Prospects for diversifying temperate pasture systems in south-eastern Australia with serradella (Ornithopus spp.)
L. E. Goward A B * , C. K. Revell C , D. M. Peck
A B * , C. K. Revell C , D. M. Peck  D , A. W. Humphries D , R. C. Hayes
D , A. W. Humphries D , R. C. Hayes  E , R. J. Simpson
E , R. J. Simpson  A , R. E. Haling
A , R. E. Haling  A , B. Penrose
A , B. Penrose  B F and R. W. Smith
B F and R. W. Smith  B
B
A
B
C
D
E
F
Abstract
There is potential for the wider use of serradella (Ornithopus spp.) in south-eastern Australian permanent pastures where the legume component has historically been based on subterranean clover (Trifolium subterraneum L.). Serradella is a genus of annual legumes native to the Mediterranean region and central and north-western Europe. Cultivar development in Australia has largely focused on the yellow (O. compressus L.) and French serradella (O. sativus Brot.) species, with slender (O. pinnatus (Mill.) Druce) and common birds-foot (O. perpusillus L.) serradella of minor importance. Serradellas have been shown to be productive on deep, sandy, acidic soils in Mediterranean climates where they have demonstrated equal or higher production than has subterranean clover. Recent research has highlighted a broader adaptation zone for serradella, including the cooler regions of the temperate pasture zones of south-eastern Australia with acidic, duplex soil types. Diversifying the feedbase with serradellas offers benefits, including low incidence of pest and disease, improved drought resilience, low bloat risk, low oestrogenic activity, and tolerance of acidic and P-deficient soils with the potential to reduce P fertiliser inputs for pastures by 30%. Key challenges for broad-scale adoption of serradellas in these new environments includes selection and commercialisation of cultivars with appropriate flowering and seed traits, effective introduction of serradella rhizobia and improved options for controlling weeds. This paper reviews the traits of serradellas that make them a viable legume option for south-eastern Australia, along with progress in cultivar and agronomic development.
Keywords: adaptation, annual legume, cultivars, pasture species diversification, resilient farming systems, serradella, sustainable grazing systems, temperate pastures.
Introduction
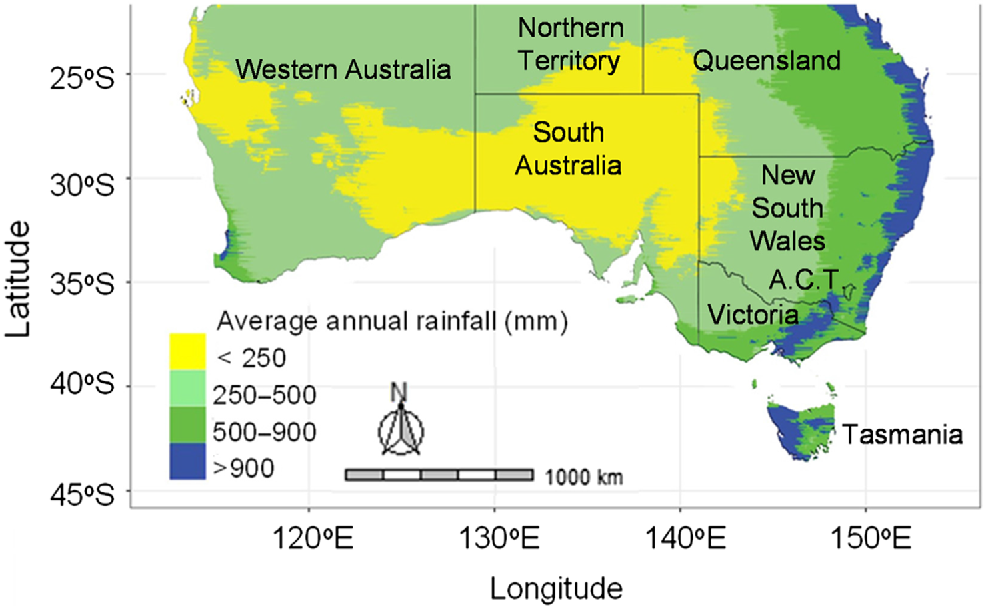
In southern Australia, large areas of annual pasture legumes are used as a protein- and energy-rich feed source for grazing livestock and are often the sole source of nitrogen inputs (via nitrogen (N) fixation) to the pasture system that typically also includes grasses and other companion species (Ledgard 2001; Hayes et al. 2017). The region includes Mediterranean and temperate climatic zones (average annual rainfall (AAR) 250–1200 mm, Fig. 1). Effective rainfall is typically concentrated in the winter months (Dear and Ewing 2008) and growing season length is commonly estimated from an assessment of monthly rainfall. Plants with an annual life cycle are suited to much of the area (3.5–9 month growing seasons, Nichols et al. 2013), avoiding growth over the hot and dry conditions of summer months. Annual pasture legumes typically develop a soil seed bank to persist from year to year and from which they self-regenerate to begin a new pasture phase. Seed dormancy traits such as hardseededness (a physical dormancy where impermeability of the seed coat prevents water uptake) and physiological (or embryo) dormancy (where viable seeds fail to germinate after imbibition) regulate germination within and among years (Taylor 2005). The expression of these traits and rate of dormancy release are critical for persistence of annual legumes in environments with variable growing-season rainfall and occasional droughts (Cocks et al. 1980) and in years when the pastures are cropped as part of a ley-farming system. They also contribute to tolerance of ‘false breaks’ of the growing season, a common feature of southern Australia where germination after early autumn rains is followed by several weeks of dry conditions and a high proportion of seedlings may die (Taylor 2005).
Average annual rainfall zones of southern Australia (AAR, Australian Bureau of Meteorology 1981–2010). State and Territory borders are shown by black lines. Map was produced using standard R packages, ozmaps (Sumner et al. 2021) and raster (Hijmans et al. 2022) packages.
Subterranean clover (Trifolium subterraneum L.) is the most widely grown annual legume in southern Australia (Morley 1961; Donald 1970, 2012; Nichols et al. 2013) and has been estimated to have been sown over 29 million hectares (Nichols et al. 2013). However, reviews of legume use across this region have highlighted many potential advantages for diversification of the legume base and reducing the reliance on subterranean clover in pasture systems (Howieson et al. 2000; Loi et al. 2005; Nichols et al. 2012).
The potential of yellow serradella was first recognised in the 1960s with the release of cultivar ‘Pitman’. Since the 1980s, a collective research effort has evaluated alternative legume species, including serradella species, for potential use in areas where subterranean clover is grown, comparing their performance with that of subterranean clover (Loi et al. 2005; Nichols et al. 2007, 2012). Of the species tested, yellow serradella (Ornithopus compressus L.) and French serradella (Ornithopus sativus Brot.) have been among the most widely developed and adopted alternatives to subterranean clover (Nichols et al. 2012). On appropriate soil types, serradella can achieve seasonal dry-matter production and nutritive value similar to subterranean clover (Rossiter et al. 1985; Loi et al. 2005; Hackney et al. 2021; Norman and Masters 2023). Serradellas have an indeterminate flowering habit (Revell et al. 1999; Goward et al. 2024a), which means that senescence may be delayed if late season rainfall occurs, benefiting livestock production (Wilmot et al. 2023). Serradellas are also recognised for their adaptation to low fertility soils, with a lower critical requirement for soil phosphorus (Sandral et al. 2019) and, for many cultivars, an improved tolerance of low soil pH and high aluminium concentrations than for subterranean clover (Condon et al. 2021a; Kidd et al. 2021). Unlike for subterranean clover-based pastures, there have been no reports of animal health issues such as harmful plant secondary compounds (e.g. isoflavones; Wilmot et al. 2023) or bloat (Mueller-Harvey 2006; Badgery et al. 2023) from livestock grazing serradella. Serradellas may also offer some potential to reduce methane emissions compared with subterranean clover, with herbage of French serradella, in particular, observed to contain high concentrations of condensed tannins (Li et al. 2025). All species of serradella are aerial-seeding and development of cultivars suited to direct header harvesting (cf. suction harvesting of subterranean clover, which buries its seed; Moss et al. 2021) has opened up opportunities for cheaper and easier seed production (Loi et al. 2005; Nutt et al. 2020).
In Australia, serradella adoption has largely occurred on low phosphorus, sandy acidic soils in low–medium rainfall zones (250–500 mm AAR, Fig. 1) of Western Australia (WA) (Gladstones and McKeown 1977a; Gillespie 1990; Michalk and Revell 1993) and central-northern New South Wales (NSW) (Freebairn 1990). In WA, it is commonly sown as a pasture phase or fodder crop between cropping phases (Nichols et al. 2012), with a survey of WA growers and seed cleaning businesses estimating that up to 1 million hectares may have been sown to French serradella (cultivar ‘Cadiz’) and up to 60,000 ha to yellow serradella (Ewing 2002). A later national feed-base survey reported that the presence of serradella relative to subterranean clover in sown pastures was approximately 10% in WA and 4% in NSW (Donald 2012). Since 2012, increased adoption of serradellas in the low–medium rainfall mixed-cropping zones of south-eastern Australia has been facilitated by the release of hardseeded French serradella cultivars and research identifying the benefits of establishing serradellas via summer sowing of pod (Nutt et al. 2021). There remain large areas of acidic soils across southern Australia on which serradellas are potentially suitable. However, adoption in some regions is likely to be constrained by higher soil pH (>7), such as the neutral to alkaline soils of the South Australian (SA) and Victorian (Vic) Mallee that may be more suited to other legume species (Ballard et al. 2020). Considering that the potential ecological suitability of serradella is comparable to subterranean clover (Hill 1996), there is a major opportunity for its broader use. In particular, recent research has highlighted opportunities to extend the use of serradella into the cooler regions of the temperate pasture zones of south-eastern Australia where serradellas have been demonstrated to be adapted to the acidic, duplex soil types that are commonly found in these regions (Boschma et al. 2019; Sandral et al. 2019; Haling et al. 2022; Newell et al. 2022; Hayes et al. 2023).
This review examines the breeding and agronomic factors affecting the past and future adoption of serradella into southern Australian farming systems. A case is made for the wider adoption of serradella species in medium–high-rainfall temperate climates such as the NSW Tablelands permanent pasture region (~ 7.7 million hectares, Donald 2012).
The Ornithopus genus
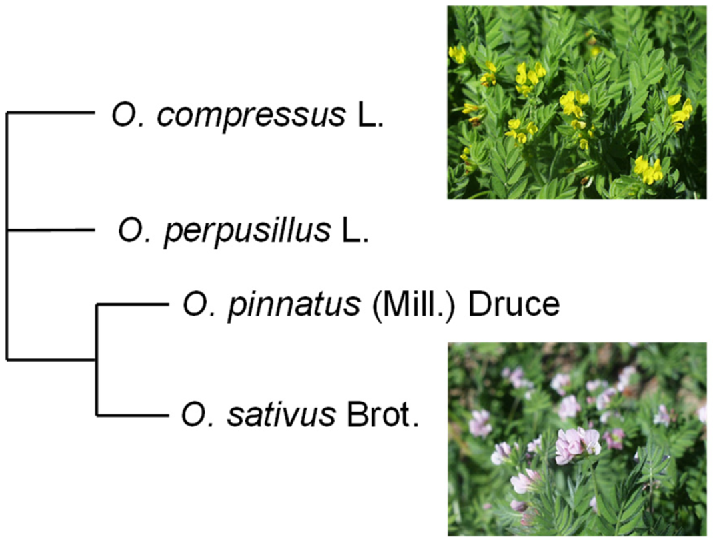
The latest classification of Ornithopus species was documented in a comprehensive assessment of the phylogeny of the Leguminosae family, with the four most common species being O. compressus L. (yellow serradella), O. perpusillus L. (common birds-foot serradella), O. pinnatus (Mill.) Druce (slender serradella) and O. sativus Brot (pink or French serradella) (The Legume Phylogeny Working Group 2017; Fig. 2). Subspecies of O. sativus Brot. include subsp. isthmocarpus Coss. (Snowball 1996; Visnevschi-Necrasov et al. 2012), previously referred to as Moroccan serradella (Gladstones and McKeown 1977b; Snowball 1996), and subsp. sativus, to which modern French serradella cultivars are attributed (Nutt et al. 2020).
Ornithopus species are predominately of Mediterranean or central and north-western European origins (Gladstones and McKeown 1977b; Nutt 2008; Snowball 1996; Smith et al. 2021) and the Australian Pastures Genebank (APG) contains >2800 Ornithopus accessions (Australian Pastures Genebank 2025), mainly collected from these regions. These accessions provide an important genetic resource for future use of serradellas. Yellow serradella is the most widely collected Ornithopus species in the APG (over 2100 accessions), having been collected over a broad range of climatic conditions similar to those in which annual legumes are grown in southern Australia (Smith et al. 2021; Fig. 1, Table 1). In contrast, French serradella (~350 accessions) has been collected from a more limited geographic distribution, with fewer extremes in the maximum elevation and latitude (Table 1). Considerably fewer accessions of other serradella species have been collected.
| Geographic and climate variables | Ornithopus compressus (n = 2267) | Ornithopus sativus (n = 340) | |||
|---|---|---|---|---|---|
| Minimum | Maximum | Minimum | Maximum | ||
| Elevation (m) | 1903 | 531 | |||
| Average annual rainfall (mm) | 312 | 1297 | 322 | 986 | |
| Latitude (°) | 28.10 | 49.65 | 32.50 | 39.98 | |
| Average temperature of warmest month (°C) | 22 | 36 | 28 | 35 | |
| Average temperature of coldest month (°C) | −5 | 14 | 2 | 9 | |
| Average temperature of warmest quarter (°C) | 17 | 27 | 21 | 27 | |
| Average temperature of coldest quarter (°C) | −1 | 17 | 8 | 14 | |
Development and adoption of serradella in Australian farming systems
The timeline of serradella cultivar releases in Australia is shown in Table 2 and illustrates the key traits targeted in the development of cultivars adapted to Australian farming systems. Breeding and selection have mainly focused on yellow serradella and French serradella. In contrast, only one slender serradella (O. pinnatus (Mill.) Druce) cultivar (‘Jebala’) and no O. perpusillus L. cultivars have been released (Table 2). Prior to 2004, serradella cultivars were mostly commercialised without intellectual property (IP) protection and were freely accessible (i.e. ‘public’ cultivars). Since that time, more cultivars have been registered with IP protection under plant breeders rights (PBR), a practice favoured by the seed industry but not always conducive to widespread adoption (Revell et al. 2013).
| Cultivar A | Origin | Year | Reference | Adaptation | |
|---|---|---|---|---|---|
| O. compressus | |||||
| Pitman | Naturalised | 1966 | Oram (1992) | First serradella line found naturalised in Waroona, WA. Suited to medium–high rainfall environments. However, too late flowering for the larger potential growing area that was in low–medium-rainfall WA environments. | |
| Uniserra | EMS Mutation of Pitman | 1970 | Gladstones and Devitt (1972) | Pitman mutant that is earlier flowering than Pitman but still too late for low–medium-rainfall zones in WA. | |
| Avila | Introduction (Spain) | 1987 | Drew (1989) | Similar maturity to Pitman but more vigorous and productive than Pitman and Pitman mutants. Best of late material when evaluated on acid sands and loams in higher-rainfall regions in NSW, Victoria (Vic) and South Australia (SA). | |
| Elgara | Introduction (Morocco) | 1988 | Freebairn and Drew (1989) | Earlier flowering than prior releases. Most vigorous and productive of the early maturing types tested in drier regions of NSW at the time. | |
| Eneabba | EMS Mutation of Pitman | 1988 | Gladstones and Bolland (1989b) | Similar flowering date to Uniserra but superior early winter vigour and greater regeneration/persistence. | |
| Madeira | Introduction (Portugal) | 1988 | Gladstones et al. (1989) | Performed well in sandy soils in south-eastern Queensland (Qld) in terms of early vigour, regeneration and dry-matter production but less tolerant of aluminium than other cultivars. | |
| Tauro | Introduction (Italy) | 1988 | Oram (1987) | Selected for SA non-calcereous sandy soils in rainfall zones of >400–450 mm AAR. | |
| Paros | Introduction (Greece) | 1990 | Gladstones et al. (1990) | Earlier maturing than prior yellow serradella cultivars. Superior aluminium soil tolerance to Madeira. Suited to low-rainfall (300–375 mm AAR) regions of WA. | |
| Yellotas | Introduction and selection (Greece) | 1991 | Martin et al. (2023) | Developed in higher-rainfall regions of Vic and observed to have superior regeneration and dry-matter production to Avila and Tauro in Tasmania (Tas). Similarly rapid rate of hard-seed breakdown comparable to Pitman and well adapted subterranean clover cultivars. Good grazing tolerance. | |
| King | Introduction and selection (Greece) | 2001 B | Nichols et al. (2007) | Early maturity, selected for use in low–medium-rainfall regions of central to northern NSW. | |
| Charano | Introduction and selection (Greece) | 2004 C | Nutt (1997) | (PBR terminated) Similar flowering date to Paros but straighter pods more suited to bulk handling. | |
| Santorini | Introduction and selection (Greece) | 2004 C | Nutt (1997) | (PBR terminated) Non-segmenting straight pods and improved pod retention, more suited to direct header harvest and improved ease of dehulling. Relatively high aluminium tolerance. | |
| Yelbini | Introduction and selection (Greece) | 2005 C | Nutt (2003) | (PBR expired) Earliest maturing of the serradella cultivars, important for reducing risk of budworm attack. Straight pods for ease of bulk handling. | |
| SerraMax | Introduction and selection (Greece) | 2022 C | Smith (2022) | (PBR) Selected for its relatively rapid softening of hard seeds, early maturity, rapid germination, and straight pod characteristics. | |
| O. sativus | |||||
| Grasslands Koha | Introduction and selection (Europe) | 1989 C | de Lautour (1988) | (PBR terminated) First French serradella registered in Australia. Mid–late flowering. Developed in New Zealand (NZ) and selected from European lines that were evaluated between 1966 and 1975 for growth vigour, disease tolerance and flowering characteristics. Distinct from O. compressus and O. pinnatus in having segmenting pods. >95% softseeded. | |
| Cadiz | Introduction (South Africa) | 2002 C | Nutt (1997) | (PBR expired) Naturalised strain collected in South Africa. Much earlier flowering than previous material evaluated and suited to low–medium-rainfall target area at the time. >95% softseeded. | |
| Margurita | Selection from Cadiz | 2005 C | Nutt (2004a) | (PBR to expire June 2025) Along with Erica, first of the hardseeded French serradella cultivars developed. Erect growth habit suited to direct header harvest. | |
| Erica | Selection from Cadiz | 2005 C | Nutt (2004b) | (PBR terminated) Sister line to Margurita with a more prostrate growth habit and therefore more grazing tolerant but seed production not continued as Margurita preferred because of greater ease of harvesting for on farm seed production. | |
| Eliza | Selection from Cadiz | 2015 C | Collins (2011) | (PBR) Earlier flowering selection from Cadiz, >95% softseeded. | |
| Fran2o | Crossbred (Eliza × ‘EHS1.10’) | 2021 C | Harrison (2022) | (PBR pending) Eliza parent for earlier flowering and crossed with hardseeded line. | |
| O. pinnatus | |||||
| Jebala | Introduction and selection (Morocco) | 1988 | Gladstones and Bolland (1989a) | Earliest flowering line of this species. At time of release was considered to have greater tolerance to infertile acid soils than other Ornithopus species and was also thought to have greater waterlogging tolerance. As such, was recommended for use in higher-rainfall regions which have soils that are highly acidic and/or subject to waterlogging. | |
| O. sativus × O. compressus hybrid | |||||
| Grasslands Spectra | Crossbred (Pitman × ‘G19’) | 1997 C | Stratton (1996) | (PBR terminated) Attempt to have cultivar with hardseeded traits of yellow serradella and pod characteristics of French serradella. Evaluated in NZ and Tas. Seed available in Margot Forde Forage Germplasm Centre. | |
EMS, ethyl methanesulfonate.
The first significant research effort occurred in WA and focused on yellow serradella. It was largely driven by a need for hardseeded annual pasture legumes adapted to deep sandy, acidic soils where subterranean clover, lucerne (Medicago sativa L.) and annual medics (Medicago spp.) along with their specific rhizobia did not persist (Gillespie 1990; Michalk and Revell 1993). The hardseed characteristic of most yellow serradella accessions made them immediately amenable for use as a self-regenerating annual legume in southern Australia. The initial cultivars of yellow serradella such as ‘Pitman’ were found to be too late maturing for the lower-rainfall zones (250–500 mm AAR, Fig. 1) (Gladstones and Devitt 1971; Gladstones 1976), so germplasm collected from the Mediterranean region was evaluated and found to be better adapted to the shorter, milder winters in these lower-rainfall zones. This led to the release of multiple early to mid-season maturing cultivars in the 1980s (Table 3). Adoption of serradella in regions of southern WA and central-western NSW, in which these early–mid-season maturity types were suited, was reported by the early 1990s (Freebairn 1990; Revell 1992). Continued selection for early maturity in yellow serradella led to the release of cultivars such as ‘Charano’ (early) and eventually ‘Yelbini’ (very early) (Tables 2, 3), which enabled their use in regions with as little as 300 mm annual rainfall (Revell et al. 1998a).
| Cultivar | Mean days to flowerA | Maturity class | |
|---|---|---|---|
| O. compressus | |||
| Pitman | 130 | Late | |
| Avila | 125–130 | Late | |
| Yellotas | 123 | Mid–late | |
| Tauro | 115–124 | Early–mid | |
| Eneabba | 120 | Early–mid | |
| Uniserra | 110 | Early–mid | |
| Madeira | 97–112 | Early–mid | |
| Elgara | 100–109 | Early–mid | |
| Santorini | 95–108 | Early–mid | |
| Charano | 85–98 | Early | |
| Paros | 96–100 | Early | |
| SerraMax | 85–97 | Early | |
| Yelbini | 78–82 | Very early | |
| O. pinnatus | |||
| Jebala | 118 | Early–mid | |
| O. sativus | |||
| Grasslands Koha | 154 | Late | |
| Cadiz | 103–114 | Mid | |
| Erica | 109–110 | Mid | |
| Margurita | 106–108 | Mid | |
| Fran2o | 94 | Early | |
| Eliza | 93 | Very early–early | |
| O. sativus × O. compressus hybrid | |||
| Grasslands Spectra | 146 | Late |
Sources: Snowball (1990); Nutt (2004a); Boschma et al. (2019); Harrison (2022). Unregistered cultivars Rosa and Serratas (115 and 129 days to flower respectively) have approximately mid–late- and late-maturity classifications (Boschma et al. 2019; Simpson 2020).
A second driving factor for cultivar development in yellow serradellas was improved pod traits. Yellow serradella seed develops in a woody pod and, in some cultivars, a hook on the terminal beak and limited segmentation (i.e. pods that do not readily break into segments) complicated its bulk handling to the point that seed production became expensive and unprofitable (Harrison et al. 2024). Straighter pods were therefore targeted for cultivar releases from the 2000s onward (e.g. ‘Charano’, Table 2) to improve compatibility with bulk-handling equipment. Easily segmented pods (e.g. ‘Serramax’, Loi et al. 2021) that are more amenable to flow through machinery were also selected. However, because segmenting pods are harder to dehull (the process of extracting seed from the pods), cultivar development simultaneously targeted more rapid hardseed breakdown patterns to alleviate the need for dehulling (Revell et al. 1998a, 1998b).
Yellow serradellas typically produce a high level of initial hardseed that is very slow to soften (Revell et al. 1998b, 1999; Newell et al. 2022). This is generally an advantage for long-term persistence and regeneration after cropping phases. However, it can lead to poor regeneration in the year after sowing (Howieson et al. 2021; Hayes et al. 2023). A more rapid hardseed breakdown pattern was a key selection criterion in the development of ‘SerraMax’ (Table 2; Revell et al. 1998b; Loi et al. 2021; Howieson et al. 2021) and is also observed in other cultivars including ‘Pitman’ and ‘Yellotas’ (Newell et al. 2022; Martin et al. 2023). These pod- and seed-trait improvements have helped elevate the utility of yellow serradellas in farming systems in southern Australia.
Renewed interest in French serradella emerged in the mid-1990s, with the distinct advantages that pods could be easily harvested in a single pass by using a conventional cereal harvester and pods broke into segments with minimal processing. Therefore, individual pod segments (containing a single seed) could be sown without further processing (Nutt 1993; Nutt and Paterson 1997; Loi et al. 2005), thereby reducing the cost of seed and subsequent pasture establishment. The release of ‘Cadiz’ (Table 2) provided a mid-season maturity (Table 3) cultivar suitable for a comparable region to which many yellow serradella cultivars had been developed (Nichols et al. 2012). However, as for most French serradella genotypes that had been tested prior to 2002, ‘Cadiz’ was almost entirely softseeded (Bolland 1982; Snowball 1996). To address this issue, breeders developed the first hardseeded cultivars of French serradella, ‘Margurita’ and ‘Erica’, both of which had mid-season maturity (Table 3; Nutt 2008; Harrison et al. 2024). This novel hardseeded trait in French serradellas enabled broader use in ley farming systems and, by 2005, the area sown to French serradella in WA eclipsed yellow serradella by more than two-fold (Nichols et al. 2007). Most recently, an early-maturity hardseeded cultivar, ‘Fran2o’, was released (Harrison et al. 2024). The latest estimate of land area sown to the older and more widely adopted ‘Margurita’ is over 1 million hectares (Harrison et al. 2024).
Opportunities and limitations for wider use of serradella species in new environments
Opportunity exists to expand the use of yellow and French serradella into environments and systems in which they have not previously been adopted in Australia. Serradellas are potentially suited to large areas of south-eastern Australia, including, but not limited, to the NSW Tablelands and Monaro, Vic Gippsland and Tasmanian (Tas) Midlands regions. These regions typically have a cooler climate, with longer growing seasons and higher AAR than in the regions of WA and NSW where serradellas have traditionally been grown. In addition, these regions are typically dominated by permanent pasture systems rather than pasture phases grown in rotation with grain crops. Here, we explore a range of key plant adaptation traits and agronomic considerations to determine the scope for development and adoption of serradellas into permanent pasture systems in south-eastern Australia as alternative legume species to subterranean clover.
There are several adaptations of serradellas that make them a promising legume alternative to subterranean clover in south-eastern Australia, including traits associated with root development and function, stress tolerance, seed morphology and seeding habits. Other traits, such as the specificity of their rhizobial association and herbicide tolerance are challenges to adoption of serradellas in south-eastern Australian pasture systems.
Soil acidity and aluminium toxicity tolerance
Soil acidity is a major issue in mixed farming and permanent pasture enterprises in south-eastern Australia (Scott et al. 2000a; Li et al. 2019; Condon et al. 2021b). Soil acidity can be alleviated by surface applications of lime (Li et al. 2019), but for many parts of south-eastern Australia, standard application rates of surface-applied lime have not kept pace with acidification rates, resulting in very acidic subsoil layers. In the permanent pasture regions, acid-tolerant species are commonly used in low input grazing enterprises because applications of lime are constrained by a limited return on investment (Scott et al. 2000b). This is particularly the case in medium- to high-rainfall regions (>500 mm AAR) of temperate south-eastern Australia, where highly acidic soils (pHCa < 4.8) are common (Figs 1, 3). Serradellas have an optimal pH range starting at a pHCa of 4.0, compared with 4.5 for subterranean clover (Condon et al. 2021a). Consequently, serradella has a distinct advantage where soil pHCa is 4.0–4.5. The upper end of the pHCa range for serradella is 7.0 (Condon et al. 2021a), above which other species, such as annual medics, tend to produce more biomass for forage and seed yield for regeneration (Ballard et al. 2020; Latta and Moodie 2022).
Surface soil pHCa (0–5 cm, Soil and Landscape Grid of Australia 2021). Map was produced using standard R packages, ozmaps (Sumner et al. 2021) and raster (Hijmans et al. 2022). Collected and interpolated raster data have a spatial resolution of approximately 90 m.
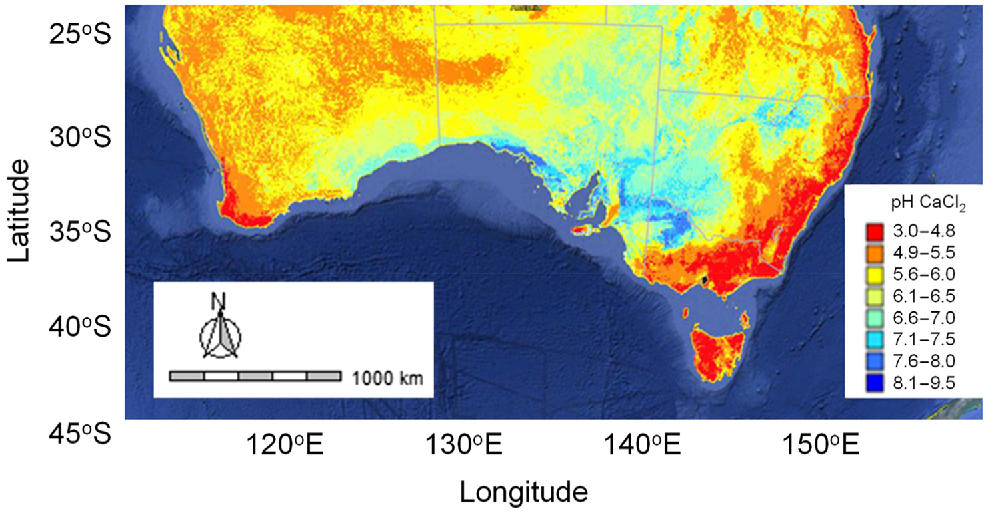
Aluminium (Al) toxicity is a significant risk to productivity on acidic soils and increases with a decreasing soil pH (Dowling et al. 2025). Many (temperate) pasture legume species used in Australia are more sensitive to Al than are companion grass species (Mackay et al. 1991). Screening of 18 forage species found French serradella to be relatively more tolerant of Al than is subterranean clover (Mackay et al. 1991). However, within serradella species, some cultivars are less tolerant than are others and this could limit shoot growth for a few serradella genotypes under very acidic soil conditions (pHCa <4.5, Kidd et al. 2021). This supports broad suitability of serradellas to acidic soils, but highlights that consideration may need to be given to cultivar selection to achieve optimum productivity.
A major benefit of yellow and French serradella is their lower requirement for soil P to achieve maximum yields than that of subterranean clover (Paynter 1992; Paynter and Bolland 1993; Sandral et al. 2019). This lower ‘critical P’ requirement for serradella, than for subterranean clover, is a potentially valuable trait for southern Australia where P deficiency is the largest and most widely encountered soil nutrient constraint after nitrogen supply (Angus et al. 2019). It is estimated that serradella-based permanent pasture systems could achieve near-maximum production at an Olsen P of 10 mg/kg soil, whereas subterranean clover-based pastures would require an Olsen P of 15 mg/kg for equivalent production (Sandral et al. 2019). This indicates potential to reduce both the economic and environmental cost of P fertiliser (Simpson et al. 2014; Simpson and Haling 2021). The superior capacity of serradella to acquire soil P, in comparison to clover species, is due to root morphological traits such as relatively long, thin roots and long root hairs, which provide a large soil interface for P acquisition (Haling et al. 2016; Sandral et al. 2018). The colonisation of roots of serradellas and subterranean clovers by arbuscular mycorrhizal fungi can assist with the acquisition of soil P, but does not change the relative difference in P acquisition efficiency of the two species (Sandral et al. 2019; McLachlan et al. 2020).
French and yellow serradellas are comparatively deeper-rooted species than is subterranean clover, with reports of rooting depths on sandy, acidic soils (unrestricted by moisture deficit, chemical or physical barriers) of 1.7–1.95 m for serradellas compared with 0.6–1.2 m for subterranean clover (Hamblin and Hamblin 1985; Carr et al. 1999; Loi et al. 2005). Expression of this deep rooting trait has been linked to enhanced ability to scavenge deeper soil water reserves later into the season (Hackney et al. 2019, 2021; Monjardino et al. 2022), being particularly valuable on soils with a low water holding capacity, and has also been linked to improved K acquisition (Ozanne et al. 1965; Asher and Ozanne 1966; Hamblin and Hamblin 1985; Sandral et al. 2006). In south-eastern Australia, expression of this trait may be constrained on the widely-occurring duplex soils where high bulk density in the heavy clay subsoil may constrain root penetration to depth (Simpson 2020).
Periodic waterlogging can occur in many parts of the landscape used for pasture systems in south-eastern Australia. In these regions, the risk of waterlogging is likely to be higher than on sandy soils, owing to the combination of higher rainfall and finer soil textures. Laboratory screening of a range of yellow, French and slender serradella genotypes for waterlogging tolerance showed that most of the genotypes could recover from transient waterlogging (<2 weeks) but were unsuited to longer periods of exposure to anerobic conditions (Kidd et al. 2020). Field evaluations in NSW (Sandral et al. 2019; Hackney et al. 2021), South Australia (Ballard et al. 2020) and Victoria (Latta and Moodie 2022) have supported the use of serradella on heavier-textured soils than have been used historically. However, the risk of waterlogging is likely to increase with increasingly finer-textured soils and this may limit adoption. Slender serradella (O. pinnatus) has been observed anecdotally to be more tolerant of waterlogging than is yellow serradella (Freebairn 1990) and has been recommended for use in mixtures in higher-rainfall areas. However, in more detailed investigations, slender serradella was found to have no more direct tolerance of waterlogging than did other serradella species (Kidd et al. 2020). It is assumed, therefore, that its persistence in paddocks that are prone to waterlogging is due more to avoidance, than physiological tolerance of waterlogging, whereby slow growth during winter is followed by a flush of growth in spring when waterlogging has dissipated.
Nitrogen fixation is a critically important attribute of annual pasture legumes. Serradella forms an effective symbiosis with Bradyrhizobium species, which belong to a different genus of rhizobia than those required by other pasture legumes grown in Australia (Rigg et al. 2021; Farquharson et al. 2022). Successful introduction of compatible rhizobia will be essential to the establishment of serradellas in new environments. There have been multiple observations of nodulation failure when sowing serradella into new environments in NSW and TAS (R. Haling and R. Smith, pers. comm.) and, as such, consideration needs to be given to the range of factors that may influence nodulation and symbiotic and saprophytic competence, including paddock history, inoculant type and soil conditions.
The current recommended commercial strain for serradella is inoculant Group S (WSM471), but serradella also nodulates well with the Group G strain for lupins (WU425) (Farquharson et al. 2022). If well-nodulated serradella or lupins have not been previously grown, then Group G or S rhizobia must be introduced when sowing serradella. If paddock history is unknown, it is advisable to refer to soil tests for lupin/serradella (e.g. rNod, a DNA-based soil testing service) that check for rhizobial strains (Farquharson et al. 2022) or inoculate to remove the risk of nodulation failure.
Peat-based inoculants are the oldest and most common form of inoculant used in Australia, typically applied to the surface of the seed as a wet slurry and followed by the addition of lime to help dry the seed and increase rhizobial survival (Farquharson et al. 2022). Higher water volumes are required when inoculating podded seed than with seed alone. Early research with agar cultures of rhizobia found deleterious effects of lime pelleting on serradella nodulation, but no effect when peat-cultured rhizobia were used (Shipton and Parker 1967). Hartley et al. (2004) found that lime pelleting serradella increased rhizobia numbers 24 h after inoculation by 90%, nodulation by 57% and shoot dry matter by 28% compared with unpelleted seeds and is the basis for the general recommendation to lime pellet serradella in all states except WA (Farquharson et al. 2022). Application technologies for inoculants have developed over the past 10–15 years to include dry clay (bentonite) or peat-based granules, freeze-dried powder and liquid formulations (Farquharson et al. 2022). Dry granules and freeze-dried formulations are available for serradella and further evaluation of these forms of rhizobia application for sowing in new environments is warranted.
Acid soil tolerance affects the performance of host plants and the host-specific rhizobia, which enables the capacity of plants to fix nitrogen (Sanford et al. 1994; Howieson and Ballard 2004; Guo et al. 2012). Rhizobia are often more acid sensitive than are the host species (Condon et al. 2021a). The lower end of the optimal pHCa range for serradella rhizobia (Bradyrhizobium spp.) is 5.0 compared with 5.5 for subterranean clover (Rhizobium sp.) (Condon et al. 2021a). However, there are few reports (Sanford et al. 1994; Peoples et al. 2012) of the amount of nitrogen fixed by serradella plants to quantify the impact of suboptimal pH (pHCa <5) on N fixation. In WA, Sanford et al. (1994) found that 76% of fixed N was derived from atmospheric N2 (Ndfa), whereas in NSW, estimates were lower at 26–31% Ndfa (Peoples et al. 2012). Further estimates of N fixation across a broader range of environments and among serradella species are recommended to assess the effectiveness of the rhizobial symbiosis in serradella and whether there is value in the development of more acid-tolerant serradella rhizobia.
Dry sowing of serradella seeds in pod is a novel and revolutionary pasture establishment practice that can deliver multiple benefits (Nutt et al. 2021). Benefits include timely sowing operations, enabling producers to shift pasture sowing to summer, before the main winter-crop sowing window. This early sowing also allows for the early germination of serradella with the season’s first rainfalls leading to increased dry matter production and higher seed yields (Nutt et al. 2021). A major challenge with establishing serradella through the sowing of pod (cf. bare seed) is achieving successful nodulation. The rhizobia need to survive desiccation and high temperatures prior to seed softening and autumn rainfall (Bowman et al. 1995). If establishing serradella as part of a pasture and crop rotation, growing a lupin crop within a cropping phase before sowing serradella is one way of establishing a background population of compatible rhizobia. Such a strategy is less feasible in permanent pasture systems. Applying a peat slurry with methyl cellulose adhesive to hulled pod segments of yellow serradella and sowing into dry soil, with germination rains occurring 77 days later resulted in only 22% of plants being nodulated in the first year, which increased to 72% in the following year (Bowman et al. 1995). Bowman et al. (1995) concluded that rhizobia applied to dry sown seed can survive in sufficient numbers to initiate seedling nodulation and to become established in the soil. However, high nodulation in the first year is desired to maximise pasture production, first-year seed set and subsequent pasture production. In a ley pasture system, it is also important to build up the rhizobia population to a level that will ensure survival during the cropping phase. Peat slurry application at double rates is recommended for pulses when dry sowing (Farquharson et al. 2022) and, therefore, double to quadruple inoculation rates may improve nodulation outcomes when sowing serradella in late summer to early autumn.
Research has shown that granular formulations of rhizobia at the standard rate resulted in nodulation in pulses similar to, or greater than, double rates of peat on seed when the dry period exceeded 7 days and the granular product had high rhizobia numbers (Farquharson et al. 2022). Inoculation with dry granules has been used in WA when summer-sowing dormant serradella seeds (hard seeds) into hot, dry soils (Nutt et al. 2020b). Dorji et al. (2023) sowed hardseeded pod segments of French Serradella and bentonite clay granules at four sites in SA in March and April 2023, with opening rains occurring 1–1.5 months later. However, they reported poor nodulation status at all sites without a lupin history, with a mean across sites of 41% plants with nodules. It is possible that the difference between the WA and SA results is the history of lupins being widely grown in WA but not in SA. Supporting this, Dorji et al. (2023) reported high levels of nodulation on their sites with a lupin history and that these sites could be identified using the rNod test. Delaying the sowing of hard seed in a summer sowing system will assist rhizobia survival, but too much delay will reduce the time needed for seed softening, leading to lower plant numbers at the break of season. For pulses, peat granules have provided similar nodulation as peat slurry for lupins (Denton et al. 2009, 2017); so, using this technology when dry sowing serradella deserves further evaluation.
Spraying soil with rhizobia ahead of planting was proposed as an alternative inoculation strategy in lupins and faba bean (Evans et al. 2001). Adapting this strategy for dry sowing systems in serradella (spraying the inoculum after the break of season) may be possible, although rhizobia numbers in proximity to the seed are likely to be lower and nodulation could be compromised.
In permanent pasture systems, the timing of annual regeneration varies between years owing to seasonal conditions. Seed ecology and dormancy traits play a key role in regulating germination, helping optimise seedling regeneration. The main form of seed dormancy in serradella is hardseededness, the primary, physical dormancy that results from the development of an impermeable seed coat at maturity (Taylor 2005). The development of cultivars that vary in both the initial level of hard seed and the rate of hard-seed breakdown (dormancy release) has revolutionised the use of serradella in Australia. Intraspecific variation exists in the expression of hardseededness in yellow (Bolland 1985) and French (Harrison et al. 2024) serradella, with cultivars of yellow serradella typically producing higher levels of hard seed at maturity than those of French serradella. The first French serradella cultivars (released prior to 2005, Table 2) were almost entirely softseeded and this was sometimes problematic because it led to high seedling mortality following ‘false breaks’ to the growing season (e.g. Dear et al. 2002).
Differences in initial levels of hard seed and hard-seed breakdown patterns may have significant implications on the success of establishing serradella pastures in new environments and the prospect for longer-term persistence. Differences in the patterns of hard-seed breakdown have been observed among cultivars (Revell et al. 1998b, Newell et al. 2022; Harrison et al. 2024) and can be influenced by environmental conditions experienced during the period of seed softening (Newell et al. 2022) and by seed burial (Revell et al. 1998b; Taylor 2005). For example, Newell et al. (2022) suggested that in permanent pastures, serradellas that soften slowly over several years are likely to have limited regeneration in the year after pasture establishment, resulting in a high potential for weed invasion. However, where weeds can be managed, this may be an advantageous trait for long-term persistence once a seedbank is established.
A premature end to the growing season with low soil moisture conditions during seed maturation has been shown to substantially reduce seed production and the viability of hard seeds in yellow serradella (Revell et al. 1999) and can result in lower initial hardseededness in French serradella seeds (Nutt 2008). Yellow serradella seeds become hard as they dehydrate and full impermeability is reached at a seed moisture content of about 5–7% (Revell et al. 1999). Rainfall experienced during seed maturation can potentially reduce the level of initial hardseededness (Taylor 2005) if seeds that are normally in a drying phase rehydrate and rupture the seed coat. This may be an issue for temperate environments with long growing seasons in eastern Australia because full hardseededness in both French and yellow serradellas may not be reached, affecting the persistence of seedbanks and levels of seedling regeneration.
Hard-seed breakdown in annual legume species is generally explained by a two-stage conceptual model where the first or preconditioning stage takes place with time at a rate that increases with increasing temperature, followed by the softening stage, which is determined by response to diurnal fluctuations within a particular range (Taylor 2005). Temperature fluctuations for the second stage appear to be quite specific to each legume species and can vary among cultivars (Taylor 2005). Seven cycles of 48°C/15°C successfully softened preconditioned seeds of ‘Serramax’ (formerly known as GEH72-1A) but were less successful for ‘Santorini’ and ‘Charano’ (Taylor and Revell 2002). The softening response to shallow burial of seed in yellow serradella (Revell et al. 1998b, 1999) has been explained by an inhibitory effect of light on the second stage of softening that is overcome by soil cover. The second stage of seed softening of yellow serradella ‘SerraMax’ was inhibited in some seeds by light levels as low as 0.3% of daylight, and in all seeds at a light level between 5% and 25% (Taylor and Revell 1999). Both specific temperature fluctuations and conditions of darkness need to be satisfied before seed softening occurs.
Hard seeds of French serradella (‘Margurita’ and ‘Erica’) have also been shown to soften more rapidly with shallow burial early in summer (Howieson et al. 2021). Nutt (2008) suggested that the two-stage model of seed softening does not fully explain hard-seed breakdown patterns in all French serradella cultivars and offered other factors such as humidity and the exceedance of critical thresholds for seed moisture content as being important.
The influence of seed burial on patterns of hard-seed breakdown in serradella has implications for the use of serradella in permanent pastures. We suggest that further studies of hard-seed breakdown in serradella are required in permanent pasture environments where there is limited opportunity to bury pods mechanically. It should be noted that seed storage is an important consideration in studies of physical dormancy, with reports of faster hard-seed breakdown in seed that has been stored for an extended period than in fresh seed (Harrison et al. 2020). Storing pods for several years prior to sowing may be one way to increase initial emergence rates without the costs of dehulling the seed, but such a practice requires further testing.
Most field-softened seeds of Mediterranean annual pasture legumes fully imbibe within 1 or 2 days of being placed in contact with water (Taylor 2005). However, delayed imbibition is quite marked in some cultivars of yellow serradella (Taylor and Revell 2002; Taylor 2004, 2005). For example, some field-softened seeds of yellow serradella ‘Santorini’ can take up to 6–7 weeks of moist conditions to complete germination (Loi et al. 2021), whereas other cultivars will germinate over 1–5 weeks of moist conditions (Revell et al. 1998b; Taylor and Revell 2002; Loi et al. 2021; Newell et al. 2022). These genotypic differences in the rate of imbibition appear to be a function of differences in seed structures regulating moisture uptake in yellow (Taylor 2004) and French serradella (Nutt 2008) and can be influenced by temperature and state of seed hydration (Taylor 2004). Hydrothermal time models may offer a framework for modelling serradella germination, but need further development (Bradford 2002). A spread of germination has ecological merit, providing a balance between gaining a competitive advantage from an early germination of some seeds (Ross and Harper 1972) while also providing some insurance against seedling mortality in ‘false breaks’ of season (Taylor 2005). In cultivars such as ‘Yellotas’ that display a narrow spread of germination (Martin et al. 2023), long-term persistence may be compromised if there is insufficient residual seed remaining in the seedbank to ensure regeneration in the following year (Newell et al. 2022; Hayes et al. 2023).
Physiological dormancy is a protective mechanism to prevent germination of newly ripened seed during late spring and early summer and is potentially important in temperate environments receiving rainfall during seed formation. Barrett-Lennard and Gladstones (1964) found no evidence of physiological dormancy in seeds of yellow serradella ‘Pitman’, but they did suggest that a water-soluble substance was present in the pod wall and inhibited germination during maturation of the seed. Peck et al. (2023) found that at least one French serradella cultivar (‘Margurita’) has physiological dormancy, but factors influencing its expression are unclear. In subterranean clover, physiological dormancy of newly ripened seed dissipates over time, with higher temperatures increasing the rate of breakdown (Taylor 1970). Artificial techniques to overcome dormancy and enhance germination in subterranean clover include exposure to cool temperatures (1–10°C), atmospheres of CO2 and ethylene, and flushing seeds with water (Ballard 1961; Esashi and Leopold 1969; Taylor 1970). Genetic variation in the existence and requirements needed to breakdown physiological dormancy in serradella is a potential area of future research.
Management of serradella for south-eastern Australian farming systems
Adoption of serradellas in new regions will rely on successful sowing strategies suited to the system and environment. To achieve even establishment in the first year of a serradella pasture, seed has been traditionally sown in autumn as bare seed, which has been dehulled and scarified (i.e. fracturing the hard seed coat to allow seed to be permeable to moisture). Post-harvest seed-processing methods have been developed for serradella, which involve mechanical threshing that dehull and scarify the seed (Weeldenburg and Smith 1969).
More recently, techniques for the establishment of serradella have exploited the ease of on-farm seed production and its patterns of hard-seed breakdown. Unprocessed pod segments of suitable cultivars can be sown in late summer–early autumn, soften over autumn and germinate on opening seasonal rainfall (Howieson et al. 2021; Nutt et al. 2021).
Timing is critical in Mediterranean climates. If sowing is delayed to mid-autumn (mid-April), the amount of softened (germinable) seed can be reduced by up to 56% relative to a mid-March sowing date, and by as much as 86% following a mid-May sowing, requiring much higher sowing rates that are unlikely to be economic (Howieson et al. 2021). Late summer–early autumn sowing allows for increased early feed and increased seed set in years with a dry spring (Nutt et al. 2021). The yellow serradella ‘SerraMax’ is particularly suited to sowing in late summer to early autumn (Howieson et al. 2021; Loi et al. 2021). ‘Twin sowing’, where serradella pods are undersown with a cereal or oilseed crop, is a variation of the summer sowing technique. Minimal germination of hardseeded cultivars occurs under the crop in the first year, but during the subsequent summer–autumn, breakdown of hard seeds occurs under the crop stubble to regenerate the pasture on the next break of season rains (Loi et al. 2012). For mixed farming enterprises, summer sowing and twin sowing have allowed farmers to sow pastures at a time of year that does not interfere with crop sowing operations (Loi and Yates 2020). These procedures need to be accompanied by appropriate weed management but offer considerable farm management advantages.
Aerial sowing is a method for introducing improved pasture species into non-arable country and has been used previously to establish pastures on non-arable soils in the rangelands of central NSW (Michalk and Campbell 2002). Michalk and Campbell (2002) reported that a pod:scarified seed mixture of 75:25 was the most suitable ratio for establishing yellow serradella when broadcasting into native grasslands on hardsetting, non-arable, acidic hill country. However, they also reported nodulation failure. Experimentation with the use of clay-based granular inoculation techniques or other rhizobia application methods may warrant investigation for aerial sowing. Hard seeds stored for several years in a laboratory environment (20°C ± 5°C) soften much more readily than do fresh seeds (Harrison et al. 2020). It is possible that storing serradella pods of hardseeded cultivars for 2–3 years may assist their establishment when they are broadcast. Movement of serradella with livestock may be another way of introducing the species into non-arable country. Livestock may move undigested legume seed through landscapes, but Edward et al. (1998) showed that the fraction of undigested seed in serradella was low (10%) relative to other small-seeded legume species. Nevertheless, where high seed loads exist, this still may be a feasible option over time, but the introduction of compatible rhizobia may still be a challenge.
The expansion of serradella adoption into longer season environments will need to consider management of pests and diseases. Serradellas have been reported to have a low incidence of disease infection relative to other annual pasture legumes (Taylor et al. 1979), but have been observed to have foliar diseases such as brown leaf spot (Pleiochaeta setosa), grey mould (Botrytis cinerea), Sclerotinia (Sclerotinia sclerotiorum) and unspecified fungal diseases of the genera Colletotrichum (anthracnose) and Cercospora (NSW DPI 2025), as well as the root disease Rhizoctonia solani (Mackie et al. 1999; You and Lancaster 2008). Diseases caused by viral infections from alfalfa mosaic (AMV) and pea seed-borne mosaic (PSbMV) viruses (Latham and Jones 2001) have also been recorded. The screening of serradella germplasm for disease resistance and the development of disease management packages will be of benefit when considering adaptation to higher-rainfall regions.
Native budworm (Helicoverpa punctigera Wllgr.) is a major pest that causes damage to podded crops and pastures in southern Australia. When the timing of the life cycle of the caterpillar phase of this pest coincides with serradella pod development, there is a risk of seed damage. Early completion of the sensitive seed/pod filling stage before budworm populations build up can minimise seed losses and this was one of the reasons that early maturing cultivars were a primary focus of breeding programs in WA (Revell 1992; Loi et al. 2021). Budworm damage was one of the key problems associated with poor persistence of French serradella ‘Cadiz’ relative to subterranean clover cultivars in southern NSW (Dear et al. 2002). Tolerance to blue green aphid (Acyrthosiphon kondoi Shinji) is high in yellow serradellas but tolerance to red-legged earth mites (RLEM, Halotydeus destructor Tucker) is low–moderate (Revell et al. 1998a). Chemical control options are available to manage RLEM (as well as budworm and aphids) but ongoing work to determine the optimal timing of use around shifting life cycle patterns of the insects may be required, considering changes in temperature and rainfall patterns (Maino et al. 2024).
There are few herbicide options for use on serradella, with only five herbicide active ingredients (flumetsulam, imazamox, imazethapyr, clethodim and 2, 4-DB) being registered specifically for use on serradella in Australia (Australian Pesticides and Veterinary Medicines Authority 2025). In the past, the use of grass-selective herbicides in serradella stands has been shown to help control problem weeds in cropping phases (Revell and Thomas 2004). Research into the tolerance of serradella to broadleaf herbicides by Blake (2002) and Rogers and Lauritsen (2004) showed considerable variation among and within serradella species. Lockley and Wu (2008) demonstrated that French serradella ‘Erica’ recovered well from early applications (3–4-leaf stage) of flumetsulam (2, 4-DB, clethodim, haloxyfop-R, imazamox and imazethapyr), but was severely damaged by MCPA (terbutryn, diflufenican, or bromoxynil). In a separate evaluation in southern NSW, Sandral and Dear (2005) found French serradella ‘Margurita’ to be among the most sensitive of the annual legume species tested to a wide range of herbicides used to control broadleaf weeds. Further research into novel chemistries is needed, and when growing serradella in mixed-species pastures, herbicide selection should be guided by label recommendations specific to each species.
Weed wipers (using glyphosate) have been used effectively in serradella for selective control of some broadleaf weeds such as wild radish (Raphanus raphanistrum L.). The ability to spray-top early-maturing cultivars, such as yellow serradella ‘Yelbini’, with knockdown herbicides when weeds are flowering also provides an opportunity to control herbicide-resistant grasses in cropping rotations. For yellow serradella cultivars such as ‘Santorini’ and ‘Yelbini’, a slow rate of germination (as long as 40–60 days) (Howieson et al. 2021) may also allow knockdown herbicides to be used 10–14 days after opening rains to achieve effective early weed control (Taylor 2005), but the effect on early pasture production owing to the loss of early germinating plants has not been reported. Currently, a lack of registered herbicides is a potential limitation for ensuring effective establishment because of competition from weeds. An increased number of registered herbicides paired with research that investigates the efficacy of a suite of herbicides in improving establishment (and subsequent pasture management) would be valuable.
Grazing management can significantly affect the productivity and persistence of a pasture because of effects on seed production, flowering and interspecific competition within a mixed pasture sward. Because serradellas are aerial-seeded, it is commonly recommended that serradella be rested or at least have reduced grazing pressure during flowering every few years to promote seed production and replenish soil seed reserves (Gladstones and Devitt 1971; Freebairn et al. 1997).
Grazing has been observed to delay the reproductive development of yellow serradella by up to ~2 weeks compared with undefoliated swards (Gladstones and Devitt 1971). There are limited data on the effect of the timing of grazing and grazing intensity on the herbage and seed production of serradella in mixed pasture swards in permanent pasture systems. A study comparing the effect of grazing intensity (light or heavy) and timing (winter, winter–spring or late spring) on seed set of yellow serradella ‘Avila’ and subterranean clover cultivars ‘Karridale’, ‘Clare’ and ‘Trikkala’ found that seed yield was reduced by 30–55% in ‘Avila’ and 30–48% for ‘Clare’ in late spring grazing treatments relative to an ungrazed control (Conlan et al. 1994). In contrast, seed yields of subterranean clover cultivars ‘Karridale’ and ‘Trikkala’ were not different from that of the ungrazed control, which was explained by the capacity of these cultivars to bury their burrs (Conlan et al. 1994). Studies in south-eastern Australia comparing various annual pasture legumes, including French serradella (‘Cadiz’), over a 3-year period concluded that frequent grazing hampered the aerial seeding legume species and provided a competitive advantage to the background subterranean clover populations (Dear et al. 2002).
The nutritive value of yellow and French serradella plant material is comparable to that of subterranean clover (Rossiter et al. 1985; Wilmot et al. 2023), with protein concentrations of green forage in excess of 25% and in vitro dry matter digestibility values of 70–75%. These attributes of feed quality are key drivers of suitability of serradella as an annual pasture legume in temperate Australian environments (Nichols et al. 2012). Sustained late season forage quality in years with extended growing seasons is considered a valuable attribute of serradella pastures (Monjardino et al. 2022). An indeterminate flowering habit is one reason that has been suggested for the late dry-matter accumulation and sustained nutritive value of serradella relative to other annual pasture legumes (Lloveras and Iglesias 1998; Hackney et al. 2021; Monjardino et al. 2022). However, there is inevitably a decline in pasture feed quality as plants mature and leaflets are shed, as observed by declining dry-matter digestibility over time (Iglesias and Lloveras 2000; Norman and Masters 2023). Norman and Masters (2023) reported a more rapid decline in herbage quality of ‘Santorini’ yellow serradella than of ‘Antas’ subclover, but this may simply reflect the earlier maturity of the serradella in that WA environment. Consequently, timing the reproductive phase to ensure a long grazing window with highly digestible forage will remain an important strategy for maximising the value of serradella-based pastures (Thomas et al. 2021).
Yellow and French serradellas are generally regarded as being safe for livestock consumption and may have some relative advantages compared with subterranean clover (Freebairn 1990; Freebairn et al. 1997; Wilmot et al. 2023). Isoflavones are phytoestrogenic compounds that are found in some older subterranean clover cultivars (Wilmot et al. 2023) and have been problematic for sheep reproduction. Recent research has measured low isoflavone concentrations in French serradella ‘Erica’ relative to an oestrogenic clover reference (Wilmot et al. 2023). In a comprehensive screening of a range of yellow and French serradella accessions (n = 129 and 175 respectively) the isoflavone, formononetin, was measured to be 7.4 ± 0.4 and 18.7 ± 0.4 mg/kg dry matter (mean ± s.d.) (Visnevschi-Necrasov et al. 2012), which is below thresholds that have been recorded to cause ewe infertility in subterranean clover pastures (Nichols et al. 2013). These data have provided support for the view that serradellas are safe for the grazing of sheep.
The herbage of yellow serradella ‘Avila’, French serradella ‘Margurita’ and subterranean clover cultivars ‘Coolamon’ and ‘Seaton Park’ was recently compared by Li et al. (2025). The serradella cultivars were shown to have higher concentrations of some plant secondary compounds (PSCs), including tannins and saponins, than did subterranean clover, but similar concentrations of flavonoids and phenols. Plant secondary compounds were once considered anti-nutritional factors but are now widely recognised for their roles in both plant defence and as bioactives for livestock productivity. The higher level of tannins in serradella relative to subterranean clover is believed to reduce the risk of bloat in sheep and cattle (Mueller-Harvey 2006). It remains unclear whether higher concentrations of some PSCs will result in lower emissions of enteric methane in grazed serradella pastures, but early research seems promising (Badgery et al. 2023; Li et al. 2025).
A key principle for cultivar selection is choosing cultivars with a maturity type that is suited to the the length of growing season (e.g. subterranean clover, Dear et al. 2007; Nichols et al. 2013). This ensures that flowering occurs at a time of year when the risk of abiotic stresses (frost, heat, drought) causing damage to flowers and developing seeds is minimised. Flowering as late as possible maximises the length of the vegetative period of growth and, thus, high-quality herbage production for forage (Norman and Masters 2023). However, flowering still needs to occur early enough for seed production to be completed before the growing season ends. In a permanent pasture, a suitable match of flowering date (maturity type) balances herbage production with the need for reliable seed production for pasture regeneration and persistence (Donald 1970).
Most of the development of serradella in Australia has focussed on early-maturing cultivars (Table 3). This has led to rapid adoption of serradella in low-rainfall areas. However, uptake in permanent pastures in colder and higher-rainfall (500–900 mm AAR, Fig. 1) climatic zones has been limited. In part, this can be attributed to a lack of cultivars with suitably adapted flowering traits. For instance, there are few mid- and later-flowering cultivars that are adapted to the colder climate and generally higher-rainfall environments. Suitable maturity is integral for serradellas being a viable alternative to subterranean clover. It ensures competitiveness with well-adapted subterranean clover cultivars and the other botanical components of the mixed-species pastures that are used in these regions (Hayes et al. 2023).
The indeterminate flowering habit of pasture legumes such as serradella can delay senescence and maintain forage quality for longer in the growing season. However flowering is not infinite and many cultivars display a peak period of flowering (Dodd and Orr 1995; Goward et al. 2024a). Ensuring that peak flowering is timed to occur when the risks of abiotic stresses that adversely affect seed production are low assists the reliability of seed production (Aitken 1974). This is crucial for the maintenance of a soil seedbank for subsequent regeneration and for longer-term persistence (Dear et al. 2002).
The late maturing yellow serradella ‘Avila’ has shown promising persistence in permanent pasture systems in the higher-rainfall zones of south-eastern Australia (Drew 1989; Simpson 2020; Hayes et al. 2023). ‘Avila’ is one of three late maturing yellow serradella cultivars released along with ‘Yellotas’ and ‘Pitman’ (Table 3). ‘Avila’ is reported to have greater seedling vigour and production than has ‘Pitman’ (Drew 1989), but is also considered to have a more desirable hard-seed breakdown pattern than do both ‘Pitman’ and ‘Yellotas’ for persistence in permanent pasture systems (Newell et al. 2022). Both ‘Pitman’ and ‘Yellotas’ exhibit a rapid seed softening pattern that can result in the vast majority of hard seeds softening in the initial year after they are set, leaving them at risk of depleting their bank of hard seeds. In contrast, the hard seed of ‘Avila’ softens over several years (Newell et al. 2022). However, high levels of residual hard seeds present an issue when sowing a pasture for the first time because insufficient seeds may soften to germinate in the autumn of the second year. In a permanent pasture, this can hamper establishment where there is a high weed seedbank. For the French serradellas, existing hardseeded cultivars exhibit a gradual seed softening pattern, which is likely to assist in longer-term persistence, but use of these cultivars is limited by a lack of later maturing types with hard seeds (Tables 2, 3). Cultivars of this nature are currently being developed (Haling et al. 2022).
The number of days to first flower (Table 3) is a common metric used to assign maturity type (Nichols et al. 2013) and inform cultivar selection for a given region. The functionality of this metric is reliant on the ranking of cultivars, according to days to first flower, being consistent across environments, as is largely the case for subterranean clover cultivars (Lodge and Harden 2007; Boschma et al. 2019). However, recent studies across four diverse environments (Perth, WA; Tamworth, NSW; Cowra, NSW; and Canberra, Australian Capital Territory (ACT)) suggested that the ranking of certain serradella cultivars can vary among locations (Boschma et al. 2019; Simpson 2020; Simpson et al. 2025). Thus, the current maturity type classification may be insufficient for predicting flowering time of some early–mid-flowering serradella cultivars grown in cool temperate climates.
Strong associations between the requirement for vernalisation and/or photoperiod (‘long-days’) and late-maturity (Goward et al. 2023), and the requirement for vernalisation and flowering date stability (Goward et al. 2024b) have been identified as key factors in the future selection and breeding of serradella cultivars adapted to cool temperate environments (Goward et al. 2023, 2024b). Similar to the development of lupins suitable to low-rainfall zones in WA, it is likely that flowering responsiveness to vernalisation may have been largely bred out of serradella cultivars adapted to low-rainfall environments (Francis and Gladstones 1974; Goward et al. 2023). In contrast, the ‘quantitative long day’ and ‘facultative’ vernalisation responses of late maturing yellow serradella ‘Avila’ and late maturing French serradella ‘Serratas’ have been observed to have similarities with subterranean clover cultivars adapted to longer-season environments (Aitken 1974; Goward et al. 2023).
The rate of flower production (days between flowering on successive nodes) is a key seed yield determinant of annual pasture legumes (Francis and Gladstones 1974), and substantial variation has been observed among serradella cultivars (Goward et al. 2024a). The rate at which seeds mature, defined as the time from first flower to production of a viable (germinable) seed, varies among serradella cultivars and is worthy of consideration, because it influences the estimation of optimal flowering and safe grazing periods (Francis and Gladstones 1974; Thomas et al. 2021). Evaluation of 107 serradella accessions highlighted variation among genotypes in the rate at which seeds mature, with up to 40-day difference between the fastest- and slowest-maturing genotypes when grown under field conditions (Fu et al. 1994). Working with two cultivars of yellow serradella, Revell et al. (1999) found that seed viability and the formation of the impermeable hard seed coat in yellow serradella ‘Avila’ occurred approximately 21–29 days from flowering. Maximum seed weight was achieved about 45 days after flowering in an irrigated environment, but was nearly 10 days earlier (and seeds were smaller) under conditions of moisture stress.
Self-regenerating pastures in south-eastern Australia are subject to a wide window of potential germination dates among years for a given location (Pook et al. 2009; Unkovich 2010; Flohr et al. 2021). The capacity of a given genotype to flower at approximately the same target date irrespective of their germination date (i.e. have stable flowering dates) ensures that plants will utilise the full length of the growing season that the germination date permits. Several studies (Bolland 1982, 1985; Fu et al. 1994; Snowball 1996) have compared the flowering dates of serradella genotypes for one environment and one sowing date, but few (Gladstones and Devitt 1971; Goward et al. 2024b) have closely examined the causes for differences in flowering dates arising from different germination times in modern serradella cultivars. Recent field evaluations (Boschma et al. 2019; Simpson 2020; Haling et al. 2022; Simpson et al. 2025) measured flowering date instability by comparing the start to flowering of cultivars sown in early autumn (March) with those sown later in autumn (May). In these studies, commercially available serradella cultivars were grown alongside subterranean clover cultivars spanning a maturity range of ‘very early’ through to ‘late’. Serradella cultivars were observed to express more flowering date instability than were subterranean clover cultivars of comparable maturity type. That is, for a given location, subterranean clovers most often flowered at a similar date regardless of sowing date. In contrast, serradella cultivars (e.g. ‘Margurita’) often flowered earlier when sown earlier than when sown late (Boschma et al. 2019; Simpson 2020; Haling et al. 2022). Quantification of the vernalisation requirements required for flowering date stability of cultivars of these species for different environments is required, as has been demonstrated for one location (Canberra, ACT) that is representative of a cold NSW Tablelands environment (Goward et al. 2024b). Goward et al. (2024b) showed that cultivars that require less vernalisation to initiate flowering tended to be associated with greater flowering date instability. A modelled sensitivity analysis of vernalisation and photoperiod drivers for target environments could assist in understanding the suitability of existing cultivars for new regions, as well as identifying the combinations of responses to photoperiod and vernalising temperatures that can deliver cultivars of differing maturity types, in combination with stable flowering dates.
Most of the existing cultivars of French serradella commercialised in Australia have either been direct releases of accessions or selections from within the early–mid-maturity cultivars (suggesting a narrow genetic base). Expanding the genetic diversity in serradella can be generated from further exploitation of historical plant collections or through targeted breeding programs. Given the altitudes and latitudes from which French serradella accessions have been collected (Table 1), it is expected that there is scope for selecting more later-maturing cultivars but the development of efficient breeding methods will become increasingly important as more traits (e.g. hardseededness) are desired to be stacked into new cultivars. Nutt et al. (2020a) reported that French serradella is a species with autogamous self-pollination, but with 25% cross-pollination in the field. The percentage of outcrossing in yellow serradella is unknown, but is expected to be lower becauses of the smaller flower size.
Outcrossing could be highly advantageous in breeding programs when combined with traditional methods employed by plant breeders working with inbreeding species. For example, one strategy to stack different traits into a single plant could be to create a diverse population by growing several accessions together, encouraging outcrossing with natural pollinators, followed by selection for desirable traits and selfing of individual plants (to fix the genetic trait) by using speed-breeding methods. Further introgression of traits between yellow and French serradella (such as hardseededness) could be possible through the development of hybrids (as in the example ‘Grasslands Spectra’ (Williams et al. 1987), in a similar way that traits have been transferred between strand (Medicago littoralis Rhode ex Loisel.) and barrel medics (Peck and Damin 2022). Analysis of the breeding system of French serradella has suggested that early flowering may be a dominant trait and that natural selection pressure may result in a genetic shift towards earlier-flowering types (Nutt et al. 2020a; Harrison et al. 2024), although a later-maturing trait could presumably be fixed through recurrent selection.
New speed breeding methodologies allow breeding to be conducted more efficiently than with conventional systems (Pazos-Navarro et al. 2017; Ghosh et al. 2018; Watson et al. 2018; Peck and Damin 2022). Serradella with its mainly autogamous self-pollination (Nutt et al. 2020a), an efficient hybridising method (Veerappan et al. 2014), and low level of physiological seed dormancy (Peck et al. 2023) is expected to be well suited to speed-breeding methods. Currently, only two cultivars have been released in Australia as the result of either inter- or intraspecific crossing programs (‘Grasslands Spectra’ and ‘Fran2o’, Table 2, Harrison et al. 2024), but recent advances in crossbreeding methods developed in other temperate annual pasture species are expected to make the development of crossbred serradella cultivars more efficient. Harrison et al. (2024) reported 14 successful hybridisations of 97 attempts by using the method of Nutt et al. (2020a), where keel petals are removed and emasculated using fine forceps. Veerappan et al. (2014) reported that in barrel medic, the ‘keel petal incision’ method achieves ~80% success rate, with the flower looking similar to an unopened flower bud before crossing. The method prevents pollen from dislodging from the stigma during subsequent movement of the plant and protects the stigma and pollen from desiccation. As the general structure of a serradella flower is similar to an annual medic flower, it is likely that the ‘keel petal incision method’ can achieve high rates of hybridisation in serradella.
Barrel medic and subterranean clover reference genomes have been sequenced (Rose 2008; Hirakawa et al. 2016; Kaur et al. 2017; Kang et al. 2020; Shirasawa et al. 2023) and as serradella species are of increasing importance in Australia, it is recommended that both yellow and French serradella genomes be sequenced. Molecular markers have been developed for several important traits in annual medics (Oldach et al. 2008; Bogacki et al. 2013; Oldach et al. 2014; Peck et al. 2021) and marker-assisted selection has been used to develop a barrel medic cultivar (Peck et al. 2015). Sequencing a serradella genotype will assist the development of markers for key traits, which in turn will assist breeding and selection, including the use of speed-breeding methods.
Core collections that maximise genetic diversity in a limited number of accessions have been developed for a range of key annual legume species in Australia (Genesys-PGR; Nichols et al. 2013; Smith et al. 2021) but not for serradella. Core collections are a useful starting point when screening a large germplasm collection of a species for the presence of a particular trait. It is suggested that a core collection combining the yellow and French serradella species be developed because of the possibility of interspecific hybridisation and the broad range of genetic diversity that exists across these species in the APG.
Conclusions and future research
Serradellas are versatile annual pasture legumes that have already provided a valuable alternative to subterranean clover in WA and increasingly in NSW farming systems. There is the prospect for the wider use of serradella, particularly in permanent pasture systems of south-eastern Australia. In these systems, serradellas provide opportunity to augment legume productivity on low-fertility, acidic soils. Diversifying the legume feedbase in these regions may also increase system resilience by mitigating against pest/disease and animal health risks associated with traditional species. This review has considered the suite of factors that have led to the successful development and adoption of serradellas in Australia and includes not only the benefits that serradellas can deliver for system productivity, but the cultivar and agronomic development that has been required to realise these benefits. Expanding serradella into new environments will require evaluation of cultivars and germplasm to identify those that are better adapted to permanent pasture systems in these longer-growing season and cooler-climate environments. This will need to be combined with refinement of existing agronomic knowledge (e.g. sowing timing, seed type, rhizobia, grazing management) to suit these systems. The collective engagement of producers, cross-discipline researchers and the seed industry will be critically important for success.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
AW Howard Memorial Trust Research Fellowship and Australian Sustainable Agriculture Scholarships (University of Tasmania and CSIRO) have provided financial support to the corresponding author (Laura Goward). Preparation of this review was supported in part by Meat and Livestock Australia project B.PAS.0361 ‘Serradellas for New Environments’.
Acknowledgements
We thank Kirsten Verburg and Jody Biggs for their invaluable assistance in developing the R script used to generate Fig. 1. Their support and expertise greatly contributed to the clarity and accuracy of the visual representation.
References
Angus J, Bell M, McBeath T, Scanlan C (2019) Nutrient-management challenges and opportunities in conservation agriculture. In ‘Australian Agriculture in 2020: from conservation to automation’. (Eds J Pratley, J Kirkegaard) pp. 221–236. (Agronomy Australia and Charles Sturt University: Wagga Wagga, NSW, Australia)
Asher CJ, Ozanne PG (1966) Root growth in seedlings of annual pasture species. Plant and Soil 24(3), 423-436.
| Google Scholar |
Australian Bureau of Meteorology (1981–2010) Australia annual rainfall gridded data. Available at http://www.bom.gov.au/
Australian Pastures Genebank (2025) APG Australian Pastures Genebank. Available at https://www.genesys-pgr.org/partners/64c0a36d-cc85-4754-8d17-c282e314e1bb [Accessed 21 February 2025]
Australian Pesticides and Veterinary Medicines Authority (2025) Public Chemical Registration Information System Search. Australian Pesticides and Veterinary Medicines Authority. Available at https://portal.apvma.gov.au/pubcris [Accessed 5 January 2025]
Badgery W, Li G, Simmons A, Wood J, Smith R, Peck D, Ingram L, Durmic Z, Cowie A, Humphries A, Hutton P, Winslow E, Vercoe P, Eckard R (2023) Reducing enteric methane of ruminants in Australian grazing systems – a review of the role for temperate legumes and herbs. Crop & Pasture Science 74(8), 661-679.
| Crossref | Google Scholar |
Ballard LAT (1961) Studies of dormancy in the seeds of subterranean clover (Trifolium subterraneum L.) II. The interaction of time, temperature, and carbon dioxide during passage out of dormancy. Australian Journal of Biological Sciences 14, 173-186.
| Crossref | Google Scholar |
Ballard RA, Peck D, Flohr B, Llewellyn R, Hill J, Scholz N, Tomney F, Gunn J, Richter I, McBeath T, Davoren B, Shoobridge W, Hackney B, Moodie M, Howie JH (2020) New pasture opportunities to boost productivity of mixed farms in low/medium rainfall areas. Grains Research Development Corporation. Available at https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2020/02/new-pasture-opportunities-to-boost-productivity-of-mixed-farms-in-lowmedium-rainfall-areas
Barrett-Lennard RA, Gladstones JS (1964) Dormancy and hard-seededness in Western Australian serradella (Ornithopus compressus L.). Australian Journal of Agricultural Research 15(6), 895-904.
| Crossref | Google Scholar |
Bogacki P, Peck DM, Nair RM, Howie J, Oldach KH (2013) Genetic analysis of tolerance to Boron toxicity in the legume Medicago truncatula. BMC Plant Biology 2013 13, 54.
| Crossref | Google Scholar | PubMed |
Bolland MDA (1985) Serradella (Ornithopus sp.): maturity range and hard seed studies of some strains of five species. Australian Journal of Experimental Agriculture 25, 580-587.
| Crossref | Google Scholar |
Boschma S, Kidd D, Newell M, Stefanski A, Haling R, Hayes R, Ryan M, Simpson R (2019) Flowering time responses of serradella cultivars. In ‘Proceedings of the 19th Australian Society of Agronomy Conference’, 25–29 August 2019, Wagga Wagga, NSW, Australia. (Australian Society of Agronomy) Available at http://www.agronomyaustraliaproceedings.org/
Bowman AM, Hebb DM, Munnich D, Rummery GL, Brockwell J (1995) Field persistence of Bradyrhizobium sp. (Lupinus) inoculant for serradella (Ornithopus compresssus L. Australian Journal of Experimental Agriculture 35, 357-365.
| Crossref | Google Scholar |
Bradford KJ (2002) Applications of hydrothermal time to quantifying and modeling seed germination and dormancy. Weed Science 50(2), 248-260.
| Crossref | Google Scholar |
Carr SJ, Loi A, Howieson JH, Porqueddu C (1999) Attributes of Biserrula pelecinus L. (biserrula): a new pasture legume for sustainable farming on acidic sandy soils in Mediterranean environments. Cahiers Options Mediterraneennes 39, 87-90.
| Google Scholar |
Collins D (2011) Eliza. Plant Varieties Journal 24(3), 50-51.
| Google Scholar |
Condon J, Conyers M, Burns H, Hackney B, Orgill S, Jenkins L, Beange L (2021a) ‘Soil acidity and liming.’ 4th edn. AgFact Ac. 19. (NSW Department of Primary Industries) Available at https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0005/1366421/AC.19-4th-edition-2021.pdf
Condon J, Burns H, Li G (2021b) The extent, significance and amelioration of subsurface acidity in southern New South Wales, Australia. Soil Research 59, 1-11.
| Crossref | Google Scholar |
Conlan DJ, Dear BS, Coombes NE (1994) Effect of grazing intensity and number of grazings on herbage production and seed yields of Trifolium subterraneum, Medicago murex, and Ornithopus compressus. Australian Journal of Experimental Agriculture 34, 181-188.
| Crossref | Google Scholar |
de Lautour G (1988) Grasslands Koha pink serradella (Ornithopus sativus Brot.) cultivar registration. Plant Varieties Journal 1(4), 16-19.
| Google Scholar |
Dear BS, Ewing MA (2008) The search for new pasture plants to achieve more sustainable production systems in southern Australia. Australian Journal of Experimental Agriculture 48(4), 387-396.
| Crossref | Google Scholar |
Dear BS, Wilson BCD, Rodham CA, McCaskie P, Sandral GA (2002) Productivity and persistence of Trifolium hirtum, T. michelianum, T. glanduliferum and Ornithopus sativus sown as monocultures or in mixtures with T. subterraneum in the south-eastern Australian wheat belt. Australian Journal of Experimental Agriculture 42, 549.
| Crossref | Google Scholar |
Dear BS, Sandral GA, Virgona JM, Swan AD, Orchard BA, Cocks PS (2007) Lucerne, phalaris, and wallaby grass in short-term pasture phases in two eastern Australian wheatbelt environments. 2. Effect of perennial density and species on subterranean clover populations and the relative success of 3 clover cultivars of different maturity. Australian Journal of Agricultural Research 58(2), 123-135.
| Crossref | Google Scholar |
Denton MD, Pearce DJ, Ballard RA, Hannah MC, Mutch LA, Norng S, Slattery JF (2009) A multi-site field evaluation of granular inoculants for legume nodulation. Soil Biology and Biochemistry 41, 2508-2516.
| Crossref | Google Scholar |
Denton MD, Phillips LA, Peoples MB, Pearce DJ, Swan AD, Mele PM, Brockwell J (2017) Legume inoculant application methods: effects on nodulation patterns, nitrogen fixation, crop growth and yield in narrow-leaf lupin and faba bean. Plant and Soil 419, 25-39.
| Crossref | Google Scholar |
Dodd MB, Orr SJ (1995) Seasonal growth, flowering patterns, and phosphate response of 18 annual legume species grown in a hill-country soil. New Zealand Journal of Agricultural Research 38, 21-32.
| Crossref | Google Scholar |
Dorji T, Peck D, Philp J, Smith A, Lee S, Griffiths H, Wright A, Scholz N, Denton M (2023) Early autumn sowing demonstrations of hardseeded pod segments of French serradella on sandy soils. In ‘Eyre Peninsula Farming Systems Summary 2023’. pp. 155–159. (Minnipa Agricultural Centre, SARDI) Available at https://airep.com.au/wp-content/uploads/2023/04/EPFS_2023_26_earlyautumnsowingdemonstrationofhardseededpods_DORJIPECK.pdf
Dowling PM, Vimpany IA, Conyers MK, Millar GD, Helyar KR, Michalk DL, Nicol HI, Bradley J, Milham PJ, Hayes RC (2025) Changes in pasture and soil properties with liming and superphosphate application on five soils in the Central Tablelands of New South Wales over 12 years. Crop & Pasture Science 76(5), CP24336.
| Crossref | Google Scholar |
Drew TP (1989) Ornithopus compressus L. (yellow serradella) cv. Avila. Australian Journal of Experimental Agriculture 29, 302-303.
| Crossref | Google Scholar |
Edward AY, Ewing MA, Revell CK (1998) Fate of serradella, medic and biserrula seeds in pods ingested by sheep. In ‘Proceedings of the 9th Australian Agronomy Conference’, Wagga Wagga, NSW, Australia. (Eds DL Michalk, JE Pratley) pp. 199–202. (Australian Society of Agronomy: Wagga Wagga, NSW, Australia)
Esashi Y, Leopold AC (1969) Dormancy regulation in subterranean clover seeds by ethylene. Plant Physiology 44, 1470-1472.
| Crossref | Google Scholar | PubMed |
Evans J, Eberbach P, Luckett D, Cormack S (2001) Pre-Season soil establishment of legume inoculant rhizobia is not effective for the nodulation of lupin and faba bean crops in acidic soils. Australian Journal of Experimental Agriculture 41(8), 1149-1160.
| Crossref | Google Scholar |
Ewing M (2002) UWA255 – Economic and environmental benefits of serradella adoption on highly acidic, low rainfall soils. Grains Research and Development Corporation. Available at https://grdc.com.au/research/reports/ [accessed 5 February 2025]
Farquharson EA, Ballard RA, Herridge DF, Ryder MH, Denton MD, Webster A, Yates RJ, Seymour NP, Deaker RJ, Hartley E, Gemmel LG, Hackney B, O’Hara GW (2022) Inoculating Legumes: Practice and Science. Grains Research and Development Corporation, Australia. Available at https://www.alosca.com.au/wp-content/uploads/2023/05/Inoculating-Legumes-Practice-and-Science.pdf
Flohr BM, Ouzman J, McBeath TM, Rebetzke GJ, Kirkegaard JA, Llewellyn RS (2021) Redefining the link between rainfall and crop establishment in dryland cropping systems. Agricultural Systems 190, 103105.
| Crossref | Google Scholar |
Francis CM, Gladstones JS (1974) Relationships among rate and duration of flowering and seed yield components in subterranean clover (Trifolium subterraneum). Australian Journal of Agricultural Research 25(3), 435-442.
| Crossref | Google Scholar |
Freebairn RD, Drew TP (1989) Ornithopus compressus L. (yellow serradella) cv. Elgara. Australian Journal of Experimental Agriculture 29, 305-306.
| Crossref | Google Scholar |
Freebairn RD, Mullen CL, Matthews BA (1997) Pastures for acid soils in the north and central west. In ‘Proceedings Twelfth Annual Conference Grasslands Society NSW’, 28–30 July 1997, Dubbo, NSW, Australia. pp. 33–37. (Grasslands Society of NSW Inc.) Available at https://www.pgnsw.com.au/documents/conference-proceedings/1997/Freebairn-Mullen-Matthews-1997.pdf
Fu SM, Hampton JG, Williams WM (1994) Description and evaluation of serradella (Ornithopus L.) accessions. New Zealand Journal of Agricultural Research 37, 471-479.
| Crossref | Google Scholar |
Ghosh S, Watson A, Gonzalez-Navarro OE, Ramirez-Gonzalez RH, Yanes L, Mendoza-Suárez M, Simmonds J, Wells R, Rayner T, Green P, Hafeez A, Hayta S, Melton RE, Steed A, Sarkar A, Carter J, Perkins L, Lord J, Tester M, Osbourn A, Moscou MJ, Nicholson P, Harwood W, Martin C, Domoney C, Uauy C, Hazard B, Wulff BBH, Hickey LT (2018) Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nature Protocols 13, 2944-2963.
| Crossref | Google Scholar | PubMed |
Gillespie DJ (1990) Roles for new pasture legume species in Southern Australia. In ‘Agronomy Australia Proceedings’, Katanning Symposium’, WA, Australia, 1989. (Australian Society of Agronomy). Available at http://agronomyaustraliaproceedings.org/images/sampledata/1989/invited/katanning-symposium/p-05.pdf
Gladstones JS (1976) Observations on the distribution and ecology in Iberia and North Africa of some annual legumes adapted to neutral and acid soils. Australian Plant Introduction Review 11, 9-23.
| Google Scholar |
Gladstones JS, Bolland MDA (1989a) Ornithopus Pinnatus (Miller) Druce (slender Serradella) cv. Jebala. Australian Journal of Experimental Agriculture 29, 303-304.
| Crossref | Google Scholar |
Gladstones JS, Bolland MDA (1989b) Ornithopus compressus L. (yellow serradella) cv. Eneabba. Australian Journal of Experimental Agriculture 29, 303-304.
| Crossref | Google Scholar |
Gladstones JS, Devitt AC (1971) Breeding and testing early-flowering strains of yellow-flowered serradella (Ornithopus compressus). Australian Journal of Experimental Agriculture and Animal Husbandry 11, 431-439.
| Crossref | Google Scholar |
Gladstones JS, McKeown NR (1977a) Serradella: a pasture legume for sandy soils. Journal of the Department of Agriculture, Western Australia 18(4), 11-14 Available at https://researchlibrary.agric.wa.gov.au/journal_agriculture4/vol18/iss1/5.
| Google Scholar |
Gladstones JS, McKeown NR (1977b) Botany and origins of serradella. Journal of the Department of Agriculture, Western Australia 18(2), 4.
| Google Scholar |
Gladstones JS, Bolland MDA, Lloyd DL (1989) Ornithopus compressus L. (yellow serradella) cv. Madeira. Australian Journal of Experimental Agriculture 29, 304-305.
| Crossref | Google Scholar |
Gladstones JS, Bolland MDA, Revell CK (1990) (a) Ornithopus compressus L. (yellow serradella) cv. Paros. Australian Journal of Experimental Agriculture 30, 443-444.
| Crossref | Google Scholar |
Goward LE, Haling RE, Smith RW, Penrose B, Simpson RJ (2023) Flowering responses of serradella (Ornithopus spp.) and subterranean clover (Trifolium subterraneum L.) to vernalisation and photoperiod and their role in maturity type determination and flowering date stability. Crop & Pasture Science 74, 769-782.
| Crossref | Google Scholar |
Goward LE, Haling RE, Smith RW, Penrose B, Simpson RJ (2024a) Developmental patterns of flowers and pods and the effect on seed number in French serradella (Ornithopus sativus) and yellow serradella (Ornithopus compressus) cultivars. Crop & Pasture Science 75, CP23324.
| Crossref | Google Scholar |
Goward L, Haling RE, Stefanski A, Smith RW, Penrose B, Simpson RJ (2024b) Stable flowering dates among pasture legumes are associated with flowering regulation that requires longer vernalisation. In ‘Proceedings of the 21st Australian Society of Agronomy Conference’, 21–24 October 2024, Albany, WA, Australia. (Australian Society of Agronomy Inc.: Albany, WA, Australia) Available at http://www.agronomyaustraliaproceedings.org/
Guo YJ, Li GD, Hayes RC, Dear BS, Price A (2012) Tolerance of the annual legumes Biserrula pelecinus, Ornithopus sativa, Trifolium spumosum, T. vesiculosum and T. subterraneum to soil acidity. New Zealand Journal of Agricultural Research 55, 1-14.
| Crossref | Google Scholar |
Hackney B, Flinn S, McCormick J, Piltz J, Orgill S (2019) The role of indeterminate growth, rooting depth and maturity time in establishing a legume seedbank under drought conditions. In ‘Proceedings of the 19th Australian Agronomy Conference’, 25–29 August 2019, Wagga Wagga, NSW, Australia. pp. 1–4. (Australian Society of Agronomy) Available at https://www.agronomyaustraliaproceedings.org/images/sampledata/2019/2019ASA_Hackney_Belinda_112.pdf
Hackney B, Rodham C, Dyce G, Piltz J (2021) Pasture legumes differ in herbage production and quality throughout spring, impacting their potential role in fodder conservation and animal production. Grass and Forage Science 76, 116-133.
| Crossref | Google Scholar |
Haling RE, Yang Z, Shadwell N, Culvenor RA, Stefanski A, Ryan MH, Sandral GA, Kidd DR, Lambers H, Simpson RJ (2016) Root morphological traits that determine phosphorus-acquisition efficiency and critical external phosphorus requirement in pasture species. Functional Plant Biology 43, 815.
| Crossref | Google Scholar | PubMed |
Haling RE, Goward L, Stefanski A, Simpson RJ (2022) Variation in flowering time and flowering date stability within a cultivar of French serradella. Crop & Pasture Science 74, 756-768.
| Crossref | Google Scholar |
Hamblin A, Hamblin J (1985) Root characteristics of some temperate legume species and varieties on deep, free-draining entisols. Australian Journal of Agricultural Research 36, 63.
| Crossref | Google Scholar |
Harrison R (2022) Fran2o. Plant Varieties Journal 35(2), 98-100.
| Google Scholar |
Harrison RJ, Howieson JG, Yates RJ, Nutt BJ (2020) Long-term storage of forage legumes greatly alters the hard seed breakdown pattern in situ. Grass and Forage Science 76(1), 72-81.
| Crossref | Google Scholar |
Harrison RJ, Nutt BJ, Yates RJ, Hackney BF, Howieson JG (2024) The de-domestication of Ornithopus sativus Brot. to develop cultivars with physical dormancy (hardseed). Grass and Forage Science 79(3), 366-380.
| Crossref | Google Scholar |
Hartley E, Gemell LG, Herridge DF (2004) Lime pelleting inoculated serradella (Ornithopus spp.) increases nodulation and yield. Soil Biology & Biochemistry 36, 1289-1294.
| Crossref | Google Scholar |
Hayes RC, Li GD, Sandral GA, Swan TD, Price A, Hildebrand S, Goward L, Fuller C, Peoples MB (2017) Enhancing composition and persistence of mixed pasture swards in southern New South Wales through alternative spatial configurations and improved legume performance. Crop & Pasture Science 68, 1112.
| Crossref | Google Scholar |
Hayes RC, Newell MT, Li GD, Haling RE, Harris CA, Culvenor RA, Badgery WB, Munday N, Price A, Stutz RS, Simpson RJ (2023) Legume persistence for grasslands in tableland environments of south-eastern Australia. Crop & Pasture Science 74, 712-738.
| Crossref | Google Scholar |
Hijmans RJ, van Etten J, Mattiuzzi M, Braverman MR, et al. (2022) raster: Geographic Data Analysis and Modeling. R package version 3.5-21. Available at https://CRAN.R-project.org/package=raster
Hill MJ (1996) Potential adaptation zones for temperate pasture species as constrained by climate: a knowledge-based logical modelling approach. Australian Journal of Agricultural Research 47, 1095-1117.
| Crossref | Google Scholar |
Hirakawa H, Kaur P, Shirasawa K, Nichols P, Nagano S, Appels R, Erskine W, Isobe SN (2016) Draft genome sequence of subterranean clover, a reference for genus Trifolium. Scientific Reports 6, 30358.
| Crossref | Google Scholar | PubMed |
Howieson J, Ballard R (2004) Optimising the legume symbiosis in stressful and competitive environments within southern Australia: some contemporary thoughts. Soil Biology and Biochemistry 36, 1261-1273.
| Crossref | Google Scholar |
Howieson JG, O’Hara GW, Carr SJ (2000) Changing roles for legumes in Mediterranean agriculture: developments from an Australian perspective. Field Crops Research 65(2), 107-122.
| Crossref | Google Scholar |
Howieson JG, Harrison RJ, Yates RJ, Hackney B, Loi A, Nutt BJ (2021) Hard seed breakdown patterns of unprocessed forage legume seed sown into dry soil in summer in southern Australia. Grass and Forage Science 76, 82-92.
| Crossref | Google Scholar |
Iglesias I, Lloveras J (2000) Forage production and quality of serradella in mild winter areas in north-west Spain. New Zealand Journal of Agricultural Research 43, 35-40.
| Crossref | Google Scholar |
Kang Y, Li M, Sinharoy S, Verdier J (2020) A snapshot of functional genetic studies in Medicago truncatula. In ‘The model legume Medicago truncatula’. (Ed. F de Bruijn) pp. 7–30. (John Wiley & Sons) doi:10.1002/9781119409144.ch02
Kaur P, Bayer PE, Milec Z, Vrána J, Yuan Y, Appels R, Edwards D, Batley J, Nichols P, Erskine W, Doležel J (2017) An advanced reference genome of Trifolium subterraneum L. reveals genes related to agronomic performance. Plant Biotechnology Journal 15, 1034-1046.
| Crossref | Google Scholar | PubMed |
Kidd DR, Di Bella CE, Kotula L, Colmer TD, Ryan MH, Striker GG (2020) Defining the waterlogging tolerance of Ornithopus spp. for the temperate pasture zone of southern Australia. Crop & Pasture Science 71, 506.
| Crossref | Google Scholar |
Kidd DR, Ryan MH, Colmer TD, Simpson RJ (2021) Root growth response of serradella species to aluminium in solution culture and soil. Grass and Forage Science 76, 57-71.
| Crossref | Google Scholar |
Latham LJ, Jones RAC, McKirdy SJ (2001) Cucumber mosaic cucumovirus infection of cool-season crop, annual pasture, and forage legumes: susceptibility, sensitivity, and seed transmission. Australian Journal of Agricultural Research 52(6), 683.
| Crossref | Google Scholar |
Latta R, Moodie M (2022) Development of new pasture systems in NW Victoria. In ‘Proceedings of the 20th Agronomy Australia Conference’, 18–22 September 2022, Toowoomba, Qld, Australia. (Australian Society of Agronomy) Available at https://agronomyaustraliaproceedings.org/images/sampledata/2022/Pastures/ASAmoodie_m_423s.pdf
Ledgard SF (2001) Nitrogen cycling in low input legume-based agriculture, with emphasis on legume/grass pastures. Plant and Soil 228, 43-59.
| Crossref | Google Scholar |
Li GD, Conyers MK, Helyar KR, Lisle CJ, Poile GJ, Cullis BR (2019) Long-term surface application of lime ameliorates subsurface soil acidity in the mixed farming zone of south-eastern Australia. Geoderma 338, 236-246.
| Crossref | Google Scholar |
Li GD, Newell MT, Boschma SP, Meyer R, Wood JA, Badgery WB, Hayes RC (2025) Agronomic performance, herbage quality, methane yield and methane emission potential of pasture mixtures. Crop & Pasture Science 76(4), 1-21.
| Crossref | Google Scholar |
Lloveras J, Iglesias I (1998) Accumulation of dry matter and evolution of nutritive value in serradella (Ornithopus sativus Brot.). Agronomy Journal 90, 59-63.
| Crossref | Google Scholar |
Lodge GM, Harden S (2007) Evaluation of annual pasture legumes in northern New South Wales. 2. Trifolium and Medicago spp. and other legumes. Australian Journal of Experimental Agriculture 47, 563-574.
| Crossref | Google Scholar |
Loi A, Yates R (2020) Twin sowing and summer sowing: alternative techniques to introduce annual legumes into pastures. Available at https://www.agric.wa.gov.au/pasture-establishment/twin-sowing-and-summer-sowing-alternative-techniques-introduce-annual-legumes.
Loi A, Howieson JG, Nutt BJ, Carr SJ (2005) A second generation of annual pasture legumes and their potential for inclusion in Mediterranean-type farming systems. Australian Journal of Experimental Agriculture 45, 289.
| Crossref | Google Scholar |
Loi A, Nutt BJ, Flower K (2012) Twin sowing: a new technique to reduce the costs of pasture establishment. In ‘Proceedings of the 14th Australian Agronomy Conference’, 21–25 September 2008, Adelaide, SA, Australia. (Australian Society of Agronomy) Available at http://agronomyaustraliaproceedings.org/images/sampledata/2008/concurrent/new-pastures-forage-options/5580_loia.pdf
Loi A, Revell C, Nutt B (2021) Technical dossier for SerraMax Yellow Serradella (Ornithopus compressus L.). Available at https://www.agric.wa.gov.au/sites/gateway/files/Technical%20dossier%20for%20SerraMax%20-%20Final%20version%207%20Dec%20%28A5516500%29.pdf
Mackie JM, Lloyd DL, Ryley MJ, Irwin JG (1999) Fungal diseases of temperate annual pasture legumes in southern Queensland. Australian Journal of Experimental Agriculture 39(6), 699-707.
| Crossref | Google Scholar |
Maino JL, Umina PA, Pavri C, Cheng X, Ridsdill-Smith J (2024) Adapting pest management strategies to changing climates for the redlegged earth mite, Halotydeus destructor. Scientific Reports 14, 16939.
| Crossref | Google Scholar | PubMed |
Martin G, Smith RW, Hall EJ, Newell MT, Haling R, Hayes RC (2023) Yellotas: a unique yellow serradella cultivar with potential for permanent pasture environments. In ‘Proceedings of the Pasture Legumes for Sustainable Productive Systems Symposium’, 4–6 July 2023, Perth, WA, Australia. Australian Grassland Association Research Series No. 6. (Ed. B Cullen) pp. 35–39. (Australian Grassland Association Inc.) Available at https://australiangrassland.org.au/aga/wp-content/uploads/2025/04/AGA-Pasture-legumes-for-sustainable-productive-systems-2023.pdf
McLachlan JW, Becquer A, Haling RE, Simpson RJ, Flavel RJ, Guppy CN (2020) Intrinsic root morphology determines the phosphorus acquisition efficiency of five annual pasture legumes irrespective of mycorrhizal colonisation. Functional Plant Biology 48, 156-170.
| Crossref | Google Scholar |
Michalk DL, Campbell MH (2002) Effect of surface-sown pod:seed mixtures on serradella establishment and persistence on rangelands in central New South Wales. Australian Journal of Experimental Agriculture 42(2), 143-148.
| Crossref | Google Scholar |
Michalk DL, Revell CK (1993) Serradella (Ornithopus spp.) research: An Australian overview. In ‘Alternative Pasture Legumes 1993: Proceedings of the Second National Alternative Pasture Legumes Workshop’, Adelaide, SA, Australia, 1993. Technical Report 219. (Eds DL Michalk, AD Craig, WJ Collins) pp. 39–46. (Department of Primary Industries, South Australia)
Monjardino M, Loi A, Thomas DT, Revell CK, Flohr BM, Llewellyn RS, Norman HC (2022) Improved legume pastures increase economic value, resilience and sustainability of crop–livestock systems. Agricultural Systems 203, 103519.
| Crossref | Google Scholar |
Moss WM, Guzzomi AL, Foster KJ, Ryan MH, Nichols PGH (2021) Harvesting subterranean clover seed – current practices, technology and issues. Crop & Pasture Science 72, 223-235.
| Crossref | Google Scholar |
Mueller-Harvey I (2006) Unravelling the conundrum of tannins in animal nutrition and health. Journal of the Science of Food and Agriculture 86(13), 2010-2037.
| Crossref | Google Scholar |
Newell MT, Haling RE, Hayes RC, Stefanski A, Li GD, Simpson RJ (2022) Hard seed breakdown patterns of serradella (Ornithopus spp.) in two contrasting environments of south-eastern Australia. Crop & Pasture Science 74(8), 700-711.
| Crossref | Google Scholar |
Nichols PGH, Loi A, Nutt BJ, Evans PM, Craig AD, Pengelly BC, Dear BS, Lloyd DL, Revell CK, Nair RM, Ewing MA, Howieson JG, Auricht GA, Howie JH, Sandral GA, Carr SJ, de Koning CT, Hackney BF, Crocker GJ, Snowball R, Hughes EJ, Hall EJ, Foster KJ, Skinner PW, Barbetti MJ, You MP (2007) New annual and short-lived perennial pasture legumes for Australian agriculture – 15 years of revolution. Field Crops Research 104, 10-23.
| Crossref | Google Scholar |
Nichols PGH, Revell CK, Humphries AW, Howie JH, Hall EJ, Sandral GA, Ghamkhar K, Harris CA (2012) Temperate pasture legumes in Australia: their history, current use, and future prospects. Crop & Pasture Science 63, 691 725.
| Crossref | Google Scholar |
Nichols PGH, Foster KJ, Piano E, Pecetti L, Kaur P, Ghamkhar K, Collins WJ (2013) Genetic improvement of subterranean clover (Trifolium subterraneum L.). 1. Germplasm, traits and future prospects. Crop & Pasture Science 64, 312-346.
| Crossref | Google Scholar |
Norman HC, Masters DG (2023) Livestock preference and feeding value as key determinants for forage improvement – why not ask the consumers? Animal Production Science 63, 1161-1176.
| Crossref | Google Scholar |
NSW DPI (2025) Serradella – Part D Introduction. Available at https://www.dpi.nsw.gov.au/agriculture/pastures-and-rangelands/species-varieties/serradella/part-d [accessed 5 January 2025]
Nutt BJ (1997) Cadiz French serradella (Ornithopus sativus Brot.) cultivar registration. Plant Varieties Journal 10(2), 12-14.
| Google Scholar |
Nutt B (2003) Yelbini. Plant Varieties Journal 17(1), 547-549.
| Google Scholar |
Nutt B (2004a) Margurita. Plant Varieties Journal 17(3), 311-312.
| Google Scholar |
Nutt B (2004b) Erica. Plant Varieties Journal 17(3), 313-315.
| Google Scholar |
Nutt BJ, Paterson J (1997) Cadiz French serradella: a new pasture variety for deep acid soils (Farmnote) 12/97. Department of Agriculture and Food, Western Australia. Available at https://irwinhunter.com.au/wp-content/uploads/2014/02/cadiz_serradella_publication.pdf
Nutt BJ, Harrison RJ, McComb JA, Howieson JG (2020) The breeding system of Ornithopus sativus Brot. subsp. sativus. Grass and Forage Science 76, 3-9.
| Crossref | Google Scholar |
Nutt BJ, Loi A, Hackney B, Yates RJ, D’Antuono M, Harrison RJ, Howieson JG (2021) “Summer sowing”: A successful innovation to increase the adoption of key species of annual forage legumes for agriculture in Mediterranean and temperate environments. Grass and Forage Science 76, 93-104.
| Crossref | Google Scholar |
Oldach KH, Peck DM, Cheong J, Williams KJ, Nair RM (2008) Identification of a chemically induced point mutation mediating herbicide tolerance in annual medics (Medicago spp.). Annals of Botany 101, 997-1005.
| Crossref | Google Scholar | PubMed |
Oldach KH, Peck DM, Nair RM, Sokolova M, Harris J, Bogacki P, Ballard R (2014) Genetic analysis of tolerance to the root lesion nematode Pratylenchus neglectus in the legume Medicago littoralis. BMC Plant Biology 14, 100.
| Crossref | Google Scholar |
Oram R (1987) Serradella, Ornithopus compressus L. (yellow serradella), cv. Tauro. Journal of the Australian Institute of Agricultural Science 53, 312-313.
| Google Scholar |
Ozanne PG, Asher CJ, Kirton DJ (1965) Root distribution in a deep sand and its relationship to the uptake of added potassium by pasture plants. Australian Journal of Agricultural Research 16, 785-800.
| Crossref | Google Scholar |
Paynter BH (1992) Comparison of the phosphate requirements of burr medic and yellow serradella with subterranean clover in the low rainfall wheatbelt of Western Australia. Australian Journal of Experimental Agriculture 32, 1077-1086.
| Crossref | Google Scholar |
Paynter B, Bolland M (1993) Comparison of the Phosphate Requirements of Burr Medic and Yellow Serradella with that of Wheat in the Low Rainfall Wheat-Belt of Western-Australia. Australian Journal of Experimental Agriculture 33, 145-157.
| Crossref | Google Scholar |
Pazos-Navarro M, Castello M, Bennett RG, Nichols P, Croser J (2017) In vitro-assisted single-seed descent for breeding-cycle compression in subterranean clover (Trifolium subterraneum L.). Crop & Pasture Science 68, 958-966.
| Crossref | Google Scholar |
Peck D, Damin T (2022) Speed breeding methodologies delivers two new barrel medic cultivars to farmers within 6.5 years of breeding commencing. In ‘Proceedings of the 20th Australian Agronomy Conference’, 18–22 September 2022, Toowoomba, Qld, Australia. (Australian Society of Agronomy) Available at http://agronomyaustraliaproceedings.org/images/sampledata/2022/Pastures/ASApeckXXX__d_341s.pdf
Peck D, Palmer R, Hawkey D, Ballard R, Oldach K, Sutton T, Howie J (2015) The development of a mid-season barrel medic (Medicago truncatula Gaertn.) cultivar with tolerance to sulfonylurea herbicide residues. In ‘Proceedings of the 17th Australian Agronomy Conference’, 20–24 September 2015, Hobart, Tas, Australia. (Australian Society of Agronomy) Available at agronomyaustraliaproceedings.org
Peck DM, Michelmore S, Sutton T (2021) Genetic analysis of boron tolerance in burr medic (Medicago polymorpha L.). Crop & Pasture Science 72(9), 634-640.
| Crossref | Google Scholar |
Peck DM, Humphries AW, Ballard RA (2023) Development of methods to overcome physiological seed dormancy of temperate annual pasture legumes to assist speed breeding. Crop & Pasture Science 74(8), 797-808.
| Crossref | Google Scholar |
Peoples MB, Brockwell J, Hunt JR, Swan AD, Watson L, Hayes RC, Li GD, Hackney B, Nuttall JG, Davies SL, Fillery IRP (2012) Factors affecting the potential contributions of N2 fixation by legumes in Australian pasture systems. Crop & Pasture Science 63, 759.
| Crossref | Google Scholar |
Pook M, Lisson S, Risbey J, Ummenhofer CC, McIntosh P, Rebbeck M (2009) The autumn break for cropping in southeast Australia: trends, synoptic influences and impacts on wheat yield. International Journal of Climatology 29, 2012-2026.
| Crossref | Google Scholar |
Revell C (1992) New yellow serradella varieties for low rainfall pastures. Journal of the Department of Agriculture, Western Australia, Series 4 33(3), Article 9 Available at https://library.dpird.wa.gov.au/journal_agriculture4/vol33/iss3/9.
| Google Scholar |
Revell C, Nutt B, Ewing M (1998a) Success with Serradella in the wheatbelt. Journal of the Department of Agriculture, Western Australia, Series 4 39(1), Article 6 Available at https://library.dpird.wa.gov.au/journal_agriculture4/vol39/iss1/6.
| Google Scholar |
Revell CK, Taylor GB, Cocks PS (1998b) Long-term softening of surface and buried hard seeds of yellow serradella grown in a range of environments. Australian Journal of Agricultural Research 49, 673-686.
| Crossref | Google Scholar |
Revell CK, Taylor GB, Cocks PS (1999) Effect of length of growing season on development of hard seeds in yellow serradella and their subsequent softening at various depths of burial. Australian Journal of Agricultural Research 50, 1211-1223.
| Crossref | Google Scholar |
Revell CK, Ewing MA, Real D, Nichols PGH, Sandral GA (2013) Commercialisation and impacts of pasture legumes in southern Australia – lessons learnt. In ‘Revitalising Grasslands to Sustain Our Communities: Proceedings of the 22nd International Grassland Congress’, 15–19 September 2013, Sydney, NSW, Australia. (Eds DL Michalk, GD Millar, WB Badgery, KM Broadfoot) (New South Wales Department of Primary Industries: Orange, NSW, Australia) Available at https://uknowledge.uky.edu/igc/22/1-3/2
Rigg JL, Webster AT, Harvey DM, Orgill SE, Galea F, Dando AG, Collins DP, Harris CA, Newell MT, Badgery WB, Hayes RC (2021) Cross-host compatibility of commercial rhizobial strains for new and existing pasture legume cultivars in south-eastern Australia. Crop & Pasture Science 72, 652-665.
| Crossref | Google Scholar |
Rose RJ (2008) Medicago truncatula as a model for understanding plant interactions with other organisms, plant development and stress biology: past, present and future. Functional Plant Biology 35, 253 264.
| Crossref | Google Scholar | PubMed |
Ross MA, Harper JL (1972) Occupation of biological space during seedling establishment. Journal of Ecology 60(1), 77-88.
| Crossref | Google Scholar |
Rossiter RC, Collins WJ, Klein L (1985) Winter growth and nutritive quality of serradella (Ornithopus spp.). Australian Journal of Experimental Agriculture 25, 362-366.
| Crossref | Google Scholar |
Sandral GA, Dear BS, Virgona JM, Swan AD, Orchard BA (2006) Changes in soil water content under annual- and perennial-based pasture systems in the wheatbelt of southern New South Wales. Australian Journal of Agricultural Research 57(3), 321.
| Crossref | Google Scholar |
Sandral GA, Haling RE, Ryan MH, Price A, Pitt WM, Hildebrand SM, Fuller CG, Kidd DR, Stefanski A, Lambers H, Simpson RJ (2018) Intrinsic capacity for nutrient foraging predicts critical external phosphorus requirement of 12 pasture legumes. Crop & Pasture Science 69, 174-182.
| Crossref | Google Scholar |
Sandral GA, Price A, Hildebrand SM, Fuller CG, Haling RE, Stefanski A, Yang Z, Culvenor RA, Ryan MH, Kidd DR, Diffey S, Lambers H, Simpson RJ (2019) Field benchmarking of the critical external phosphorus requirements of pasture legumes for southern Australia. Crop & Pasture Science 70, 1080-1096.
| Crossref | Google Scholar |
Sanford P, Pate JS, Unkovich MJ (1994) A survey of proportional dependence of subterranean clover and other pasture legumes on N2 fixation in south-west Australia utilizing 15N natural abundance. Australian Journal of Agricultural Research 45(1), 165-181.
| Crossref | Google Scholar |
Scott JF, Lodge GM, McCormick LH (2000a) Economics of increasing the persistence of sown pastures: costs, stocking rate and cash flow. Australian Journal of Experimental Agriculture 40, 313-323.
| Crossref | Google Scholar |
Scott BJ, Ridley AM, Conyers MK (2000b) Management of soil acidity in long-term pastures of south-eastern Australia: a review. Australian Journal of Experimental Agriculture 40, 1173-1198.
| Crossref | Google Scholar |
Shipton QA, Parker CA (1967) Nodulation of lime pelleted lupins and serradella when inoculated with peat and agar cultures. Australian Journal of Experimental Agriculture and Animal Husbandry 7(26), 259-262.
| Crossref | Google Scholar |
Shirasawa K, Moraga R, Ghelfi A, Hirakawa H, Nagasaki H, Ghamkhar K, Barrett BA, Griffiths AG, Isobe SN (2023) An improved reference genome for Trifolium subterraneum L. provides insight into molecular diversity and intra-specific phylogeny. Frontiers in Plant Science 14, 1103857.
| Crossref | Google Scholar |
Simpson R (2020) B.PSP.0018 – Phosphorus efficient pastures: delivering high nitrogen and water use efficiency, and reducing cost of production across southern Australia – Evaluation report. Meat & Livestock Australia. Available at https://www.mla.com.au/research-and-development/reports/2023/b.psp.0018---phosphorus-efficient-pastures-delivering-high-nitrogen-and-water-use-efficiency-and-reducing-cost-of-production-across-southern-australia--evaluation-report/
Simpson RJ, Haling RE (2021) Delivering improved phosphorus acquisition by root systems in pasture and arable crops. Available at https://www.bdschapters.com/webshop/a-z-chapters/d/delivering-improved-phosphorus-acquisition-by-root-systems-in-pasture-and-arable-crops/.
Simpson RJ, Richardson AE, Nichols SN, Crush JR (2014) Pasture plants and soil fertility management to improve the efficiency of phosphorus fertiliser use in temperate grassland systems. Crop & Pasture Science 65, 556-575.
| Crossref | Google Scholar |
Simpson RJ, Boschma SP, Kidd DR, Newell MT, Stefanski A, Haling RE, Ryan MH, Hayes RC, Goward L (2025) Maturity type and flowering date instability among serradella (Ornithopus spp.) cultivars. Crop Science 65(3), e70083.
| Crossref | Google Scholar |
Smith L (2022) Serradella (Ornithopus compressus) cv. Serramax. Plant Varieties Journal 35(1), 170-172.
| Google Scholar |
Smith RW, Harris CA, Cox K, McClements D, Clark SG, Hossain Z, Humphries AW (2021) A history of Australian pasture genetic resource collections. Crop & Pasture Science 72, 591-612.
| Crossref | Google Scholar |
Snowball R (1996) The Australian germplasm of pink flowering serradella species. In ‘Proceedings of the 8th Australian Agronomy Conference’, 30 September–3 October 1996, Toowoomba, Qld, Australia. (Australian Society of Agronomy) Available at https://agronomyaustraliaproceedings.org/images/sampledata/1996/contributed/506snowball.pdf
Stratton (1996) Hybrid serradella (Ornithopus sativus × Ornithopus compressus) cv. Grasslands Spectra. Plant Varieties Journal 9(4), 30-31.
| Google Scholar |
Sumner M, Cook D, Herenu D (2021) ozmaps: Australia Maps. R package version 0.4.5. Available at https://cran.r-project.org/web/packages/ozmaps/index.html
Taylor GB (1970) The germinability of soft seed of a number of strains of subterranean clover. Australian Journal of Experimental Agriculture and Animal Husbandry 10, 293-297.
| Crossref | Google Scholar |
Taylor GB (2004) Effect of temperature and state of hydration on rate of imbibition in soft seeds of yellow serradella. Australian Journal of Agricultural Research 55(1), 39-45.
| Crossref | Google Scholar |
Taylor GB (2005) Hardseededness in Mediterranean annual pasture legumes in Australia: a review. Australian Journal of Agricultural Research 56, 645-661.
| Crossref | Google Scholar |
Taylor GB, Revell CK (1999) Effect of pod burial, light, and temperature on seed softening in yellow serradella. Australian Journal of Agricultural Research 50, 1203-1209.
| Crossref | Google Scholar |
Taylor GB, Revell CK (2002) Seed softening, imbibition time, and seedling establishment in yellow serradella. Australian Journal of Agricultural Research 53, 1011-1018.
| Crossref | Google Scholar |
Taylor AO, Hughes KA, Hunt BJ, Latch GCM (1979) I. A survey of lines for yield and disease resistance at Kaitaia and Palmerston North. New Zealand Journal of Experimental Agriculture 7, 141-147.
| Crossref | Google Scholar |
The Legume Phylogeny Working Group (2017) A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. TAXON 66, 44-77.
| Crossref | Google Scholar |
Thomas DT, Flohr BM, Monjardino M, Loi A, Llewellyn RS, Lawes RA, Norman HC (2021) Selecting higher nutritive value annual pasture legumes increases the profitability of sheep production. Agricultural Systems 194, 103272.
| Crossref | Google Scholar |
Unkovich M (2010) A simple, self adjusting rule for identifying seasonal breaks for crop models. In ‘Proceedings of the 15th Agronomy Conference 2010’, Lincoln, New Zealand. (Australian Society of Agronomy: Lincoln, NZ) Available at https://www.regional.org.au/au/asa/2010/crop-production/sequence/7129_unkovich.htm#TopOfPage
Veerappan V, Kadel K, Alexis N, Scott A, Kryvoruchko I, Sinharoy S, Taylor M, Udvardi M, Dickstein R (2014) Keel petal incision: a simple and efficient method for genetic crossing in Medicago truncatula. Plant Methods 10, 11.
| Crossref | Google Scholar | PubMed |
Visnevschi-Necrasov T, Harris DJ, Faria MA, Pereira G, Nunes E (2012) Phylogeny and genetic diversity within Iberian populations of Ornithopus L. and Biserrula L. estimated using ITS DNA sequences. Spanish Journal of Agricultural Research 10(1), 149-154.
| Crossref | Google Scholar |
Watson A, Ghosh S, Williams MJ, Cuddy WS, Simmonds J, Rey M-D, Asyraf Md Hatta M, Hinchliffe A, Steed A, Reynolds D, Adamski NM, Breakspear A, Korolev A, Rayner T, Dixon LE, Riaz A, Martin W, Ryan M, Edwards D, Batley J, Raman H, Carter J, Rogers C, Domoney C, Moore G, Harwood W, Nicholson P, Dieters MJ, DeLacy IH, Zhou J, Uauy C, Boden SA, Park RF, Wulff BBH, Hickey LT (2018) Speed breeding is a powerful tool to accelerate crop research and breeding. Nature Plants 4, 23-29.
| Crossref | Google Scholar | PubMed |
Weeldenburg JR, Smith RW (1969) Dehulling and scarifying serradella seed. Journal of Agriculture 10(5), 230-231.
| Google Scholar |
Williams WM, De LG, Williams EG (1987) A hybrid between Ornithopus sativus and O. compressus cv. pitman obtained with the aid of ovule–embryo culture. Australian Journal of Botany 35, 219-226.
| Crossref | Google Scholar |
Wilmot MG, Norman HC, Hendry J, Young P, Hulm E, Toovey A, Speijers J, Harrison R (2023) Sheep grazing Trigonella balansae had productivity, health and meat quality similar to sheep grazing subterranean clover or French serradella. Animal Production Science 63, 152-167.
| Crossref | Google Scholar |
You MP, Lancaster B (2008) Cross-pathogenicity of Rhizoctonia solani strains on pasture legumes in pasture-crop rotations. Plant and Soil 302, 203-211.
| Crossref | Google Scholar |