Prevalence of invasive cancer in a large general practice patient population in New Zealand
Dong Hyun Kim 1 , Lynne Chepulis 2 , Rawiri Keenan 2 , Chunhuan Lao 2 , Fraser Hodgson 3 , Chris Bullen 4 , Ross Lawrenson 2 5 61 School of Medicine, University of Auckland, Auckland, New Zealand
2 Waikato Medical Research Centre, University of Waikato, Private Bag 3105, Hamilton, New Zealand
3 Pinnacle Health, Hamilton, New Zealand
4 School of Population Health, University of Auckland, Auckland, New Zealand
5 Strategy and Funding, Waikato District Health Board, Hamilton, New Zealand
6 Corresponding author. Email: Ross.Lawrenson@waikatodhb.health.nz
Journal of Primary Health Care 12(3) 215-224 https://doi.org/10.1071/HC19113
Published: 9 September 2020
Journal Compilation © Royal New Zealand College of General Practitioners 2020 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
INTRODUCTION: The prevalence of cancer in the community is likely to be increasing due to an ageing population, implementation of cancer screening programmes and advances in cancer treatment.
AIM: To determine the prevalence of primary invasive cancers in a large general practice patient population in New Zealand and to characterise the health-care status of these cancer patients.
METHODS: Data were sourced from the patient management system of a large general practice (n = 11,374 patients) in a medium-sized Waikato town and from the New Zealand Cancer Registry dataset to identify patients diagnosed with cancer between January 2009 and December 2018.
RESULTS: There were 206 cancer diagnoses in 201 patients; 35 cancers were diagnosed in 1887 Māori patients (1.9%) and 171 in 9487 non-Māori patients (1.8%). The age-standardised prevalence was 3092/100,000 in Māori patients and 1971/100,000 in non-Māori patients. The most prevalent cancers were breast, male genital organ, digestive organ and skin cancers. In May 2019, 81 of 201 (40.8%) patients with cancer were receiving only usual care from their general practitioner, whereas 66 (32.8%) were having their cancer managed in secondary care. Comorbidities were common, including hypertension (38.8%), gastrointestinal disorders (29.9%) and mood disorders (24.4%).
DISCUSSION: Results suggest that there may be disparities in cancer prevalence between Māori and non-Māori patients, although this needs to be confirmed in other general practices. Furthermore, primary care appears to be responsible for most of the care in this patient cohort and workloads should be planned accordingly, particularly with the high incidence of comorbidities.
KEYwords: Cancer prevalence, New Zealand, cancer survivors, primary health care, registries
| WHAT GAP THIS FILLS |
| What is already known: There is increasing workload in primary care related to care of cancer survivors. There are many ways primary care can be involved in the care of cancer survivors. |
| What this study adds: This is the first New Zealand study reporting on the prevalence of cancer in primary care. It describes the characteristics of the care needs of cancer survivors in primary care. |
Introduction
The prevalence of cancer in primary care is expected to increase, with more cancer survivors requiring ongoing treatment, surveillance and end-of-life care.1 The reasons for this are multifactorial: an ageing population means the number of people developing cancer is increasing; advances in cancer treatment means that people are living longer; and implementation of cancer screening programmes means they are being diagnosed earlier in the cancer pathway, which in turn makes curative treatment more likely.2,3 However, there is only limited reporting of the number of patients in New Zealand primary care who have a current or previous diagnosis of cancer. Recently, a review of linked health data from 1995 to 2013 indicated that the age-standardised prevalence of cancer in New Zealand was 2.8% in people aged ≥15 years and was highest in older people, Māori women and people living in the least deprived areas.4 However, although this study provides a robust analysis of existing data, the data used are now 7 years old and the report does not include primary care determinants of health (eg comorbidities) or general practice management needs.
General practice is an essential part of cancer care as it is directly involved in all stages of the cancer continuum, from screening, diagnosis and referral to secondary care, to ongoing support post-treatment. A systematic review of 15 studies highlighted the importance of general practitioners (GPs) in providing support for cancer information, recovery, treatment and ‘life-adjustment’ issues, noting also that the primary care needs of cancer survivors are often not met.5 During active cancer treatment, many cancer patients receive treatment from secondary care services with no active involvement of their GP during this time.6 As a result, when patients are later discharged back into the care of their GP, they may be uncertain about who is responsible for ongoing cancer-related care, with resulting deficits in overall follow-up care.6 As survivors are at risk of cancer recurrence or may have cancer- or treatment-related problems, they need thorough and ongoing patient follow up in general practice.7
GPs can also monitor emerging conditions related to cancer treatment; for example, osteoporosis may develop following endocrine therapies;8 heart disease with chemotherapy or radiotherapy;9,10 and venous thromboembolism11 or secondary cancers may occur.12,13 Additionally, cancer disproportionately affects the elderly, who are more likely to present with comorbidities.14 Unlike younger patients, where cancer treatment can be life-saving, treatment of elderly patients may worsen geriatric symptoms and this must be appropriately managed by both primary and secondary care services.15
For GPs to provide effective care to cancer patients at all stages of the cancer continuum, they need to be aware of the likely cancer prevalence in their patients and the typical health-care management needs of these patients. Patients’ needs may be weightier in younger patients and in patients with particular types of cancer (eg breast). Supportive care needs are also different for male and female cancer patients.16 New Zealand GPs should be aware of marked inequities in cancer care17,18 and outcomes19–23 for Māori cancer patients and survivors that occur along the entire cancer care pathway, largely attributable to the westernised model of health care widely used in New Zealand not catering to the needs of many Māori patients.24
The aim of this study was to determine the prevalence of primary invasive cancer in a large general practice patient population and to measure differences in cancer prevalence between Māori and non-Māori patients.
Methods
This study was completed in a large general practice with 11,374 registered patients in May 2019. The practice is located in a small town (population 17,500 in 2019) in the Waikato region. Ethics approval for this study was granted by the Health and Disability Ethics Committee (ref NZ/1/1C32112).
Data collection
To identify cancer cases in the general practice, two strategies were used. First, the practice’s electronic Patient Management System (MedTech 32, https://medtechglobal.com) database was queried using the query function for all cases matching the Read code clinical terminology system: B* (Neoplasms) and ‘History of Disease’ with a date of classification between January 2009 and December 2018. Second, a list of National Health Index (NHI) numbers of all enrolled patients was cross-referenced against the New Zealand Cancer Register to identify additional cases of cancer recorded in the same time frame (the date of the latest cancer registration was used). These two datasets were then combined for analysis. Only invasive cancers (excluding non-melanoma skin cancers) were included in this study.
Next, the clinical records of each cancer patient were retrieved from the Practice Management System and we extracted data relating to age, gender, ethnicity, date of cancer diagnosis, cancer type, Surveillance Epidemiology and End Result (SEER) summary stage at diagnosis,25 cancer treatment modalities and the current health-care status of the patient (as at May 2019). Patient ethnicity was categorised as Māori or non-Māori according to the records, using prioritisation to manage multiple ethnicities. The health-care status of patients was defined as being under standard care by GPs; being in the care of secondary care after completion of treatment; currently receiving active cancer treatment from secondary care (where ‘secondary care’ refers to the medical, surgical and radiation oncology services provided by Waikato District Health Board); receiving active cancer treatment from the general practice; or receiving palliative care. Additional information collected included comorbidities (at any time), defined using the C3 databased index.26
Data analysis
Data are reported for all cancer patients, and for Māori and non-Māori separately. Other ethnic groups are not separately reported due to low numbers. Differences between Māori and non-Māori were examined by using Chi-squared tests, with a P-value <0.05 considered significant. Patient age is reported as age at the time of the data audit (30 May 2019). The age-standardised prevalence of cancer, and the prevalence of key cancers were calculated using the World Health Organization Standard Population as a reference.27 Statistical analyses were conducted using IBM SPSS Statistics (SPSS Inc., Chicago, IL, USA).
Results
The primary care database search identified 180 primary invasive cancer diagnoses during the study period, including five patients having two primary invasive cancers diagnosed during this time. All cancer cases were recorded in the New Zealand Cancer Register, except for the 32 cases diagnosed in 2018 due to Register data being complete only until December 2017 at the time of this study. A further 21 cancer diagnoses were recorded in the New Zealand Cancer Register (2009–17), but not in the primary care dataset. These included 14 cases that had not been recorded and seven cancers that were coded in the Patient Management System as procedures rather than as a cancer diagnosis (eg mastectomies and prostatectomies). The combined primary care and Cancer Register dataset therefore contained 206 primary invasive cancers in 201 patients (aged ≥15 years), giving an overall crude 10-year cancer prevalence rate of 1.8% (1.9% for Māori and 1.8% for non-Māori).
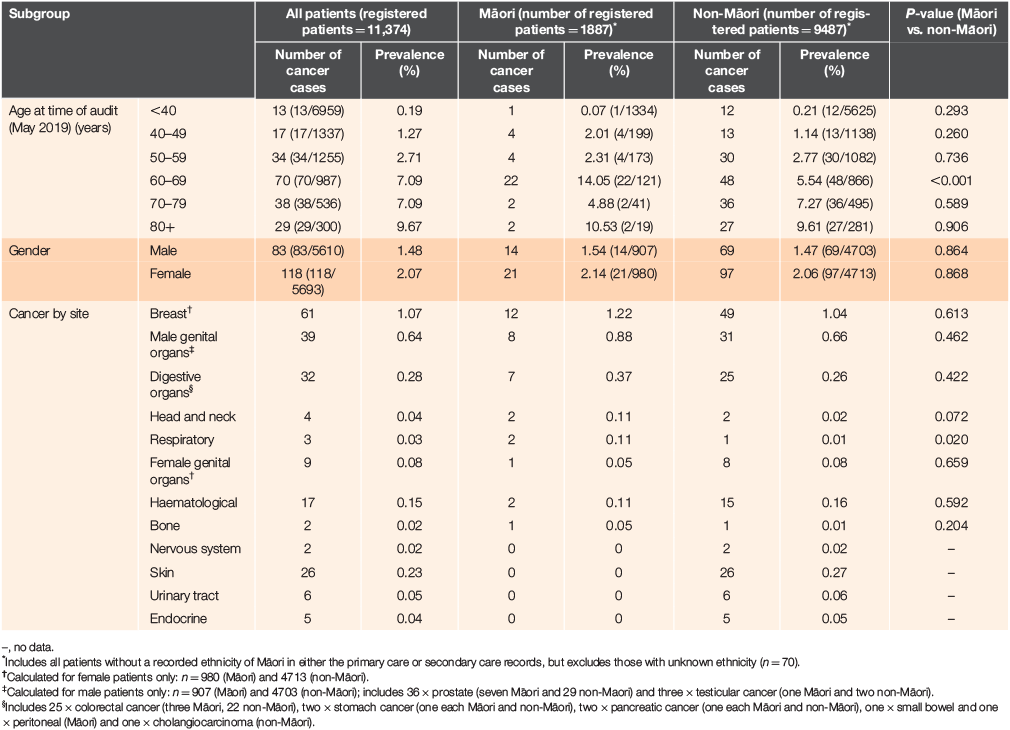
Of patients with a cancer diagnosis, 79.6% (160) were New Zealand European, 17.4% (35) were Māori, 1.0% (2) Pasifika and 2.0% (4) were of other ethnicity. The mean age of patients with cancer was 61.7 years for Māori and 64.3 years for non-Māori. The prevalence of invasive cancer in Māori and non-Māori by age, gender and cancer site is reported in Table 1.
|
Overall, a greater proportion of cancer patients were female (58.7% vs. 41.3% male; P < 0.0001) and the ratio of female-to-male patients was slightly higher for Māori (1:1.5) than non-Māori patients (1:1.4). Cancers were most frequently reported in patients aged >60 years, with the highest prevalence (9.7%) in patients aged >80 years. However, in Māori patients, cancer prevalence was highest in patients aged 60–69 years (14.1%; Table 1).
The most common cancers were breast (n = 61, 1.1% of female patients), male genital (prostate, n = 36 [0.6%]; and testicular, n = 3 [0.1%] of male patients), digestive (colorectal [n = 25], stomach [n = 2], pancreatic [n = 2], other [n = 3]; 0.3% of all patients) and skin cancer (all melanoma, n = 26, 0.2%). The prevalence of respiratory cancer was significantly higher in Māori patients (0.1% vs. 0.01%; P = 0.020) and was numerically higher for head and neck cancers (0.11% vs. 0.02%; P = 0.072).
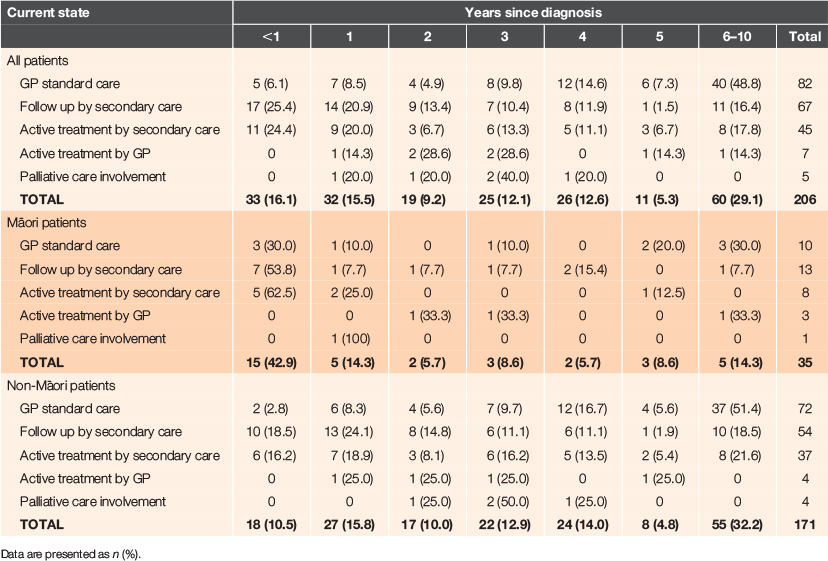
Table 2 summarises the number of years since diagnosis of cancer and the current healthcare status of these cancer cases. Nearly one-third of all cancer cases in this practice (29.1%) had been diagnosed 6–10 years ago, but this proportion differed for Māori and non-Māori patients (32.2% vs. 14.3% respectively). Significantly more Māori patients were diagnosed within the previous 12 months (P = 0.001). At the time of auditing this information (May 2019), half (54.4%) of all patients were still under specialist care, but many (n = 82, 39.8%) were receiving standard GP follow-up care only. Seven cancer cases (five breast and two prostate; 3.3%) were currently receiving active treatment from their GP (endocrine therapy after specialist discharge) and five cases (2.4%) were receiving palliative care. Similar proportions of Māori and non-Māori cancer cases were being followed up by secondary care (37.1% vs. 31.6%), as were the proportion receiving active treatment from secondary care (22.9% vs. 21.6%), although Māori patients were less likely to be under usual follow-up care from their GP (28.6% vs. 42.1%), but more likely to be receiving active treatment from the GP (8.6% vs. 2.3%).
|
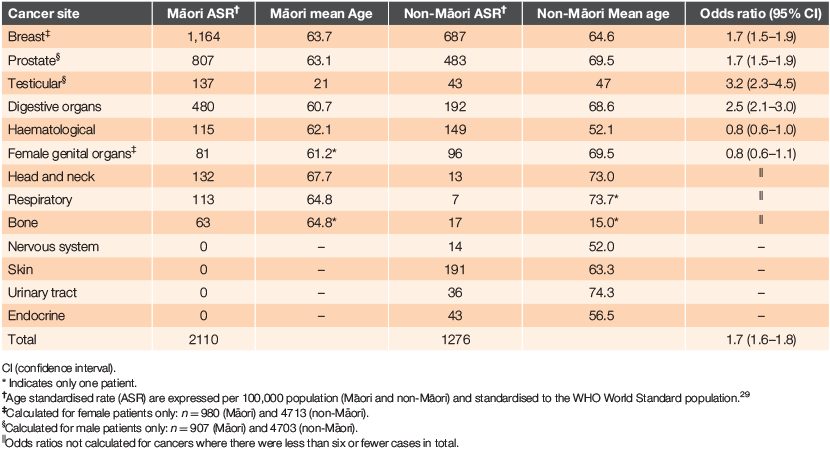
Table 3 summarises the age-standardised rate (ASR) for invasive cancers by site. Overall, the age-standardised 10-year prevalence rate was 3092/100,000 for Māori and 1971/100,000 for non-Māori. Breast, digestive, male reproductive, head and neck, bone and respiratory cancers were more prevalent in Māori patients, whereas the ASR of haematological, skin, urinary and endocrine cancers was higher in non-Māori patients. Māori patients had a 10-fold increase in the risk of head and neck cancer and a 16-fold increase in risk of respiratory cancer (Table 3).
|
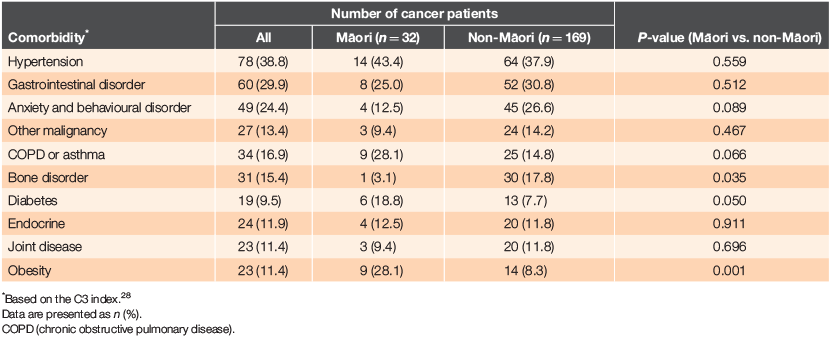
The comorbidities of cancer patients are shown in Table 4. The most common comorbidities were hypertension (n = 78, 38.8%), followed by gastrointestinal disorders (n = 60, 29.9%), anxiety and behavioural disorders (n = 49, 24.4%) and other malignancies (n = 27, 13.4%). Obesity (28.1% vs. 8.3%; P = 0.001) and diabetes (18.8% vs 7.7%; P = 0.05) were more common in Māori than non-Māori cancer patients, whereas bone disorders were significantly less common in Māori (3.1% vs. 17.8%; P = 0.035). Differences in other comorbidities did not reach statistical significance, though chronic obstructive pulmonary disease or asthma was twice as common in Māori patients (28.1% vs. 14.8%; P = 0.066). The number of patients with zero (11.4% vs. 13.9%, P = 0.700), one (22.9 vs. 21.7; P = 0.900), two (17.1 vs. 16.9, P = 1.000) and three comorbidities (51.5 vs. 47.2%, P = 0.700) was also similar in Māori and non-Māori patients.3
|
Discussion
Using a combined dataset of primary care and New Zealand Cancer Register data, the crude 10-year prevalence of cancer in this practice was 1.8%, representing 201 patients from a current enrolled population of 11,374. A predictive modelling study in the United Kingdom suggested that a practice with 2000 patients would be expected to have ~70 invasive cancer patients (3.5%),2 and similar modelling in Australia estimated 3.6% of the population were patients with invasive cancer.28 The prevalence in our study is lower than in these countries, but higher than the 1% of Israeli primary care patients reported to have cancer in 1990.29 The prevalence of cancer in our study is also lower than the 18.5-year cancer prevalence of 2.8% reported in New Zealand previously.4 The differences may be due to the shorter time period covered by our study or to the characteristics of the study practice not being representative of the national prevalence.4
Further, studies reporting on cancer incidence or prevalence from national health datasets rely on the use of cancer registrations and thus report primarily on new and current cases of cancer, rather than the number of patients who are now post-treatment and ‘surviving’ their cancer diagnosis. Such data make it difficult to comprehensively understand the burden of cancer in primary care in New Zealand, particularly as cancer patients now survive longer because of earlier detection and improved treatment regimes. The use of the national statistics data to estimate cancer prevalence is welcome, but it would be useful to also know the number of cancer patients who are now in remission or have survived their diagnosis but still require treatment. Data from the US found that 64% of cancer patients now survive for at least 5 years and 40% for at least 10 years after their diagnosis.3 Thus, even using crude national estimates of incidence for the most common cancers (prostate, colorectal, melanoma and lung),30 we can estimate that ~10,000–15,000 New Zealanders survived their cancer diagnosis during this 5-year period and will now be requiring ongoing cancer care.
General practitioners are traditionally the doctors most likely to look after cancer survivors. In one Dutch study, the number of primary care visits by survivors of breast and prostate cancer compared to control patients without cancer was significantly increased by 24%, with the greatest increase in the number of visits recorded 2–5 years after diagnosis.31 These data suggest that GPs should be prepared to deal with the increased amount of aftercare that cancer survivors require, particularly as other studies report that cancer survivors may have a strong preference for follow up from secondary rather than primary care.32 Reasons cited for this have included a perceived lack of cancer expertise by GPs and their lack of involvement with original cancer care.32 In one US study, only 40% of GPs reported that they were confident in their own knowledge of testing for cancer recurrence and 60% of oncologists doubted that primary care doctors had the skills required for appropriate testing of cancer recurrence.33 Although perceptions of the capability of medical staff in New Zealand for follow-up cancer care are unknown, a small number of studies do report that good relationships with GPs are essential for expediting the process of cancer diagnosis.34–36 However, as the results of our study suggests, the number of primary care patients who are diagnosed with cancer is low, although these rates will differ in practices in other regions. This warrants further study.
We also showed in this study that although the prevalence of cancer in Māori patients was only slightly higher than that for non- Māori patients (1.9% vs. 1.8%), there was a significantly higher 10-year ASR (3092 vs. 1971/100,000 population). This aligns with the recent national review of cancer prevalence4 and with other studies reporting that both the incidence and survival of many cancers are disproportionately worse in Māori.23,37,38 This may be attributable, at least in part, to the New Zealand primary health-care system not meeting the needs of the Māori population.24,39 However, we also note that in our limited dataset, Māori were younger at diagnosis for many of these cancers. This may explain why the ASR was higher for Māori than for non-Māori, but crude rates were similar. This needs exploring further, preferably with a larger dataset.
The difference in ASRs between Māori and non-Māori may also be explained by nearly half of all Māori cancer cases (15/35) in this practice being diagnosed <12 months ago, and this may have skewed this dataset. The reasons for this recent increase in the number of Māori cancers is unknown, but may be due to a recent increase in Māori enrolment at the practice or a shift of Māori patients away from this practice whereby patients diagnosed earlier are no longer enrolled.
For GPs to provide holistic patient care, it is important to manage the comorbidities of cancer patients as well as the original cancer diagnosis.14 As our study reports, many cancer patients had comorbidities, including hypertension (38.8%), gastrointestinal issues (29.9%) and anxiety and mood disorders (24.4%). Mood disorders are especially important to recognise and treat in cancer patients, as they can affect adherence to treatment.40,41 The prevalence of mood disorders in our study is similar to that observed in a study in Germany (31.8%),40 but lower than the 41.6% reported for combined anxiety and depression in 10,153 patients screened for mental health disorders.41 Furthermore, a systematic review of 66 studies showed that the prevalence of depression alone can be as high as 49% in cancer patients having palliative care. Currently, specialist psychosocial support for cancer patients in New Zealand is available only by referral from secondary care, and primary care referrals are limited to supportive care programmes only.42 However, GPs can refer patients to generalist psychological support specialist services and it could be worthwhile to screen all patients in primary care with a current or past diagnosis of cancer for depression and anxiety risk.
Although we found similar numbers of comorbidities in Māori and non-Māori cancer patients, there were differences in the types of comorbid conditions; for example, Māori patients were more likely to have a concomitant diagnosis of diabetes and obesity, possibly reflecting the higher prevalence of these conditions in Māori.43,44 However, cancer incidence is correlated with diabetes and antihyperglycemic medications,45 so patients with diabetes in primary care should also be monitored for increased cancer risk.
We found that the New Zealand Cancer Register was the most accurate source of primary care patient cancer data; 100% of cases were recorded in the Register, but 10% of cases in the Register were incorrectly coded in the practice records. Extracting these data from the GP database was also time-consuming, requiring evaluation of individual patient records, whereas the New Zealand Cancer Register data provided a virtually complete dataset of patient and disease characteristics. We therefore recommend the New Zealand Cancer Register as the simpler and more accurate dataset to use for determining the prevalence of cancer in primary care, although this does need to be validated in other practice settings in both rural and urban areas. However, a disadvantage of using Register data to report on prevalence is that there is a 12- to 18-month lag in accessing recent data. This could be problematic if current primary care practice prevalence data were required.
Conclusions
Our analysis suggests the prevalence of cancer in primary care is low (1.8%) and that there may be disparities in cancer prevalence between Māori and non-Māori patients. We found most cancer patients were receiving treatment or follow up by secondary care, but approximately one-third of all cancer patients had been discharged back to their GP for follow-up care. GPs have a range of responsibilities in managing cancer patients – many have comorbidities and so there is a need for shared care for patients receiving ongoing specialist care. GPs must also manage the survival of cancer patients who have treatment sequelae and, finally, some patients will be receiving palliative care.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
This research was supported by The University of Auckland and The University of Waikato. The authors wish to thank the Mahoe Medical Centre for their support with this project.
References
[1] Rubin G, Berendsen A, Crawford SM, et al. The expanding role of primary care in cancer control. Lancet Oncol. 2015; 16 1231–72.| The expanding role of primary care in cancer control.Crossref | GoogleScholarGoogle Scholar | 26431866PubMed |
[2] Maddams J, Utley M, Møller H. Projections of cancer prevalence in the United Kingdom, 2010–2040. Br J Cancer. 2012; 107 1195–202.
| Projections of cancer prevalence in the United Kingdom, 2010–2040.Crossref | GoogleScholarGoogle Scholar | 22892390PubMed |
[3] de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomark Prev. 2013; 22 561–70.
| Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care.Crossref | GoogleScholarGoogle Scholar |
[4] Brewer N, Atkinson J, Guilford P, et al. An estimate of limited duration cancer prevalence in New Zealand using ‘big’ data. N Z Med J. 2020; 133 49–62.
| 32379739PubMed |
[5] Hoekstra RA, Heins MJ, Korevaar JC. Health care needs of cancer survivors in general practice: a systematic review. BMC Fam Pract. 2014; 15 94
| Health care needs of cancer survivors in general practice: a systematic review.Crossref | GoogleScholarGoogle Scholar | 24885266PubMed |
[6] Grunfeld E, Earle CC. The interface between primary and oncology specialty care: treatment through survivorship. J Natl Cancer Inst Monogr. 2010; 2010 25–30.
| The interface between primary and oncology specialty care: treatment through survivorship.Crossref | GoogleScholarGoogle Scholar | 20386051PubMed |
[7] Richards M, Corner J, Maher J. The National Cancer Survivorship Initiative: new and emerging evidence on the ongoing needs of cancer survivors. Br J Cancer. 2011; 105 S1–4.
| The National Cancer Survivorship Initiative: new and emerging evidence on the ongoing needs of cancer survivors.Crossref | GoogleScholarGoogle Scholar | 22048027PubMed |
[8] Michaud LB. Managing cancer treatment-induced bone loss and osteoporosis in patients with breast or prostate cancer. Am J Health Syst Pharm. 2010; 67 S20–30.
| Managing cancer treatment-induced bone loss and osteoporosis in patients with breast or prostate cancer.Crossref | GoogleScholarGoogle Scholar | 20332495PubMed |
[9] Seicean S, Seicean A, Plana JC, et al. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol. 2012; 60 2384–90.
| Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study.Crossref | GoogleScholarGoogle Scholar | 23141499PubMed |
[10] Rehammar JC, Jensen M-B, McGale P, et al. Risk of heart disease in relation to radiotherapy and chemotherapy with anthracyclines among 19,464 breast cancer patients in Denmark, 1977–2005. Radiother Oncol. 2017; 123 299–305.
| Risk of heart disease in relation to radiotherapy and chemotherapy with anthracyclines among 19,464 breast cancer patients in Denmark, 1977–2005.Crossref | GoogleScholarGoogle Scholar | 28365142PubMed |
[11] Louzada ML, Carrier M, Lazo-Langner A, et al. Development of a clinical prediction rule for risk stratification of recurrent venous thromboembolism in patients with cancer-associated venous thromboembolism. Circulation. 2012; 126 448–54.
| Development of a clinical prediction rule for risk stratification of recurrent venous thromboembolism in patients with cancer-associated venous thromboembolism.Crossref | GoogleScholarGoogle Scholar | 22679142PubMed |
[12] Beaugerie L, Carrat F, Colombel J-F, et al. Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer. Gut. 2014; 63 1416–23.
| Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer.Crossref | GoogleScholarGoogle Scholar | 24162591PubMed |
[13] Czarnecki D, Staples M, Mar A, et al. Recurrent nonmelanoma skin cancer in Southern Australia. Int J Dermatol. 1996; 35 410–2.
| Recurrent nonmelanoma skin cancer in Southern Australia.Crossref | GoogleScholarGoogle Scholar | 8737875PubMed |
[14] Koroukian SM, Murray P, Madigan E. Comorbidity, disability, and geriatric syndromes in elderly cancer patients receiving home health care. J Clin Oncol. 2006; 24 2304–10.
| Comorbidity, disability, and geriatric syndromes in elderly cancer patients receiving home health care.Crossref | GoogleScholarGoogle Scholar | 16710028PubMed |
[15] Chen RC, Royce TJ, Extermann M, et al., editors. Impact of age and comorbidity on treatment and outcomes in elderly cancer patients. Seminars in Radiation Oncology. Elsevier; 2012.
[16] Burg MA, Adorno G, Lopez ED, et al. Current unmet needs of cancer survivors: analysis of open-ended responses to the American Cancer Society Study of Cancer Survivors II. Cancer. 2015; 121 623–30.
| Current unmet needs of cancer survivors: analysis of open-ended responses to the American Cancer Society Study of Cancer Survivors II.Crossref | GoogleScholarGoogle Scholar | 25581252PubMed |
[17] Walker T, Signal L, Russell M, et al. The road we travel: Māori experience of cancer. N Z Med J. 2008; 121 27–35.
| 18709045PubMed |
[18] Slater T, Matheson A, Davies C, et al. ‘It’s whanaungatanga and all that kind of stuff’: Maori cancer patients’ experiences of health services. J Prim Health Care. 2013; 5 308–14.
| ‘It’s whanaungatanga and all that kind of stuff’: Maori cancer patients’ experiences of health services.Crossref | GoogleScholarGoogle Scholar | 24294619PubMed |
[19] Teng AM, Atkinson J, Disney G, et al. Ethnic inequalities in cancer incidence and mortality: census-linked cohort studies with 87 million years of person-time follow-up. BMC Cancer. 2016; 16 755
| Ethnic inequalities in cancer incidence and mortality: census-linked cohort studies with 87 million years of person-time follow-up.Crossref | GoogleScholarGoogle Scholar | 27669745PubMed |
[20] Hill S, Sarfati D, Robson B, et al. Indigenous inequalities in cancer: what role for health care? ANZ J Surg. 2013; 83 36–41.
| Indigenous inequalities in cancer: what role for health care?Crossref | GoogleScholarGoogle Scholar | 23253098PubMed |
[21] Soeberg M, Blakely T, Sarfati D. Trends in ethnic and socioeconomic inequalities in cancer survival, New Zealand, 1991–2004. Cancer Epidemiol. 2015; 39 860–2.
| Trends in ethnic and socioeconomic inequalities in cancer survival, New Zealand, 1991–2004.Crossref | GoogleScholarGoogle Scholar | 26651447PubMed |
[22] Tin Tin S, Elwood JM, Brown C, et al. Ethnic disparities in breast cancer survival in New Zealand: which factors contribute? BMC Cancer. 2018; 18 58
| Ethnic disparities in breast cancer survival in New Zealand: which factors contribute?Crossref | GoogleScholarGoogle Scholar | 29310606PubMed |
[23] Robson B, Cormack D, Purdie G. Unequal impact II: Māori and Non-Māori cancer statistics by deprivation and rural-urban status 2002–2006: Te Rōpū Rangahu Hauora a Eru Pōmare. Wellington: University of Otago; 2010.
[24] Elers P. Māori health: issues relating to health care services. Te Kaharoa. 2014; 7 163–72.
| Māori health: issues relating to health care services.Crossref | GoogleScholarGoogle Scholar |
[25] Ruhl J, Callaghna C, Hurlbut A, et al. Summary stage 2018: codes and coding instructions. Bethesda, MD. Available from: https://seer.cancer.gov/tools/ssm/2018-Summary-Stage-Manual.pdf.
[26] Sarfati D, Gurney J, Stanley J, et al. Cancer-specific administrative data–based comorbidity indices provided valid alternative to Charlson and National Cancer Institute Indices. J Clin Epidemiol. 2014; 67 586–95.
| Cancer-specific administrative data–based comorbidity indices provided valid alternative to Charlson and National Cancer Institute Indices.Crossref | GoogleScholarGoogle Scholar | 24582212PubMed |
[27] Ahmad OB, Boschi-Pinto C, Lopez AD, et al. Age standardization of rates: a new WHO standard. Vol. 9(10). Geneva: World Health Organization; 2001.
[28] Australian Institute of Health and Welfare Cancer survival and prevalence in Australia: period estimates from 1982 to 2010. Asia Pac J Clin Oncol. 2013; 9 29–39.
| Cancer survival and prevalence in Australia: period estimates from 1982 to 2010.Crossref | GoogleScholarGoogle Scholar | 23418847PubMed |
[29] Spenser T. Cancer prevalence in Israeli family practice: The Cancer Research Group. Scand J Prim Health Care. 1994; 12 20–3.
| Cancer prevalence in Israeli family practice: The Cancer Research Group.Crossref | GoogleScholarGoogle Scholar |
[30] Ministry of Health. Selected Cancer 2015, 2016, 2017. [cited 2019 October 10]. Available from: https://www.health.govt.nz/publication/selected-cancers-2015-2016-2017
[31] Heins M, Schellevis F, Rijken M, et al. Determinants of increased primary health care use in cancer survivors. J Clin Oncol. 2012; 30 4155–60.
| Determinants of increased primary health care use in cancer survivors.Crossref | GoogleScholarGoogle Scholar | 23071230PubMed |
[32] Hudson SV, Miller SM, Hemler J, et al. Adult cancer survivors discuss follow-up in primary care:‘not what I want, but maybe what I need’. Ann Fam Med. 2012; 10 418–27.
| Adult cancer survivors discuss follow-up in primary care:‘not what I want, but maybe what I need’.Crossref | GoogleScholarGoogle Scholar | 22966105PubMed |
[33] Potosky AL, Han PK, Rowland J, et al. Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med. 2011; 26 1403–10.
| Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors.Crossref | GoogleScholarGoogle Scholar | 21785923PubMed |
[34] Cassim S, Chepulis L, Keenan R, et al. Patient and carer perceived barriers to early presentation and diagnosis of lung cancer: a systematic review. BMC Cancer. 2019; 19 25
| Patient and carer perceived barriers to early presentation and diagnosis of lung cancer: a systematic review.Crossref | GoogleScholarGoogle Scholar | 30621616PubMed |
[35] Walton L, McNeill R, Stevens W, et al. Patient perceptions of barriers to the early diagnosis of lung cancer and advice for health service improvement. Fam Pract. 2013; 30 436–44.
| Patient perceptions of barriers to the early diagnosis of lung cancer and advice for health service improvement.Crossref | GoogleScholarGoogle Scholar | 23377608PubMed |
[36] Slater T, Matheson A, Davies C, et al. The role and potential of community-based cancer care for Māori in Aotearoa/New Zealand. N Z Med J. 2016; 129 29–38.
| 26914420PubMed |
[37] Hill S, Sarfati D, Blakely T, et al. Survival disparities in indigenous and non-indigenous New Zealanders with colon cancer: the role of patient comorbidity, treatment and health service factors. J Epidemiol Community Health. 2010; 64 117–23.
| Survival disparities in indigenous and non-indigenous New Zealanders with colon cancer: the role of patient comorbidity, treatment and health service factors.Crossref | GoogleScholarGoogle Scholar | 20056966PubMed |
[38] Dickson G, Cunningham CW, Parry S. The prevalence of colorectal adenomas in Māori and New Zealand Europeans parallels colorectal cancer rates. N Z Med J. 2010; 123 45–9.
| 20720602PubMed |
[39] Kidd J, Gibbons V, Lawrenson R, et al. A whanau ora approach to health care for Maori. J Prim Health Care. 2010; 2 163–4.
| A whanau ora approach to health care for Maori.Crossref | GoogleScholarGoogle Scholar | 20690309PubMed |
[40] Mehnert A, Brähler E, Faller H, et al. Four-week prevalence of mental disorders in patients with cancer across major tumor entities. J Clin Oncol. 2014; 32 3540–6.
| Four-week prevalence of mental disorders in patients with cancer across major tumor entities.Crossref | GoogleScholarGoogle Scholar | 25287821PubMed |
[41] Linden W, Vodermaier A, MacKenzie R, et al. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012; 141 343–51.
| Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age.Crossref | GoogleScholarGoogle Scholar | 22727334PubMed |
[42] Ministry of Health. Cancer psychological and social support initiative. Wellington, NZ: Ministry of Health. [cited 2019 November 20]. Available from https://www.health.govt.nz/our-work/diseases-and-conditions/national-cancer-programme/cancer-initiatives/cancer-psychological-and-social-support-initiative
[43] Ministry of Health. Obesity statistics. Wellington, NZ: Ministry of Health. [cited 2019 November 20]. Available from: https://www.health.govt.nz/nz-health-statistics/health-statistics-and-data-sets/obesity-statistics
[44] Ministry of Health. Diabetes. Wellington, NZ: Ministry of Health. [cited 2019 November 20]. Available from: https://www.health.govt.nz/our-work/populations/maori-health/tatau-kahukura-maori-health-statistics/nga-mana-hauora-tutohu-health-status-indicators/diabetes
[45] Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010; 60 207–21.
| Diabetes and cancer: a consensus report.Crossref | GoogleScholarGoogle Scholar | 20554718PubMed |


