Monitoring the use of dabigatran etexilate for stroke prevention: compliance with renal function guidelines
Bryan H. Simpson 1 3 , David M. Reith 2 , Natalie J. Medlicott 1 , Alesha J. Smith 11 School of Pharmacy, University of Otago, Dunedin, New Zealand
2 Otago Medical School, University of Otago, Dunedin, New Zealand
3 Corresponding author. Email: bryan.simpson@postgrad.otago.ac.nz
Journal of Primary Health Care 12(4) 327-334 https://doi.org/10.1071/HC19115
Published: 9 December 2020
Journal Compilation © Royal New Zealand College of General Practitioners 2020 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
INTRODUCTION: Dabigatran etexilate has become widely used in New Zealand, but information relating to when renal function monitoring is being undertaken is lacking.
AIM: To investigate if clinically appropriate renal function monitoring is being undertaken in New Zealand primary care for stroke prevention in non-valvular atrial fibrillation patients prescribed dabigatran etexilate.
METHODS: New Zealand non-valvular atrial fibrillation patients’ prescription and primary care health data were extracted from national administrative databases for the period 1 July 2011 to 31 December 2015. The proportion of patients who had serum creatinine measurements at close proximity to treatment initiation and 12-months post initiation were assessed with 95% confidence intervals (CIs) and compared with Fisher’s exact test. Log-rank tests for univariate analysis (gender, age, ethnicity and deprivation) effects on serum creatinine testing at dabigatran etexilate treatment initiation and 12-months post initiation were performed.
RESULTS: Overall, 1,948 patients who had been dispensed dabigatran etexilate with available primary care health data were identified. A total of 1,752 (89.9% [CI: 88.5–91.2]) patients had a renal function test at dabigatran etexilate initiation. There were 929 (72.8% [CI: 70.2–75.2]) patients who received ≥1 year supply of dabigatran etexilate and of these 207 (22.3% [CI: 19.6.6–25.1]) had a serum creatinine test 1 year after initiation. Demographic univariate analysis yielded insignificant log-rank tests for association with having serum creatinine measurements, except for Pacific Peoples.
DISCUSSION: There appears to be sub-optimal adherence to renal function monitoring for non-valvular atrial fibrillation patients who receive more than 12-months’ treatment with dabigatran etexilate in New Zealand primary care.
Keywords: dabigatran etexilate, non-valvular atrial fibrillation, renal function, stroke
| WHAT GAP THIS FILLS |
| What is already known: There has been rapid adoption of dabigatran etexilate use in New Zealand since its subsidy in 2011. Dabigatran etexilate now accounts for 51% of the patients receiving oral anticoagulation. Renal function is an important determinant of dabigatran etexilate dosing. |
| What this study adds: Information from New Zealand primary care showing that sub-optimal adherence to renal function monitoring for patients receiving dabigatran etexilate. |
Introduction
The direct thrombin inhibitor, dabigatran etexilate, has become widely used in New Zealand, with 51% of patients receiving oral anticoagulation now being treated with this medication.1 Approximately 80% of dabigatran etexilate is excreted renally,2 so it is important to ensure that appropriately timed renal function testing is undertaken to reduce the risk of bleeding due to drug accumulation.3–5
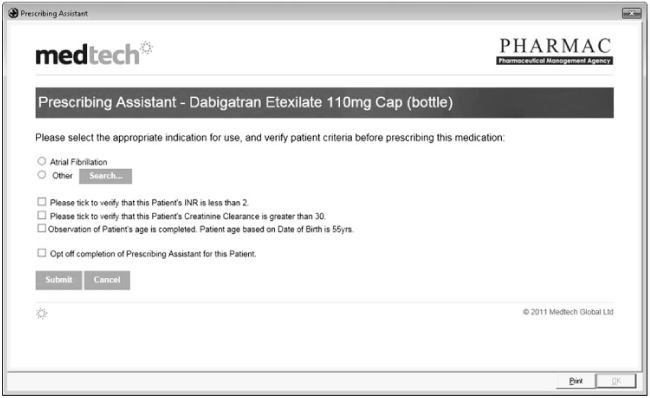
When dabigatran etexilate gained full subsidy in New Zealand in 2011, the national pharmaceutical management organisation (PHARMAC) commissioned the major primary care electronic health record (EHR) system provider to generate a computerised prescribing assistant. This prescribing assistant, among other functions, prompted prescribers to determine their patients’ renal function status at treatment initiation. The prescribing assistant was only activated for the first dabigatran etexilate prescription and not for any that followed (Figure 1).
|
The present study investigates renal function monitoring in patients with non-valvular atrial fibrillation who have had dabigatran etexilate treatment initiated by primary care physicians. Patients with non-valvular atrial fibrillation often have multiple co-morbidities, including reduced renal function,6,7 so renal function status is an important consideration when dabigatran etexilate dosing decisions are being made.8 Plasma concentrations of dabigatran etexilate are highly correlated with renal function and levels outside the optimum range are potentially associated with higher risk of adverse outcomes.5,9 Renal function is easily accessed during routine clinical care by estimation of creatinine clearance following a serum creatinine test.10 Currently, in New Zealand, there is no single dataset relating to adherence to appropriately timed renal function testing for dabigatran etexilate.
Previous published audits of New Zealand dabigatran etexilate patients have shown 100% adherence to renal function testing protocols at treatment initiation and between 63 and 90% adherence in the first year for patients being treated for >1 year.11,12 In relation to the testing in the first year, audits have investigated if patients receiving dabigatran etexilate had a renal function test undertaken in the 12 months following treatment initiation.13 Current clinical guidance recommends that patients undergo tests every 12 months or more often if clinically indicated.14–17 Therefore, as these audits do not account for the proximity of the test to the 12-month anniversary of treatment initiation, it is not possible to determine if testing is being undertaken at clinically appropriate times. Therefore, there may be misrepresentation of the number of patients undergoing renal function testing at clinically appropriate times. Furthermore, there is a lack of published research describing when clinicians undertake renal function testing for patients prescribed dabigatran etexilate.
The aim of this population study was to investigate if clinically appropriate renal function testing recommendations were being undertaken in primary care for non-valvular atrial fibrillation patients prescribed dabigatran etexilate.
Methods
Study cohort
This was a retrospective cohort study using administrative health data. The databases accessed were the New Zealand Ministry of Health Pharmaceutical Collection (PC)18 and the Best Practice Intelligence (BPI) database, operated by Best Practice Advocacy Centre Clinical Solutions, New Zealand.19 The PC contains prescription details about pharmaceutical dispensing claims for dabigatran etexilate along with other prescribed medicines, as well as information on gender, date of birth, age, ethnicity, frequency of dispensings and quantity dispensed for all New Zealanders.11,12 The BPI database is a secure, internet-based, reporting tool that uses data downloaded from the enrolled general practice patient EHRs, covers ~20% of the New Zealand population and contains patient health data such as diagnosis and laboratory results. The study cohort included primary care patients with a diagnosis of atrial fibrillation made by a general practitioner (READ codes G573, G5730, G5731, G5732, G573z) who were aged ≥18 years, and had at least one dispensing of dabigatran etexilate during the study period between 1 July 2011 (when dabigatran etexilate became available in New Zealand) and 31 December 2014. The datasets were linked using each patient’s encrypted National Health Index number (NHI number; a life-long unique identifier for all interactions with the New Zealand health system) to ensure patient anonymity. Ethical approval was obtained from the University of Otago Ethics Committee (Reference: HD15/054).
Patient covariates
Patient covariate data of dispensed medications and patient demographics (including gender, age, ethnicity, deprivation score) were extracted from the PC and BPI databases for patients who met the inclusion criteria. Patients were categorized into age groups of <65 years, 65 – 74 years, 75 – 79 years and >80 years to align to both regulatory agencies and the categories used to guide dosing.15,20 The treatment period with dabigatran etexilate was determined by the number of days supplied for each series of continuous treatment. Continuous dabigatran etexilate use was defined as one or more dispensings recorded in the PC with <120 days between dispensing (prescriptions in New Zealand for dabigatran etexilate typically supply 90 days, which are dispensed in 30-day amounts). When ≥120 days elapsed between dabigatran etexilate prescriptions, patients were considered to have ceased dabigatran etexilate treatment. Patients were considered new in the study if they restarted dabigatran etexilate treatment after ≥120 days had elapsed.
Serum creatinine measurements
Evidence reflecting measurement of renal function at any time during dabigatran etexilate treatment were extracted and analysed. Results of all serum creatinine tests reported in close proximity to the current clinical guidance were included. Close proximity was defined as a completed serum creatinine measurement within 1 month either side of the specified time.21,22 If multiple measurements were recorded in close proximity to the specified time, only the measurement closest to the testing date was used for the analysis.
Compliance to recommended renal function testing protocols was examined. Compliance was defined as meeting the minimum recommended renal function monitoring of having a renal function test undertaken at treatment initiation and at 12 months post dabigatran etexilate treatment initiation. Patients who had dabigatran etexilate initiation after 31 December 2013 were excluded from the follow-up serum creatinine test analysis as their data were outside the study time frame.
Statistical analysis
Statistical analyses were performed using Stata/IC (Version 14.2; StataCorpLP, College Station, TX, USA). Continuous variables were tested for normal distribution by the skewness and kurtosis test. Normally distributed data are presented as the mean ± standard deviation (s.d.) and non-normally distributed data as the median and interquartile range. The proportion of patients who had serum creatinine measurements at close proximity to dabigatran etexilate treatment initiation and 12-months post initiation were assessed with the 95% confidence interval (CI, Clopper–Pearson method) and were compared with Fisher’s exact test. The proportion of patients who had serum creatinine measurements at any time in the first 12 months of treatment were assessed (CI, Clopper–Pearson method) to allow for comparison with other studies.11,12 A box and whisker plot was used to investigate the distribution of serum creatinine tests in relation to 12-months post treatment initiation. Log-rank tests for univariate analysis (gender, age, ethnicity and deprivation index score) based on serum creatinine testing at dabigatran etexilate treatment initiation and 12-months post initiation were performed to investigate their influence on testing. The number of days that dabigatran etexilate was supplied was right-censored if a serum creatinine was not undertaken at treatment initiation or if a second test was not undertaken. Results were considered statistically significant if P < 0.05.
Results
Cohort characteristics
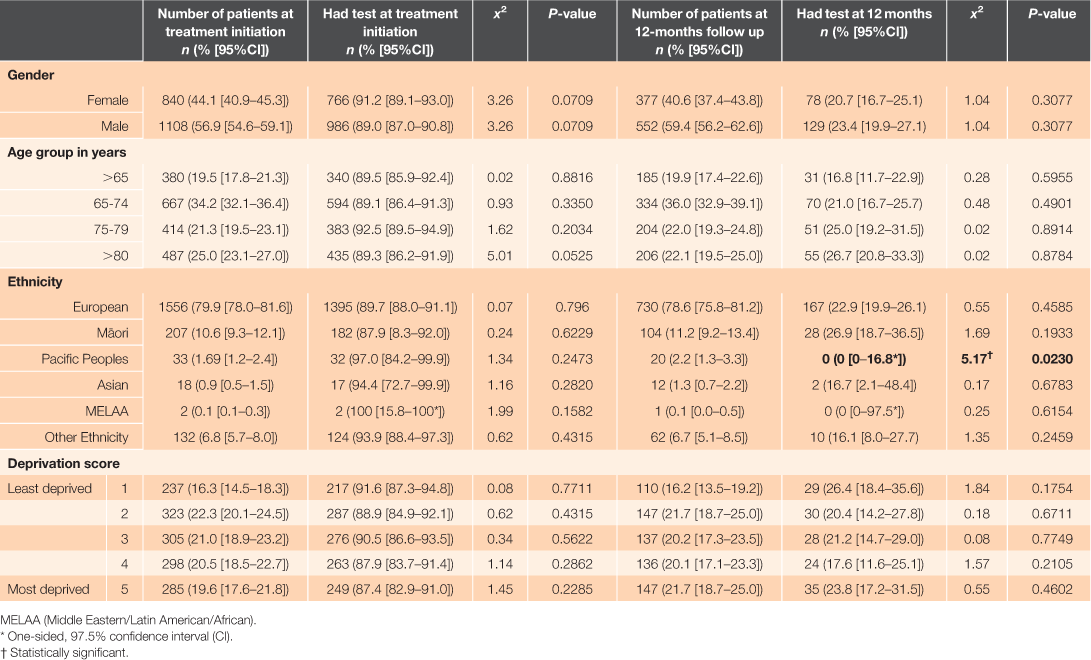
In the databases, 1,948 patients with a diagnosis of non-valvular atrial fibrillation and aged ≥18 years who had been dispensed dabigatran etexilate prescribed in primary care were identified. The median age of patients in this cohort was 74 years (IQR: 67–79.5). The median supply of dabigatran etexilate for participants was 594 days (IQR: 190–1050 days). There were 929 patients who received 12 months or more supply of dabigatran etexilate, with treatment initiated in the time frame that allowed for adequate follow up in the BPI database. A summary of the demographic characteristics of the patients at treatment initiation and 12 months is presented in Table 1.
|
Serum creatinine measurements
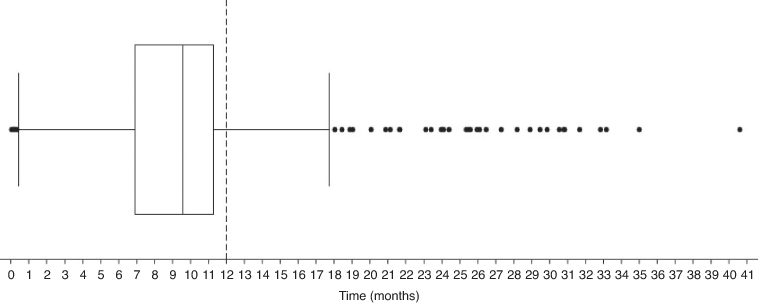
A total of 1,752 (89.9% [CI; 88.5–91.2]) patients had a serum creatinine test at close proximity to dabigatran etexilate treatment initiation. Of patients initiated on dabigatran etexilate, a total of 868 (93.4% [CI; 91.6–94.9]) had undergone at least one serum creatinine test at any time during their following treatment. The median time to testing post treatment initiation was 287 days (IQR: 208–339 days). Figure 2 shows the minimum, median, first quartile, third quartile, and maximum days tests were undertaken in relation to the closest test to 12-months post treatment initiation (the recommended follow-up testing time). Of the patients who had a supply for 12 months, there were significantly fewer patients who had a serum creatinine test in close proximity to the 12-month follow-up date, with a total of 207 patients (22.3% [CI; 19.6–25.1]; P < 0.0001). A total of 823 patients (88.6% [CI; 86.4–90.6]) had undergone at least one serum creatinine test at any time in the first 12 months of follow up. For these patients, the median number of tests in the first 12 months was two (IQR: 1–4). Univariate analysis of demographic characteristics yielded insignificant log-rank tests for association with having serum creatinine measurements taken both at treatment initiation and at close proximity to 12-months post initiation for all categories, except for Pacific Peoples, with none having a measurement recorded at 12 months (χ2 = 5.17; P = 0.0230) (Table 1).
Discussion
Our findings suggest that compliance with the recommended 12-month follow-up monitoring of renal function is sub-optimal for primary care non-valvular atrial fibrillation patients receiving >12 months of treatment with dabigatran etexilate.
This cohort showed similar results to other New Zealand studies when the stated rules relating to renal function testing compliance were observed.11,12 Most patients had a serum creatinine test at treatment initiation with most having a test at any time in the following 12 months. Although the Best Practice Advocacy Centre’s audit tool is useful to encourage prescribers to actively monitor their non-valvular atrial fibrillation patients, it does not investigate if the monitoring is being undertaken at the recommended clinically appropriate time frame of 12 months. Therefore, we investigated how many of the patients had a serum creatinine test at treatment initiation and at 12 months. Our findings show that there was sub-optimal adherence to renal function testing at 12 months for these patients, with only 22.3% having a test recorded. Additionally, it has previously been reported that deprivation and inequalities lead to worse outcomes for patients treated with dabigatran etexilate.1 The present study adds further data to this situation as it indicates that there is inequality for Pacific Peoples for not receiving the follow-up renal testing.
It is unclear why there is a large discrepancy between the proportion of patients undergoing renal function testing at treatment initiation and at 12 months. One possibility that is highlighted in this study is that the prescribing assistant computer software commissioned by PHARMAC may have encouraged prescribers to assess patients’ renal function when the medication is first prescribed but did not activate for any subsequent prescriptions. During the time period of this study, no other computerised clinical decision support tools for monitoring renal function relating to dabigatran etexilate treatment were available for prescribers. Therefore, prescribers were not prompted to reassess their patients’ renal function near the recommended 12-month time. Further investigation into whether clinical decision support can improve renal function monitoring and patient outcomes is required. Also, consideration is needed to ensure that any such clinical decision support initiatives should be designed to ensure that they meet the needs of New Zealand’s diverse ethnic population.
Other reasons for deviating from the recommended renal function regimen are difficult to isolate and can be varied. For example, clinicians may be using alternative guidelines or following their own experiences with dabigatran etexilate. They may be assessing patients at the time of prescribing and using their professional judgement to determine not to undertake renal function monitoring when patients look otherwise well, or possibly judge that a previous test was adequate for assessment. Also, clinicians may have become reliant on computerised systems providing prompts for other clinical situations23 and therefore inadvertently omit requesting or undertaking the recommended renal function testing due to absence of an automated reminder. Additionally, prescribers may have requested the test to be undertaken, but patients may have decided against having the test done or have lost the laboratory request form. Finally, the test may have been undertaken but the results not been entered or entered incorrectly into the EHR.
Undertaking routine estimation of renal function for non-valvular atrial fibrillation patients is an important consideration during their treatment with dabigatran etexilate. Approximately 80% of dabigatran etexilate is eliminated via renal excretion2 and with renal function decreasing significantly with age in patients with non-valvular atrial fibrillation,7 there is potential for patients to be exposed to supratherapeutic plasma levels and thus experience adverse drug reactions such as haemorrhage, which may be life threatening.5
The limitations of this study include that although a serum creatinine is recorded in EHRs, it is not possible to ascertain if this was collected for the purpose of renal function estimation for dabigatran etexilate treatment. Also, the data did not allow for investigation into patient outcomes related to compliance to renal function testing. Further research is required to investigate the clinical importance of renal function testing compliance. The main strength of this study is its inclusion of a large cohort of patients with a similar demographic profile to that previously described for a nationwide cohort,1 with sufficient sample size to provide adequate information about renal function monitoring for an entire population.
Improved adherence to guideline-directed stroke prevention therapy with oral anticoagulation drugs improves patient outcomes.24 In New Zealand patients, this could be achieved by the possible inclusion of a clinical decision support tool that provides a timely reminder prompting clinicians to reassess renal function according to guideline-specified times. For serum creatinine measurements recently recorded in the EHR, the estimated renal function could be reported to clinicians for consideration and possible dose modifications could be investigated. This would be especially useful in situations where ad hoc testing, possibly unrelated to dabigatran etexilate treatment, had been performed and showed a change in renal function that warranted a dose modification; this could be highlighted to prescribers for consideration outside regular review periods. Additionally, if there were no recent serum creatinine tests recorded at specified treatment time frames (eg every 12 months or more frequently for higher-risk patients), this could be brought to the attention of prescribers for consideration at the time of generating a new prescription.
Conclusion
There appears to be sub-optimal adherence to renal function monitoring for non-valvular atrial fibrillation patients who receive more than 12 months treatment with dabigatran etexilate in New Zealand primary care. This may lead to incorrect dose adjustments for this frequently used medication and poorer outcomes for patients. Further investigation is required into whether an automated clinical decision support tool that reminds clinicians when to undertake renal function monitoring at clinically appropriate times can improve adherence to guidelines and patient outcomes.
Competing interests
The authors declare no competing interests.
Funding
This research did not receive any specific funding.
References
[1] Simpson BH, Reith D, Medlicott NJ, Smith A. Deprivation and inequalities lead to worse outcomes with dabigatran etexilate. J Prim Health Care. 2018; 10 303–11.| Deprivation and inequalities lead to worse outcomes with dabigatran etexilate.Crossref | GoogleScholarGoogle Scholar | 31039959PubMed |
[2] Ezekowitz MD, Connolly S, Parekh A, et al. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009; 157 805–810.e2.
| Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran.Crossref | GoogleScholarGoogle Scholar | 19376304PubMed |
[3] Hankey GJ, Eikelboom JW. Dabigatran etexilate: a new oral thrombin inhibitor. Circulation. 2011; 123 1436–50.
| Dabigatran etexilate: a new oral thrombin inhibitor.Crossref | GoogleScholarGoogle Scholar | 21464059PubMed |
[4] Douxfils J, Ageno W, Samama CM, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost. 2018; 16 209–19.
| Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians.Crossref | GoogleScholarGoogle Scholar | 29193737PubMed |
[5] Simpson BH, Reith DM, Medlicott NJ, et al. Exposure response supports therapeutic drug monitoring for dabigatran etexilate in patients with atrial fibrillation. TH Open. 2019; 3 e210–5.
| Exposure response supports therapeutic drug monitoring for dabigatran etexilate in patients with atrial fibrillation.Crossref | GoogleScholarGoogle Scholar | 31328179PubMed |
[6] Apostolakis S, Guo Y, Lane DA, et al. Renal function and outcomes in anticoagulated patients with non-valvular atrial fibrillation: the AMADEUS trial. Eur Heart J. 2013; 34 3572–9.
| Renal function and outcomes in anticoagulated patients with non-valvular atrial fibrillation: the AMADEUS trial.Crossref | GoogleScholarGoogle Scholar | 23966309PubMed |
[7] Simpson BH, Reith DM, Medlicott NJ, Smith AJ. Choice of renal function estimator influences adverse outcomes with dabigatran etexilate in patients with atrial fibrillation. TH Open. 2018; 2 e420–7.
| Choice of renal function estimator influences adverse outcomes with dabigatran etexilate in patients with atrial fibrillation.Crossref | GoogleScholarGoogle Scholar | 31249970PubMed |
[8] Drouet L, Bal dit Sollier C, Steiner T, et al. Measuring non-vitamin K antagonist oral anticoagulant levels: when is it appropriate and which methods should be used? Int J Stroke. 2016; 11 748–58.
| Measuring non-vitamin K antagonist oral anticoagulant levels: when is it appropriate and which methods should be used?Crossref | GoogleScholarGoogle Scholar | 27412190PubMed |
[9] Gong IY, Kim RB. Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban. Can J Cardiol. 2013; 29 S24–33.
| Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban.Crossref | GoogleScholarGoogle Scholar | 23790595PubMed |
[10] Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16 31–41.
| Prediction of creatinine clearance from serum creatinine.Crossref | GoogleScholarGoogle Scholar | 1244564PubMed |
[11] Thorne K, Dee S, Jayathissa S. Prescriber compliance with renal function monitoring in patients taking dabigatran (Pradaxa). N Z Med J. 2015; 128 83–88.
| 26913911PubMed |
[12] McBain L, Kyle A. Renal function monitoring in patients prescribed dabigatran in the Compass Health Primary Health Organisation: a quality improvement audit. N Z Med J. 2018; 131 40–47.
| 29518798PubMed |
[13] Best Practice Advocacy Centre. (bpacnz). Clinical audit: renal function testing in people taking dabigatran. Dunedin: bpacnz; 2012. [cited 2019 Sep 5]. Available from: http://www.bpac.org.nz/Audits/Dabigatran-renal.aspx
[14] Best Practice Advocacy Centre. (bpacnz) The use of dabigatran in general practice: a cautious approach is recommended. Best Pract J. 2011; 38 10–27.
[15] Boehringer Ingelheim (N.Z.) Limited. Pradaxa: New Zealand Datasheet. Wellington: Medsafe; 2017. [cited 2017 Nov 1]. Available from: http://www.medsafe.govt.nz/other/copyright.asp
[16] Medsafe Pharmacovigilance Team Monitor renal function in elderly patients taking dabigatran. Prescriber Update. 2015; 36 55
[17] Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018; 39 1330–93.
| The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation.Crossref | GoogleScholarGoogle Scholar | 29562325PubMed |
[18] New Zealand Ministry of Health. Pharmaceutical Collection. Wellington, New Zealand: Ministry of Health; 2016.
[19] Best Practice Inc. Decision support for health professionals. Dunedin: bpac Inc.; 2013. [cited 2018 Mar 12]. http://www.bestpractice.net.nz/
[20] Committee for Human Medicinal Products (CHMP). Adequacy of guidance on the elderly regarding medicinal products for human use. Amsterdam: European Medicines Agency; 2007.
[21] Graham MR, Wright MA, Manley HJ. Effectiveness of an amiodarone protocol and management clinic in improving adherence to amiodarone monitoring guidelines. J Pharm Technol 2004; 20 5–10.
| Effectiveness of an amiodarone protocol and management clinic in improving adherence to amiodarone monitoring guidelines.Crossref | GoogleScholarGoogle Scholar |
[22] Schmidt M, Mansfield KE, Bhaskaran K, et al. Adherence to guidelines for creatinine and potassium monitoring and discontinuation following renin–angiotensin system blockade: a UK general practice-based cohort study. BMJ Open. 2017; 7 e012818
| Adherence to guidelines for creatinine and potassium monitoring and discontinuation following renin–angiotensin system blockade: a UK general practice-based cohort study.Crossref | GoogleScholarGoogle Scholar | 29150471PubMed |
[23] Tomlin A, Dovey S, Gauld R, et al. Better use of primary care laboratory services following interventions to ‘market’ clinical guidelines in New Zealand: a controlled before-and-after study. BMJ Qual Saf. 2011; 20 282
| Better use of primary care laboratory services following interventions to ‘market’ clinical guidelines in New Zealand: a controlled before-and-after study.Crossref | GoogleScholarGoogle Scholar | 21228434PubMed |
[24] Piccini JP, Xu H, Cox M, et al. Adherence to guideline-directed stroke prevention therapy for atrial fibrillation is achievable. Circulation. 2019; 139 1497–506.
| Adherence to guideline-directed stroke prevention therapy for atrial fibrillation is achievable.Crossref | GoogleScholarGoogle Scholar | 30700141PubMed |