) are the most commonly occurring weeds in New South Wales cropping fields. Agronomy 12(12), 2914.
| Crossref | Google Scholar |
Broster JC, Boutsalis P, Gill GS, Preston C (2023a) Frequency of herbicide resistance in wild oats (Avena spp.), brome grass (Bromus spp.) and barley grass (Hordeum spp.) as determined by random surveys across south-eastern Australia. Crop & Pasture Science 74(12), 1193-1200.
| Crossref | Google Scholar |
Broster JC, Jalaludin A, Widderick MJ, Chambers AJ, Walsh MJ (2023b) Herbicide resistance in summer annual weeds of Australia’s northern grains region. Agronomy 13(7), 1862.
| Crossref | Google Scholar |
Chauhan BS, Gill G, Preston C (2006a) Seedling recruitment pattern and depth of recruitment of 10 weed species in minimum tillage and no-till seeding systems. Weed Science 54(4), 658-668.
| Crossref | Google Scholar |
Chauhan BS, Gill G, Preston C (2006b) Influence of environmental factors on seed germination and seedling emergence of Oriental mustard (Sisymbrium orientale). Weed Science 54(6), 1025-1031.
| Crossref | Google Scholar |
Chauhan BS, Gill G, Preston C (2006c) Factors affecting seed germination of annual sowthistle (Sonchus oleraceus) in southern Australia. Weed Science 54(5), 854-860.
| Crossref | Google Scholar |
Cheam AH (1986) Seed production and seed dormancy in wild radish (Raphanus raphanistrum L.) and some possibilities for improving control. Weed Research 26(6), 405-414.
| Crossref | Google Scholar |
Cirujeda A, Recasens J, Taberner A (2003) Effect of ploughing and harrowing on a herbicide resistant corn poppy (Papaver rhoeas) population. Biological Agriculture & Horticulture 21(3), 231-246.
| Crossref | Google Scholar |
Cook A, Davidson W, Miller R (2014) A new glyphosate resistant weed species confirmed for northern New South Wales and the world: common sowthistle (Sonchus oleraceus). In ‘Proceedings of the 19th Australasian weeds conference’, 1–4 September 2014, Hobart, Tasmania, Australia. (Ed. M Baker) pp. 206–209. (Tasmanian Weed Society)
Dang HT, Malone JM, Boutsalis P, Gill G, Preston C (2017) Identification of a target-site mutation conferring resistance to triazine herbicides in oriental mustard (Sisymbrium orientale L.) from Australia. Weed Biology and Management 17(4), 153-160.
| Crossref | Google Scholar |
Dang HT, Malone JM, Boutsalis P, Gill G, Preston C (2018) The mechanism of diflufenican resistance and its inheritance in oriental mustard (Sisymbrium orientale L.) from Australia. Pest Management Science 74(6), 1279-1285.
| Crossref | Google Scholar | PubMed |
Derksen DA, Anderson RL, Blackshaw RE, Maxwell B (2002) Weed dynamics and management strategies for cropping systems in the northern Great Plains. Agronomy Journal 94(2), 174-185.
| Crossref | Google Scholar |
Eslami SV, Gill GS, Bellotti B, McDonald G (2006) Wild radish (Raphanus raphanistrum) interference in wheat. Weed Science 54(4), 749-756.
| Crossref | Google Scholar |
Fried G, Norton LR, Reboud X (2008) Environmental and management factors determining weed species composition and diversity in France. Agriculture, Ecosystems & Environment 128(1–2), 68-76.
| Crossref | Google Scholar |
Hashem A, Bowran D, Piper T, Dhammu H (2001) Resistance of wild radish (Raphanus raphanistrum) to acetolactate synthase-inhibiting herbicides in the Western Australia wheat belt. Weed Technology 15(1), 68-74.
| Crossref | Google Scholar |
Hawes C, Squire GR, Hallett PD, Watson CA, Young M (2010) Arable plant communities as indicators of farming practice. Agriculture, Ecosystems & Environment 138(1–2), 17-26.
| Crossref | Google Scholar |
Heap IM (2024) International herbicide-resistant weeds database. Available at www.weedscience.org [accessed 6 September 2024]
Hudson D, Richards R (2014) Evaluation of the agronomic, environmental, economic, and coexistence impacts following the introduction of GM canola to Australia (2008–2010). AgBioForum 17(1), 1-12.
| Google Scholar |
Krishnan M, Petrovic T, Schwerdt JG, Merriam AB, Hereward JP, Preston C (2025) A novel mutation in SoIAA20 confers cross-resistance to 2,4-Dichlorophenoxyacetic acid and other auxinic herbicides in Sonchus oleraceus. Pest Management Science 81(1), 141-148.
| Crossref | Google Scholar | PubMed |
Long W, Malone J, Boutsalis P, Preston C (2019) Diversity and extent of mutations endowing resistance to the acetolactate synthase (AHAS)-inhibiting herbicides in Indian hedge mustard (Sisymbrium orientale) populations in Australia. Pesticide Biochemistry and Physiology 157, 53-59.
| Crossref | Google Scholar | PubMed |
Lu Y-Q, Baker J, Preston C (2007) The spread of resistance to acetolactate synthase inhibiting herbicides in a wind borne, self-pollinated weed species, Lactuca serriola L. Theoretical and Applied Genetics 115, 443-450.
| Crossref | Google Scholar | PubMed |
Merriam AB, Boutsalis P, Malone J, Gill G, Preston C (2018) Extent of herbicide resistant common sowthistle (Sonchus oleraceus) in southern Australia. In ‘Proceedings of 21st Australasian weeds conference’, 9–13 September 2018, Sydney, NSW, Australia. (Eds S Johnson, L Weston, H Wu, B Auld) pp. 16–19. (Weed Science Society of New South Wales)
Murphy CE, Lemerle D (2006) Continuous cropping systems and weed selection. Euphytica 148, 61-73.
| Crossref | Google Scholar |
Owen MJ, Martinez NJ, Powles SB (2014) Multiple herbicide-resistant Lolium rigidum (annual ryegrass) now dominates across the Western Australian grain belt. Weed Research 54(3), 314-324.
| Crossref | Google Scholar |
Owen MJ, Martinez NJ, Powles SB (2015a) Multiple herbicide-resistant wild radish (Raphanus raphanistrum) populations dominate Western Australian cropping fields. Crop & Pasture Science 66(10), 1079-1085.
| Crossref | Google Scholar |
Owen MJ, Martinez NJ, Powles SB (2015b) Herbicide resistance in Bromus and Hordeum spp. in the Western Australian grain belt. Crop & Pasture Science 66(5), 466-473.
| Crossref | Google Scholar |
Owen MJ, Powles SB (2016) The frequency of herbicide-resistant wild oat (Avena spp.) populations remains stable in Western Australian cropping fields. Crop & Pasture Science 67(5), 520-527.
| Crossref | Google Scholar |
Preston C, Powles SB (2002) Evolution of herbicide resistance in weeds: initial frequency of target site-based resistance to acetolactate synthase-inhibiting herbicides in Lolium rigidum. Heredity 88, 8-13.
| Crossref | Google Scholar | PubMed |
Preston C, Dolman FC, Boutsalis P (2013) Multiple resistance to acetohydroxyacid synthase–inhibiting and auxinic herbicides in a population of oriental mustard (Sisymbrium orientale). Weed Science 61(2), 185-192.
| Crossref | Google Scholar |
Reeves TG, Code GR, Piggin CM (1981) Seed production and longevity, seasonal emergence and phenology of wild radish (Raphanus raphanistrum L.). Australian Journal of Experimental Agriculture and Animal Husbandry 21(112), 524-530.
| Crossref | Google Scholar |
Schultz JL, Chatham LA, Riggins CW, Tranel PJ, Bradley KW (2015) Distribution of herbicide resistances and molecular mechanisms conferring resistance in Missouri waterhemp (Amaranthus rudis Sauer) populations. Weed Science 63(1), 336-345.
| Crossref | Google Scholar |
Sheldon JC, Burrows FM (1973) The dispersal effectiveness of the achene–pappus units of selected Compositae in steady winds with convection. New Phytologist 72(3), 665-675.
| Crossref | Google Scholar |
Van der Meulen A, Chauhan BS (2017) A review of weed management in wheat using crop competition. Crop Protection 95, 38-44.
| Crossref | Google Scholar |
Walsh MJ, Duane RD, Powles SB (2001) High frequency of chlorsulfuron-resistant wild radish (Raphanus raphanistrum) populations across the Western Australian wheatbelt. Weed Technology 15(2), 199-203.
| Crossref | Google Scholar |
Walsh MJ, Powles SB, Beard BR, Parkin BT, Porter SA (2004) Multiple-herbicide resistance across four modes of action in wild radish (Raphanus raphanistrum). Weed Science 52(1), 8-13.
| Crossref | Google Scholar |
Walsh M, Newman P, Powles S (2013) Targeting weed seeds in-crop: a new weed control paradigm for global agriculture. Weed Technology 27(3), 431-436.
| Crossref | Google Scholar |
Widderick MJ, Walker SR, Sindel BM, Bell KL (2010) Germination, emergence, and persistence of Sonchus oleraceus, a major crop weed in subtropical Australia. Weed Biology and Management 10(2), 102-112.
| Crossref | Google Scholar |
Yu Q, Powles SB (2014) Resistance to AHAS inhibitor herbicides: current understanding. Pest Management Science 70(9), 1340-1350.
| Crossref | Google Scholar | PubMed |


