Genitalic morphology and phylogenomic placement of the Australian spider Paraplectanoides crassipes Keyserling, 1886 (Araneae, Araneidae) with a discussion on the classification of the family Araneidae
Gustavo Hormiga A * , Siddharth Kulkarni
A * , Siddharth Kulkarni  B , Miquel Arnedo
B , Miquel Arnedo  C , Dimitar Dimitrov
C , Dimitar Dimitrov  D , Gonzalo Giribet
D , Gonzalo Giribet  E , Robert J. Kallal
E , Robert J. Kallal  A and Nikolaj Scharff
A and Nikolaj Scharff  F *
F *
A
B
C
D
E
F
Abstract
We complement and expand the existing descriptions of the Australian araneid spider Paraplectanoides crassipes Keyserling, 1886, and provide the first detailed analysis of the male palpal homologies to include examination of the expanded organ and scanning electron micrographs of the palpal sclerites. We study the placement of Paraplectanoides and the classification of the family Araneidae by combining ultraconserved elements with Sanger markers. We also added Sanger sequences of the Australian araneid genus Venomius to the molecular dataset of Scharff et al. (2020) to explore the phylogenetic placement and implications for classification of the family. We evaluate a recent proposal on the classification of the family Araneidae by Kuntner et al. (2023) in which a new family is erected for P. crassipes. Paraplectanoides is monotypic. Examination of the type material shows that Paraplectanoides kochi O. Pickard-Cambridge, 1877 is misplaced in the genus and the name is a senior synonym of the araneid Isoxya penizoides Simon, 1887 (new synonymy) that results in the new combination Isoxya kochi (O. Pickard-Cambridge, 1877). The classification of Araneidae is revised and the following nomenclatural acts are introduced: Paraplectanoididae Kuntner, Coddington, Agnarsson and Bond, 2023 is a junior synonym of Araneidae Clerck, 1757 new synonymy; phonognathines and nephilines are subfamilies of Araneidae (Subfamily Phonognathinae Simon, 1894 rank resurrected; and Subfamily Nephilinae Simon, 1894 rank resurrected). The results of our analyses corroborate the sister group relationship between Paraplectanoides and the araneid subfamily Nephilinae. Venomius is sister to the Nephilinae + Paraplectanoides clade. The placement of the oarcine araneids and Venomius renders the family Araneidae non-monophyletic if this were to be circumscribed as in Kuntner et al. (2023). In light of the paucity of data that the latter study presents, and in absence of a robust, stable and more densely sampled phylogenetic analysis of Araneidae, the changes and definitions introduced by that classification are premature and could lead to a large number of new families for what once were araneid species if the maximum-crown-clade family definitions were to be used. Consequently, we argue for restoring the familial and subfamilial classification of Araneidae of Dimitrov et al. (2017), Scharff et al. (2020) and Kallal et al. (2020).
Keywords: Arachnida, Araneae, Australia, comparative morphology, Linnaean ranks, molecular phyogenetics, monophyly, phylogeny, taxonomy.
The question, ‘Precisely how large is the scope of a genus, a family, or an order?’ is not much more determinate than the question, ‘Precisely how far is up?’ [George G. Simpson 1945, p. 16].
Those are my principles, and if you don’t like them… well, I have others. [Groucho Marx, attributed].
Introduction
The superfamily Araneoidea is a clade of 17 ecribellate spider families known for diverse capture webs (Hormiga and Griswold 2014; Dimitrov et al. 2017). Although many araneoids build iconic orb webs, others showcase a remarkable diversity of sheet webs and irregular webs, and there are even species that do not build foraging webs at all. This diversity has intrigued arachnologists for over two centuries, influencing decisions on taxonomic classification and interpretation of the evolutionary history. Araneidae is the third most speciose spider family, and includes several common and widespread species, some with pronounced sexual size dimorphism that build mainly two-dimensional orb webs. The Australian araneid spider Paraplectanoides crassipes Keyserling, 1886 can be placed, without hesitation, among the strangest members of Araneoidea. The only known species of Paraplectanoides lives in Queensland (Qld), New South Wales (NSW), Tasmania (Tas.), South Australia (SA) and Western Australia (WA). Although this species was first described by Keyserling in 1886 in his monograph on Australian arachnids based on two specimens from Queensland, knolwedge of the biology of P. crassipes can be ascribed to the careful long-term studies of Tasmanian zoologist Vernon Victor Hickman (1894–1984). This araneid species is very rarely found and specimens are therefore scarce in museum collections. Hickman (1976, p. 166) noted the rarity, commenting that ‘during the past 50 years I have found only 18 mature females and one mature male in the field’. There are many unusual features in Paraplectanoides, including somatic morphology (Fig. 1–3) and peculiar web (Hickman 1976, fig. 1) but perhaps the more atypical features are those that relate to the life cycle. Females live for at least 6 years (but likely longer, up to nine), are not mature until the third year of life, and are able to store sperm for at least 6 years and still produce a high percentage of fertile eggs (Hickman 1976). Hickman (1976) redescribed P. crassipes and described the minute, extremely sexually dimorphic male for the first time. Davies (1988) illustrated the habitus and genitalia of both sexes of this species in the guide to the orb weaving genera of Australia and considered Paraplectanoides an ‘araneine because it has transverse furrows on the epigastric plates, a paramedian apophysis and a radix in the male palp’. No additional data on the biology of this species have been published since these two studies were undertaken.
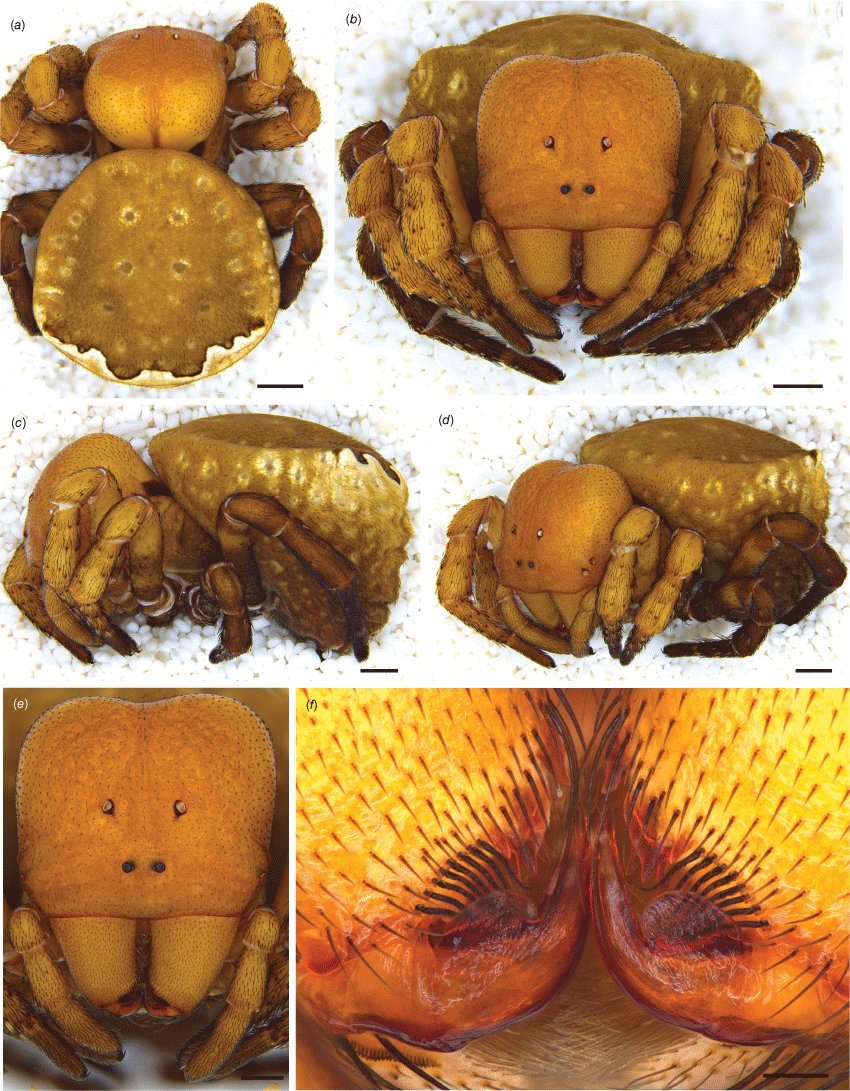
Paraplectanoides crassipes, live habitus. (a–d) Adult female from Biamanga National Park (NSW) (GH1810). (e, f) Juvenile female from Risdon (Tas.) (GH2864). Photos G. Hormiga.

Paraplectanoides crassipes, adult female from Kangaroo Island (SA). (a) Dorsal view; (b) anterior view; (c) lateral view; (d) anterolateral view; (e) prosoma, anterior; and (f) cheliceral fang. Scale bars: a, 1 mm; b–d, 750 μm; e, 1 mm; f, 100 μm.
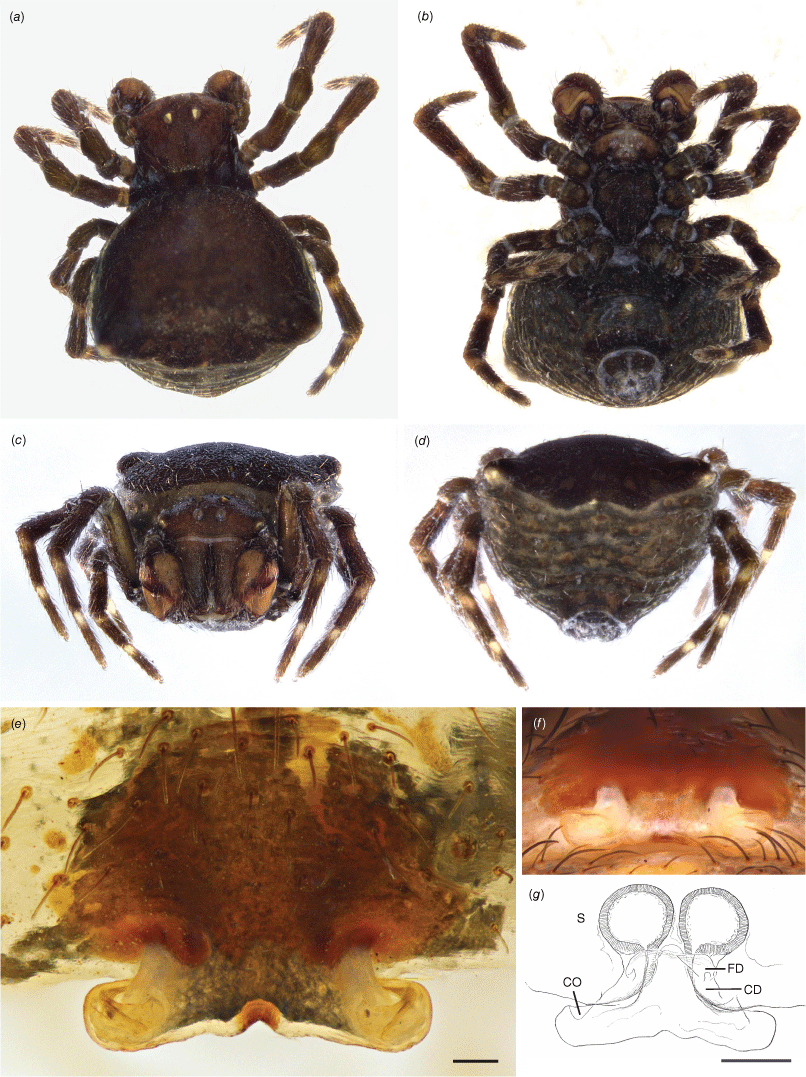
Paraplectanoides crassipes, (a–d) adult male habitus (from East Risdon, Tas.). (e–g) Epigynum (Mount Barker, WA); (a) dorsal view; (b) ventral view; (c) anterior view; (d) caudal view; (e) ventral view; (f) caudal view; (g) ventral view (cleared; S, spermatheca; CD, copulatory duct; FD, fertilisation duct; CO, copulatory opening). Scale bars: e, 75 μm; f, 100 μm; g, 0.2 mm.
Scharff et al. (2020) discovered the genealogical propinquity between nephiline araneids and Paraplectanoides in a phylogenetic analysis using DNA Sanger sequencing data from five genes, namely 16S rRNA (16S), 18S rRNA (18S), 28S rRNA (28S), cytochrome c oxidase subunit I (COI) and histone H3 (H3). This intriguing and remarkable sister group relationship has also been corroborated with phylogenetic analyses of data from six genetic markers, including the aforementioned five and 12S rRNA (12S) (Kallal and Hormiga 2018, 2019), ultraconserved elements (UCEs) (Kulkarni et al. 2020, 2021) and UCE sequences combined with Sanger data (Kulkarni et al. 2023). Despite the robust, key phylogenetic placement of Paraplectanoides in Araneidae, recent comparative and biogeographic studies have omitted this taxon and the publicly available genomic data (e.g. Kuntner et al. 2019; Turk et al. 2020; see also comments in Kallal et al. 2020).
Although quite detailed and accurate, Hickman’s (1976) description of P. crassipes is almost half a century old and somewhat outdated. In this paper we update and expand the morphological description of this species, provide a detailed assessment of the male palpal sclerites based on the expanded palp and using scanning electron microscopy (SEM) for the first time (Fig. 1–7), and discuss the systematic placement and classification. Finally, based in part on the findings reported here, we evaluate, discuss and reject a recent proposal on the classification of the family Araneidae by Kuntner et al. (2023) in which, among other things, a new family is erected for this single species.
Materials and methods
Specimens were examined and illustrated using a Leica M205A stereoscopic microscope equipped with a Leica DFC425 camera and Leica Application Suite X software (LAS X, ver. 1.4, Leica Microsystems, https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/) or with a camera lucida. Further details were studied and illustrated using a Leica DMXRE compound microscope with a camera lucida. Single stereoscope images were stacked with Helicon Focus (ver. 6.7.1, see www.heliconsoft.com) software from Helicon Soft Ltd to increase depth of field. Left structures (i.e. palps or legs) are depicted unless otherwise stated. Most setae and macrosetae are not depicted in the final palp and epigynum drawings. Drawings were rendered using a graphite pencil, and scanned and edited using Adobe Photoshop. Plates were assembled using Adobe Illustrator. Morphological measurements were taken using the LAS X Live Measurement module in the dissecting microscope. All morphological measurements are expressed in millimetres. Female genitalia were excised using surgical blades or sharpened needles. Epigyna and male palps were transferred to methyl salicylate (Holm 1979) for examination under the microscope. Male palps were expanded by immersion in a bath of a concentrated solution of potassium hydroxide for ~2 min followed by immersion in distilled water. The female spinneret spigots of Paraplectanoides were examined with a Leica M205A stereoscope at 160×. SEM images were captured using the Zeiss EVO10 at the Department of Biological Sciences of The George Washington University. Specimens were critical point dried, sputter-coated in a gold-palladium alloy and mounted as described in Álvarez-Padilla and Hormiga (2007). Label data from museum specimens are reported verbatim in the ‘Specimens examined’ section.
Field work in Australia was made possible by research permits FA19008 (Department of Primary Industries, Parks, Water and Environment, Tas., Australia) and SL10324 (NSW National Parks & Wildlife Service, NSW, Australia).
Museum repositories of the specimens studied in this work are abbreviated as follows: AM, Australian Museum (Sydney, NSW, Australia); MCZ, Museum of Comparative Zoology, Harvard University (Cambridge, MA, USA); QM, Queensland Museum (Brisbane, Qld, Australia); TMAG, Tasmanian Museum and Art Gallery (Hobart, Tas., Australia); WAM, Western Australian Museum (Perth, WA, Australia); and NHMD, Natural History Museum of Denmark, University of Copenhagen (Copenhagen, Denmark).
Anatomical abbreviations used in text and figures: ALS, anterior lateral spinneret; BH, basal haematodocha; CD, copulatory duct; E, embolus; FD, fertilisation duct; MA, median apophysis; MH, median haematodocha; P, paracymbium; PMS, posterior median spinneret; PLS, posterior lateral spinneret; R, radix; S, spermatheca; ST, subtegulum; T, tegulum.
Genomic data and phylogenetic analysis
To explore the phylogenetic placement of Paraplectanoides in Araneidae, we combined phylogenomic data (UCE sequences) with Sanger sequencing data. These data were taken from a more inclusive study that aimed to examine the relationships of the families of Araneae, with emphasis on araneomorphs (Kulkarni et al. 2023). A subset of the data, including the araneid terminals and some outgroups, allows for a more thorough examination of tree space in the search for the optimal topology. Although our araneid phylogenomic dataset is the most extensive to date, the goal is not to provide a detailed hypothesis for araneid relationships that would require an even deeper taxon sampling given the large size of the family but to offer a phylogenetic context for our discussion on classification. We included 46 terminals, some newly sequenced UCEs from Kulkarni et al. (2023) and others from previous studies (see Supplementary Table S1 for sequence data sources), representing 43 Araneidae terminals, 1 Synotaxidae and 2 linyphioids that were used to root the tree. Outgroup taxa were selected based on the phylogenetic hypothesis of Kulkarni et al. (2023). We included additional taxa from the datasets of Fernández et al. (2018), Kulkarni et al. (2021, 2023) and Kallal et al. (2021). Phylogenetic analyses were performed on the unpartitioned nucleotide data using IQ-TREE (ver. 2.1.3, see http://iqtree.org; Nguyen et al. 2015). Model selection was allowed for each unpartitioned dataset using the TEST function (Kalyaanamoorthy et al. 2017; Hoang et al. 2018). Nodal support was estimated by 1000 ultrafast bootstrap replicates (Hoang et al. 2018) with 2000 iterations, appended with the -bnni flag. According to this command, the ultrafast bootstrap optimises each bootstrap tree using a hill-climbing nearest neighbour interchange (NNI) search based on the corresponding bootstrap alignment (Hoang et al. 2018). We compiled another dataset that included 67 terminals generated by combining the UCE dataset with six publicly available Sanger sequenced loci, 12S, 16S and COI genes, three nuclear genes – the protein-coding H3, and small and large subunits of ribosomal RNA genes (18S and 28S respectively). COI and H3 markers were aligned using MACSE (Ranwez et al. 2011) with the invertebrate mitochondrial code followed for COI. The remaining markers (12S, 16S, 18S and 28S) were aligned using MAFFT (ver. 7.52, see http://mafft.cbrc.jp; Katoh and Standley 2013). Trimming was performed on all UCE alignments using trimAL (ver. 1.2, see http://trimal.cgenomics.org; Capella-Gutiérrez et al. 2009) with -gappyout setting.
In addition, we added sequences of 16S, H3 and COI from the recently described Australian araneid Venomius tomhardyi Rossi, Castanheira, Baptista & Framenau, 2023 to the dataset of Scharff et al. (2020) that is the largest published araneid dataset including data from 16S, 18S, 28S, H3 and COI for 158 araneids and outgroups. Venomius sequences (COI, 16S and H3) were generated from a female specimen from Flinders Island (Tas., deposited at the TMAG collection; voucher GH2512, RJKDNA011; Supplementary Fig. S2) following the protocols described in Kallal et al. (2018) and deposited at GenBank (see Table S1 for accession numbers). After the addition of these sequences the corresponding gene matrices were aligned using MAFFT (ver. 7.520, see http://mafft.cbrc.jp; Katoh and Standley 2013) using the L-INS-i algorithm. Aligned matrices were combined in a supermatrix and partitioned following Scharff et al. (2020) with each gene in a separate partition, and 28S split into a conserved and more variable partition. The final combined dataset included 159 terminals and 4355 bp. Maximum likelihood phylogenetic analyses were carried out in IQ-TREE (ver. 2.2.2.3, see http://iqtree.org; Minh et al. 2020). Likelihood search and model selection were undertaken in the same run as IQ-TREE implements model selection using ModelFinder (see http://www.iqtree.org/ModelFinder/; Kalyaanamoorthy et al. 2017). Support was evaluated using ultrafast bootstrap (Hoang et al. 2018) with 10 000 bootstrap replicates and SH-aLRT branch test (Guindon et al. 2010) with 1000 replicates. Best-fit models of sequence evolution were as follows TVMe + I + G4 (H3); TIM3 + F + I + G4 (28S – conservative); TVM + F + I + G4 (28S – variable); GTR + F + I + G4 (16S); TNe + I + G4 (18S) and GTR + F + I + G4 (COI). The resulting tree was visualised in FigTree (ver. 1.4.4, see http://tree.bio.ed.ac.uk/software/figtree).
Results
Phylogenetic analysis
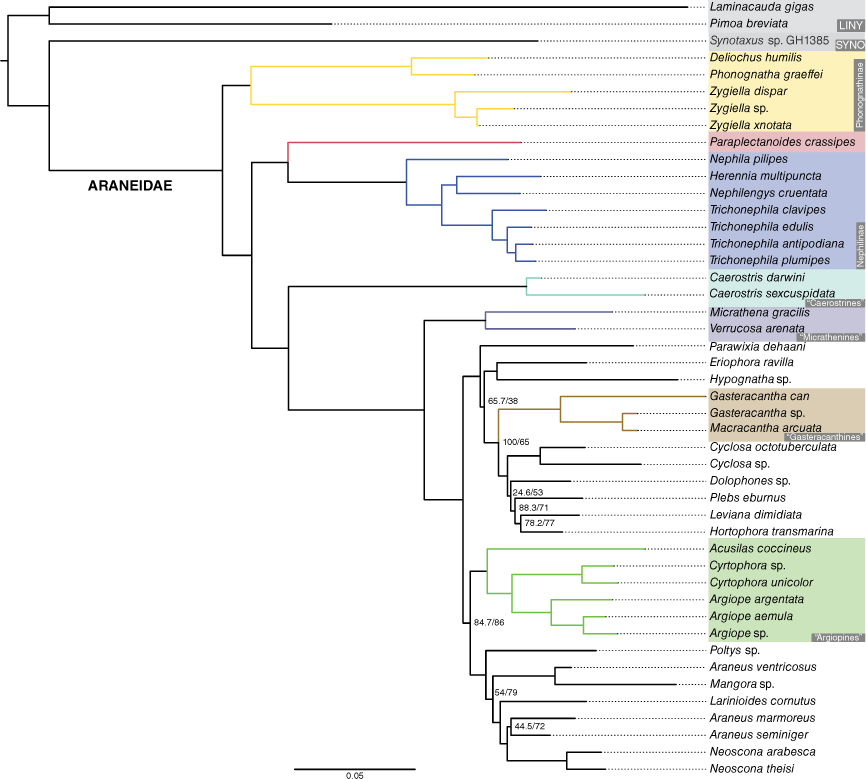
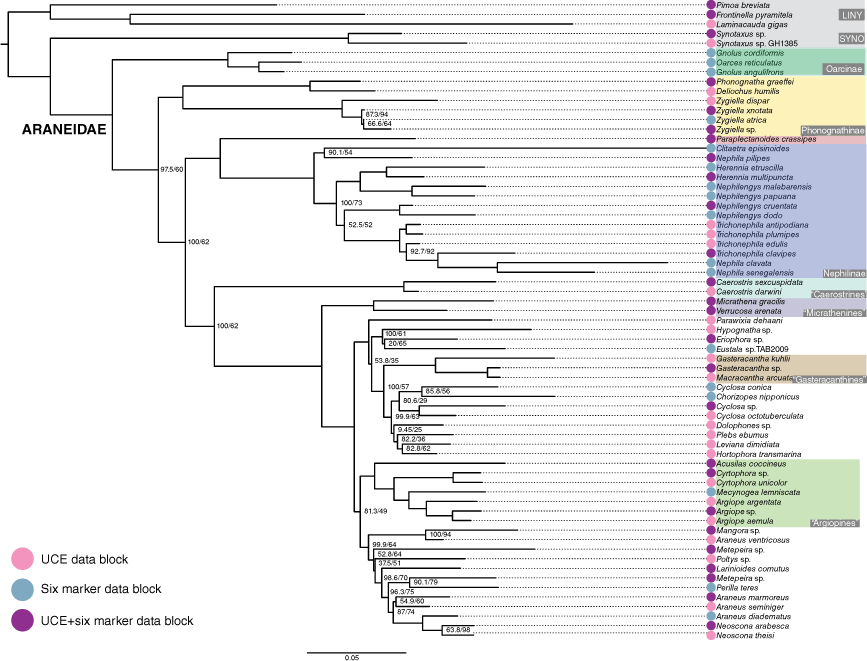
Our UCE data set included 452 loci and the combined data set included six additional Sanger sequenced markers. The phylogeny was rooted to Linyphiidae + Pimoidae (linyphioids) and Synotaxidae was the sister group of Araneidae. In the UCE phylogeny (Fig. 8), Phonognathinae (represented with five terminals in the genera Phonognatha, Deliochus and Zygiella) was the sister group of a clade including Paraplectanoides + Nephilinae and remaining Araneidae. In the remaining Araneidae clade, Caerostris Thorell, 1868 (represented by two species) was the sister group of other araneids. The combined data phylogeny (Fig. 9) echoed similar relationships with a notable difference: the Oarcinae lineage, three species of the genera Gnolus Simon, 1879 and Oarces Simon, 1879 with only Sanger data, was sister group to all the remaining araneids (oarcines were not represented in the UCE-only dataset). The optimal maximum likelihood tree (best score −129 669.781) resulting from the analysis of the expanded Scharff et al. (2020) dataset placed the araneid Venomius tomhardyi as the sister group of the clade formed by Paraplectanoides + Nephilinae rendering Araneidae non-monophyletic (Fig. 10, Supplementary Fig. S1).
Systematics
Family ARANEIDAE Clerck, 1757
Type: Araneus Clerck, 1757.
Type species: Araneus angulatus Clerck, 1757.
PARAPLECTANOIDIDAE Kuntner, Coddington, Agnarsson & Bond, 2023, new synonymy.
Type species: Paraplectanoides crassipes Keyserling, 1886.
Subfamily NEPHILINAE Simon, 1894, rank resurrected
Type: Nephila Leach, 1815.
Type species: Aranea pilipes Fabricius, 1793.
Nephilinae Simon, 1894. Dimitrov et al. (2017), Scharff et al. (2020), Kallal et al. (2020).
Nephilidae Simon, 1894. Kuntner (2006), Kuntner et al. (2019, 2023).
Subfamily PHONOGNATHINAE Simon, 1894, rank resurrected
Type: Phonognatha Simon, 1894.
Type species: Epeira graeffei Keyserling, 1865.
Phonognatheae Simon, 1894.
Zygielleae Simon, 1929.
Zygiellidae Simon, 1929. Wunderlich (2004).
Zygiellinae Wunderlich, 2004. Gregorič et al. (2015), Kallal and Hormiga (2018), Kallal et al. (2018), Scharff et al. (2020).
Phonognathidae Simon, 1894. Kuntner et al. (2019, 2023).
Phonognathinae Simon, 1894. Kallal et al. (2020).
Paraplectanoides Keyserling, 1886
Type: Paraplectanoides crassipes Keyserling, 1886; gender feminine.
Paraplectanoides crassipes Keyserling, 1886
Paraplectanoides crassipes Keyserling, 1886, p. 112, pl. 9, f. 1 (Df).
P. crassipes Simon, 1895, p. 871.
P. crassipes Hickman, 1976, p. 166, f. 2–5, 7–13 (f, Dm).
P. crassipes Davies, 1988, p. 308, f. 30 (mf).
Types: Two adult female syntypes from Gayndah (Qld, Australia), housed at the Universität Hamburg Zoological Museum (Germany). We have studied high resolution images of the syntypes taken by Nadine Dupérré, including both the habitus and dissected epigyna (Fig. 7a–d).
In the original description Keyserling (1886, p. 114) reports ‘Sydney, Gayndah’ as the type locality of P. crassipes. Although there is no indication in the description that Keyserling examined more than one specimen, both syntype labels have ‘Gayndah’ as the collecting locality and the reason why Keyserling wrote ‘Sydney, Gayndah’ is unclear. The town of Gayndah is in the North Burnett region (Qld), ~250 km north-west of Brisbane and therefore far from Sydney.
Hickman (1976) provides a detailed description of the somatic morphology that is expanded here with a description of the male palp, epigynum and spinneret spigots, and images of the somatic morphology and of live females (from NSW and Tas.).
Sustentaculum absent (Fig. 7e, f).
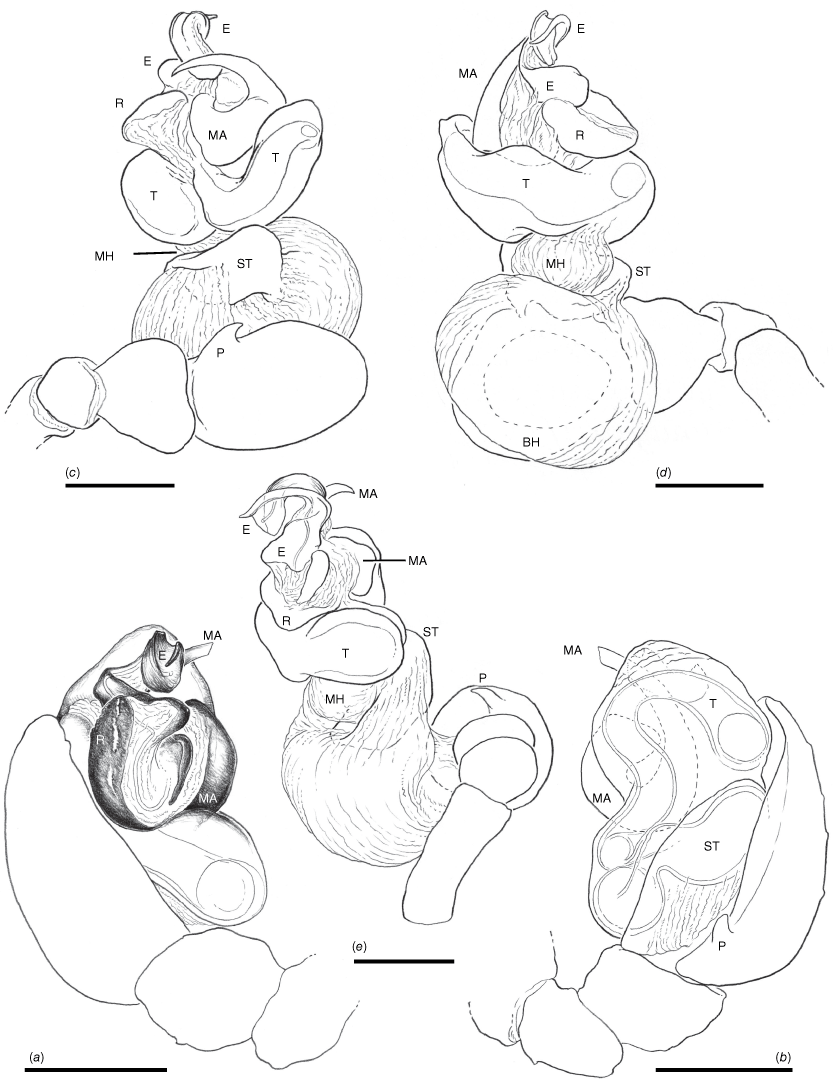
Male palp (Tas., East Risdon, 2/7/1944, V.V. Hickman, KS0050, AM, one male) (Fig. 4–6). Patella with a single macroseta, in dorsal position (Fig. 5a). Tibia with one ectal and one dorsal trichobothrium. Cymbium apically blunt, with a small pointed basal paracymbium (Fig. 6c, d). Tegulum prominent in ectal view, discoid and less sclerotised apically (Fig. 4e, 5e, f). Radix clearly visible mesally, ventrally membranous with a blade-like sclerotised area adjacent to the base of the median apophysis (Fig. 4a, 5f). Median apophysis hook shaped, with a large base exposed in ventral view (Fig. 4c). Apical region of median apophysis curved onto terminal area of embolus (Fig. 5d, 6b). Embolus distally curved and bifurcated, an enlarged base visible on the anterior part of the radix (Fig. 4a, e, 5d–f). A membranous sac-like structure is visible between the apical ends of the embolus and median apophysis; this is an extension of the membrane that connects the embolus to the radix (Fig. 4e).
Epigynum (syntypes from Qld; WA, Mount Barker, 34.38S: 117.40E, 24 Jan. 1990, A.F. Longbottom, T75728, WAM, one female) (Fig. 3e–g, 7). A short scape protruding caudally as an inverted-T structure, lightly sclerotised (Fig. 3e). Spermathecae varying in shape from ovoid (Fig. 7a–d; Qld, Tas.) to spherical (Fig. 3; WA), copulatory ducts opening onto the lateral ends of the T-shaped scape; fertilisation ducts medially oriented (Fig. 3g). We interpret the variation in spermathecal shape as intraspecific in the absence of any evidence to the contrary (e.g. we do not observe any variation in male palpal morphology across the distribution range in the available specimens).
Spinnerets (Tas., Queen’s Domain, 12/2/1974, V.V. Hickman, KS28535, AM, one female, bred from egg; Tas., Risdon, 2/12/1943, V.V. Hickman, KS28530, AM, one female). ALS with numerous piriform spigots and one major ampullate with a nubbin. PLS with five to six aciniform spigots anteriorly placed, one cylindrical and a minor ampullate with a nubbin. PLS with two cylindrical spigots, 11–13 aciniform and two aggregate spigots that share a common base. Flagelliform spigots absent. Colulus fleshy, with 10–12 setae.
South Australia, Kangaroo Island, Western River Wilderness Protection Area, Waterfall Creek Trail near Waterfall (ARP C#77), 35°41′44″S 136°54′37″E (WGS84), 9–10 May 2010, M.G. Rix, D. Harms, Sifting/beating, esp. low Xanthorrhoea, WAM T102787, 1 F (Leica images, Tarsus IV images).
Western Australia, Mt Barker, 34.38S 117.40E, 24 Jan 1990, Af. Longbottom S. 323, 16 Osborne St., on shrub at front, WAM T75728, 1 F.
Queensland, Eidsvold K33449 (no date) AM KS15753, 1F.
Queensland, Tinaru Ck, Mareeba, 17°00′S 145°526E, 24 Sept 1972, R. Mascord, on foliage, AM KS107295, 1 subF.
New South Wales, Cowan, 33°34′55″S 151°10′05″E, 11 Dec 19??, S. Maddick, beating tray, AM KS105944, 1M (GH SEM).
New South Wales, Biamanga N.P. off Field Buckets Rd, 161 m, S36.46652 E149.89529, 6.iv.2014, deep into Xanthorrhoea tussock, G. Hormiga & N. Scharff, MCZ, F (GH1810) (live photos GH DSC_1218-1230/7.iv.2014, 1253-1286/7.iv.2014, 1330-1351/9.iv.2014); habitat NS DSC_0028-0035/6.iv.2014, live photos NS DSC_0046-0063/7.iv.2014, 0094-0107/7.iv.2014, 0135-0150/9.iv.2014).
Tasmania: Risdon, S 42.82877, E 147.34500, 38 m, 24.ii.2019, G. Hormiga, M. Arnedo & S. Kulkarni, MCZ, juvenile female (GH2864) (live photos GH DSC_1610-1692/24.ii.2019).
Tasmania, Risdon, 2/12/1943, V.V. Hickman AM KS28530, 1F (in nest with eggsac).
Tasmania, East Risdon, 12/7/1944, V.V. Hickman, in nest of female AM KS0050, 1M.
Tasmania, 12/2/1974, V.V. Hickman, bred from eggsac of female from Domain, AM KS28535, 1F.
Tasmania, East Risdon, 3/7/1944, V.V. Hickman, in nest of female AM KS28531, 1F.
Tasmania, East Risdon, 23/9/1975, V.V. Hickman, ex grass tussock AM KS28532, 1M.
Tasmania, Glen Dhu. Launceston, Sept. 1923, V.V. Hickman, in a sac-like nest under a stone among brambles AM KS28529, 1F.
Paraplectanoides crassipes, male palp from East Risdon (Tas.). (a) Mesal view; (b) ectal view; (c) expanded, ectal view; (d) expanded, anteromesal view; (e) ectoventral view. Scale bars: 0.2 mm.
Paraplectanoides crassipes, male palp from Cowan (NSW), scanning electron micrographs. (a) Ectal view; (b) anteroventral view; (c) mesal view; (d) radix and embolus, mesal view; (e) ventral view; (f) mesoventral view. Scale bars: a, c, 100 μm; b, 50 μm; d, f, 30 μm, e, 40 μm.
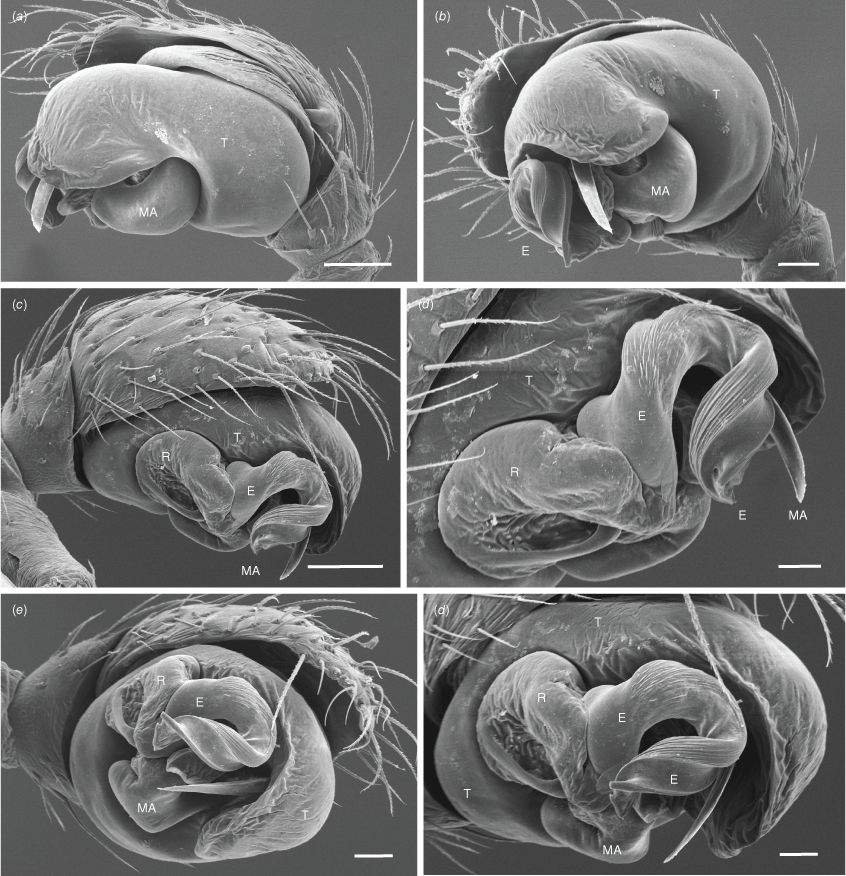
Paraplectanoides crassipes, male palp from Cowan (NSW), scanning electron micrographs. (a) Ectal view; (b) embolus and median apophysis, mesoventral view; (c) paracymbium, ectal view; (d) paracymbium, dorsoectal view. Scale bars: a, 50 μm; b, 30 μm; c, d, 10 μm.

Paraplectanoides crassipes, epigynum of the syntypes from Gayndah (Qld; images courtesy of Nadine Dupérré, LIB, Museum of Nature Hamburg, Zoology). (a, c) Ventral view; (b, d) dorsal view. (e, f) Scanning electron micrographs of the left fourth tarsus of a female of P. crassipes from Kangaroo Island (SA, WAM T102787). Scale bars: a–d, 0.1 mm; f, 100 μm; f, 40 μm).
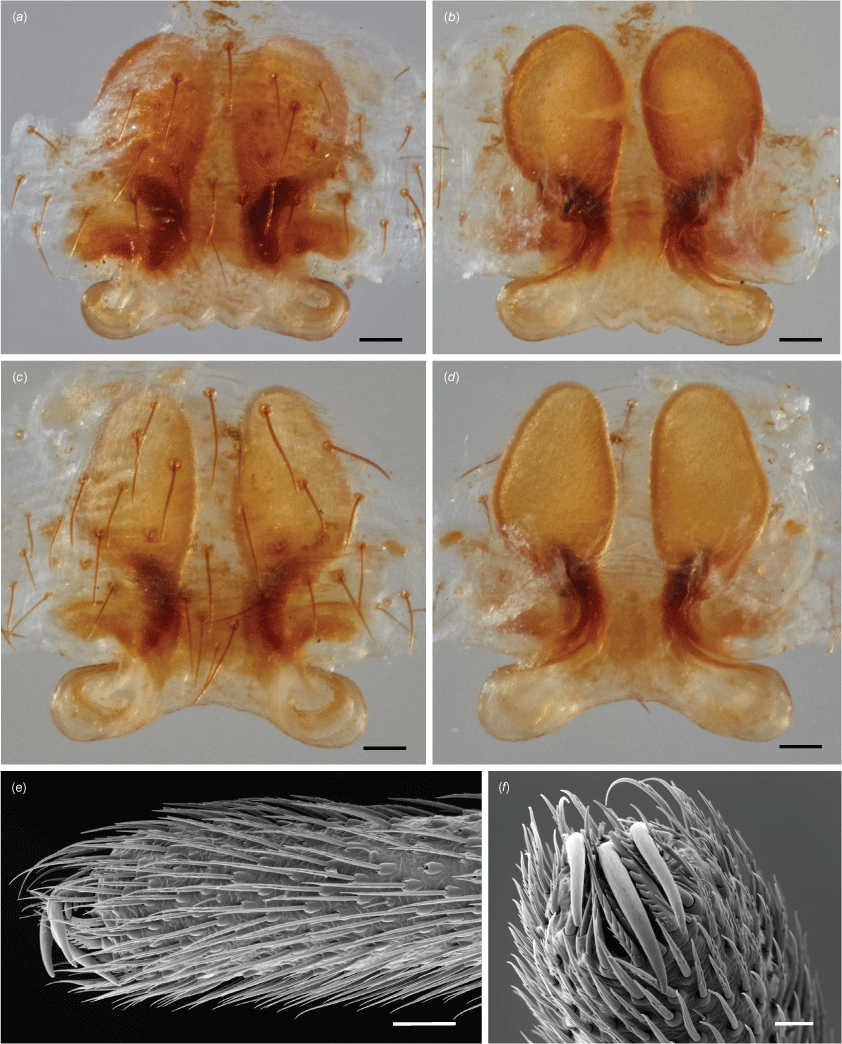
Isoxya kochi (O. Pickard-Cambridge, 1877), new combination
Paraplectana kochii O. Pickard-Cambridge, 1877, p. 35, pl. 7, f. 10. Holotype at Oxford University Museum of Natural History (examined).
Isoxia penizoides Simon, 1887, p. 269. New synonymy.
Paraplectanoides kochi Simon, 1895, p. 871.
Gasteracantha penizoides Simon, 1895, p. 843, fig. 892.
Plectana penizoides Thorell, 1899, p. 64.
Gasteracantha penizoides Simon, 1907, 1907, p. 302.
Isoxya penizoides Benoit, 1962, p. 20. Emerit (1982), p. 162, pl. 1B.
Pickard-Cambridge (1877) described this species based on a single adult female from Cape York (Australia). The description of the somatic morphology is reasonably detailed, and illustrated with dorsal and lateral views of the specimen but no description of the epigynum was provided. Unaware of Pickard-Cambridge’s (1877) description, Simon (1887) described this species as Isoxia penizoides Simon, 1887. Subsequently, Simon (1895, p. 871) transferred Paraplectana kochi to the genus Paraplectanoides but did not provide any justification for the new combination other than stating that ‘It differs in particular from Anepsia, sec. Keyserling, with a much wider clypeus, the area of the middle eyes much narrower anteriorly than posteriorly, and not contiguous with the lateral eyes on either side’. Scharff et al. (2020) suggested that this species could be a member of the arkyid genus Demadiana but examination of the type of Paraplectana kochii O. Pickard-Cambridge, 1877 shows that this is conspecific with Isoxia penizoides Simon, 1887 and therefore the latter species is a junior synonym of the former.
No additional specimens of this species have been recorded from Australia but the species is widely distributed in West and Central Africa. The jar with the type specimen in the Oxford University Museum also included a second tube with a non-type specimen of the same species. This also came from the O. Pickard-Cambridge collection and had also been identified as Paraplectana kochi. According to the label, the specimen was collected in ‘R. Coanza’. This could be Cuanza River in Angola that is also spelled Coanza, Kwanza or Quanza (Zoe Simmons, Oxford University Museum of Natural History, pers. comm. to N. Scharff). The type specimen could therefore possibly have been mislabelled.
Discussion
The morphology of Paraplectanoides
The somatic features of P. crassipes are highly autapomorphic (and therefore the species is readily identifiable due to the unique morphological features such as prosoma shape), unlike the morphological features of the genitala that are generally similar to those of many other araneids, except for the absence of a conductor. The male palps of araneids are very complex and especially difficult to interpret when unexpanded. Araneid palps are basically equipped with only three sclerites. The embolic division is inserted on the tegulum and is often subdivided into multiple subsclerites that are partly responsible for the superficial complexity. The embolic division may consist only of the embolus but this can also have the following sclerites in addition to the terminal embolus: a radix (in most araneids), a stipes (in some araneids), a number of terminal or subterminal sclerites sitting on a distal haematodocha and the terminal embolus. All the different subsclerites are connected by membranes but the entire embolic division has only a single attachment point to the tegulum. In addition to the embolic division, there is commonly also a conductor and a median apophysis, each with a separate membranous connection to the tegulum. The base of the median apophysis is often close to the embolic division and may share membranes with the latter. In addition to the three sets of sclerites, there can also be a fourth sclerite, the paramedian apophysis, that is inserted separately on the tegulum. The palpal sclerites and subsclerites provide an important source of phylogenetically informative morphological data (e.g. Scharff and Coddington 1997; Cabra-García and Hormiga 2020). Establishing hypotheses of homology can be challenging in many cases, especially due to the extensive diversity in the number and morphological characteristics of embolic division subsclerites, coupled with the presence of character homoplasy (e.g. Kallal and Hormiga 2019). Our description of the palpal morphology of Paraplectanoides (Fig. 4–6) uses the sclerite terminology of Scharff and Coddington (1997). Davies (1988, p. 308, fig. 30) illustrated an unexpanded palp and labelled a structure adjacent to the radix of P. crassipes a ‘paramedian apophysis’, and Kallal and Hormiga (2019, fig. 8) followed this interpretation in phylogenetic reconstructions of araneid palpal morphology. Given such palpal complexity, Levi labelling the median apophysis of this species as the radix in an unexpanded palp drawing (H. W. Levi, unpublished drawings of araneids available at the Museum of Comparative Zoology, Harvard University) is hardly surprising. Detailed examination of the sclerites (Fig. 4e, 5d–f) reveals that this structure is part of the radix (most conspicuously seen in Fig. 5e) and not a homologue of the paramedian apophysis. The latter sclerite is inserted exclusively on the tegulum (Comstock 1910; Scharff and Coddington 1997). Paraplectanoides lacks a conductor that, although unusual, is also absent in other araneids, such as Witica O. Pickard-Cambridge, 1895 (Levi 1986).
The epigynum of P. crassipes projects posteriorly into a structure than can be interpreted as a short scape (Fig. 3e, f). The epigynal scape is a structure with highly variable morphological characteristics that is common in many other araneids. The copulatory openings are located at the ends of the lateral epigynal extensions (Fig. 3e, g).
The chelicerae of P. crassipes have several unusual structures. As originally reported by Hickman (1976, fig. 9), there is a robust mesal lobe that runs along the longitudinal axis of the paturon in females (whether this structure is also present in males is unknown). In both sexes, the cheliceral fang has a conspicuous blade-like flange in an anterobasal position (Fig. 2f). Davies (1988, p. 308, fig. 30) described the presence of a comb of macrosetae on the cheliceral promargin (Fig. 2f). The distal parts of these macrosetae (eight on each paturon) are partially covered by the cheliceral fang flange. There is no cheliceral boss (condyle) in either sex of P. crassipes, as noted by Hickman (1976, p. 172). We have dissected out male and female chelicerae and corroborated the absence of the boss in both sexes. The statement of Kuntner et al. (2023) that ‘the cheliceral condyle (boss) in Paraplectanoides is smooth and is not striated’ is incorrect (no boss can be seen in the image of the male chelicerae provided in their supplementary fig. S6). These authors did not use SEM to assess this character but given the minute size of the adult males, ~2 mm in body length, the presence of cuticular striae (first described for nephilines by Hormiga et al. 1995) can only be accurately evaluated through electron microscopy.
Following Kallal and Hormiga (2018, p. 1075) we have interpreted the absence of a sustentaculum in Paraplectanoides as secondary. The sustentaculum is also absent in other araneids such as Micrathena Sundevall, 1833 or Bertrana Keyserling, 1884 (see Scharff and Coddington 1997).
The absence of flagelliform silk gland spigots is consistent with a lack of viscid sticky silk in the web of Paraplectanoides, reported by Hickman (1976, p. 167), nevertheless a pair of aggregate spigots sharing a common base is present in the PLS. Although the functional and behavioural significance of this trait is unknown, other araneids lack sticky silk but still build capture webs, including some cyrtophorines that may lack the triplet altogether (e.g. Cyrtophora citricola (Forsskål, 1775)) or have only the flagelliform spigot absent (Mecynogea lemniscata (Walckenaer, 1841)) (Coddington 1989). Flagelliform spigots are also absent in Demadiana Strand, 1929 and Arkys Walckenaer, 1837 (Arkyidae) that do not build foraging webs but have aggregate spigots (Framenau et al. 2010).
Paraplectanoides and the classification of the family Araneidae
Kuntner et al. (2019) recently attempted to justify a Linnaean family rank for nephilines using the age of origin of groups as the main criterion to assign classification ranks. This proposal was later rejected by Kallal et al. (2020) because the proposed groups rendered Araneidae paraphyletic and because time banding, when applied across other spider groups, produced non-sensical classifications. In the Kuntner et al. (2019) proposal, monophyly of the concept of Araneidae dismissed Paraplectanoides, the inclusion of which was relevant, given that this is the sister group of nephilines. This latter hypothesis was published by Scharff et al. (2020).
Use of the age of origin for the assignment of ranks is widely perceived as impractical as this would necessitate ‘wholesale nomenclatural changes’ that would ‘exacerbate the stability problem’ of biological classification (Avise and Mitchell 2007; Avise and Liu 2011) or as Simpson (1937) more bluntly expressed this, ‘quickly leads to confusion and to absurdity’.
Later on Kuntner et al. (2023) attempted to reclassify araneids based on a new set of criteria and included Paraplectanoides in the study. The sister group relationship of Paraplectanoides and the nephiline clade is far from controversial, as several analyses using Sanger sequence data (Kallal and Hormiga 2018, 2019), ultraconserved elements (UCEs) (Kulkarni et al. 2020, 2021) and a combination of UCE, transcriptomic and Sanger sequences (Kulkarni et al. 2023) had corroborated the original hypothesis of Scharff et al. (2020).
The results of the phylogenomic analysis presented here (Fig. 8, 9) also support the Nephilinae–Paraplectanoides sister group hypothesis. The new version of araneid classification presented by Kuntner et al. (2023) brings the novelty of creating a new family exclusively for P. crassipes. We understand that the assignment of ranks to monophyletic groups is a subjective endeavour (for example, see Giribet et al. 2016), but we favour stability over the method chosen by Kuntner et al. (2023) who opted for assigning new families to any araneid that is placed more basally phylogenetically than the split between nephilines and the remaining Araneidae. This latter approach has resulted in one monospecific family but could potentially lead to many more small families (e.g. a family rank would be needed to exclusively accommodate V. tomhardyi; Fig. 10, Supplementary Fig. S1), adding little useful information to the classification system of spiders. There is ample published empirical support for the monophyly of nephilines, phonognathines and araneids, a fact that may not be readily apparent to readers of Kuntner et al. (2023) due to the absence of citations to other relevant studies that have conducted far more comprehensive phylogenetic analyses (such as Kallal et al. 2018, 2021 and Kulkarni et al. 2021).
A maximum-likelihood phylogeny constructed using the ultraconserved elements (UCEs) of Kulkarni et al. (2023) for Araneidae. Nodal values were mostly robust (>95) for the ultrafast bootstrap and Shimodaira–Hasegawa approximate likelihood ratio test (SH-aLRT) and remaining nodal values are shown.
A maximum-likelihood phylogeny constructed using the concatenated dataset (ultraconserved elements (UCEs) combined with six standard markers) of Kulkarni et al. (2023) for Araneidae. Nodal values were mostly robust (>95) for ultrafast bootstrap and Shimodaira–Hasegawa approximate likelihood ratio test (SH-aLRT) and remaining nodal values are shown. Coloured circles indicate informative data partitions.
Simplified version of the optimal maximum likelihood tree (best score –129 669.781) resulting from of the expanded Scharff et al. (2020) dataset (see Supplementary Fig. S1 for full tree). The position of Venomius is highlighted in red. Nodal support values are those of the Shimodaira–Hasegawa approximate likelihood ratio test (SH-aLRT) followed by the ultrafast bootstraps (uBS). See text for additional details.

The crux of the matter at hand does not lie in competing phylogenetic hypotheses of araneid relationships but rather in how a particular taxonomic ranking scheme is justified and defended. Ideally, such changes in classification are made with thorough diagnoses and descriptions, and supported by a robust phylogenetic position of the organism in question. It is thus useful to explore some of the principles that Kuntner et al. (2023) offer to settle what are ultimately subjective decisions about ranks to determine whether or not such views ‘eclipse the arguments put forth by Kallal et al. (2020)’.
A notable aspect of Kuntner et al. (2023) is the paucity of new data, especially considering that a new spider family is proposed and that a scarcity of information has been published regarding the sole species. In bracing the readers for the proposal of a new family for a single species, Kuntner et al. (2023) comment on the many examples of monotypic representatives that are ‘sister clades to more speciose ones’. The justification used by Kuntner et al. (2023) only considers extant species, such as Gingko biloba or Sphenodon punctatus thereby providing an incomplete perspective on the systematics of those groups, disregarding the valuable insights offered by the fossil record that should not be overlooked. When fossil taxa are considered part of the tree of life, Gingko biloba is not the sole representative of Ginkgoales, the group also includes four to five extinct families (Zhou 2009); the tuatara (Sphenodon punctatus) is not the only rhynchocephalian (the clade includes at least other 18 genera, all of which are extinct); and Sphenodon is not the sister group to Squamata but to a clade of three extinct genera, namely Cynosphedon, Zapatodon and Oenosaurus (Rauhut et al. 2012). Examples of sister group asymmetries in species composition can be found in two recently erected monospecific families within Araneae: Trogloraptoridae (Griswold et al. 2012) and Myrmecicultoridae (Ramírez et al. 2019). In both cases a single species is hypothesised to be sister group to a large clade: Trogloraptor is sister group to the superfamily Dysderoidea (Michalik et al. 2019) and Myrmecicultor is sister group to a clade that includes Dionycha and the Oval Calamistrum clade (Ramírez et al. 2019). Due to the limited and fragmentary nature of the spider fossil record, the lack of closely related fossil relatives known for either group is unsurprising. Both studies justify in detail, with morphological and molecular data, the erection of these two families and document the absence of a logical phylogenetic placement for a single species in any of the existing families. For example, Ramírez et al. (2019, p. 5) explain this as follows:
Our phylogenetic analysis indicates that Myrmecicultor chihuahuensis is a member of the RTA clade, but it is not nested in any of its main groups, namely the dionychans, the Oval Calamistrum clade, or the zodarioids. For this reason, we create a new family.
These two examples stand in stark contrast with the case of the family proposal for P. crassipes of Kuntner et al. (2023) due to the scant data that these provide and the existence of a family placement for this species (that is, Araneidae) based on the results of several phylogenetic analyses of considerably larger data matrices. Kuntner et al. (2023) argue that the classification proposed is superior to that of Kallal et al. (2020) that follows Dimitrov et al. (2017) and Scharff et al. (2020), based on a better supported phylogeny, improved diagnosability, higher information content, higher utility and superior logic. We shall examine the validity and relevance of these five arguments.
A better supported phylogeny
Kuntner et al. (2023) contended that Kallal et al. (2020) ‘presented a weakly supported topology not confirmed by any subsequent study’. The ‘weakly supported’ phylogenetic hypothesis was that of Kallal and Hormiga (2018) that used Sanger sequencing of six genetic markers combined with 235 morphological and behavioural characters scored for 80 araneid terminals (plus outgroups), representing 49 genera, including Paraplectanoides. The reason why Kuntner et al. (2023) carried out a miniature phylogenetic analysis of araneids to propose a classification when there were published analyses with significantly more extensive and relevant taxonomic representation of araneids is unclear. These authors contended that the analysis undertaken would ‘stabilize’ the Araneidae topology and ‘further test’ the sister group relationship of Paraplectanoides and the nephiline clade; and stated that the sample of araneids was ‘selected to maximize subfamily coverage’. Excluding nephilines and phonognathines (two groups whose monophyly is far from contentious due to corroboration in multiple phylogenetic analyses), the ‘extensive taxon sampling’ (verbatim) of Kuntner et al. (2023) consisted of seven araneid species. At the time of publication of the study, the World Spider Catalog (ver. 24, Natural History Museum Bern, see http://wsc.nmbe.ch, accessed 6 June 2023) listed 189 genera and over 3100 species for the group. Naturally, given the small number of araneid terminals in the analysis, this sample lacks representatives of well recognised and well diagnosed subfamilies, such as oarcines, mastophorines or gasteracanthines, to name a few lineages. The phylogenomic analyses of Kallal et al. (2018, 2021) included transcriptomes of 18 and 21 non-nephilines araneids respectively and the UCE analysis of Kulkarni et al. (2021) included 10 non-nephiline araneids. How employing such a limited taxon sample can reasonably strive to ‘stabilize’ the topology of the large, diverse family Araneidae and how an analysis based on such a small sample size can be used to propose a new, satisfactory, stable classification is perplexing. Any conclusions about the intrafamilial relationships of araneids based on such a small, unbalanced taxon sample must be treated with extreme caution, as our results show (Fig. 8, 9, 10). For example, analysis of the combined dataset places oarcines (an araneid lineage that is not represented in Kuntner et al. 2023) as sister to all other araneids (Fig. 8), albeit with low support, resulting in a tree topology that would again render Araneidae paraphyletic if the classification of Kuntner et al. (2023) is followed, unless yet another new family is erected to accommodate Oarces and Gnolus. Scharff et al. (2020) found that Gnolus was nested in a clade with Caerostris and Testudinaria Taczanowski, 1879, an early split of the ARA Clade. Our analysis of the expanded molecular dataset of Scharff et al. (2020) places the recently described Australian araneid genus Venomius (Rossi et al. 2023) as sister group to the lineage formed by Paraplectanoides + Nephilinae (Fig. 10, Supplementary Fig. S1), therefore rendering Araneidae non-monophyletic (unless yet another new family is erected to accommodate the monotypic genus Venomius). The oarcines and Venomius illustrate, once more, the importance of taxon sampling not only for phylogenetic inference but also building robust and stable classifications. This also highlights how the addition of new araneid species, in the absence of a stable phylogenetic hypothesis, can potentially disrupt the monophyly of higher taxa and therefore require changes in classification. In the absence of such a robust hypothesis to provide a stable classification, circumscribing Araneidae and subfamilies thereof as in Kallal et al. (2020) offers a more stable solution to a problem that is yet to be satisfactorily resolved. Given the present state of knowledge of araneid relationships and how much diversity remains to be discovered and described (e.g. the Australasian fauna), splitting Araneidae into multiple small families is premature. Drawing any relevant conclusions from the comparison of support values of the trees in Kallal and Hormiga (2018), and the tree in Kuntner et al. (2023) because of the difference in the numbers of characters (Sanger sequences plus morphology versus UCEs) and taxa (80 versus 18 araneids) is difficult. Kallal et al. (2020) used the most informative hypothesis at the time to address the issues in question. The phylogenetic tree of Kuntner et al. (2023) does not diminish the subjectivity of the choice of Linnaean ranks.
Diagnosability of higher rank taxa
Although the diagnosability of taxa is important, the value does not reside in the rank or hierarchical position of the taxon being diagnosed, as Kuntner et al. (2023) suggest. In our view there is no reason to attribute differential value to diagnostic characters based on the rank of the taxon because ranks are arbitrary and subjective (e.g. see Giribet et al. 2016). For example, we believe that the diagnosis of a family rank taxon has the same importance as the diagnosis of subfamilies or superfamilies. Perhaps a more fruitful approach to understand the principles of Kuntner et al. (2023) may be to examine some of the diagnoses proposed. Phonognathinae and Nephilinae have been well characterised morphologically in earlier studies (e.g. Kallal and Hormiga 2018 and Kuntner et al. 2008). For the family Araneidae (as circumscribed in Dimitrov et al. 2017 and Kallal et al. 2021), Kuntner et al. (2019) created the rankless taxon name ‘Orbipurae’ (sometimes calling this ‘Araneidae sensu lato’). Although the family Araneidae might be difficult to diagnose given the diversity (e.g. Kallal and Hormiga 2018, p. 1075; Scharff et al. 2020, p. 9), taxonomists do not express significant difficulties, if any, in assigning taxa and new species to this family. For example, in 2022 arachnologists described 40 new species of araneids (in 13 genera) (World Spider Catalog, see http://wsc.nmbe.ch). Even if in most of these 40 cases the new species were placed in the family without an explicit phylogenetic analysis, the taxonomists did not report any difficulties in assigning these taxa to Araneidae. Kuntner et al. (2023) diagnosed ‘Orbipurae’ as having two- or three-dimensional webs with orb web elements (with numerous modifications or losses), the presence of a cheliceral chilum, book lung covers usually grooved, lateral eyes usually on tubercles and the male palpal median apophysis sharing a haematodocha with the embolic division. These are characters with numerous exceptions. For example, orb webs (both two- or three-dimensional) are found in several other araneoid families (e.g. Tetragnathidae or Anapidae), as are the lateral eyes on tubercles. Neither the sustentaculum nor the radix, both hypothesised to be araneid synapomorphies by Scharff and Coddington (1997) are discussed. A subclade of Araneidae labelled ‘Araneidae sensu stricto’ in Kuntner et al. (2023) (the newly recircumscribed Araneidae in the classification that is equivalent to the ‘ARA Clade’ of Scharff et al. 2020) is diagnosed by having two- or three-dimensional webs with orb web elements (the same diagnostic feature as in ‘Orbipurae’), the smooth (not striated) cheliceral boss (as in all non-nephiline spiders with a condyle), the squat shape of the male palpal tibia, the reasonably globular tegulum, the lateral eyes widely separated from the medians, and possibly the presence of an epigynal scape ‘or its homologs’. Again, most of these allegedly diagnostic characters are also found in other araneoid groups, for example the globular tegulum and separated lateral eyes are found in many tetragnathids. It is unclear what the ‘squat shape of the male palpal tibia’ is and no definition or reference is provided by these authors. The diagnosis of Paraplectanoididae is the same as that of the single species and, as explained above, but note that one of the diagnostic characters that the authors present for this monotypic genus, the smooth cheliceral boss, is erroneous, since there is no boss (see Hickman 1976). Given how highly autapomorphic P. crassipes is, we doubt that any arachnologist would have any difficulties diagnosing this apart from other araneids, irrespective of family placement. To claim that ‘The last comprehensive araneid classification was Simon (1895) – taxonomists have been amending it ever since’ (Kuntner et al. 2023) is misleading, particularly to those who are not familiar with the history of spider systematics, because this confounds the circumscription of the superfamily Araneoidea with that of the family Araneidae. Simon’s (1895, p. 593) concept of Araneidae, for which the name ‘Famille Argiopidae’ was used is similar to what is currently circumscribed as Araneoidea (a lineage of 17 families with more than 13 000 species described; Hormiga and Griswold 2014). Essentially, in Simon’s (1895) classification all araneoid families were grouped under the family name Argiopidae (see Scharff and Coddington 1997, table 1), except Theridiidae and Mimetidae that had unique families. However, Simon (1895) grouped the bulk of the genera currently classified in Araneidae (including Paraplectanoides but excluding nephilines that had a unique subfamilial group) under the argiopid subfamily Argiopinae (Scharff and Coddington 1997). Contrary to what Kuntner et al. (2023) imply, Simon’s (1895) Argiopinae (=Araneidae) did not include the modern families Cyatholipidae, Linyphiidae, Mimetidae, Nesticidae and Tetragnathidae. Naturally there are small discrepancies between the modern circumscriptions of families and those of 1895 (e.g. nesticids and cyatholipids were classified under the argiopoid subfamily Tetragnathinae) but when focusing on the overall scheme, Simon’s (1895) classification shows that more than a century and a quarter ago the family Araneidae was recognised as a group under the name Argiopinae, and was both large and diverse. Unsurprisingly, ever since that time, arachnologists have been able to diagnose the family Araneidae (e.g. Wiehle 1931) although the exact composition has changed with the increase in data and advances in systematic methodologies.
A convincing attempt to tackle the difficult problem of morphologically diagnosing Araneidae and dividing this into additional higher taxa (such as families or subfamilies) requires a systematic study across a wide range of species in the family to test existing diagnostic features such as the sustentaculum and to discover new features.
Information content
Kuntner et al. (2023) repeatedly state that their classification is superior to that of Kallal et al. (2020) on account of its higher information content, a claim that was also made in Kuntner et al. (2019). It is therefore important to understand what exactly is meant by ‘information content’ and how it is measured, so that competing classifications can be contrasted, and how one of them can be deemed to be more informative than the alternatives. What exactly is then ‘information content’? Although these authors do not define ‘information content’, they do cite Mickevich and Platnick’s (1989) classic paper, ‘On the information content of classifications’. Mickevich and Platnick (1989) reviewed several measures of agreement between different classifications based on different datasets or obtained by different analytical methods. They also examined how different cladogram topologies can convey different amounts of information for the same number of terminal taxa and how such information can be quantified. Mickevich and Platnick (1989, p. 46) proposed the product of two proportions as a measure of information: the maximal total information and the maximal number of prohibited Adams resolutions. We fail to see how the proposed information measure of Mickevich and Platnick (1989) relates to the arguments of Kuntner et al. (2023) given that the classification argument in question relates to how to assign ranks to an existing phylogenetic hypothesis and not contrasting alternative phylogenetic trees. Nevertheless, Mickevich and Platnick (1989, p. 34) present an explicit argument based on information content against erecting a new family for a single species: ‘groups including only one terminal taxon do not convey any information because they too are predefined by that initial problem’. In summary, in the absence of an explicit definition and measurement of information content, we find that the proposal of Kuntner et al. (2023) is unconvincing at best.
In assessing the value of classifying Araneidae as a single family (e.g. as in Scharff et al. 2020) or as multiple families (Kuntner et al. 2023), there are two additional aspects to consider that relate to the type of information that these different classification schemes convey and to the testability of monophyly. In a phylogenetic system, classifying Araneidae as a single family (including Nephilinae, Phonognathinae, etc.) implies a shared evolutionary history of the component subfamilies, a history not shared with any other spider families, with subsequent diversification. This type of information on common ancestry implied by the ranked categorical name is absent when the various araneid subfamilies are ranked as families (reference to the tree topology would be needed to obtain that information), unless the rankless name ‘Orbipurae’ (Kuntner et al. 2019) would be used for such a clade. Replacing the name ‘Araneidae’ (sensu Scharff et al. 2020) with ‘Orbipurae’ seems unnecessary and could lead to confusion, especially for non-systematists who are already familiar with the name Araneidae. Perhaps for this reason the name ‘Orbipurae’ yields only four results in Google Scholar, all of which are self-citations except for a paper critical of the multi-familial classification system of Araneidae.
Although use of the PhyloCode ‘node-based’ definitions had been promoted (e.g. Kuntner 2006 or Kuntner et al. 2019), Kuntner et al. (2023) currently use the PhyloCode ‘maximum-crown-clade’ concept to define families. For example, Kuntner et al. (2019, p. 563) recently defined Araneidae ‘as the least inclusive clade containing Araneus, Argiope, Caerostris, Cyclosa, Cyrtophora and Verrucosa’. Araneidae is later defined as ‘the most inclusive crown clade that contains the common ancestor of Araneus, but not of Phonognatha and not of Paraplectanoides and not of Nephila’ (Kuntner et al. 2023, p. 969). Knowing the reason why this change is needed would be helpful but the authors offer no explanation for why one type of definition should be preferred over the other, although the implications for classification seem substantial. For example, if new data were to robustly show that the nephiline lineage is the sister group of Argiope, this subfamily could not be ranked as a family if Araneidae were to be circumscribed as a monophyletic group using the node-based definition. In contrast, if the maximum-crown-clade definition is used, the family rank for nephilines would be possible, but only at the cost of a greatly reduced in content the family Araneidae and of incurring in the likely need of new families for those terminals that fall outside the now reduced Araneidae. In a more concrete empirical case, under the new definition of Araneidae, based on the phylogenetic tree illustrated in Fig. 10 and Supplementary Fig. S1, the recently described araneid V. tomhardy would require yet another new family to accommodate this single species (notably, Rossi et al. (2023) expressed no doubts about Venomius membership in Araneidae when describing this new species). However non-monophyly of Araneidae would not be problematic were the Kuntner et al. (2023) family definition followed, because the genus Venomius would not be a member of the family and therefore Araneidae would remain monophyletic despite this discordant placement. Such a family definition questions whether there is a phylogenetic tree topology that could possibly falsify Araneidae monophyly if the family is defined as in Kuntner et al. (2023). This would not be the case because any araneid species or lineage that could possibly render the family non-monophyletic by nature of phylogenetic placement, similarly to the oarcines (Fig. 9) or Venomius (Fig. 10), would automatically be deemed not to be an araneid under the maximum-crown-clade family definition. Monophyly of the families as defined by Kuntner et al. (2023) is not open to refutation as this cannot be falsified. The family definitions of Kuntner et al. (2023) are offered to promote stability but this is achieved by stabilising the spelling of the name irrespective of the species content of the named group.
Utility of higher rank names
Kuntner et al. (2023) repeatedly praise their classification proposal based on the higher ‘utility.’ Implicit in the assessment is the premise that the family rank is more ‘useful’ than the subfamily rank. The assumption of this premise is not warranted. The World Spider Catalog (see http://wsc.nmbe.ch) is invoked as an example of the importance, in the view of the authors, of receiving family rank. Whether treated as a nephiline or nephilid makes no difference in terms of finding the relevant taxonomic publications on, for example, Nephila cornuta (Pallas, 1772); the catalog is equally useful regardless of the rank of nephilines. Notably, the authors do not follow the World Spider Catalog’s family ranks in publications (e.g. Turk et al. 2020). For example, in Agnarsson et al. (2023), the same authors state that ‘The circumscription of the family Araneidae follows Kuntner et al. (2019) and not the World Spider Catalogue [sic.]’. As another example of the utility of the family rank, Kuntner et al. (2023) state that ‘Nephilidae is the only spider family for which all species have been scored for IUCN threat status (https://www.iucnredlist.org/) and retaining this as a family adds value to conservation biology’. The reason for a family rank being more valuable than a subfamily rank to a conservation biologist remains to be explained but as in the case of the World Spider Catalog, species conservation status data are available regardless of the familial assignment. Incidentally, not all valid species names of nephilines (namely, those provided in the World Spider Catalog, see http://wsc.nmbe.ch) are provided on the IUCN website. As noted by Kallal et al. (2020), several valid nephiline species are ‘deemed’ to be junior synonyms but in the absence of a modern published revision on Nephila and formal synonymies, these names remain valid in the sense of the International Commission on Zoological Nomenclature (1999). The importance of the ‘utility’ of family ranks is not only entirely subjective but seems to emanate directly from the particular views on classification: ‘the classical supergeneric ranks (family, class, order, phylum, kingdom) should also be retained, but intermediate ranks should not’ (Kuntner and Agnarsson 2006, p. 682) (see also a critique of their ‘combination approach’ to classification in Dimitrov et al. 2009, pp. 308–309). Logically, family rank is deemed to be very useful if the assumption is made a priori that subfamilies should not be used and that families should be retained but this argument is somewhat tautological.
Logical considerations
Kallal et al. (2020) did not commit a modal scope fallacy when classifying Paraplectanoides as an araneid and did not assume or argue that this genus ‘will always be an araneid’ because ‘it is currently catalogued as an araneid’, as Kuntner et al. (2023, p. 967) claim. All previous authors have treated P. crassipes as an araneid (e.g. Simon 1895, Hickman 1976, Davies 1988 and Framenau et al. 2014) and therefore this family placement was an empirically based starting point for the study of Kallal et al. (2020), not a modal scope fallacy. Furthermore, if the results of our phylogenetic analyses had placed Paraplectanoides as sister group to the genus Tetragnatha Latreille, 1804, for example, the genus would have been classified as a member of Tetragnathidae, irrespective of any other prior familial assignments.
In Ernst Mayr’s Evolutionary Taxonomy (e.g. Mayr 1974), the nephilines could be recognised as a family while accepting the paraphyly of Araneidae, using the same arguments that had been used to deny Aves being dinosaurs. To rank nephilines as a family required the elevation of several groups to family rank, some of which were monotypic. This is unnecessary from a phylogenetic system perspective and not informative from an ‘information content’ point of view, and rank changes could have no end. For example, the nephiline genus Herennia is highly autapomorphic and one could remove this from the ‘Nephilidae’, splitting the family into multiple families: Nephilidae s. str. and Herenniidae, and this would necessitate adding Nephilengyidae, Nephilingidae, Clitaetridae and Trichonephilidae. This hypothetical classification would be possible and entirely consistent with the principles exposed in the proposal of Kuntner et al. (2023) but this approach of excessive splitting to favour a taxonomic rank would be an unending and meritless exercise, and would destabilise spider classification.
Conclusions
We have added new morphological data on P. crassipes, a species that despite being highly autapomorphic in many biological aspects, has genitalic features consistent with other members of the family Araneidae. Our phylogenetic analyses corroborate Nephilinae as the sister group of Paraplectanoides and provide a cladistic context to classify araneids as in the studies of Dimitrov et al. (2017), Scharff et al. (2020) and Kallal et al. (2020). In that sense, the family name Araneidae conveys the shared evolutionary trajectory of the component subfamilies prior to the remarkable diversification of the group and this information is lost when Araneidae is split into multiple families. Until a robust, stable higher-level classification of Araneidae is reached and for the sake of nomenclatural stability, we cannot adopt the recent classification of Kuntner et al. (2023). Given the sensitivity of the tree topology to the addition taxa, as empirically shown by the placement of Oarcinae and Venomius, dividing Araneidae into families is not only unnecessary but also premature, and very likely to lead to additional nomenclatural instability and the creation of new families simply to accommodate species that do not fit the authors’ definition of Araneidae. Although ongoing discussions once again demonstrate the arbitrary and subjective nature of the assignment of Linnaean ranks to monophyletic groups, not all possible phylogenetic classifications are equally desirable. We believe that the allegedly objective criteria used by Kuntner et al. (2023) to prefer a particular set of rank labels fall short of eclipsing the views of Kallal et al. (2020).
Data availability
All new molecular data that were used in this study have been deposited in GenBank and are publicly available.
Conflicts of interest
Gonzalo Giribet is the Editor-in-Chief of Invertebrate Systematics but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Invertebrate Systematics encourages editors to publish in the journal and they are kept totally separate from the decision-making processes for their manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
D. Dimitrov received support from the Norwegian Metacenter for Computational Science (NOTUR; project NN9601K). G. Hormiga acknowledges support from the US National Science Foundation (grants DEB 1754289, 2154246). G. Hormiga’s work at the University of Copenhagen (Natural History Museum of Denmark) was supported by a scholarship from Danmarks Nationalbank.
Acknowledgements
We are very grateful to the following individuals and institutions for making specimens available for study: Robert J. Raven, Michael G. Rix and Owen Seeman (Queensland Museum, Brisbane), Mark S. Harvey and Julianne Waldock (Western Australia Museum, Perth), Graham Milledge (Australian Museum, Sydney), and Catherine Byrne and Kirrily Moore (Tasmanian Museum and Art Gallery, Hobart). We thank Nadine Dupérré (Museum of Nature Hamburg, Zoology) for taking outstanding images of the syntypes of P. crassipes and allowing us to use some of these images in this publication. We also thank Zoë Simmons (Oxford University Museum of Natural History) for access to the type material of Paraplectana kochii O. Pickard-Cambridge, 1877 and additional material in the O. Pickard-Cambridge spider collection. Jesús Ballesteros Chavez, Charles Griswold, Nicolás Hazzi and Jimmy Cabra-García provided insightful comments on an earlier version of this paper. We are grateful to Associate Editor Andy Austin and two anonymous reviewers for comments on this work.
References
Agnarsson I, Starrett J, Babbitz Z, Bond JE, Gregorič M, Christian Raberahona O, Williams S, Kuntner M (2023) Discovery and genetic characterization of single cohort adult colonies with male aggregations, and preliminary evidence for lekking in a malagasy kite spider (Isoxya, Gasteracanthinae). Insect Systematics and Diversity 7, 3.
| Crossref | Google Scholar |
Álvarez-Padilla F, Hormiga G (2007) A protocol for digesting internal soft tissues and mounting spiders for scanning electron microscopy. Journal of Arachnology 35, 538-542.
| Crossref | Google Scholar |
Avise JC, Liu J-X (2011) On the temporal inconsistencies of Linnean taxonomic ranks: temporal inconsistencies. Biological Journal of the Linnean Society 102, 707-714.
| Crossref | Google Scholar |
Avise JC, Mitchell D (2007) Time to standardize taxonomies. Systematic Biology 56, 130-133.
| Crossref | Google Scholar | PubMed |
Benoit PLG (1962) Les Araneidae-Nephilinae africains. Revue de Zoologie et de Botanique Africaines 65, 217-231 [In French].
| Google Scholar |
Cabra-García J, Hormiga G (2020) Exploring the impact of morphology, multiple sequence alignment and choice of optimality criteria in phylogenetic inference: a case study with the Neotropical orb-weaving spider genus Wagneriana (Araneae: Araneidae). Zoological Journal of the Linnean Society 188, 976-1151.
| Crossref | Google Scholar |
Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972-1973.
| Crossref | Google Scholar | PubMed |
Coddington JA (1989) Spinneret silk spigot morphology: evidence for the monophyly of orbweaving spiders, Cyrtophorinae (Araneidae), and the group Theridiidae plus Nesticidae. Journal of Arachnology 17, 71-95.
| Google Scholar |
Comstock JH (1910) The palpi of male spiders. Annals of the Entomological Society of America 3, 161-185.
| Crossref | Google Scholar |
Davies VT (1988) An illustrated guide to the genera of orb-weaving spiders in Australia. Memoirs of the Queensland Museum 25, 273-332.
| Google Scholar |
Dimitrov D, Benjamin SP, Hormiga G (2009) A revised phylogenetic analysis for the spider genus Clitaetra Simon, 1889 (Araneae, Araneoidea, Nephilidae) with the first description of the male of the Sri Lankan species Clitaetra thisbe Simon, 1903. Bulletin of the Museum of Comparative Zoology 159, 301-323.
| Crossref | Google Scholar |
Dimitrov D, Benavides LR, Arnedo MA, Giribet G, Griswold CE, Scharff N, Hormiga G (2017) Rounding up the usual suspects: a standard target‐gene approach for resolving the interfamilial phylogenetic relationships of ecribellate orb‐weaving spiders with a new family‐rank classification (Araneae, Araneoidea). Cladistics 33, 221-250.
| Crossref | Google Scholar | PubMed |
Emerit E (1982) Collections européennes peu connues de gastéracanthes d’Afrique et de Madagascar (Araneidae, Gasteracanthinae). Bulletin du Muséum National d’Histoire Naturelle de Paris 4(4(A)), 153-164 [In French].
| Google Scholar |
Fernández R, Kallal RJ, Dimitrov D, Ballesteros JA, Arnedo MA, Giribet G, Hormiga G (2018) Phylogenomics, diversification dynamics, and comparative transcriptomics across the Spider Tree of Life. Current Biology 28, 1489-1497.e5.
| Crossref | Google Scholar | PubMed |
Framenau VW, Scharff N, Harvey MS (2010) Systematics of the Australian orb-weaving spider genus Demadiana with comments on the generic classification of the Arkyinae (Araneae: Araneidae). Invertebrate Systematics 24(2), 139-171.
| Crossref | Google Scholar |
Giribet G, Hormiga G, Edgecombe GD (2016) The meaning of categorical ranks in evolutionary biology. Organisms Diversity & Evolution 16, 427-430.
| Crossref | Google Scholar |
Gregorič M, Agnarsson I, Blackledge TA, Kuntner M (2015) Phylogenetic position and composition of Zygiellinae and Caerostris, with new insight into orb-web evolution and gigantism: orb web evolution and gigantism. Zoological Journal of the Linnean Society 175, 225-243.
| Crossref | Google Scholar |
Griswold CE, Audisio T, Ledford JM (2012) An extraordinary new family of spiders from caves in the Pacific Northwest (Araneae, Trogloraptoridae, new family). ZooKeys 215, 77-102.
| Crossref | Google Scholar | PubMed |
Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59, 307-321.
| Crossref | Google Scholar | PubMed |
Hickman VV (1976) On Paraplectanoides crassipes Keyserling (Araneae: Araneidae). Bulletin of the British Arachnological Society 3, 166-174.
| Google Scholar |
Hoang DT, Chernomor O, Von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35, 518-522.
| Crossref | Google Scholar | PubMed |
Holm Å (1979) A taxonomic study of European and east African species of the genera Pelecopsis and Trichopterna (Araneae, Linyphiidae), with descriptions of a new genus and two new species of Pelecopsis from Kenya. Zoologica Scripta 8, 255-278.
| Crossref | Google Scholar |
Hormiga G, Griswold CE (2014) Systematics, phylogeny, and evolution of orb-weaving spiders. Annual Review of Entomology 59, 487-512.
| Crossref | Google Scholar | PubMed |
Hormiga G, Eberhard W, Coddington J (1995) Web-construction behavior in Australian Phonognatha and the phylogeny of nephiline and tetragnathid spiders (Araneae, Tetragnathidae). Australian Journal of Zoology 43, 313-364.
| Crossref | Google Scholar |
Kallal RJ, Hormiga G (2018) Systematics, phylogeny and biogeography of the Australasian leaf-curling orb-weaving spiders (Araneae: Araneidae: Zygiellinae), with a comparative analysis of retreat evolution. Zoological Journal of the Linnean Society 184, 1055-1141.
| Crossref | Google Scholar |
Kallal RJ, Hormiga G (2019) Evolution of the male palp morphology of the orb-weaver hunting spider Chorizopes (Araneae : Araneidae) revisited on a new phylogeny of Araneidae, and description of a third species from Madagascar. Invertebrate Systematics 33, 473-487.
| Crossref | Google Scholar |
Kallal RJ, Fernández R, Giribet G, Hormiga G (2018) A phylotranscriptomic backbone of the orb-weaving spider family Araneidae (Arachnida, Araneae) supported by multiple methodological approaches. Molecular Phylogenetics and Evolution 126, 129-140.
| Crossref | Google Scholar | PubMed |
Kallal RJ, Dimitrov D, Arnedo MA, Giribet G, Hormiga G (2020) Monophyly, taxon sampling, and the nature of ranks in the classification of orb-weaving spiders (Araneae: Araneoidea). Systematic Biology 69, 401-411.
| Crossref | Google Scholar | PubMed |
Kallal RJ, Kulkarni SS, Dimitrov D, Benavides LR, Arnedo MA, Giribet G, Hormiga G (2021) Converging on the orb: denser taxon sampling elucidates spider phylogeny and new analytical methods support repeated evolution of the orb web. Cladistics 37, 298-316.
| Crossref | Google Scholar | PubMed |
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14, 587-589.
| Crossref | Google Scholar | PubMed |
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30(4), 772-780.
| Crossref | Google Scholar |
Keyserling E (1886) ‘Die Arachniden Australiens, nach der Natur beschrieben und abgebildet’, Zweiter Theil [Lieferung 33-34]. pp. 87–152, pl. 7–12. (Bauer & Raspe: Nürnberg, German Empire) [In German] 10.5962/bhl.title.121660
Kulkarni S, Wood H, Lloyd M, Hormiga G (2020) Spider‐specific probe set for ultraconserved elements offers new perspectives on the evolutionary history of spiders (Arachnida, Araneae). Molecular Ecology Resources 20, 185-203.
| Crossref | Google Scholar | PubMed |
Kulkarni S, Kallal RJ, Wood H, Dimitrov D, Giribet G, Hormiga G (2021) Interrogating genomic-scale data to resolve recalcitrant nodes in the Spider Tree of Life. Molecular Biology and Evolution 38, 891-903.
| Crossref | Google Scholar | PubMed |
Kulkarni S, Wood HM, Hormiga G (2023) Advances in the reconstruction of the Spider Tree of Life: a roadmap for spider systematics and comparative studies. Cladistics 39(6), 479-532.
| Crossref | Google Scholar | PubMed |
Kuntner M (2006) Phylogenetic systematics of the Gondwanan nephilid spider lineage Clitaetrinae (Araneae, Nephilidae). Zoologica Scripta 35, 19-62.
| Crossref | Google Scholar |
Kuntner M, Agnarsson I (2006) Are the Linnean and phylogenetic nomenclatural systems combinable? Recommendations for biological nomenclature. Systematic Biology 55(5), 774-784.
| Google Scholar |
Kuntner M, Coddington JA, Hormiga G (2008) Phylogeny of extant nephilid orb-weaving spiders (Araneae, Nephilidae): testing morphological and ethological homologies. Cladistics 24, 147-217.
| Crossref | Google Scholar |
Kuntner M, Hamilton CA, Cheng R-C, Gregorič M, Lupše N, Lokovšek T, Lemmon EM, Lemmon AR, Agnarsson I, Coddington JA, Bond JE (2019) Golden orbweavers ignore biological rules: phylogenomic and comparative analyses unravel a complex evolution of sexual size dimorphism. Systematic Biology 68, 555-572.
| Crossref | Google Scholar | PubMed |
Kuntner M, Čandek K, Gregorič M, Turk E, Hamilton CA, Chamberland L, Starrett J, Cheng R-C, Coddington JA, Agnarsson I, Bond JE (2023) Increasing information content and diagnosability in family-level classifications. Systematic Biology 72, 964-971.
| Crossref | Google Scholar | PubMed |
Levi HW (1986) The orb-weaver genus Witica (Araneae: Araneidae). Psyche: a Journal of Entomology 93, 35-46.
| Crossref | Google Scholar |
Mayr E (1974) Cladistic analysis or cladistic classification? Zeitschrift fűr Zoologische Systematik und Evolutionforschung 12(1), 94-128.
| Google Scholar |
Michalik P, Kallal R, Dederichs TM, Labarque FM, Hormiga G, Giribet G, Ramírez MJ (2019) Phylogenomics and genital morphology of cave raptor spiders (Araneae, Trogloraptoridae) reveal an independent origin of a flow‐through female genital system. Journal of Zoological Systematics and Evolutionary Research 57, 737-747.
| Crossref | Google Scholar |
Mickevich MF, Platnick NI (1989) On the information content of classifications. Cladistics 5, 33-47.
| Crossref | Google Scholar | PubMed |
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, Von Haeseler A, Lanfear R (2020) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37, 1530-1534.
| Crossref | Google Scholar | PubMed |
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32, 268-274.
| Crossref | Google Scholar | PubMed |
Pickard-Cambridge O. (1877) II.—On some new genera and species of Araneidea. Annals and Magazine of Natural History 19(109), 26-39.
| Crossref | Google Scholar |
Ramírez MJ, Grismado CJ, Ubick D, Ovtsharenko V, Cushing PE, Platnick NI, Wheeler WC, Prendini L, Crowley LM, Horner NV (2019) Myrmecicultoridae, a new family of myrmecophilic spiders from the Chihuahuan Desert (Araneae: Entelegynae). American Museum Novitates 2019, 1-24.
| Crossref | Google Scholar |
Ranwez V, Harispe S, Delsuc F, Douzery EJP (2011) MACSE: multiple alignment of coding sequences accounting for frameshifts and stop codons. PLoS One 6, e22594.
| Crossref | Google Scholar | PubMed |
Rauhut OWM, Heyng AM, López-Arbarello A, Hecker A (2012) A new rhynchocephalian from the Late Jurassic of Germany with a dentition that is unique amongst tetrapods. PLoS One 7, e46839.
| Crossref | Google Scholar | PubMed |
Rossi GF, Castanheira PS, Baptista RLC, Framenau VW (2023) Venomius, a new monotypic genus of Australian orb-weaving spiders (Araneae, Araneidae). Evolutionary Systematics 7, 285-292.
| Crossref | Google Scholar |
Scharff N, Coddington JA (1997) A phylogenetic analysis of the orb-weaving spider family Araneidae (Arachnida, Araneae). Zoological Journal of the Linnean Society 120, 355-434.
| Crossref | Google Scholar |
Scharff N, Coddington JA, Blackledge TA, Agnarsson I, Framenau VW, Szűts T, Hayashi CY, Dimitrov D (2020) Phylogeny of the orb‐weaving spider family Araneidae (Araneae: Araneoidea). Cladistics 36, 1-21.
| Crossref | Google Scholar | PubMed |
Simon E (1887) Etudes arachnologiques. 19e Mémoire. XXVII. Arachnides recueillis à Assinie (Afrique occidentale) par MM. Chaper et Alluaud. Annales de la Société Entomologique de France 6(7), 261-276 [In French].
| Google Scholar |
Simon E (1907) Arachnides recueillis par L. Fea sur la côte occidentale d’Afrique. 1re partie. Annali del Museo Civico di Storia Naturale di Genova 43, 218-323 [In French].
| Google Scholar |
Simpson GG (1937) Supra-specific variation in nature and in classification from the view-point of paleontology. The American Naturalist 71, 236-267.
| Crossref | Google Scholar |
Simpson GG (1945) The principles of classification and a classification of mammals. Bulletin of the American Museum of Natural History 85, 1-350.
| Google Scholar |
Thorell T (1899) Araneae Camerunenses (Africae occidentalis) quas anno 1891 collegerunt Cel. Dr Y. Sjöstedt aliique. Bihang till Kongliga Svenska Vetenskaps-Akademiens Handlingar 25(IV, 1), 1-105 [In Latin].
| Google Scholar |
Turk E, Čandek K, Kralj‐Fišer S, Kuntner M (2020) Biogeographical history of golden orbweavers: chronology of a global conquest. Journal of Biogeography 47, 1333-1344.
| Crossref | Google Scholar |
Wheeler WC, Coddington JA, Crowley LM, Dimitrov D, Goloboff PA, Griswold CE, Hormiga G, Prendini L, Ramírez MJ, Sierwald P, Almeida-Silva L, Alvarez-Padilla F, Arnedo MA, Benavides Silva LR, Benjamin SP, Bond JE, Grismado CJ, Hasan E, Hedin M, Izquierdo MA, Labarque FM, Ledford J, Lopardo L, Maddison WP, Miller JA, Piacentini LN, Platnick NI, Polotow D, Silva-Dávila D, Scharff N, Szűts T, Ubick D, Vink CJ, Wood HM, Zhang J (2017) The spider tree of life: phylogeny of Araneae based on target-gene analyses from an extensive taxon sampling. Cladistics 33(6), 574-616.
| Crossref | Google Scholar | PubMed |
Wiehle H (1931) Spinnentiere oder Arachnoidea. 27. Familie. Araneidae. Die Tierwelt Deutschlands 23, 47-136 [In German].
| Google Scholar |
Wunderlich J (2004) The fossil spiders (Araneae) of the families Tetragnathidae and Zygiellidae n. stat. in Baltic and Dominican amber, with notes on higher extant and fossil taxa. Beiträge zur Araneologie 3, 899-955.
| Google Scholar |
Zhou Z-Y (2009) An overview of fossil Ginkgoales. Palaeoworld 18, 1-22.
| Crossref | Google Scholar |


