Breeding commercially adoptable cotton resistant to reniform nematode
Shiming Liu A * , Dinesh Kafle
A * , Dinesh Kafle  B , Linda Smith
B , Linda Smith  B , Damien Erbacher C , Iain Wilson
B , Damien Erbacher C , Iain Wilson  D and Warwick Stiller A
D and Warwick Stiller A
A
B
C
D
Abstract
The reniform nematode has become a major constraint in central Queensland cotton regions. This prompts a need to develop resistant cotton to control its ongoing impact and spread.
This study aimed to examine the effectiveness of imported resistances to local reniform nematode and demonstrated a breeding approach to develop high-performance resistant cotton with the current genetically modified traits.
Two introduced resistance sources, in either exotic or local genotypic backgrounds, were tested in infested fields to quantify their effect on suppressing reniform population in soils. Backcrossing was employed to transfer the resistance into elite genotypic backgrounds and the resultant lines were tested and selected in a yield test system to identify high-performance lines.
In the infested fields, the resistance from Gossypium barbadense, namely Renbarb, was more capable than Renlon from G. longicalyx, in suppressing the increases of the reniform nematode population, and offered better early plant growth. Three repeated backcrosses were found to be minimally required to re-assure yield potential of the derived families and/or lines with resistance comparable to a recurrent parent. The best lines with Renbarb exhibited higher yields than the commercial varieties in non-infested field conditions and had improved fibre properties.
High-performing cotton resistant to reniform nematode was developed through incorporating the effective resistance into elite genotypic backgrounds and exploiting within-family variation.
Breeding and deploying the resistance should enhance ongoing farm productivity by minimising the impact and spread of reniform.
Keywords: backcross, fibre quality, Gossypium barbadense, G. hirsutum, lint yield, marker-assisted selection, nematode resistance, Rotylenchulus reniformis.
Introduction
Soil-borne parasitic nematodes are a major biotic constraint for global cotton production, because they can significantly reduce cotton productivity via acting alone and/or interacting with soil-borne pathogens, for example, Fusarium, Verticillium, Rhizoctonia solani and Berkeleyomyces basicola (Koenning et al. 2004). Renifom nematode (Rotylenchulus reniformis), as one of four nematode species pathogenic to cotton, is reported to be the most destructive and has increased its spread and impact (Koenning et al. 1996, 2004; Robinson 2007). In the recent two decades, for example, the reported surveys have suggested that reniform has gradually replaced its root-knot counterpart (Meloidogyne incognita) in Mississippi, Louisiana and Alabama in the USA, where the latter was historically the major concern (Singh et al. 2023). Reniform can infest the roots of cotton as early as at seed germination, resulting in stunting and dead plants and subsequently a 40–60% reduced crop yield (Robinson 2007).
The reniform nematode is a sedentary semi-endoparasite but can infect and live with a broad range of plant species, ranging from most field crops to vegetables and weeds species (Koenning et al. 2004; Khan 2005; Robinson 2007). The juveniles from hatching to the J4 stage remain inside the cuticles of the moults to grow into vermiform immature adults. After emerging free, only the females penetrate the cotton root cortex and establish a permanent feeding position in the stele of the roots to steal and compromise plant nutrients, water and carbohydrates and their transportation (Koenning et al. 2004; Robinson 2007). Reniform is highly adaptive against cold, hot and drought conditions and can live in different soil types to a depth of 1 m or more. Its ability to live and survive in deep subsoils increases the chance of its rapid return in topsoils, even under the practice of treating the topsoils with nematicides at planting (Robinson 2007). Similarly, crop rotation systems, either with poor and non-host crops, such as corn, sorghum, wheat, barley and sunnhemp or fallow, can reduce nematode population in soils but the effect was reported to be varied and/or short-lived, as the population often rebounded quickly when the fields returned to plant cotton (Davis et al. 2003; Koenning et al. 2004; Khan 2005; Starr et al. 2007; Smith et al. 2024). These abilities together with its short life cycle make it a most challenging pest to manage and control.
The search and deployment of genetic solutions have been pursued to overcome the above challenges and to find cost-saving and durable control measures for reniform nematode in cotton. The efforts led by USDA researchers concluded that moderate, but largely insufficient, resistance was present within Gossypium hirsutum (Weaver et al. 2007; Gaudin and Wubben 2021); however, there was high resistance to immunity in diploid cotton species, particularly the A, D, E and F subgenomes as well as undomesticated G. barbadense (AD2 genome) (Robinson 2007; Starr et al. 2007; Khanal et al. 2018a). Interspecific breeding was conducted to transfer different sources of resistance into G. hirsutum backgrounds and today a number of resistances are available and can be exploited in developing resistant cotton varieties (Khanal et al. 2018a; Singh et al. 2023). Of them, Renlon and Renbarb are the two most popular being studied and used extensively. The former originates from G. longicalyx (F) and was incorporated into G. hirsutum first by using a bridge-crossing approach to develop a triple-species hybrid followed by many generations of backcrossing (Robinson et al. 2007; Bell et al. 2014). The resistance is controlled by a single dominant gene and confers plant immunity from reniform infestation. However, it induces a plant intolerant response, particularly when used in heavily infested conditions and causes self-harm to plant root cells, which makes the plants themselves less able to take up water and nutrients, hence plants become stunted (Sikkens et al. 2011). Therefore, the use of Renlon becomes questionable for commercial exploitation; however, research is continuing to use it in combination with other control measures, for example, nematicide treatment (Schrimsher et al. 2014), and also even with other resistance genes, i.e. Renbarb (Wubben et al. 2017; Gaudin et al. 2020). Renbarb originates from an undomesticated G. barbadense accession, GB713 (Gutiérrez et al. 2011), and is controlled by two major quantitative trait loci (QTLs), namely, Renbarb2 and Renbarb3, with the former being a major contributor to suppress egg production (Gutiérrez et al. 2011; Wubben et al. 2017; Gaudin et al. 2020). In infested fields, Renbarb showed high resistance to the parasite without intolerant responses to hamper plant growth and development (Sikkens et al. 2011; Schrimsher et al. 2014). Renlon and Renbarb were mapped in different chromosomes in cotton and simple-sequence-repeat (SSR) markers developed (Dighe et al. 2009; Gutiérrez et al. 2011) and stacking them together did not offer any additive resistance (Gaudin et al. 2020). Cotton breeders in USA currently use Renbarb in developing and releasing new resistant cotton varieties, but agronomic performance of these resistant lines requires further improvement (Singh et al. 2021).
The backcross breeding method is most commonly used to transfer novel traits of interest in exotic germplasm into elite locally adapted varieties in plant breeding (Briggs and Allard 1953). The traits are usually simply inherited and/or highly heritable when they belong to quantitative traits. In cotton breeding, the method was used to incorporate new disease resistance genes from non-adapted to adapted germplasm (Bayles et al. 2005), to improve fibre strength (Meredith 1977), and in this transgenic cotton era, to incorporate and stack genetically modified (GM) traits into elite cotton varieties (Zhang 2015). The method was used successfully to transfer nematode resistance from other species into G. hirsutum (Dighe et al. 2009; McCarty et al. 2013, 2017; Bell et al. 2014, 2015, 2023) and to develop new varieties (Singh et al. 2023). The goal of the approach is to develop new varieties competitive to the elite recurrent parent (RP), but with the add-on of resistance from a donor parent; thus, the new version is expected to outperform susceptible commercial varieties grown in the nematode-infested farms while remaining competitive when grown in non-infested farms. Generations of backcrossing and alleles in the donor parent closely linked to the trait of interest can have an impact on the outcome of the approach (Meredith 1977; Bayles et al. 2005) and further understanding is also required on how these factors can affect the outcome in breeding cotton resistant to reniform nematode.
Two species of reniform nematode, namely, R. reniformis and R. parvus, exist in Australia (Colbran 1960). Only R. reniformis causes damage to crops, such as pineapples, vegetables, and sweet potatoes in northern Queensland. It was first detected in a cotton field in 2003, near Emerald in central Queensland, followed by confirmation of its spread and impact in cotton in the Dawson Valley since 2012 (Smith et al. 2024). After importing two resistance sources from USDA, the CSIRO cotton breeding program evaluated their effectiveness and relevance to local reniform species, meanwhile looking for the best strategy of executing breeding resistance to local reniform nematode in cotton. After a comprehensive review on the current impact state of the parasite, the remoteness of infested region to key cotton production regions and also the need of strict adherence of industry biosecurity policy, it became apparent that our breeding effort for the resistance cannot rely on a phenotyping bioassay under the controlled environment to identify resistance and susceptibility in segregating generations of breeding populations, as the other programs are doing (e.g. Robinson et al. 2007). In contrast, given the availability of molecular markers for introducing resistance, we chose an alternative approach to develop resistant cotton, which comprises first using marker-assisted selection to track, select and develop homozygous resistant lines, followed by the agronomic evaluation of the new lines in non-infested field environments to select for improved yield and fibre quality. We demonstrated that such an approach could develop new resistant varieties that are competitive in agronomic performance against the commercial varieties. Thus, when released, they would provide an effective control measure for reniform nematode infested farms and for the industry, so as to restrict and minimise the impact and spread of the parasite while not compromising farm productivity and economic return. In this study, we share the results of that breeding practice.
Materials and methods
Rectification of the applicative of introduced resistance
In the 2016/2017 summer, four introduced resistant germplasms comprising two each of Renlon and Renbarb, were tested in an infested field, along with two commercial varieties, Sicot 71 and Sicot 746B3F. Original seeds of the lines with Renlon and Renbarb resistance were kindly provided by Dr A. A. Bell at USDA-ARS, Southern Plains Agricultural Research Centre, College Station, TX 77845 (Bell et al. 2014), and Dr J. C. McCarty at USDA-ARS, Mississippi State, MS 39762 (McCarty et al. 2013) respectively. Sicot 71 is conventional and Sicot 746B3F is transgenic containing three GM traits, namely, Cry1Ac, Cry2Ab and Vip3A, for insecticidal proteins to kill lepidoptera pests plus tolerance to the herbicide Glyphosate provided by Bayer (https://www.crop.bayer.com.au/products/biotechnology-traits/bollgard-3-with-roundup-ready-flex-cotton). Both commercial varieties are fully susceptible to the infection of reniform nematode and act as the susceptible controls. The experiment was laid out according to the row and column design, with four replications (Whitaker et al. 2002). Single row plots of 5 m in length were hand-sown with a planting rate of 10 seeds/m2 on 30 August 2016. The experiment was terminated on 1 February 2017 at late flowering stage. Soil samples were taken twice from every plot, at sowing time, and at the end of the experiment, with detail given below.
In the 2022/2023 summer, two elite breeding lines with Renlon and four with Renbarb resistant gene were tested in an infested field near Theodore with two commercial and susceptible varieties, Sicot 761B3XF and Siokra 253B3XF. These lines also contain three GM traits for insecticidal proteins as described before and tolerance to glyphosate, dicamba and glufosinate herbicides (https://www.crop.bayer.com.au/products/biotechnology-traits/xtendflex-cotton). In the breeding process, tracking and selecting the resistance to reniform nematode was undertaken with DNA markers (Supplementary Table S1). For Renlon, it is a marker called GI_072641 (Bell et al. 2014). For Renbarb2, two single-nucleotide polymorphism (SNP) markers, i07592Gh and i07587Gh based on the 63K SNP array (Hulse-Kemp et al. 2015), were identified and chosen, because they flanked the Renbarb2 QTL region and exhibited polymorphism between the Australian-bred G. hirsutum and G. barbadense varieties instead of the flanking SSR markers reported in the same chromosome region for Renbarb2 (Gutiérrez et al. 2011). However, we are aware that two SNP markers for Renbarb2 are far apart (~667 kbp), which may lead to linkage drag, especially as the region is derived from G. barbadense.
How the new resistant lines were derived is detailed in the section outlining the breeding scheme. In brief, resistant single plants were first selected on the basis of the presence of the flanking KASP markers and then advanced via selfing to become homozygous lines. After confirming the resistance on the basis of status of the KASP markers, the derived new lines were tested in the regional yield test system to identify the superior lines.
In this infested field experiment, the selected lines were grown in single-row plots 12 m in length in an area of eight planting rows wide and eight plots long so that each test line was planted once in each planting row over eight replications. The experiment was sown on 30 September 2022 and terminated in early February 2023, when plants reached cut-out. Soil samples were taken from every plot twice, prior to planting and at the time of termination.
In both above experiments, plots were divided into multiple 1-m sections before soil sampling and then one sample was taken from the topsoils of up to 10 cm depth from each section. These initial samples were bulked and mixed to represent plot soil sample. They were finally placed in a cooler box with ice bricks and transported to the laboratory at Ecosciences Precinct, Dutton Park, Queensland, to process and count vermiform nematodes described in the next section. The sampling position between prior to planting and at the time of experiment termination may not be the same but should be approximately closer at plot level. At the early flowering stage, biomass samples in 1 m2 were taken from the plots of the first three replications near to head ditch, and plant height, nodes and fresh weight were measured.
Quantify the population of reniform nematode in soil samples
The reniform nematodes were extracted from the soil sample using the modified whitehead tray method developed by Whitehead and Hemming (1965). In brief, 200 mL of soil was placed onto a whitehead tray (metal sieve with tissue paper layer placed inside a tray) and water was added to submerge the soil. Trays were incubated for 3 days inside a dark cabinet. After the incubation period, the metal sieve containing the soil was removed and the remaining solution was poured through two fine sieves, one to remove soil debris (150 microns) and another finer sieve (38 micron) to collect the nematodes. The nematodes collected on fine sieve were rinsed into a vial, which was then analysed under the microscope for identification and quantification of plant-parasitic nematodes.
Breeding scheme for cotton resistance to reniform nematodes
We integrated backcrossing with marker-assisted selection to transfer reniform nematode resistance from two introduced sources into our elite genotypic backgrounds. Fig. 1 shows an example scheme we used to transfer Renlon into an elite conventional line. LONREN-2 was a donor with a normal leaf type, and it had a good performance in our initial field test; 67102OL-117 with okra leaf was used as the recurrent parent (RP). Four times of backcrossing (BC) were conducted throughout. Resistant plants were screened and selected in a segregated family of BC2F1, BC3F1 and BC4F1 generation, and advanced via selfing in the successive generations until they became homozygous. At the end, all derived lines from different BC generations were tested together in field experiments for two consecutive years from 2019/2020.
Breeding scheme and timeline for transferring reniform nematode resistance from a donor parent, LONREN-2 (Renlon), into a current parent, 67102OL-117, via backcrossing. Resistant plants were tracked and selected in each of three backcrossing generations, i.e. BC2F1, BC3F1 and BC4F1, and from them, resistant lines were derived, confirmed and advanced by selfing to become homozygous. All these lines were finally tested together in field experiments in 2019/2020 and 2020/2012. indicatess selfing.
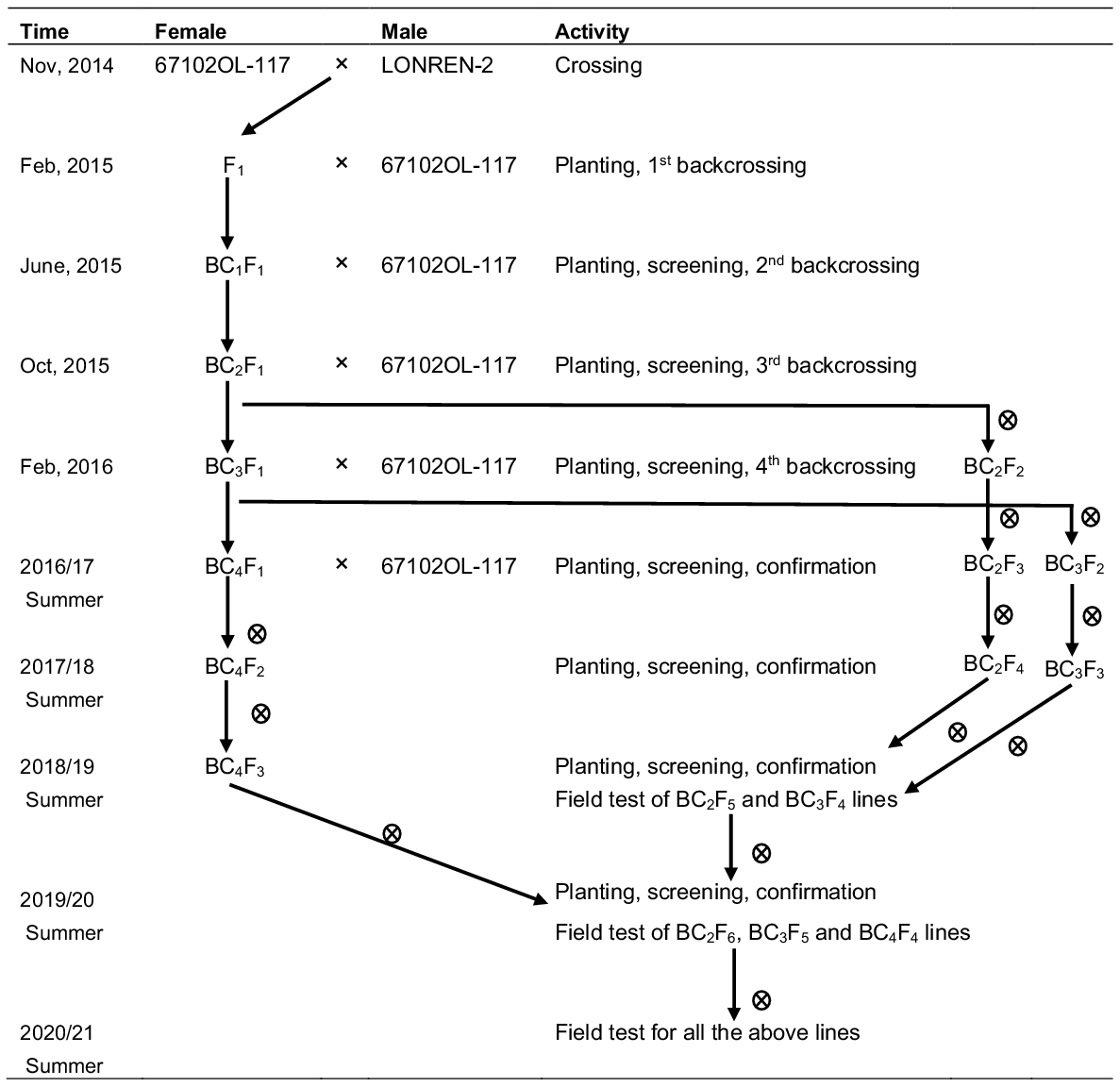
In the 2016/2017 summary, a conventional BC2F3-derived resistant line with Renlon was selected as a donor to cross with a transgenic breeding line containing the B3XF traits, to develop transgenic lines with nematode resistance. The resultant F1 hybrid was further crossed with another F1 hybrid from our B3XF breeding pipeline, to add on and enforce more genetics of our elite germplasm (Supplementary Fig. S1). The final cross was advanced through selfing up to F4 and screening was conducted in a segregating population in a similar manner as described above to select plants with homozygote for transgenic traits as well as Renlon. The seeds from resistant plants were finally harvested and bulked together, and they were space planted and grown as a population in a non-infested field. After confirming resistance status, single plants were harvested and selected on the basis of lint percentage and fibre property measurements of the high-volume instrumentation system (HVI). The derived lines from the retained individual plants were tested, culled and progressed according to the process described in backcross scheme (Fig. 1) in every successive season, poor performers were discarded based on yield and fibre properties in the field test and the retained lines were re-tested in the follow-up season. At the end, the six best candidate lines were tested in the replicate trials over 12 locations in the 2020/2021 and 2021/2022 season. Two of them were kept and used in an infested field experiment above to validate the effectiveness of the resistance.
A backcrossing scheme similar to that for Renlon was used to transfer Renbarb from M713 Ren 5 into an elite breeding line containing the B3XF traits. However, the process was complicated with the number of independent traits to be stacked together, when both two major QTLs for Renbarb2 and Renbar3 were equally targeted. To reduce the complication and save time, a decision was made to focus only on Renbarb2 (referred as Renbarb through the paper for simplicity), while leaving Renbarb3 unscreened, given the latter only attributed minor resistance (Wubben et al. 2017). Three generations of backcrossing were conducted and selection for plants with the presence of nematode resistance and GM traits was conducted in the BC2F1 and BC3F1 generations and they were advanced through selfing in glasshouse, until all traits were homozygous. Following a similar process to that used to develop transgenic lines with Renlon aforementioned, bulked seeds from single homozygous plants were space planted and screened for the confirmation of resistance in a non-infested field. The derived lines were selected and tested seasonally, and, finally, those best performers were tested through two seasons over multiple test sites across cotton production regions free from nematode infestation.
Field experiments
Table S2 provides detail of field experiments conducted for different BC generation-derived families and lines. In brief, single plant-derived lines were initially grown and tested in progeny row experiments at the Australian Cotton Research Institute (ACRI), Myall Vale, near Narrabri, NSW, Australia (30°10′S, 149°35′E). Selection was based on yield and fibre quality and the retained lines were tested in the experiments with two and three replications by using single-row plots for two consecutive seasons.
For the lines containing the B3XF traits derived from two BC generation families with Renbarb, all the lines were initially tested in progeny row experiment for selection of improved yield and fibre properties and then the retained lines were tested in replicated field trials by using three-row plots across cotton production regions free from nematode infestation for two consecutive seasons (Table S3).
All the replicated experiments were according to row and column designs to ensure that test lines were allocated within the experiments optimally and efficiently (Williams and John 1989) and commercial varieties were included as controls.
Statistical analysis
Nematode counts from soil samples were expressed in numbers per 200 mL soil and then subjected to ANOVA. Plant height, nodes and fresh biomass were also subjected to ANOVA. Means were separated using the least significant difference (l.s.d.) at P = 0.05, when genotypic main effect was significant (P ≤ 0.05) according to F-test.
Data for lint yield and fibre quality in replicated field experiments were pooled together for all BC families with Renlon and Renbarb respectively to form multiple environment trial (MET) datasets. METs were analysed with linear mixed model by using REML (Smith et al. 2005). In the analysis, spatial variations were examined and considered when they were present (Liu et al. 2015). Given that test lines were progressively reduced in the experiments after following the seasonal test and selection, only best linear unbiased estimates (BLUEs) of the lines that remained in the final season experiments were obtained for the comparison, from the appropriate models fitted.
The role of repeated backcrossing is to reconstitute the genetic background of the recurrent parent, therefore, in theory, the recovery rate of RP background is increased with the times of backcrossing conducted (Briggs and Allard 1953). In this study, yield potential exhibited progressive increase for the families derived from 2–4 BC generation with Renlon resistance. That trend allowed to estimate the observed recovery rate when using mean lint yields of each BC generation-derived family, the RP and the donor. The estimates were plotted against the theoretical recovery rate to examine the effectiveness of our current BC breeding practice.
All the analyses were processed using R4.4.0 (R Core Team 2024) and ASreml (Butler et al. 2023).
Results
Effectiveness of introduced resistance
At pre-planting, the initial reniform population was lower in the number in the field used in 2016/2017 than in 2021/2022 (Fig. 2). All four introduced resistant lines were effective in suppressing the increase of reniform population during the early crop-growing period up to mid-season when the experiment was terminated. There was a substantial increase (≥1000-fold) of reniform nematode in the soil samples planted with two susceptible commercial varieties (Fig. 2a). In comparison, LONREN-2 was less effective than LONREN-1, and also M713 Ren 1 and 5, in containing the resistance from G. barbadense.
Nematode population in soil samples prior to planting and at plant cut-out stage in field plots planted with (a) the introduced, or (b) locally bred resistant lines and susceptible varieties. Two LONREN lines carry a Renlon resistance; two M713 lines have both Renbarb2 and Renbarb3 resistance. Breeding lines, CSX3111 and 3279, have Renlon resistance; the lines, CSX3206, 3291, 1284 and 1347 have Renbarb2 from M713 Ren 5, but whether they also have Renbarb3 was unclear, because that QTL was not screened, tracked and selected; Sicot 71 and Sicot 746B3F in the upper panel (a) and Sicot 761B3XF and Siokra 253B3XF in the low panel (b) are susceptible commercial varieties. Different letters between the means of prior-sown and cut-out stage for each test lines suggest their significant difference at P = 0.05; error bars represent the standard error of the mean. B3F, Bollgard 3 Roundup Ready Flex®; and B3XF, Bollgard 3 XtendFLEX®.
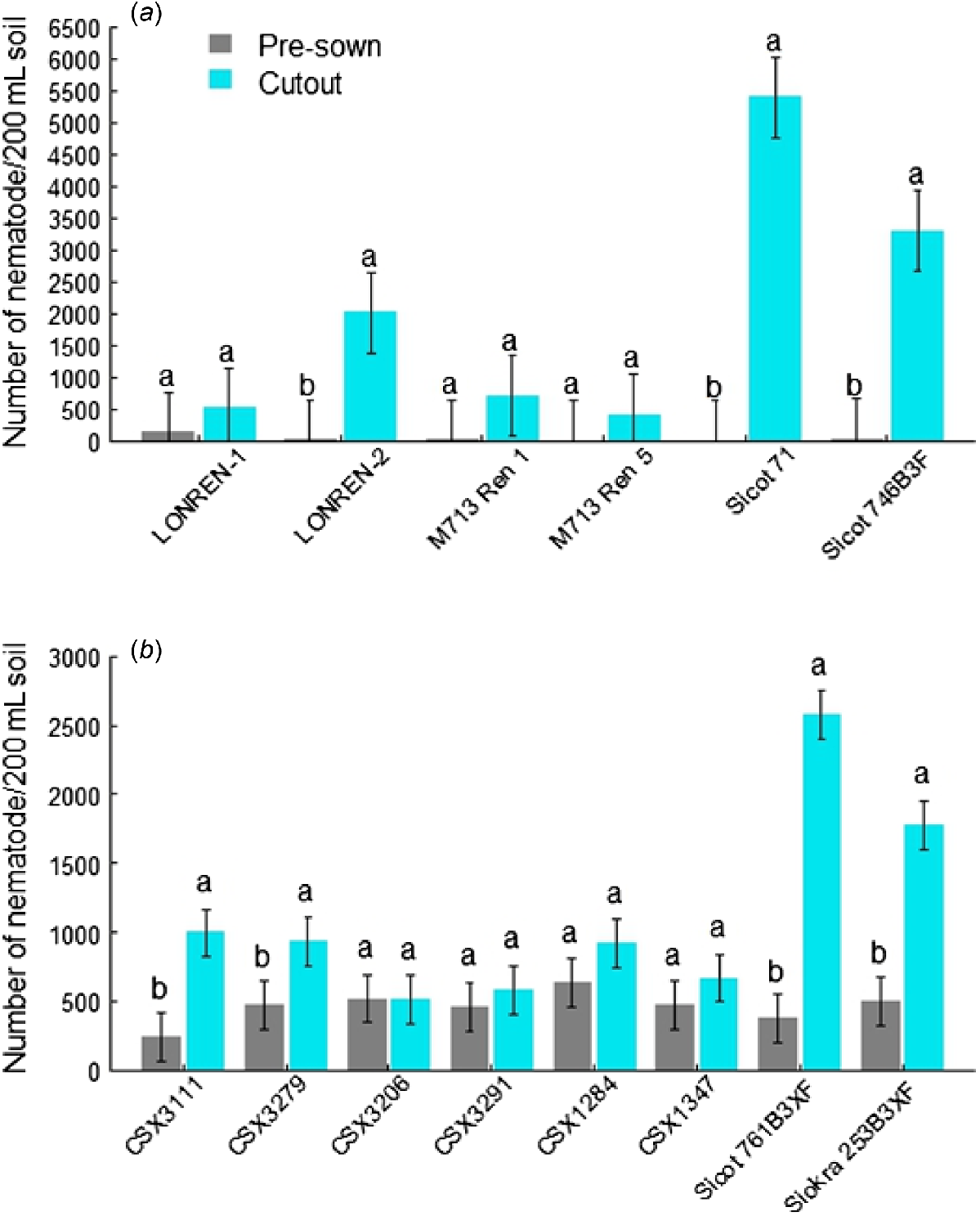
Newly bred resistant lines were similarly effective in suppressing reniform population, i.e. a significantly reduced gain of reniform population in plots grown with resistant cotton over the crop period to mid-season, in contrast to a substantial increase in plots grown with susceptible controls (Fig. 2b). This is particularly evident in four lines with Renbarb from M713 Ren 5, because the parasite population remained unchanged throughout two soil sampling times. In two lines with Renlon from LONREN-2; however, the increase was still statistically significant, but not to the same extent as what was observed in the soil samples where two susceptible controls were grown.
Plant measurements taken after 3 months of planting suggested taller plants in the most resistant lines with Renbarb (Table S4). This was also accompanied with longer internodes and higher accumulated fresh biomass/m2. In contrast, two lines with Renlon and Sicot 761B3XF had shorter plant and/or lower fresh biomass; therefore, they exhibited less early growth.
Effect of backcross generations on agronomic performance
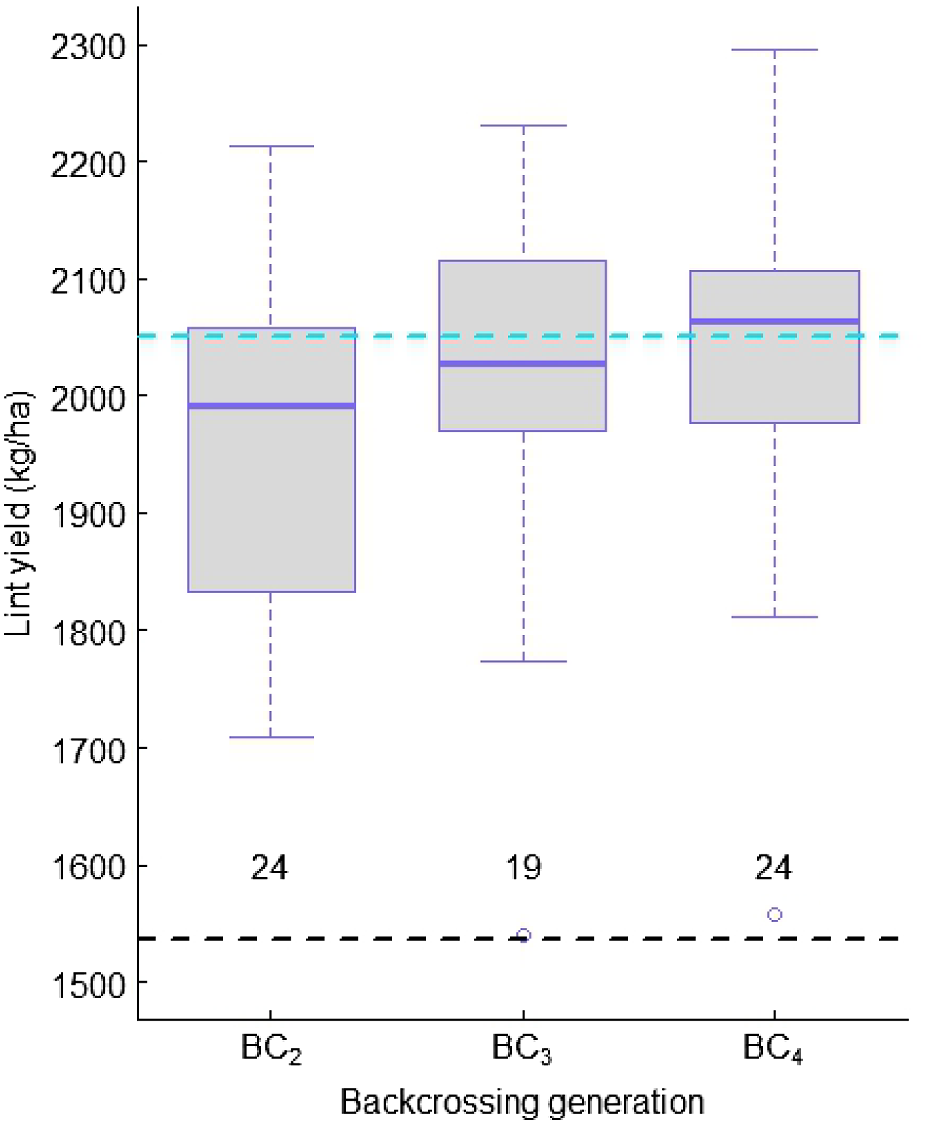
Lint yield gradually shifted higher in response to the number of times of backcrossing, whereas the variability remained comparable among the families derived from different BC generations (Fig. 3). Mean yields of BC3 and BC4 families were close to the RP, and they also contained individual lines that outyielded the RP. When yield gain across different backcross generations was converted as the recovery rate of the RP, it showed a strong relation with the theoretical (Fig. S2). The recovery rates matched each other in BC3, and were better than the theoretical in BC4, but lower than the theoretical in BC2, which may suggest some challenges achieving the expected recovery in early backcrossing generations.
Mean and variation of lint yield in three backcrossing generation-derived families with Renlon resistance when tested in non-infested fields. Blue and black dashed lines represent lint yield of a recurrent parent, 67102OL-117, and a donor, LONREN-2. The value below each boxplot represents the number of test lines.
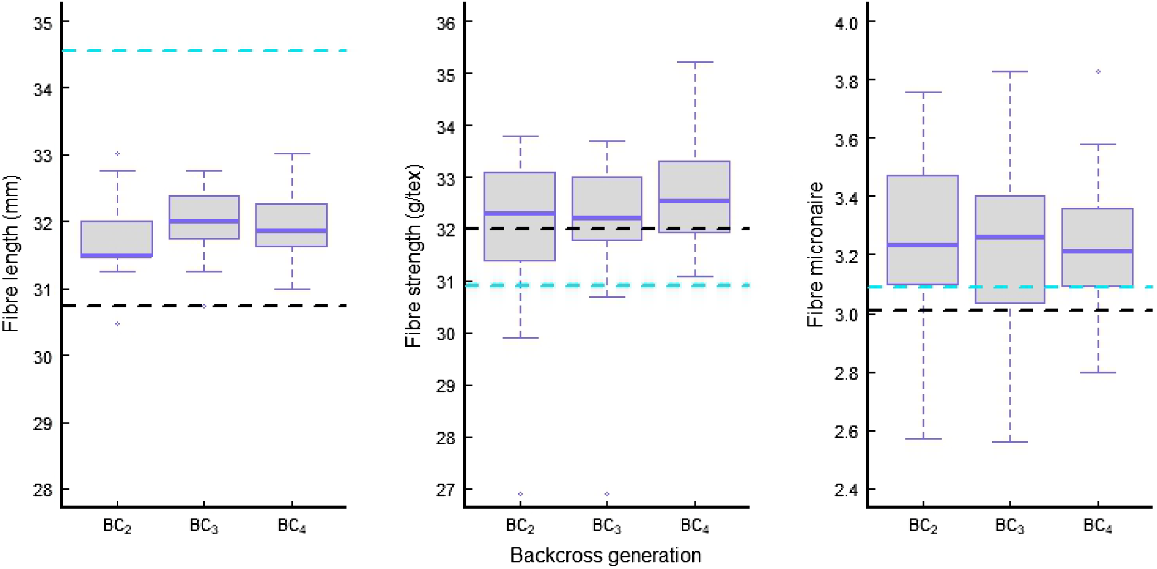
The effect of successive backcross generations on fibre properties was not as apparent as seen in lint yield (Fig. 4). Mean fibre length never achieved the level of the recurrent parent. For both fibre strength and micronaire, the RP and donor had a small difference, and there was also no apparent gain or loss with the times of repeated backcrossing; however, all BC families had higher means than their two parents. This phenomenon was more obvious for fibre strength and micronaire, when four best-yielding lines from different BC generations were compared (Table S5). Therefore, the resistant donor contributed favourable alleles to improve these fibre properties, which agreed with the previous finding that LONREN lines had better fibre strength and finer fibre (Bell et al. 2014). Our evidence also indicates that the positive contribution from the donor did not diminish with the times of repeat backcrossing, which drove a proportional reduction of donor genetics in the resultant families and lines.
Mean and variation of three fibre quality properties in the families from different backcrossing generations with Renlon resistance when tested in non-infested fields. Blue and black dashed lines represent mean fibre quality properties of a recurrent parent, 67102OL-117, and a donor, LONREN-2.
Not all transgenic lines with the resistance were derived from backcrossing, but from a cross of two hybrids sharing a common elite parent (Fig. S1). When the best lines were tested in non-infested farm sites, they exhibited 6–9% lower lint yield than the control, Sicot 761B3XF, but possessed improved fibre properties (Table S6). One explanation is linkage drag and/or pleiotropy may still act with Renlon (Robinson et al. 2007). Two of these lines also showed less early growth vigour when grown in an infested field (Table S4), which partially supports the previous finding of the association between stunted plants and the presence of Renlon (e.g. Sikkens et al. 2011). All the above findings made us conclude that continued work with Renlon was not viable; so, no further work was conducted.
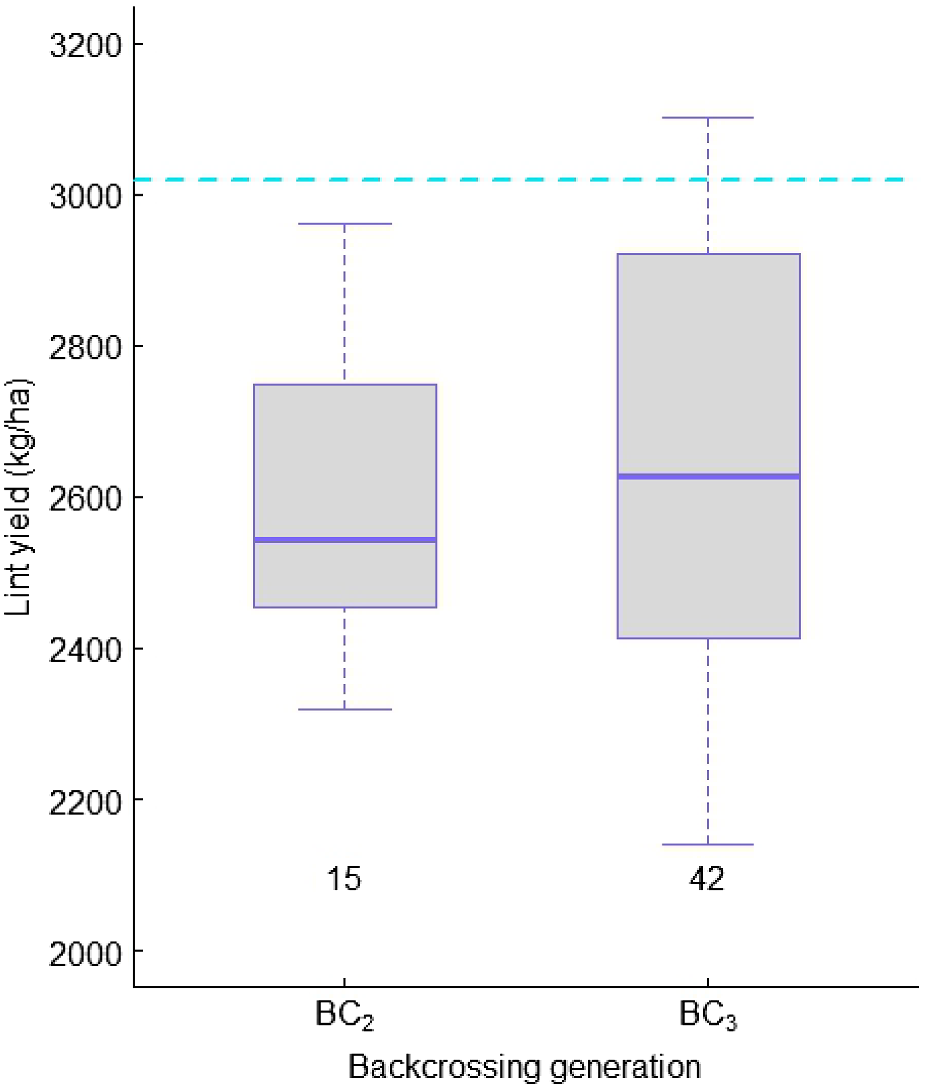
A similar number of derived lines from BC2 and BC3 were initially tested in a non-replicated progeny row experiment, after they were derived from retained individual plants. Subsequent selection resulted in a greater number of lines retained from the BC3-derived (42) than BC2-derived (15) family (Table S3); one more backcrossing, therefore, was able to improve both yield and fibre properties. When selections were tested in multiple sites over two seasons, the superiority of BC3- over BC2-derived families remained unchanged, and, more interestingly, within the BC3-derived family, there were lines that yielded as high as the best control, Sicot 619B3XF (Table 1, Fig. 5).
| Line | Backcross generation | Lint yield (kg/ha) | Lint percentage (%) | Length (mm) | Strength (g/tex) | Micronaire | |
|---|---|---|---|---|---|---|---|
| CSX3215 | BC2 | 2962a | 42.6bc | 32.1ab | 31.5b | 4.4a | |
| CSX3321 | BC2 | 2963a | 41.8cd | 32.4a | 33.2a | 4.2bc | |
| CSX3206 | BC2 | 2583b | 40.8d | 32.4ab | 33.2ab | 3.6f | |
| CSX3291 | BC2 | 2516b | 41.0d | 32.7ab | 32.6bc | 3.5f | |
| CSX1126 | BC3 | 3061a | 41.3d | 32.1ab | 33.6a | 4.1c | |
| CSX1228 | BC3 | 3068a | 43.4b | 32.0ab | 33.8a | 4.2bc | |
| CSX1443 | BC3 | 3045a | 42.2cd | 32.3a | 31.6b | 4.1c | |
| CSX1484 | BC3 | 3101a | 43.1bc | 31.8ab | 33.5a | 3.9d | |
| CSX1347 | BC3 | 2980a | 42.4bc | 33.3a | 34.4a | 3.8d | |
| CSX1284 | BC3 | 2807a | 42.6bc | 32.3ab | 32.9b | 3.8d | |
| Sicot 619B3XF | 3018a | 42.3cd | 29.9b | 32.9ab | 4.1c | ||
| Sicot 761B3XF | 3010a | 44.5a | 30.9b | 32.5ab | 4.3ab | ||
| l.s.d.0.05 | 221.7 | 1.02 | 1.30 | 1.39 | 0.20 |
B3XF, Bollgard 3 XtendFLEX®.
Different letters in the same column indicate a significant difference at P = 0.05.
Mean and variation of lint yield in the families derived from two backcrossing generations with Renbarb resistance when tested in non-infestation fields over different regions. Blue dashed line represents lint yield of the best control, Sicot 619B3XF, in the experiments. The values below each boxplot represent the number of test lines. B3XF, Bollgard 3 XtendFLEX®.
Yield and fibre properties from non-infested field test of best yielders and those tested in an infested field in 2022/2023 are given in Table 1. Except for two lines from BC2 family, other lines had comparable yield one to another and to the controls, with means of those BC3-derived lines being higher in general. Lint percentage was comparable to the control, Sicot 619B3XF, but lower than that for Sicot 761B3XF. Fibre quality was overall better than the controls.
Discussion
The invasion and establishment of reniform nematode in the northern traditional cotton production regions in Australia has signalled its potential to become a major threat to the entire industry (Stirling 2023; Smith et al. 2024). Concurrently, there is also renewed interest in developing cotton production in northern Australia, where reniform nematode has been known to exist for a long time (Stirling 2023). It is likely that both these developments will inevitably lead to accelerated parasite spill-over and spread into other non-infested regions, under today’s farm practice and logistics (Robinson 2007). Therefore, there is urgency to develop and implement a comprehensive management strategy for reniform nematode in Australia. The strategy should be composed of all aspects from farm gate hygiene, crop rotation, weed control, nematicide application to use of host plant resistance (Robinson 2007; Starr et al. 2007; Singh et al. 2023; Smith et al. 2024). In this study, we first attempted in Australia to understand the effectiveness of two popular resistance sources from overseas to local parasite and to undertake initial breeding efforts to develop high-performing reniform nematode resistant cotton.
Our results supported that both sources of resistance, either in their overseas or domestic elite genotypic backgrounds, were effective in suppressing local reniform reproduction when grown in infested field conditions. Meanwhile, Renlon from LONREN-2 was not as effective as Renbarb, which is inconsistent with previous studies in the USA (e. g. Bell et al. 2014; Gaudin et al. 2020). The observation also contradicts higher effectiveness of LONREN-1 in the same experiment. This inconsistency agrees with the findings of the study of Schrimsher et al. (2014), but disagrees with the evidence that both LONREN lines share the same breeding origin and their resistance is reported to confer plants immune to reniform (Yik and Birchfield 1984; Robinson et al. 2007; Bell et al. 2014). One explanation for that may be due to the interaction of the Renlon resistance with local reniform nematode population. The variability of reniform nematode was reported in the populations collected in different regions of the USA and other countries and the same was the case for the existence of different pathogenicity (Agudelo et al. 2005; Khanal et al. 2018b). Therefore, further studies are needed to understand the interactions of local reniform population with different resistance genes.
It is noteworthy that for the resistance carried in M713 Ren 5 background, this study focused only on tracking and selecting Renbarb2, owing to its major role behind the resistance (Wubben et al. 2017) as well as for reducing the number of traits targeted in backcrossing introgression process. It is interesting that our results confirmed that all derived resistant lines were highly effective in reducing the build-up of reniform population in an infested field. Wubben et al. (2017) demonstrated that the isogenic line with both Renbarb2 and Renbarb3 QTLs offered increased suppression on reniform egg production. Therefore, the questions remain on whether both resistant regions should be pyramided for better control on local reniform nematode and how that may affect agronomic performance of derived lines.
In this study, the backcross breeding method was used to transfer the resistance from two imported sources into our elite germplasm backgrounds. We relied on KASP marker-assisted selection to track and screen the resistance and conducted repeated backcrossing of selected resistant plants to reconstitute the genetics of the RP. This approach allowed selection of lines in non-infested conditions without the need of lab and field phenotyping for resistance, which subsequently saved time and resources. On the basis of the confirmation test of infested field experiments, KASP markers re-developed from the initial SSR markers or corresponding genomic regions for the resistance (Dighe et al. 2009; Gutiérrez et al. 2011; Bell et al. 2014) are reliable. Other researchers used an approach similar to that used for developing resistant germplasm in this study (Gutiérrez et al. 2011; McCarty et al. 2013). More interestingly, for Renbarb, the markers we used for selection covered much wider genomic region than that flanked with SSR markers (Gutiérrez et al. 2011). That may imply that resultant lines will contain a relatively large genetics of G. barbadense after introgression of the resistance. Deleterious genes in that introgressed region will no doubt affect agronomic performance of the resultant lines, if they exist. A good example is the phenomenon observed for Renlon in this study (Table S6). However, that does not appear to be the case for Renbarb, because relying on field evaluation, there was still a good chance of identifying outstanding lines with yield and fibre quality competitive to the commercial control varieties (Table 1). Nevertheless, further research is needed to confirm whether genomic region hosting Renbarb2 is benign to agronomic performance or not. Now such work should become easier to do with the recent progress made in identifying its likely candidate genes (Wubben et al. 2025).
The goal of our breeding effort is to develop reniform nematode-resistant cotton with agronomic performance similar to that of the current commercial varieties. Our approach also allowed breeding and selection focusing on agronomic performance within the BC-derived families, once the resistance was incorporated. This increased the likelihood of selecting outstanding lines as evident in our results (Tables 1, S5). However, with this approach, there were challenges. The most important one is undesired agronomic traits of the donor parents associated with the resistance. In this study, this is particularly evident for Renlon resistance. Repeated backcrossing has been suggested to facilitate the recombination of genomic regions associated with resistance, because that undesired linkage with the traits will be broken, and to increase performance of the resultant progenies. However, for Renlon resistance, this did not appear to occur; even resistant germplasm containing more local genetic background was used in transgenic breeding pipeline to develop better-performing transgenic resistant cotton. If this is due to linkage drag and/or pleiotropy, reducing the introgressed chromosome genomic segment should be attempted. Alternatively, breeders should evaluate alternate sources of resistance. In this study, we did not see any significant challenges imposed by Renbarb on agronomic performance, despite the fact that a relatively large coverage of its flanking KASP markers may potentially bring about unexpected genetics from G. barbadense affecting agronomic performance as discussed aforementioned. The other important matter for breeding is the recovery of the RP after each repeated backcross. In this study, we observed a lower-than-expected recovery for lint yield in the families from the second backcrossing with Renlon resistance, despite this was not occurring in the other BC generations. This is likely to be due to insufficient or unrepresentative progeny plants being selected for successive generation backcrossing. It is known that there is a larger segregation in early generations of backcrossing. In our current breeding practice, we still rely on phenotypic selection and empirical experience to decide the number of plants being selected for successive backcrossing, and the occurrence of the above phenomenon is expected. Frisch and Melchinger (2005) used simulation to demonstrate this risk. Therefore, a better alternative is to use a marker-assisted backcross breeding strategy guided with genomic prediction and simulation, because this will assist plant breeders to assess, identify and select individual progenies with higher genetic worth for successive BC, intermating or both (Visscher et al. 1996; Ni et al. 2023) through a look-ahead exercise, so as to rapidly achieve the pre-set breeding outcome.
Our study also demonstrated that three backcrosses were sufficient to shift lint yield as high as the RP, while transferring Renlon reniform-nematode resistance from a poor agronomic and exotic line into elite genotypic backgrounds. The fourth addition could provide additional gain at the family level, but not in terms of the best-yielding fixed lines (Table S5). For fibre-quality properties, three backcrosses were sufficient to recover the superiority of traits of the RP (Fig. 4). This is consistent with the fact that fibre properties are usually inherited simpler than lint yield in cotton (Liu et al. 2011; Campbell and Myers 2015). The minimal backcross generations required in this study were consistent with the number used for introgression of bacterial blight resistance in cotton (Bayles et al. 2005) and reported for transgenic trait introgression (Zhang 2015; Conaty et al. 2022).
Conclusions
This study demonstrated the effectiveness of two exotic resistance sources to local reniform nematode and identified the superior one, i.e. Renbarb, for breeding resistant cotton in Australia. Backcrossing integrated with marker-assisted selection for resistance was effective in transferring the resistance into elite genotypic backgrounds, but to regain yield competitiveness of the resultant families, at least three times of repeated backcrossing was required. That, plus further exploitation of within-family variation should ensure to retain and identify lines with the combination of high performance and resistance to reniform nematode. Relying on such an approach, this study successfully stacked resistance together with current transgenic traits and developed resistant transgenic lines with promising performance in both yield and fibre quality. Because they were competitive to the current commercial varieties in non-infested region in our multi-location and season yield testings, we would expect their adoption to provide a cost-effective measure to the infested farms to control and manage reniform nematode and then to ensure higher farm productivity and economic return. This measure should also minimise the future spread and impact of the parasite. We further propose more effective breeding strategies for cotton resistance to reniform nematode, under the vision of further enhanced agronomic performance as well as durable resistance.
Data availability
The data that supports the findings of this study can be provided upon reasonable request.
Declaration of funding
This study was funded through Cotton Breeding Australia, a Joint Venture between CSIRO and Cotton Seed Distributors (Wee Waa, NSW 2388, Australia), Grant Number: R-19598.
Author contributions
S. Liu, I. Wilson and W. Stiller conceived and designed the study. S. Liu, D. Kafle, L. Smith and D. Erbacher collated and analysed data. S. Liu, D. Kafle, L. Smith, I. Wilson and W. Stiller prepared the first draft. All authors commented on and revised the manuscript. All authors read and approved the final paper.
Acknowledgements
The authors acknowledge the technical support of our past and current CSIRO team members in Narrabri: Taryn Hunter, Leah Rood-England, Kellie Cooper, Jo Beckhouse, Sandra Magann, Alan Thompson, Chris Allen, Louise Zemcevicius, Demi Mackay, Susie Thompson, Heidi Clements, Kay Smith, Deon Cameron, Max Barnes, Scott McCarron, Adam Suckling, and in Canberra: Haylee Martin, Jackie Oliver and Judith Gaudron, and in Theodore: Kirk Anderson.
References
Agudelo P, Robbins RT, Stewart JM, Szalanski AL (2005) Intraspecific variability of Rotylenchulus reniformis from cotton-growing regions in the United States. Journal of Nematology 37, 105-114.
| Google Scholar | PubMed |
Bayles MB, Verhalen LM, McCall LL, Johnson WM, Barnes BR (2005) Recovery of recurrent parent traits when backcrossing in cotton. Crop Science 45, 2087-2095.
| Crossref | Google Scholar |
Bell AA, Forest Robinson A, Quintana J, Dighe ND, Menz MA, Stelly DM, Zheng X, Jones JE, Overstreet C, Burris E, Cantrell RG, Nichols RL (2014) Registration of LONREN-1 and LONREN-2 germplasm lines of upland cotton resistant to reniform nematode. Journal of Plant Registrations 8, 187-190.
| Crossref | Google Scholar |
Bell AA, Robinson AF, Quintana J, Duke SE, Starr JL, Stelly DM, Zheng X, Prom S, Saladino V, Gutiérrez OA, Stetina SR, Nichols RL (2015) Registration of BARBREN-713 germplasm line of upland cotton resistant to reniform and root-knot nematodes. Journal of Plant Registrations 9, 89-93.
| Crossref | Google Scholar |
Bell AA, Robinson AF, Quintana J, Hinze LL, Harris J, Liu J, Wagner T, Prom S, Saladino V, Zheng X, Stelly DM, Nichols RL (2023) Registration of eight germplasm lines of upland cotton (Gossypium hirsutum L.) resistant to reniform nematodes (Rotylenchulus reniformis) with elite agronomic performance. Journal of Plant Registrations 17, 536-543.
| Crossref | Google Scholar |
Briggs FN, Allard RW (1953) The current status of the backcross method of plant breeding. Agronomy Journal 45, 131-138.
| Crossref | Google Scholar |
Colbran RC (1960) Chemical control of nematodes in south Queensland pineapple fields. Queensland Journal of Agricultural Science 17, 165-173.
| Google Scholar |
Conaty WC, Broughton KJ, Egan LM, Li X, Li Z, Liu S, Llewellyn DJ, MacMillan CP, Moncuquet P, Rolland V, Ross B, Sargent D, Zhu Q-H, Pettolino FA, Stiller WN (2022) Cotton breeding in Australia: meeting the challenges of the 21st century. Frontiers in Plant Science 13, 904131.
| Crossref | Google Scholar |
Davis RF, Koenning SR, Kemerait RC, Cummings TD, Shurley WD (2003) Rotylenchulus reniformis management in cotton with crop rotation. Journal of Nematology 35, 58-64.
| Google Scholar | PubMed |
Dighe ND, Robinson AF, Bell AA, Menz MA, Cantrell RG, Stelly DM (2009) Linkage mapping of resistance to reniform nematode in cotton following introgression from Gossypium longicalyx (Hutch. & Lee). Crop Science 49, 1151-1164.
| Crossref | Google Scholar |
Frisch M, Melchinger AE (2005) Selection theory for marker-assisted backcrossing. Genetics 170, 909-917.
| Crossref | Google Scholar | PubMed |
Gaudin AG, Wubben MJ (2021) Genotypic and phenotypic evaluation of wild cotton accessions previously identified as resistant to root-knot (Meloidogyne incognita) or reniform nematode (Rotylenchulus reniformis). Euphytica 217, 207.
| Crossref | Google Scholar |
Gaudin AG, Wallace TP, Scheffler JA, Stetina SR, Wubben MJ (2020) Effects of combining Renlon with Renbarb1 and Renbarb2 on resistance of cotton (Gossypium hirsutum L.) to reniform nematode (Rotylenchulus reniformis Linford and Oliveria). Euphytica 216, 67.
| Crossref | Google Scholar |
Gutiérrez OA, Robinson AF, Jenkins JN, McCarty JC, Wubben MJ, Callahan FE, Nichols RL (2011) Identification of QTL regions and SSR markers associated with resistance to reniform nematode in Gossypium barbadense L. accession GB713. Theoretical and Applied Genetics 122, 271-280.
| Crossref | Google Scholar | PubMed |
Hulse-Kemp AM, Lemm J, Plieske J, Ashrafi H, Buyyarapu R, Fang DD, Frelichowski J, Giband M, Hague S, Hinze LL, et al. (2015) Development of a 63K SNP array for cotton and high-density mapping of intraspecific and interspecific populations of Gossypium spp. G3 Genes|Genomes|Genetics 5, 1187-1209.
| Crossref | Google Scholar | PubMed |
Khan MR (2005) Hosts and non-hosts of reniform nematode, Rotylenchulus reniformis Linford & Oliveira, 1940 – a critical review. Environment and Ecology 23, 124-140.
| Google Scholar |
Khanal C, McGawley EC, Overstreet C, Stetina SR (2018a) The elusive search for reniform nematode resistance in cotton. Phytopathology 108, 532-541.
| Crossref | Google Scholar |
Khanal C, McGawley EC, Overstreet C, Stetina SR, Myers GO, Kularathna MT, McInnes B, Godoy FMC (2018b) Reproduction and pathogenicity of endemic populations of Rotylenchulus reniformis on cotton. Nematropica 48, 68-81.
| Google Scholar |
Koenning SR, Walters SA, Barker KR (1996) Impact of soil texture on the reproductive and damage potentials of Rotylenchulus reniformis and Meloidogyne incognita on cotton. Journal of Nematology 28, 527-536.
| Google Scholar | PubMed |
Koenning SR, Wrather JA, Kirkpatrick TL, Walker NR, Starr JL, Mueller JD (2004) Plant-parasitic nematodes attacking cotton in the United States: old and emerging production challenges. Plant Disease 88, 100-113.
| Crossref | Google Scholar | PubMed |
Liu S, Llewellyn DJ, Stiller WN, Jacobs J, Lacape J-M, Constable GA (2011) Heritability and predicted selection response of yield components and fibre properties in an inter-specific derived RIL population of cotton. Euphytica 178, 309-320.
| Crossref | Google Scholar |
Liu SM, Constable GA, Cullis BR, Stiller WN, Reid PE (2015) Benefit of spatial analysis for furrow irrigated cotton breeding trials. Euphytica 201, 253-264.
| Crossref | Google Scholar |
McCarty JC, Jr., Jenkins JN, Wubben MJ, Gutierrez OA, Hayes RW, Callahan FE, Deng D (2013) Registration of three germplasm lines of cotton derived from Gossypium barbadense L. accession GB713 with resistance to the reniform nematode. Journal of Plant Registrations 7, 220-223.
| Crossref | Google Scholar |
McCarty JC, Jr., Jenkins JN, Wubben MJ, Hayes RW, Callahan FE, Deng D (2017) Registration of six germplasm lines of cotton with resistance to the root-knot and reniform nematodes. Journal of Plant Registrations 11, 168-171.
| Crossref | Google Scholar |
Meredith W, Jr. (1977) Backcross breeding to increase fiber strength of cotton. Crop Science 17, 172-175.
| Crossref | Google Scholar |
Ni Z, Moeinizade S, Kusmec A, Hu G, Wang L, Schnable PS (2023) New insights into trait introgression with the look-ahead intercrossing strategy. G3 Genes|Genomes|Genetics 13, jkad042.
| Crossref | Google Scholar |
Robinson AF (2007) Reniform in U.S. cotton: when, where, why, and some remedies. Annual Review of Phytopathology 45, 263-288.
| Crossref | Google Scholar | PubMed |
Robinson AF, Bell AA, Dighe ND, Menz MA, Nichols RL, Stelly DM (2007) Introgression of resistance to nematode Rotylenchulus reniformis into upland cotton (Gossypium hirsutum) from Gossypium longicalyx. Crop Science 47, 1865-1877.
| Crossref | Google Scholar |
Schrimsher DW, Lawrence KS, Sikkens RB, Weaver DB (2014) Nematicides enhance growth and yield of Rotylenchulus reniformis resistant cotton genotypes. Journal of Nematology 46(4), 365-375.
| Google Scholar | PubMed |
Sikkens RB, Weaver DB, Lawrence KS, Moore SR, van Santen E (2011) LONREN upland cotton germplasm response to Rotylenchulus reniformis inoculum level. Nematropica 41, 68-74.
| Google Scholar |
Singh B, Chastain D, Reddy KR, Snider J, Krutz LJ, Stetina S, Sehgal A (2021) Agronomic characterization of cotton genotypes susceptible and resistant to reniform nematode in the United States Midsouth. Agronomy Journal 113, 4280-4291.
| Crossref | Google Scholar |
Singh B, Chastain D, Kaur G, Snider JL, Stetina SR, Bazzer SK (2023) Reniform nematode impact on cotton growth and management strategies: a review. Agronomy Journal 115, 2140-2158.
| Crossref | Google Scholar |
Smith AB, Cullis BR, Thompson R (2005) The analysis of crop cultivar breeding and evaluation trials: an overview of current mixed model approaches. The Journal of Agricultural Science 143, 449-462.
| Crossref | Google Scholar |
Smith LJ, Scheikowski L, Kafle D (2024) The distribution of reniform nematode (Rotylenchulus reniformis) in cotton fields in central Queensland and population dynamics in response to cropping regime. Pathogens 13, 888.
| Crossref | Google Scholar |
Starr JL, Koenning SR, Kirkpatrick TL, Robinson AF, Roberts PA, Nichols RL (2007) The future of nematode management in cotton. Journal of Nematology 39, 283-294.
| Google Scholar | PubMed |
Stirling GR (2023) Reniform nematode (Rotylenchulus reniformis): a biosecurity threat to cotton, vegetable and horticultural industries in tropical and subtropical regions of Australia. Avaliable at https://static1.squarespace.com/static/65a72e9e0057f860a3a58c45/t/65cd98c617cfd24c004c5503/1707972806925/PSN+051+Biosecurity+Rotylenchulus+reniformis.pdf [accesed 5 September 2025].
| Google Scholar |
Visscher PM, Haley CS, Thompson R (1996) Marker-assisted introgression in backcross breeding programs. Genetics 144, 1923-1932.
| Crossref | Google Scholar | PubMed |
Weaver DB, Lawrence KS, van Santen E (2007) Reniform nematode resistance in upland cotton germplasm. Crop Science 47, 19-24.
| Crossref | Google Scholar |
Whitehead AG, Hemming JR (1965) A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Annals of Applied Biology 55, 25-38.
| Crossref | Google Scholar |
Williams ER, John JA (1989) Construction of row and column designs with contiguous replicates. Journal of the Royal Statistical Society. Series C (Applied Statistics) 38, 149-154.
| Crossref | Google Scholar |
Wubben MJ, McCarty JC, Jr, Jenkins JN, Callahan FE, Deng DD (2017) Individual and combined contributions of the Renbarb1, Renbarb2, and Renbarb3 quantitative trait loci to reniform nematode (Rotylenchulus reniformis Linford & Oliveira) resistance in upland cotton (Gossypium hirsutum L.). Euphytica 213, 47.
| Crossref | Google Scholar |
Wubben MJ, Khanal S, Gaudin AG, Callahan FE, McCarty JC, Jr, Jenkins JN, Nichols RL, Chee PW (2025) Transcriptome profiling and RNA-Seq SNP analysis of reniform nematode (Rotylenchulus reniformis) resistant cotton (Gossypium hirsutum) identifies activated defense pathways and candidate resistance genes. Frontiers in Plant Science 16, 1532943.
| Crossref | Google Scholar |
Yik CP, Birchfield W (1984) Resistant germplasm in Gossypium species and related plants to Rotylenchulus reniformis. Journal of Nematology 16, 146-153.
| Google Scholar | PubMed |


