Skin lesions suspicious for melanoma: New Zealand excision margin guidelines in practice
Tess Brian 1 , Michael B. Jameson 2 31 Department of Plastic and Reconstructive Surgery, Waikato Hospital, Hamilton, New Zealand
2 Oncology Department, Waikato Hospital, Hamilton, New Zealand
3 Waikato Clinical Campus, University of Auckland, Hamilton, New Zealand
Correspondence to: Tess Brian, Department of Plastic and Reconstructive Surgery, Waikato Hospital, Selwyn Street and Pembroke Street, Hamilton 3204, New Zealand. Email: tessbrian0@gmail.com
Journal of Primary Health Care 10(3) 210-214 https://doi.org/10.1071/HC17055
Published: 4 October 2018
Journal Compilation © Royal New Zealand College of General Practitioners 2018.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
INTRODUCTION: New Zealand guidelines for cutaneous melanoma management recommend excision biopsy specimens of suspected lesions have a 2 mm horizontal margin, and a deep margin into upper subcutis.
AIM: To assess guideline compliance of suspicious lesion biopsies taken in the community and in a hospital.
METHODS: Patients admitted to Waikato Hospital, Hamilton, for diagnostic or treatment melanoma surgery during the year ending February 2016 were retrospectively identified, and their demographic and biopsy characteristics examined.
RESULTS: In total, 140 patients had excision biopsies: 61.4% were performed outside the hospital. Biopsy data were available for 126 specimens. Mean horizontal margin was greater (P = 0.001) in hospital biopsies (4.8 mm, standard deviation (s.d.) 3.7 mm) than biopsies performed elsewhere (2.8 mm; s.d. 1.8 mm). Horizontal margins >2.0 mm occurred in 70.6% of specimens; 21.6% of ≤2.0 mm specimens had a tumour-positive margin. Subsequent wide local excision identified residual melanoma in 9.6% of specimens, which was not associated (P = 0.3) with primary horizontal margin ≤2.0 mm. Mean deep margin of hospital biopsies (6.5 mm; s.d. 2.7 mm) was greater (P < 0.001) than in other biopsies (4.1 mm; s.d. 2.7 mm). Horizontal margin >2.0 mm specimens had greater (P < 0.001) mean deep margin (5.9 mm; s.d. 2.7 mm) than specimens with horizontal margin ≤2.0 mm (mean deep margin 3.3 mm; s.d. 2.7 mm). Deep margin ≤2.0 mm (19.0%) was independently associated with the facility where biopsy was performed (P = 0.001) and horizontal margin (P < 0.001).
DISCUSSION: The New Zealand biopsy deep margin recommendation does not lend itself to meaningful audit. Compliance with the horizontal margin recommendation was low, but of uncertain clinical significance.
KEYWORDS: audit, clinical; biopsy, skin; guideline adherence; melanoma, cutaneous malignant; New Zealand
| WHAT GAP THIS FILLS |
| What is already known: New Zealand guidelines for cutaneous melanoma management recommend clearance margins for excision biopsy specimens of suspected lesions. |
| What this study adds: For biopsies taken both in and outside hospital, compliance with these recommendations is low. Revision of the guidelines may improve their utility. |
Introduction
Malignant melanoma is a significant public health problem in New Zealand. With 2366 cases in 2013 (10.7% of new cancer registrations), it was the fourth most common cancer behind prostate cancer (14.1%), colorectum cancer (13.9%) and breast cancer (13.7%). In the same year, there were 9063 cancer deaths: 356 (3.9%) were due to melanoma.1
The 2008 ‘Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand’ recommend that the optimal method for biopsy of suspected cutaneous melanoma lesions is complete excision with a 2 mm clinical horizontal margin, and a deep margin into upper subcutis.2 In 2013, this was reaffirmed in the National Melanoma Tumour Standards Working Group’s ‘Standards of Service Provision for Melanoma Patients in New Zealand – Provisional’.3
All such specimens of suspected cutaneous melanoma should be sent for formalin-fixed, paraffin-embedded histopathology.3 The result of this examination will determine the subsequent pathway of lesion and patient management.3 Where melanoma is detected in the initial biopsy, the histopathology will contribute to the staging of the disease, with implications for disease behaviour and patient survival.3,4 Wide excision at the biopsy site will be required as definitive local treatment.2,3 Other interventions, such as sentinel lymph node biopsy, may also be indicated. The horizontal margin of clearance in the initial biopsy may effect wide local excision and sentinel node biopsy procedures.
Using data from a tertiary hospital, this paper discusses the recommended horizontal and deep margins of excision biopsies taken for suspected cutaneous melanoma, and assesses compliance with the New Zealand guidelines.
Methods
Discharge coding and histopathology records were used to retrospectively identify all patients who were admitted to Waikato Hospital, Hamilton, for melanoma surgery during the year ending 16 February 2016. The histopathology reports for melanoma specimens from these patients were retrieved. If available, histological details of the cutaneous melanoma that had initiated admission for excision biopsy, wide local excision, sentinel node biopsy and complete regional lymph node dissection were noted, regardless of whether the initial biopsy had been performed at Waikato Hospital or in rooms or surgical facilities elsewhere.
Demographic and histopathological data were entered into PASW/SPSS Statistics 18.0 software (SPSS Inc., Chicago, IL, USA). Analysis of patient and cutaneous biopsy characteristics was then performed. Relationships between categorical variables were assessed using Chi-square tests or Fisher’s exact/Mid-P test if any cell frequency was less than five. Means of two groups of continuous quantitative variables were compared using t-test. Comparison of means of three or more such groups were made by using ANOVA. Where significant differences were demonstrated on univariate analysis, logistic regression was used to determine independence of association. Relative risk was calculated with 95% confidence intervals. Significance was accepted at two-sided P < 0.05.
The Health and Disability Ethics Committees of the New Zealand Ministry of Health do not require ethical approval of this low-risk observational activity.
Results
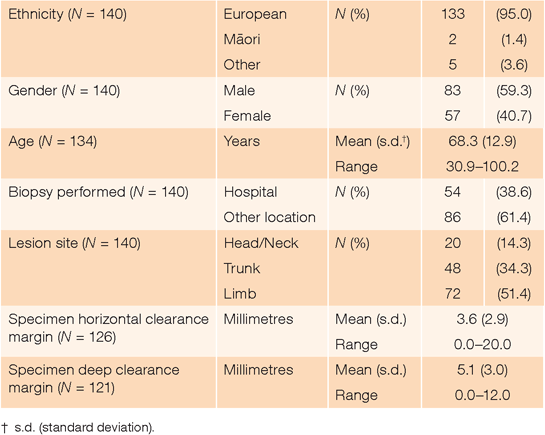
There were 143 unique patients admitted to Waikato Hospital for melanoma surgery during the year reviewed. Of these, three had melanoma diagnosed following punch or incision cutaneous biopsies. Excision biopsy was used for the remaining 140 patients (Table 1).
|
In the 126 (90.0%) excision biopsy specimens for which horizontal margin data were available, margins >2.0 mm, >3.0 mm and >5.0 mm were found in 89 (70.6%), 54 (42.9%) and 23 (18.3%) cases respectively. There was no difference in mean horizontal clearance of melanoma from incision across gender (P = 0.5), 10-year age cohorts (P = 0.6) or site of lesion (P = 0.5). Although the mean horizontal margin of biopsies taken at Waikato Hospital (4.8 mm; s.d. = 3.7 mm) was significantly greater (P = 0.001) than for biopsies performed elsewhere (2.8 mm; s.d. = 1.8 mm), the relative risk (1.3-fold; 95% confidence interval (CI) 0.8–2.4) of an excessive margin (>2.0 mm) for hospital-harvested biopsies was not significant (P = 0.3). Thirty-seven (29.4%) biopsy specimens had horizontal clearance margins ≤2.0 mm. Of these, eight (21.6%) had tumour to the edge of the specimen.
Of the 126 patients for whom biopsy horizontal margin data were available, 115 (91.3%) subsequently underwent wide local excision. Melanoma was identified in 11 (9.6%) of these specimens. While proportionately more patients had melanoma in their wider excision if their primary tumour excision margin was ≤2.0 mm (5/35, 14.3%) than if it was >2.0 mm (6/80, 7.5%), this was not statistically significant (P = 0.3).
Of the 11 (8.7%) patients with initial biopsy horizontal margin data who did not have wide local excision, four (36.4%) had very thick tumours (Breslow thickness 5.0–20.0 mm) and evidence of metastatic disease. A wide excision procedure was less likely following an initial biopsy with >5.0 mm (P < 0.001) and >3.0 mm (P = 0.04) margins, but not >2.0 mm (P = 0.4).
Sentinel node biopsy was performed in 56 (44.4%) of the 126 patients with initial biopsy horizontal margin data. The mean horizontal clearance of incision from melanoma in their initial biopsy specimens was 3.2 mm (s.d. = 2.3 mm; range 0–12.0 mm). Twenty-four (42.9%) of these specimens had a margin >3.0 mm.
In the 121 (86.4%) excision biopsy specimens for which deep margin data were available, 23 (19.0%) were ≤2.0 mm. There was no difference in mean deep clearance of melanoma from incision across gender (P = 0.6) or 10-year age cohorts (P = 0.5). However, the mean deep margin of biopsies taken at Waikato Hospital (6.5 mm; s.d. = 2.7 mm) was significantly greater (P < 0.001) than for biopsies performed elsewhere (4.1 mm; s.d. = 2.7 mm). Specimens with horizontal margins >2.0 mm also had greater (P < 0.001) deep margins (mean 5.9 mm; s.d. = 2.7 mm) than those with horizontal clearance ≤2.0 mm (mean 3.3 mm; s.d. = 2.7 mm). A significant difference (P = 0.005) in mean deep margin was also found across specimens taken from the trunk (5.8 mm; s.d. = 3.1 mm), limbs (5.3 mm; s.d. = 2.8 mm) and head or neck (3.0 mm; s.d. = 2.2 mm). On multivariate analysis, deep margin ≤2.0 mm was independently and significantly associated with the facility where biopsy had occurred (P = 0.001) and horizontal specimen clearance (P < 0.001), but not tumour site (P = 0.2). Biopsies taken outside the hospital were 8.4-fold (95% CI 2.1–34.1; P = 0.003), and those with horizontal margin ≤2.0 mm were 6.6-fold (95% CI 2.3–19.3; P = 0.001), more likely to have a deep margin ≤2.0 mm.
Discussion
Recognising the limitations of the small sample involved, this practice audit evaluated the surgical margins of excision biopsy specimens taken for suspected melanoma from patients referred to a New Zealand tertiary hospital, and compared these with the horizontal and deep clearances recommended by New Zealand guidelines. We found horizontal and deep clearances ≤2.0 mm in 29.4% and 19.0% of specimens respectively, and that, irrespective of margin clearance, residual melanoma occurred in 9.6% of specimens harvested at subsequent wide local excision.
However, the recommended 2-mm horizontal margin for excision biopsy of suspected cutaneous melanoma is a clinical macroscopic measurement.2,3 It should be measured from the edge of the lesion, using a ruler and marker pen, before excision begins. The margins considered in the current analysis are histopathological measurements. They indicate the nearest microscopic evidence of melanoma, either in situ or invasive, to the edge of a formalin-fixed specimen. Specimen shrinkage after excision and during fixation may result in a smaller histopathological margin than measured clinically. The same will be noted where microscopic tumour extends beyond clinically visible melanoma.
It is therefore clear that a horizontal histological margin >2.0 mm can only occur if the clinical margin is greater than recommended by the guidelines. Consequently, 89 (70.6%) excision biopsies for which there were data had horizontal clearance exceeding the guideline recommendation. In contrast, a histological margin ≤2.0 mm may occur if the clinical margin is less than, equal to, or greater than 2 mm. For the 37 (29.4%) specimens in this group, it is impossible to estimate the number that were excised with less than the recommended 2 mm clinical margin. However, importantly, eight (21.6% of these specimens; 6.3% of the 126 for which data were available) had a tumour-involved histological margin. This is a service-monitoring metric,3 and suggests improvements could be made.
Although a complete excision biopsy with a 2 mm horizontal margin is recommended, the evidence for this is not strong.2 Various margins have been suggested, including 2–5 mm in the 2002 United Kingdom melanoma guidelines.5 If a margin of 5 mm were accepted, only 18.3% of our reported cases would have had clearance exceeding this.
The definitive treatment for primary melanoma is wide local excision of the skin and subcutaneous tissues at the diagnostic excision biopsy site. The aim is to completely remove all in situ and invasive melanoma. The recommendations for minimum radial wide local excision margins are based on the primary melanoma’s maximum Breslow thickness, and are measured clinically from the edge of the melanoma. The recommended margin is 5 mm if only in situ melanoma is identified in the initial excision biopsy specimen. All other melanomas should have a wide excision margin of 10 or 20 mm, depending on staging.2 Therefore, if the recommended excision biopsy clinical margin was 5 mm, and histological clearance matched this in the specimen, then wide excision for in situ lesions would no longer be required as a second procedure.
However, a margin greater than 3 mm may interfere with the lymphatic mapping required for sentinel node biopsy.6 Of the cases subsequently undergoing sentinel node biopsy, 42.9% had excision biopsy margins >3.0 mm, perhaps with potential for inappropriate node sampling. This may be important because, although sentinel lymph node biopsy itself does not confer any survival advantage, it is a commonly recommended procedure in New Zealand when the primary melanoma is thicker than 1 mm, and poor execution could result in compromised staging of the cancer, with inaccurate prognostication and inappropriate management.
The New Zealand guidelines for management of suspected cutaneous melanoma recommend only that the initial excision biopsy’s deep margin should be ‘into upper subcutis’.2 The size of this margin would therefore be expected to reflect skin variation at different excision sites, with, as was noted, smaller mean clearance in the head and neck than elsewhere (P = 0.005). The imprecision of the recommendation is likely a concession to the surgical difficulties of attempting measured deep clearance from visible tumour. However, if the expectation is sufficient deep and horizontal tumour-free clearance, then this may be afforded by a deep clinical margin equivalent to that recommended for horizontal clearance (2 mm). If this were the case, then biopsies performed outside the hospital and biopsies associated with horizontal clearance ≤2 mm were likely to have inadequate deep margins (P = 0.003 and P = 0.001 respectively).
Most small suspicious cutaneous lesions are managed outside hospitals by clinicians with surgical skills to undertake excision of in situ or thin melanoma, followed by direct closure. Patients with lesions of uncertain diagnosis, thicker melanomas and lesions located where surgery is difficult are likely to be referred to a specialist centre such as Waikato Hospital. In part, this may account for the greater horizontal and deep margins noted for specimens taken at the hospital.
The New Zealand guideline recommendation of a deep biopsy margin ‘into upper subcutis’ does not lend itself to meaningful audit. This is partly because of the difficulty obtaining such information from histopathology reports. The 2 mm clinical horizontal margin recommendation is more readily assessed, but depends on the use of a proxy, the histopathological clearance measurement, as used in this audit. The result has been that a high proportion (70.6% with >2.0 mm margin, plus an unknowable percentage of those ≤2.0 mm) of the reviewed biopsies may be judged ‘non-compliant’ with the guideline. This is only important if there are clinical consequences. In most of these cases, it is unlikely that non-compliance had any clinical implications. The exceptions are biopsies where insufficient tumour clearance resulted in residual melanoma in the wide local excision specimen, and those in which excessive clearance (>3 mm) could have interfered with accurate lymphatic mapping at subsequent sentinel node biopsy. This highlights the difficulty of clinical guideline recommendations that cannot be directly audited and for which non-compliance does not necessarily change patient risk or outcomes.
Given that the aim of a clinical guideline is to maximise patient outcomes by improving and standardising practice according to available evidence, and the fact that other jurisdictions recommend different excision margins, it may be time to reconsider the New Zealand guideline. Rather than a single clinical measurement for margin from visible tumour, a clearance range may have more utility. This would allow and likely encourage practitioner discretion, depending on such variables as tumour size and location. This may also more strongly signal a minimum clearance at which the risks of tumour-positive margin and residual melanoma are acceptably low, and an upper limit at which the risk to lymphatic mapping is minimised. The excision limits may also be set to obviate the need for subsequent wide local excision of in situ and thin invasive melanomas. Where anticipated clearances are less or more than this range, a criterion for referral for biopsy to a specialist centre may be added to the guideline. However, whether the clearance recommendations are retained or amended, the imprecision remains of auditing a clinical measurement lost through specimen shrinkage at excision and formalin fixation.
COMPETING INTERESTS
None.
References
[1] New Zealand Ministry of Health. Cancer: New registrations and deaths 2013. Wellington: New Zealand Ministry of Health; 2016.[2] Australian Cancer Network Melanoma Guidelines Revision Working Party. Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand. Wellington: The Cancer Council Australia and Australian Cancer Network, Sydney and New Zealand Guidelines Group; 2008.
[3] National Melanoma Tumour Standards Working Group. Standards of Service Provision for Melanoma Patients in New Zealand - Provisional. Wellington: New Zealand Ministry of Health; 2013.
[4] Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer (AJCC), Cancer Staging Manual. 7th edn. New York: Springer; 2010.
[5] Roberts DL, Anstey AV, Barlow RJ, et al. UK guidelines for the management of cutaneous melanoma. Br J Dermatol. 2002; 146 7–17.
| UK guidelines for the management of cutaneous melanoma.Crossref | GoogleScholarGoogle Scholar |
[6] Coit DG, Thompson JA, Andtbacka R, et al. National Comprehensive Cancer Network (NCCN), Clinical Practice Guidelines in Oncology. Melanoma (Version 4.2014). Fort Washington: National Comprehensive Cancer Network; 2014.


